Experimental Verification for Numerical Simulation of Thalamic Stimulation-Evoked Calcium-Sensitive Fluorescence and Electrophysiology with Self-Assembled Multifunctional Optrode
Abstract
1. Introduction
2. Materials and Methods
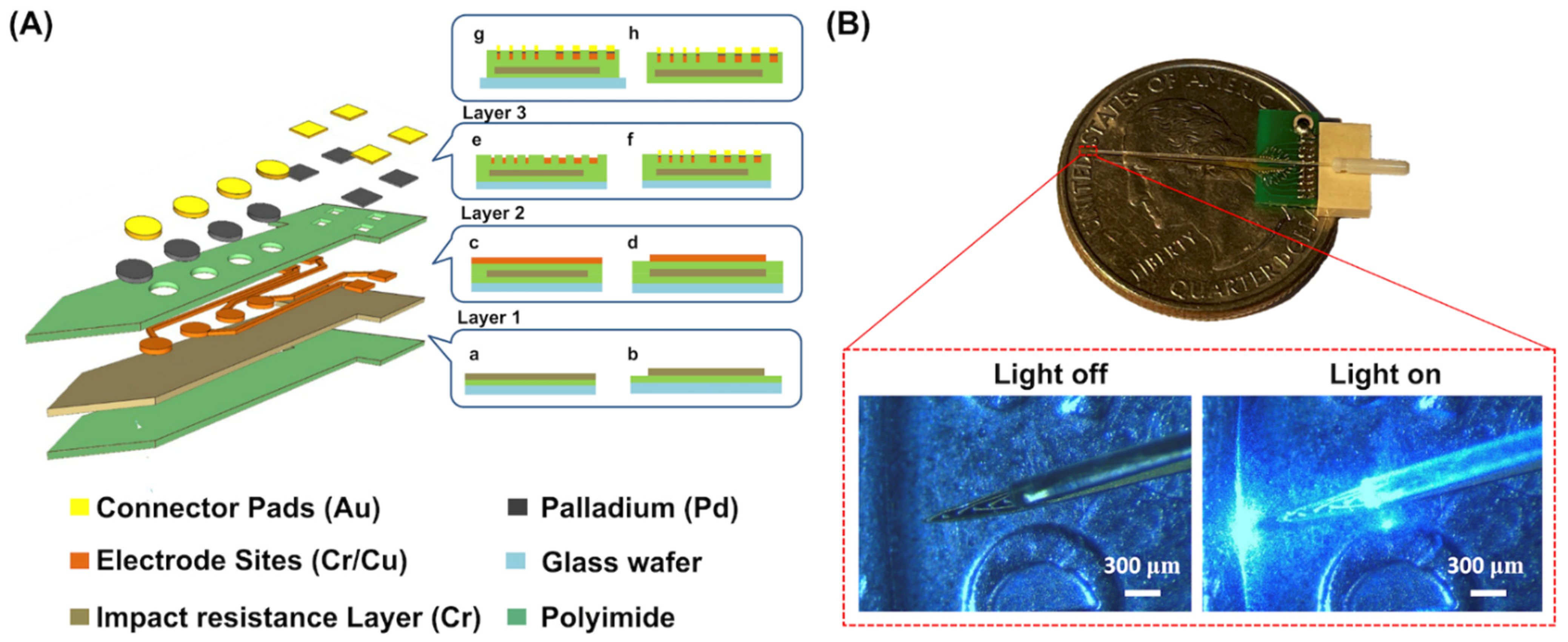
2.1. Fabrication and Design of an Optrode
2.2. Computational Modeling of the VTA in Thalamic DBS
2.3. Simulation of the Thalamic-DBS-Evoked Calcium Signal
2.4. Setup of Fiber Photometry System
2.5. Animal Preparation and Surgery
2.6. Thalamic-DBS-Induced Neuronal Activity Recording: Ca2+ Fluorescence Signals and Electrophysiology Recordings
2.7. Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Estimation of VTA Volume
3.2. Estimation of Simulated Ca2+ Fluorescence Intensity
3.3. Acute Ca2+ Fluorescence and LFP Recordings under In Vivo Thalamic Stimulation
3.4. Linear Relationship among VTA Volume, Simulated Ca2+ Fluorescence Intensity, Ca2+ Fluorescence Intensity In Vivo, and ∑LFP
4. Discussion
4.1. The Advance of Self-Assembled Optrode
4.2. The Correlation between Simulated Results and In Vivo Experiments
4.3. Comparison of the Ca2+ Photometry and Electrophysiology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormick, D.A.; Wang, Z.; Huguenard, J. Neurotransmitter Control of Neocortical Neuronal Activity and Excitability. Cereb. Cortex 1993, 3, 387–398. [Google Scholar] [CrossRef]
- Brady, S.T.; Lasek, R.J.; Allen, R.D. Fast Axonal Transport in Extruded Axoplasm from Squid Giant Axon. Science 1982, 218, 1129–1131. [Google Scholar] [CrossRef]
- Köfalvi, A.; Rodrigues, R.J.; Ledent, C.; Mackie, K.; Vizi, E.S.; Cunha, R.A.; Sperlágh, B. Involvement of Cannabinoid Receptors in the Regulation of Neurotransmitter Release in the Rodent Striatum: A Combined Immunochemical and Pharmacological Analysis. J. Neurosci. 2005, 25, 2874–2884. [Google Scholar] [CrossRef]
- Calia, A.B.; Masvidal-Codina, E.; Smith, T.M.; Schäfer, N.; Rathore, D.; Rodríguez-Lucas, E.; Illa, X.; De la Cruz, J.M.; Del Corro, E.; Prats-Alfonso, E.; et al. Full-bandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nat. Nanotechnol. 2022, 17, 301–309. [Google Scholar]
- Sassenhagen, J. How to analyse electrophysiological responses to naturalistic language with time-resolved multiple regression. Lang. Cogn. Neurosci. 2019, 34, 474–490. [Google Scholar] [CrossRef]
- Yin, P.; Liu, Y.; Xiao, L.; Zhang, C. Advanced Metallic and Polymeric Coatings for Neural Interfacing: Structures, Properties and Tissue Responses. Polymers 2021, 13, 2834. [Google Scholar] [CrossRef] [PubMed]
- Woeppel, K.M.; Cui, X.T. Nanoparticle and Biomolecule Surface Modification Synergistically Increases Neural Electrode Recording Yield and Minimizes Inflammatory Host Response. Adv. Healthc. Mater. 2021, 10, e2002150. [Google Scholar] [CrossRef]
- Buijink, A.; Piña-Fuentes, D.; Stam, M.; Bot, M.; Schuurman, P.; Munckhof, P.V.D.; van Rootselaar, A.; de Bie, R.; Beudel, M. Thalamic local field potentials recorded using the deep brain stimulation pulse generator. Clin. Neurophysiol. Pract. 2022, 7, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Sheng, H.; Zhu, L.; Zhu, J.; Zhang, H.; Liu, Y.; Zhan, L.; Wang, X.; Zhang, J.; et al. Subdural neural interfaces for long-term electrical recording, optical microscopy and magnetic resonance imaging. Biomaterials 2022, 281, 121352. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, C.; Shao, Y.; Zhou, Z.; Hou, Y.; Li, A. Partial depletion of dopaminergic neurons in the substantia nigra impairs olfaction and alters neural activity in the olfactory bulb. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Tan, L.L.; Oswald, M.J.; Heinl, C.; Romero, O.A.R.; Kaushalya, S.K.; Monyer, H.; Kuner, R. Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Constandinou, T.G. Adaptive spike detection and hardware optimization towards autonomous, high-channel-count BMIs. J. Neurosci. Methods 2021, 354, 109103. [Google Scholar] [CrossRef]
- Cummins, D.D.; Kochanski, R.B.; Gilron, R.; Swann, N.C.; Little, S.; Hammer, L.H.; Starr, P.A. Chronic Sensing of Subthalamic Local Field Potentials: Comparison of First and Second Generation Implantable Bidirectional Systems Within a Single Subject. Front. Neurosci. 2021, 15, 725797. [Google Scholar] [CrossRef]
- Kluger, D.S.; Balestrieri, E.; A Busch, N.; Gross, J. Respiration aligns perception with neural excitability. Elife 2021, 10, e70907. [Google Scholar] [CrossRef] [PubMed]
- Esghaei, M.; Daliri, M.R.; Treue, S. Attention decouples action potentials from the phase of local field potentials in macaque visual cortical area MT. BMC Biol. 2018, 16, 86. [Google Scholar] [CrossRef]
- Xu, G.; Wang, N.; Guo, M.; Zhang, T.; Tong, Y. Analysis of time-frequency characteristics and coherence of local field potentials during working memory task of rats after high-frequency repeated transcranial magnetic stimulation. J. Biomed. Eng. 2020, 37, 756–764. [Google Scholar]
- Eslami, M.; Sadeghi, B.; Goshadrou, F. Chronic ghrelin administration restores hippocampal long-term potentiation and ameliorates memory impairment in rat model of Alzheimer’s disease. Hippocampus 2018, 28, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Smart, O.; Choi, K.S.; Riva-Posse, P.; Tiruvadi, V.; Rajendra, J.; Waters, A.C.; Crowell, A.L.; Edwards, J.; Gross, R.E.; Mayberg, H.S. Initial Unilateral Exposure to Deep Brain Stimulation in Treatment-Resistant Depression Patients Alters Spectral Power in the Subcallosal Cingulate. Front. Comput. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef]
- Telkes, I.; Viswanathan, A.; Jimenez-Shahed, J.; Abosch, A.; Ozturk, M.; Gupte, A.; Jankovic, J.; Ince, N.F. Local field potentials of subthalamic nucleus contain electrophysiological footprints of motor subtypes of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E8567–E8576. [Google Scholar] [CrossRef]
- Lipstein, N.; Chang, S.; Lin, K.-H.; López-Murcia, F.J.; Neher, E.; Taschenberger, H.; Brose, N. Munc13-1 is a Ca2+-phospholipid-dependent vesicle priming hub that shapes synaptic short-term plasticity and enables sustained neurotransmission. Neuron 2021, 109, 3980–4000.e7. [Google Scholar] [CrossRef]
- Hashimoto, T.; Elder, C.M.; Vitek, J.L. A template subtraction method for stimulus artifact removal in high-frequency deep brain stimulation. J. Neurosci. Methods 2001, 113, 181–186. [Google Scholar] [CrossRef]
- Shimomura, O.; Musicki, B.; Kishi, Y.; Inouye, S. Light-emitting properties of recombinant semisynthetic aequorins and recombinant fluorescein-conjugated aequorin for measuring cellular calcium. Cell Calcium 1993, 14, 373–378. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, Y.; Wu, D.-Y.; Liu, J.-H.; Jie, W.; You, Q.-L.; Huang, L.; Hu, J.; Chu, H.-D.; Gao, F.; et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat. Commun. 2021, 12, 3321. [Google Scholar] [CrossRef]
- Bancroft, E.A.; Srinivasan, R. Emerging Roles for Aberrant Astrocytic Calcium Signals in Parkinson’s Disease. Front. Physiol. 2021, 12, 812212. [Google Scholar] [CrossRef]
- Cho, A.-N.; Bright, F.; Morey, N.; Au, C.; Ittner, L.M.; Ke, Y.D. Efficient Gene Expression in Human Stem Cell Derived-Cortical Organoids Using Adeno Associated Virus. Cells 2022, 11, 3194. [Google Scholar] [CrossRef]
- Kaszas, A.; Szalay, G.; Slézia, A.; Bojdán, A.; Vanzetta, I.; Hangya, B.; Rózsa, B.; O’Connor, R.; Moreau, D. Two-photon GCaMP6f imaging of infrared neural stimulation evoked calcium signals in mouse cortical neurons in vivo. Sci. Rep. 2021, 11, 9775. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gu, L.; Mohanty, S.K.; Chiao, J.-C. An Integrated μLED Optrode for Optogenetic Stimulation and Electrical Recording. IEEE Trans. Biomed. Eng. 2013, 60, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gu, X.-W.; Ji, B.-W.; Wang, L.-C.; Guo, Z.-J.; Yang, B.; Wang, X.-L.; Li, C.-Y.; Liu, J.-Q. Three-dimensional drivable optrode array for high-resolution neural stimulations and recordings in multiple brain regions. Biosens. Bioelectron. 2019, 131, 9–16. [Google Scholar] [CrossRef]
- Wu, F.; Stark, E.; Im, M.; Cho, I.-J.; Yoon, E.-S.; Buzsáki, G.; Wise, K.D.; Yoon, E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. J. Neural Eng. 2013, 10, 056012. [Google Scholar] [CrossRef]
- Huby, N.; Vié, V.; Renault, A.; Beaufils, S.; Lefèvre, T.; Paquet-Mercier, F.; Pézolet, M.; Bêche, B. Native spider silk as a biological optical fiber. Appl. Phys. Lett. 2013, 102, 123702. [Google Scholar] [CrossRef]
- Vázquez, G.; Valiente, R.; Gómez-Salces, S.; Flores-Romero, E.; Rickards, J.; Trejo-Luna, R. Carbon implanted waveguides in soda lime glass doped with Yb 3+ and Er 3+ for visible light emission. Opt. Laser Technol. 2016, 79, 132–136. [Google Scholar] [CrossRef]
- Son, Y.; Lee, H.J.; Kim, J.; Shin, H.; Choi, N.; Lee, C.J.; Yoon, E.-S.; Yoon, E.; Wise, K.D.; Kim, T.G.; et al. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays. Sci. Rep. 2015, 5, srep15466. [Google Scholar] [CrossRef]
- LeChasseur, Y.; Dufour, S.; Lavertu, G.; Bories, C.; Deschênes, M.; Vallée, R.; De Koninck, Y. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat. Methods 2011, 8, 319–325. [Google Scholar] [CrossRef]
- Grossman, N.; Poher, V.; Grubb, M.S.; Kennedy, G.T.; Nikolic, K.; McGovern, B.; Palmini, R.B.; Gong, Z.; Drakakis, E.M.; A A Neil, M.; et al. Multi-site optical excitation using ChR2 and micro-LED array. J. Neural Eng. 2010, 7, 16004. [Google Scholar] [CrossRef]
- McAlinden, N.; Massoubre, D.; Richardson, E.; Gu, E.; Sakata, S.; Dawson, M.D.; Mathieson, K. Thermal and optical characterization of micro-LED probes for in vivo optogenetic neural stimulation. Opt. Lett. 2013, 38, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Kwok, S.J.; Choi, M.; Yetisen, A.K.; Cho, S.; Yun, S.-H. Toward biomaterial-based implantable photonic devices. Nanophotonics 2017, 6, 414–434. [Google Scholar] [CrossRef]
- Ji, B.; Guo, Z.; Wang, M.; Yang, B.; Wang, X.; Li, W.; Liu, J. Flexible polyimide-based hybrid opto-electric neural interface with 16 channels of micro-LEDs and electrodes. Microsystems Nanoeng. 2018, 4, 27. [Google Scholar] [CrossRef]
- Xie, X.; Chorsi, H.T.; Agashiwala, K.; Chang, H.M.; Kang, J.; Chu, J.H.; Sarpkaya, I.; Htoon, H.; Schuller, J.A.; Banerjee, K. The scaling of the microLED and the advantage of 2D materials. arXiv 2021, arXiv:2101.10077. [Google Scholar]
- Lee, H.E. Novel Bio-Optoelectronics Enabled by Flexible Micro Light-Emitting Diodes. Electronics 2021, 10, 2644. [Google Scholar] [CrossRef]
- Gutruf, P.; A Rogers, J. Implantable, wireless device platforms for neuroscience research. Curr. Opin. Neurobiol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Krioukov, E.; Greve, J.; Otto, C. Performance of integrated optical microcavities for refractive index and fluorescence sensing. Sensors Actuators B Chem. 2003, 90, 58–67. [Google Scholar] [CrossRef]
- Gómez-Arribas, L.N.; Benito-Peña, E.; Hurtado-Sánchez, M.D.C.; Moreno-Bondi, M.C. Biosensing Based on Nanoparticles for Food Allergens Detection. Sensors 2018, 18, 1087. [Google Scholar] [CrossRef]
- Sagarzazu, G.; Bedu, M.; Martinelli, L.; Pelletier, N.; Safarov, V.I.; Weisbuch, C.; Gacoin, T.; Benisty, H. Quantitative analysis of enhanced light irradiance in waveguide-based fluorescent microarrays. Biosens. Bioelectron. 2009, 24, 2281–2284. [Google Scholar] [CrossRef]
- Schor, J.S.; Montalvo, I.G.; Spratt, P.W.; Brakaj, R.J.; A Stansil, J.; Twedell, E.L.; Bender, K.J.; Nelson, A.B.; Program, N.; University of California; et al. Therapeutic deep brain stimulation disrupts movement-related subthalamic nucleus activity in parkinsonian mice. Elife 2022, 11, e75253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, J.; Li, A.; Yao, M.; Liu, G.; Chen, S.; Luo, Y.; Wang, Z.; Gong, H.; Li, X.; et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat. Commun. 2022, 13, 998. [Google Scholar] [CrossRef]
- Thenaisie, Y.; Palmisano, C.; Canessa, A.; Keulen, B.J.; Capetian, P.; Jiménez, M.C.; Bally, J.F.; Manferlotti, E.; Beccaria, L.; Zutt, R.; et al. Towards adaptive deep brain stimulation: Clinical and technical notes on a novel commercial device for chronic brain sensing. J. Neural Eng. 2021, 18, 042002. [Google Scholar] [CrossRef]
- Duffley, G.; Lutz, B.J.; Szabo, A.; Wright, A.; Hess, C.W.; Ramirez-Zamora, A.; Zeilman, P.; Chiu, S.; Foote, K.D.; Okun, M.S.; et al. Home health management of Parkinson disease deep brain stimulation: A randomized clinical trial. J. Am. Med. Assoc. Neurol. 2021, 78, 972–981. [Google Scholar] [CrossRef]
- Wu, H.; Kakusa, B.; Neuner, S.; Christoffel, D.J.; Heifets, B.D.; Malenka, R.C.; Halpern, C.H. Local accumbens in vivo imaging during deep brain stimulation reveals a strategy-dependent amelioration of hedonic feeding. Proc. Natl. Acad. Sci. USA 2022, 119, e2109269118. [Google Scholar] [CrossRef]
- Jakobs, M.; Fomenko, A.; Lozano, A.; Kiening, K.L. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation—A systematic review on established indications and outlook on future developments. EMBO Mol. Med. 2019, 11, e9575. [Google Scholar] [CrossRef] [PubMed]
- Akram, H.; Sotiropoulos, S.N.; Jbabdi, S.; Georgiev, D.; Mahlknecht, P.; Hyam, J.; Foltynie, T.; Limousin, P.; De Vita, E.; Jahanshahi, M.; et al. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. Neuroimage 2017, 158, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2018, 184, 293–316. [Google Scholar] [CrossRef]
- Noecker, A.M.; Choi, K.S.; Riva-Posse, P.; Gross, R.E.; Mayberg, H.S.; McIntyre, C.C. StimVision Software: Examples and Applications in Subcallosal Cingulate Deep Brain Stimulation for Depression. Neuromodul. Technol. Neural Interface 2018, 21, 191–196. [Google Scholar] [CrossRef]
- Fritz, N.; Gulick, D.; Bailly, M.; Christen, J.M.B. Modeling optical design parameters for fine stimulation in sciatic nerve of optogenetic mice. Sci. Rep. 2021, 11, 22588. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.H.C.; Rommelfanger, N.J.; Ou, Z.; Hong, G. Bioinspired nanoantennas for opsin sensitization in optogenetic applications: A theoretical investigation. Multifunct. Mater. 2021, 4, 024002. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Q. Review of Monte Carlo modeling of light transport in tissues. J. Biomed. Opt. 2013, 18, 50902. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Molina-Luna, L.; Gautron, E.; Stephant, N.; Chauvin, A.; Du, K.; Ding, J.; Choi, C.-H.; Tessier, P.-Y.; El Mel, A.-A. The Kirkendall Effect in Binary Alloys: Trapping Gold in Copper Oxide Nanoshells. Chem. Mater. 2015, 27, 6374–6384. [Google Scholar] [CrossRef]
- Gan, C.L.; Classe, F.C.; Chan, B.L.; Hashim, U. Evolution and investigation of copper and gold ball bonds in extended reliability stressing. Gold Bull. 2014, 47, 141–151. [Google Scholar] [CrossRef]
- Gan, C.L.; Hashim, U. Reliability Assessment and Activation Energy Study of Au and Pd-Coated Cu Wires Post High Temperature Aging in Nanoscale Semiconductor Packaging. J. Electron. Packag. 2013, 135, 021010. [Google Scholar] [CrossRef]
- Ravi, R.; Paul, A. Diffusion mechanism in the gold-copper system. J. Mater. Sci. Mater. Electron. 2012, 23, 2152–2156. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Moiseenko, E.T.; Altunin, R.R. L10 ordered phase formation at solid state reactions in Cu/Au and Fe/Pd thin films. J. Solid State Chem. 2019, 269, 36–42. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Younce, J.R.; Albaugh, D.L.; Kao, Y.-C.J.; Shih, Y.-Y.I. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage 2014, 84, 11–18. [Google Scholar] [CrossRef]
- Butson, C.R.; McIntyre, C.C. Role of electrode design on the volume of tissue activated during deep brain stimulation. J. Neural Eng. 2005, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amendola, C.; Spinelli, L.; Contini, D.; De Carli, A.; Martinelli, C.; Fumagalli, M.; Torricelli, A. Accuracy of homogeneous models for photon diffusion in estimating neonatal cerebral hemodynamics by TD-NIRS. Biomed. Opt. Express 2021, 12, 1905–1921. [Google Scholar] [CrossRef]
- Chang, K.-T.; Lin, Y.-Y.; Lin, Y.-Y.; Lin, Y.-L.; Cheng, H.; Chang, Y.; Huang, M.-C. In Vivo Real-Time Discrimination Among Glioma, Infiltration Zone, and Normal Brain Tissue via Autofluorescence Technology. World Neurosurg. 2019, 122, e773–e782. [Google Scholar] [CrossRef]
- Ung, K.; Arenkiel, B. Fiber-optic implantation for chronic optogenetic stimulation of brain tissue. J. Vis. Exp. 2012, 68, e50004. [Google Scholar]
- Koss, A.M.; Alterman, R.L.; Tagliati, M.; Shils, J.L. Calculating total electrical energy delivered by deep brain stimulation systems. Ann. Neurol. 2005, 58, 168. [Google Scholar] [CrossRef]
- Marks, W.J.; Ostrem, J.L. (Eds.) Deep Brain Stimulation Management; Cambridge University Press: Cambridge, MA, USA, 2015; pp. 1–235. [Google Scholar]
- Yang, P.-F.; Chen, Y.-Y.; Chen, D.-Y.; Hu, J.W.; Chen, J.-H.; Yen, C.-T. Comparison of fMRI BOLD Response Patterns by Electrical Stimulation of the Ventroposterior Complex and Medial Thalamus of the Rat. PLoS ONE 2013, 8, e66821. [Google Scholar] [CrossRef] [PubMed]
- Siegle, J.H.; Lopez, A.C.; Patel, Y.A.; Abramov, K.; Ohayon, S.; Voigts, J. Open Ephys: An open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 2017, 14, 045003. [Google Scholar] [CrossRef]
- Zhou, H.; Neville, K.R.; Goldstein, N.; Kabu, S.; Kausar, N.; Ye, R.; Nguyen, T.T.; Gelwan, N.; Hyman, B.T.; Gomperts, S.N. Cholinergic modulation of hippocampal calcium activity across the sleep-wake cycle. Elife 2019, 8, e39777. [Google Scholar] [CrossRef] [PubMed]
- Stocke, S.K.; Samuelsen, C.L. A drivable optrode for use in chronic electrophysiology and optogenetic experiments. J. Neurosci. Methods 2020, 348, 108979. [Google Scholar] [CrossRef] [PubMed]
- Sileo, L.; Bitzenhofer, S.H.; Spagnolo, B.; Pöpplau, J.A.; Holzhammer, T.; Pisanello, M.; Pisano, F.; Bellistri, E.; Maglie, E.; De Vittorio, M.; et al. Tapered Fibers Combined With a Multi-Electrode Array for Optogenetics in Mouse Medial Prefrontal Cortex. Front. Neurosci. 2018, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Pisanello, F.; Mandelbaum, G.; Pisanello, M.; Oldenburg, I.A.; Sileo, L.; Markowitz, J.E.; Peterson, R.E.; Della Patria, A.; Haynes, T.M.; Emara, M.S.; et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 2017, 20, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Butson, C.R.; Cooper, S.E.; Henderson, J.M.; McIntyre, C.C. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 2007, 34, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Paulk, A.C.; Zelmann, R.; Crocker, B.; Widge, A.S.; Dougherty, D.D.; Eskandar, E.N.; Weisholtz, D.S.; Richardson, R.M.; Cosgrove, G.R.; Williams, Z.M.; et al. Local and distant cortical responses to single pulse intracranial stimulation in the human brain are differentially modulated by specific stimulation parameters. Brain Stimul. 2022, 15, 491–508. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Walston, S.T.; Chow, R.H.; Weiland, J.D. GCaMP expression in retinal ganglion cells characterized using a low-cost fundus imaging system. J. Neural Eng. 2017, 14, 56018. [Google Scholar] [CrossRef]
- Michelson, N.J.; Vanni, M.P.; Murphy, T.H. Comparison between transgenic and AAV-PHP. eB-mediated expression of GCaMP6s using in vivo wide-field functional imaging of brain activity. Neurophotonics 2019, 6, 25014. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Mukai, Y.; Nishimura, Y.; Takahashi, T.; Saitoh, N. Green fluorescent cAMP indicator of high speed and specificity suitable for neuronal live-cell imaging. Proc. Natl. Acad. Sci. USA 2022, 119, e2122618119. [Google Scholar] [CrossRef]
- Park, J.E.; Zhang, X.F.; Choi, S.-H.; Okahara, J.; Sasaki, E.; Silva, A.C. Generation of transgenic marmosets expressing genetically encoded calcium indicators. Sci. Rep. 2016, 6, 34931. [Google Scholar] [CrossRef] [PubMed]
- Kessi, M.; Chen, B.; Peng, J.; Yan, F.; Yang, L.; Yin, F. Calcium channelopathies and intellectual disability: A systematic review. Orphanet J. Rare Dis. 2021, 16, 219. [Google Scholar] [CrossRef]
- Eshra, A.; Schmidt, H.; Eilers, J.; Hallermann, S. Calc ium dependence of neurotransmitter release at a high fidelity synapse. Elife 2021, 10, e70408. [Google Scholar] [CrossRef]
- Trevathan, J.K.; Asp, A.J.; Nicolai, E.N.; Trevathan, J.M.; Kremer, N.A.; Kozai, T.D.; Cheng, D.; Schachter, M.J.; Nassi, J.J.; Otte, S.L.; et al. Calcium imaging in freely moving mice during electrical stimulation of deep brain structures. J. Neural Eng. 2021, 18, 026008. [Google Scholar] [CrossRef]
- Sych, Y.; Chernysheva, M.; Sumanovski, L.T.; Helmchen, F. High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat. Methods 2019, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Hammer, L.H.; Kochanski, R.B.; Starr, P.A.; Little, S. Artifact Characterization and a Multipurpose Template-Based Offline Removal Solution for a Sensing-Enabled Deep Brain Stimulation Device. Ster. Funct. Neurosurg. 2022, 100, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Legaria, A.A.; Matikainen-Ankney, B.A.; Ben Yang, B.; Ahanonu, B.; Licholai, J.A.; Parker, J.G.; Kravitz, A.V. Fiber photometry in striatum reflects primarily nonsomatic changes in calcium. Nat. Neurosci. 2022, 25, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Huang, X.; Huang, P.; Huang, L.; Feng, Z.; Xiang, X.; Chen, X.; Li, A.; Ren, C.; Li, H. Reward ameliorates depressive-like behaviors via inhibition of the substantia innominata to the lateral habenula projection. Sci. Adv. 2022, 8, eabn0193. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-W.; Lai, M.-L.; Chiu, F.-M.; Tseng, H.-Y.; Lo, Y.-C.; Li, S.-J.; Chang, C.-W.; Chen, P.-C.; Chen, Y.-Y. Experimental Verification for Numerical Simulation of Thalamic Stimulation-Evoked Calcium-Sensitive Fluorescence and Electrophysiology with Self-Assembled Multifunctional Optrode. Biosensors 2023, 13, 265. https://doi.org/10.3390/bios13020265
Liang Y-W, Lai M-L, Chiu F-M, Tseng H-Y, Lo Y-C, Li S-J, Chang C-W, Chen P-C, Chen Y-Y. Experimental Verification for Numerical Simulation of Thalamic Stimulation-Evoked Calcium-Sensitive Fluorescence and Electrophysiology with Self-Assembled Multifunctional Optrode. Biosensors. 2023; 13(2):265. https://doi.org/10.3390/bios13020265
Chicago/Turabian StyleLiang, Yao-Wen, Ming-Liang Lai, Feng-Mao Chiu, Hsin-Yi Tseng, Yu-Chun Lo, Ssu-Ju Li, Ching-Wen Chang, Po-Chuan Chen, and You-Yin Chen. 2023. "Experimental Verification for Numerical Simulation of Thalamic Stimulation-Evoked Calcium-Sensitive Fluorescence and Electrophysiology with Self-Assembled Multifunctional Optrode" Biosensors 13, no. 2: 265. https://doi.org/10.3390/bios13020265
APA StyleLiang, Y.-W., Lai, M.-L., Chiu, F.-M., Tseng, H.-Y., Lo, Y.-C., Li, S.-J., Chang, C.-W., Chen, P.-C., & Chen, Y.-Y. (2023). Experimental Verification for Numerical Simulation of Thalamic Stimulation-Evoked Calcium-Sensitive Fluorescence and Electrophysiology with Self-Assembled Multifunctional Optrode. Biosensors, 13(2), 265. https://doi.org/10.3390/bios13020265




