Abstract
Food safety is a key issue in promoting human health and sustaining life. Food analysis is essential to prevent food components or contaminants causing foodborne-related illnesses to consumers. Electrochemical sensors have become a desirable method for food safety analysis due to their simple, accurate and rapid response. The low sensitivity and poor selectivity of electrochemical sensors working in complex food sample matrices can be overcome by coupling them with covalent organic frameworks (COFs). COFs are a kind of novel porous organic polymer formed by light elements, such as C, H, N and B, via covalent bonds. This review focuses on the recent progress in COF-based electrochemical sensors for food safety analysis. Firstly, the synthesis methods of COFs are summarized. Then, a discussion of the strategies is given to improve the electrochemistry performance of COFs. There follows a summary of the recently developed COF-based electrochemical sensors for the determination of food contaminants, including bisphenols, antibiotics, pesticides, heavy metal ions, fungal toxin and bacterium. Finally, the challenges and the future directions in this field are discussed.
1. Introduction
Food safety is one of the issues of most global concern because safe food is essential for promoting human health and sustaining life [1,2]. The World Health Organization has listed food safety as one of its top 11 priorities [3]. However, with population growth, food industrialization and trade globalization, the types and quantities of food components or contaminants may increase at each stage of food production, such as crop cultivation, food production, processing, packaging, transport and storage. With the frequent occurrence of food pollution incidents around the world, food analysis, as a predominant step in food quality and safety control, has received unprecedented attention [4]. However, food analysis has faced significant challenges due to the complexity of the food sample matrix, the variety of potential interferents and the uncertainty of contaminants [5,6]. Therefore, food analysis techniques should be continuously improved to meet the needs of food testing.
In recent years, analytical techniques such as high-performance liquid chromatography [7], gas chromatography coupled with mass spectrometry [8], microfluidics [9], enzyme-linked immunosorbent assay [10], fluorescence spectroscopy [11] and nuclear magnetic resonance [12] have been performed for food quality and safety analysis. These techniques are often sensitive, accurate and selective. However, there are some drawbacks, including long analysis times, expensive or complex instrumentations and the need for skilled operators [13,14]. In addition, the food industry is more inclined to use efficient technologies to monitor food safety and quality levels. Hence, there is an urgent need to develop an accurate, simple, cost-effective, portable and fast response technique for food safety analysis. In order to meet these requirements, an electrochemical sensor has been proposed by researchers [15].
An electrochemical sensor is an electronic instrument that can conduct quantitative detection of analytes through a redox process. The electrochemical sensor is usually composed of three electrodes: working, reference and counter electrodes [16]. These three electrodes are placed in a cell containing an electrolytic solution and an analyte, and the analyte will undergo a redox reaction on the working electrode surface and generate an electrical signal proportional to the concentration of the analyte [17]. Therefore, components or contaminants in food can be immediately recognized by the electrochemical sensors. It should be mentioned that the traditional bare electrode is usually limited in terms of sensitivity and selectivity [18]. Thus, various materials have been synthesized and used as a working electrode material to improve the selectivity, sensitivity and speed of the electrochemical sensor [19,20]. Among those materials, porous materials have attracted great attention due to properties such as large surface area, many active sites, good chemical stability and fast electron transfer ability [21].
Covalent organic frameworks (COFs), as a new kind of organic porous polymer, are connected to light elements (C, H, N, B) via covalent bonds [22,23]. COFs have attracted more and more attention due to their low density, good chemical stability, permanent porosity and specific surface area [24]. Recently, COFs have been widely used in electrochemical sensing because they can improve the selectivity, sensitivity and speed of electrochemical sensors [25]. For example, COFs can prevent the agglomeration of electroactive molecules owing to their highly ordered pore structure and adjustable functional groups [26]; Moreover, they can improve the stability and reliability of the constructed electrochemical sensor [27]. However, there are still some problems that urgently need to be solved in the application of COFs to electrochemical sensing.
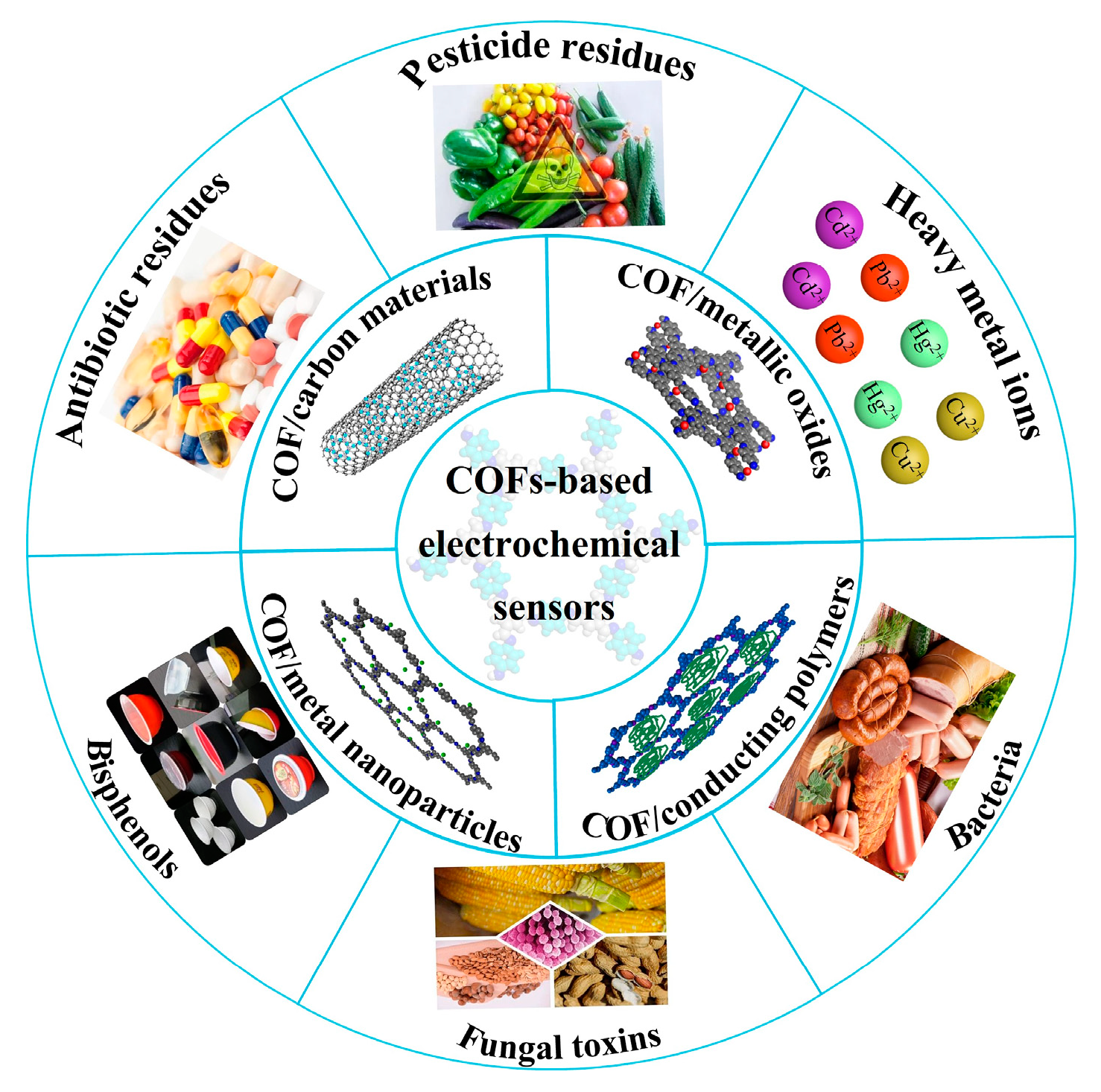
This review aims to present the recent advances of COF-based electrochemical sensors which have been reported regarding their application to food safety analysis (Figure 1). The current synthesis methods of COFs and the strategies to improve their conductivity are discussed below in detail. Then, the sensors used for food contaminant determination, including the linear range, limit of detection (LOD) and real samples, are summarized and compared. Finally, the challenges and future prospects of these electrochemical sensors in food analysis are discussed.
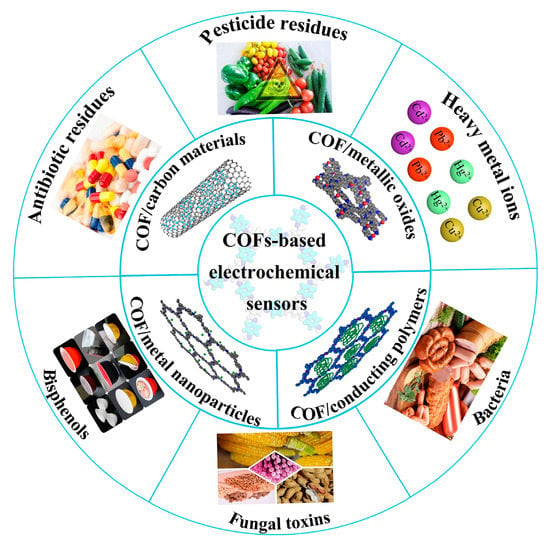
Figure 1.
COF-based electrochemical sensors for food safety analysis.
2. Preparation of COFs and Improvement to Their Electrochemistry Performance
COFs have received extensive attention due to their wide application. Thus, it is critically important to develop new synthesis technologies to improve their application range. Recently, COFs have become an attractive material in electrochemical sensing because they can improve the selectivity, sensitivity and speed of electrochemical sensors [28]. Nevertheless, the poor intrinsic conductivity and weak electrocatalysis of most COFs obviously limit their application [29]. Therefore, the combination of COFs and materials with good conductivity is an ideal method to improve the electrochemical performance of COFs.
2.1. Preparation of COFs
Up to now, researchers have reported many synthesis approaches for COFs; five typical synthesis approaches—solvothermal synthesis, mechanochemical synthesis, solvent-free synthesis, microwave-assisted synthesis and sonochemical synthesis—are summarized (Table 1) and further discussed below.

Table 1.
The synthetic approaches of COFs.
2.1.1. Solvothermal Synthesis
According to the reports, most of the prepared COFs are synthesized by the solvothermal synthesis method. In this method, monomers and solvents are added together in a Pyrex tube. After a freeze–pump–thaw cycle, the Pyrex tube is sealed and placed in an oven. The reaction often requires several days (2–9 d) under a controlled temperature (80–200 °C). After cooling to room temperature, COF is obtained through filtration, washing and drying. It should be pointed out that the solvent combinations and ratios and the reaction time have a great impact on the crystallinity and porosity of COFs in this method [56]. Since Omar M. Yaghi et al. [30] first reported the solvothermal synthesis of boronate ester-linked COF1 and COF5 in 2005, researchers synthesized more bonding types of COFs through this method, including imine- [31], olefin- [32], aminal- [33], thiazole- [34], sp2-carbon- [35] and polyimide -linked COFs [36]. Although the quality of COFs synthesized by the solvothermal method is often satisfactory, they also have limitations such as a long reaction time and organic solvents, which makes it difficult to expand them to industrial applications.
2.1.2. Mechanochemical Synthesis
Mechanochemical synthesis has become an alternative synthesis method for preparing COFs due to its being simple, timesaving, solvent-free and operable at room temperature. Rahul Banerjee et al. [37] developed this method to produce a COF, named TpPa-1. Monomers 1, 3, 5-triformylphloroglucinol and p-phenylenediamine were placed in a mortar and ground for 5 min at room temperature, and then a COF with a light yellow color was obtained. Subsequently, several COFs such as TP-COP [38], TpMA [39] and TpMaCON [40] were also synthesized by using this method. Although these examples demonstrate the feasibility of COFs using the mechanochemical synthesis method, the generalization of this method is still a challenge because of the limitations in building monomers. In addition, COFs obtained by this method usually display low surface areas and inferior crystallinity compared with those prepared by the solvothermal synthesis method.
2.1.3. Solvent-Free Synthesis
The solvent-free synthesis method has emerged as a viable method to prepare COFs owing to its environmental protection, simple operation, low cost-effectiveness and large-scale preparation [57]. Zhenjie Zhang et al. [41] first reported the solvent-free synthesis of olefin-linked COF (NKCOF-10). Under solvent-free conditions, NKCOF-10 was obtained through a benzoic-anhydride-catalyzed aldol reaction of 2, 5-dimethylpyrazine and 1, 3, 5-triformylbenzene. Subsequently, Zhenjie Zhang’s group used a similar method to synthesize various types of COFs, such as vinylene-linked COF [42], isomeric benzobisoxazole-vinylene-linked COF [43], olefin-linked COF [44,45] and C=N-linked COF [46]. Compared to the solvothermal synthesis method, this method usually requires the addition of solid-state catalytics (benzoic anhydride, benzoic acid, et al.) to improve the crystallinity and yield of COFs. The COFs obtained by this method have the advantages of high crystallinity and porosity, but it also requires high temperature and pressure, which limits its industrial application.
2.1.4. Microwave-Assisted Synthesis
The microwave-assisted synthesis method has received more attention due to its advantages of shorter reaction times, higher yields, environmental protection and lower energy consumption [58]. Andrew I. Cooper et al. first prepared boronate ester-linked COF5 using microwave as the heat source in 20 min [47]. More interestingly, COF5 obtained using the microwave method had larger Brunauer–Emmett–Teller surface area (2019 m2/g) than that prepared by the conventional solvothermal method. In addition to boronate ester-linked COFs, covalent triazine-based framework [48], melamine-based COF [49], enamine-linked COF [50], cationic COF [51] and imine-linked COF [52] have also been synthesized by this microwave-assisted method. Now, microwave-assisted synthesis has become a potential method for the industrial synthesis of COFs because it can achieve faster and cleaner synthesis.
2.1.5. Sonochemical Synthesis
The sonochemical method is capable of facilitating the homogeneity of COFs and accelerating the crystallization rate due to its use of ultrasonic wave, which can produce strikingly high pressure and local temperature, as a heat source [59]. However, the COFs prepared by the sonochemical synthesis method are still in a nascent stage. COF5 was first obtained in a sonicator unit under an adjustable power output [53]. More interestingly, COF5 processed significantly smaller crystals (50–250 nm) than that obtained by the solvothermal method. In addition, several other COFs have been synthesized using the sonochemical method, for instance, seven previously reported COFs (COF-1-COF-7) and two new COFs (SonoCOF-8, SonoCOF-9) [54,55]. The crystallinity and porosity of these COFs are comparable to or better than those of the same materials made by the solvothermal method. Sonochemical synthesis has become a potential method for the large-scale preparation of COFs owing to its super-fast synthesis rate and significantly reduced energy consumption.
The methods discussed above are used to prepare COFs. Nevertheless, the pure COFs usually have poor electrical conductivity, which limits their applications in the field of electrochemistry. Thus, new strategies that can improve the conductivity of COFs are urgently needed for their further electrochemical sensing applications.
2.2. Strategies to Improve Electrochemistry Performance of COFs
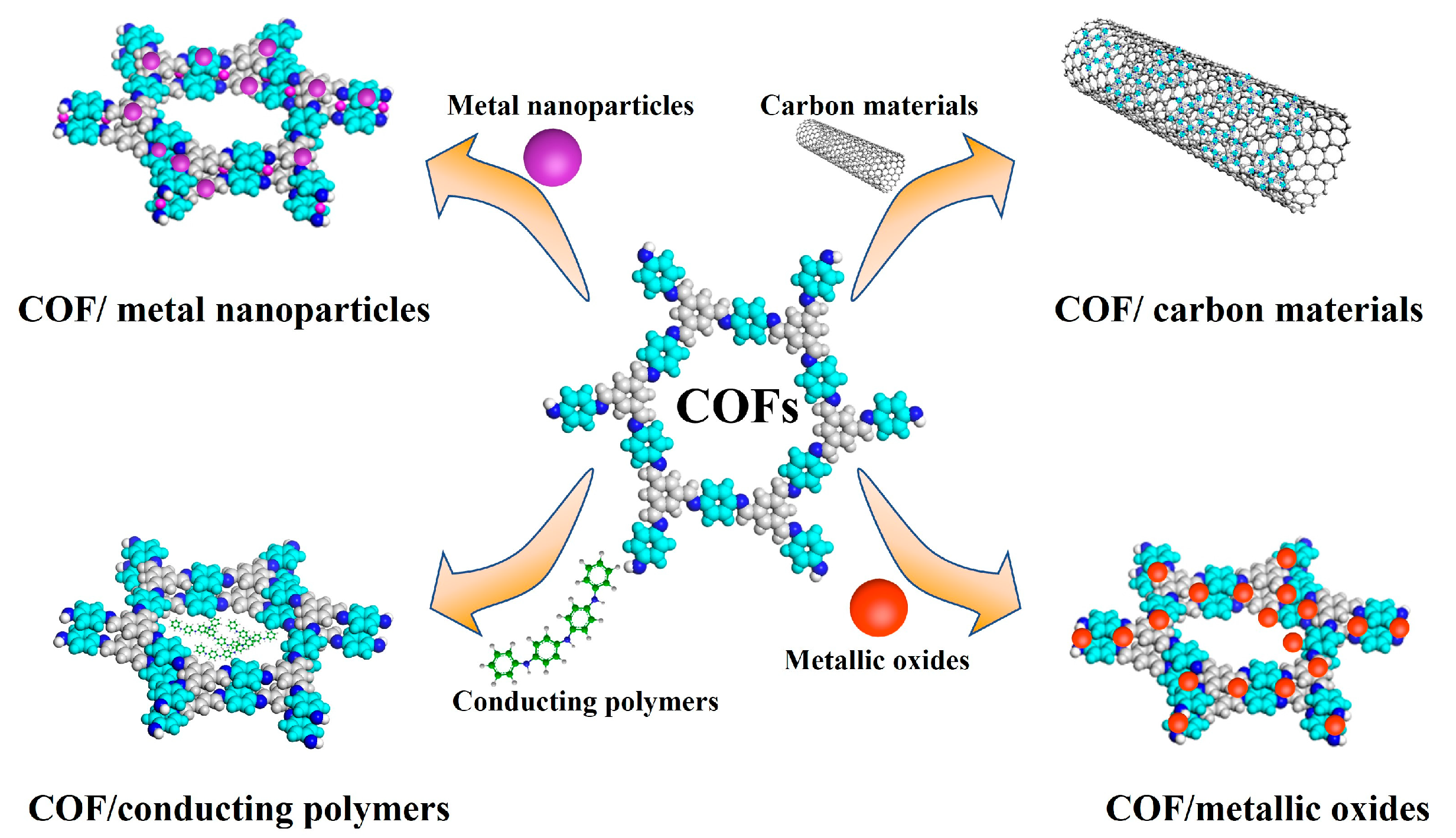
Combining COFs with some materials with specific conductivity, such as carbon materials, metal nanoparticles, metallic oxides and conducting polymers, can be considered an effective strategy to improve the electrochemistry performance of the COFs. Some of the strategies to enhance the COF electrical conductivity are described below (Figure 2 and Table 2):
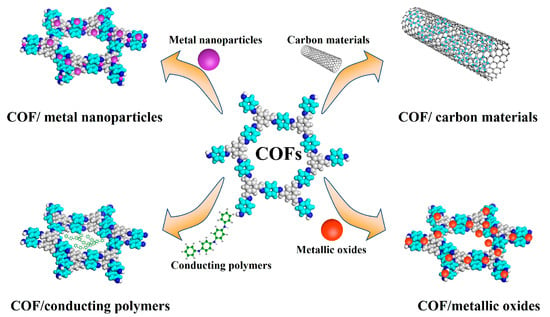
Figure 2.
Strategies to improve COFs’ electrochemistry performance.

Table 2.
Methods to improve the electrochemistry performance of COFs and their advantages and disadvantages.
2.2.1. COF/Carbon Materials
Carbon-based materials have good conductivity, high specific surface area, excellent mechanical strength and cost effectiveness [74]. Thus, combining COFs with carbon materials has become a suitable solution to enhance the electron transfer of the COFs to meet the requirements of electrochemical sensing. Up to now, the conductive carbon materials used most widely include graphene [60,61], carbon nanotubes [62], fullerenes [63], macroporous carbon [64] and graphene aerogel [65]. All of these COF/carbon materials display more excellent electrochemical performance than COFs, carbon materials or glassy carbon electrode (GCE) alone. For example, Zhixiang Xu et al. [60] prepared a graphene oxide@COF composite modified by glassy carbon electrode (GO@COF/GCE). Electrochemical characteristics showed that the GO@COF/GCE had a larger peak current and electroactive surface area than the bare GCE due to the high surface area and good electrical conductivity of the GO@COF. A similar strategy was adopted by Dawei Pan et al. [61]. They synthesized a COF with a hydrogen sulfonic functional group. After the COF was combined with graphene, the composites showed excellent electrochemical performance. These studies confirmed that the use of COFs/carbon materials is an effective method in the construction of excellent electrochemical sensors. However, the binding mechanism of COFs and carbon materials remains unclear. Therefore, a large amount of work is still necessary to analyze these problems.
2.2.2. COF/Metal Nanoparticles
The integration of metal nanoparticles (NPs) into a COF framework is another innovative method to improve COFs’ electrochemical performance. Not only can COFs provide multiple functional active sites and a large surface area for metal nanoparticles to integrate, but they can also prevent metal nanoparticle aggregation to obtain satisfactory dispersion. In addition, metal nanoparticles can improve the conductivity of COFs. Thus, the COF/metal nanoparticle composites have attracted more and more attention in the electrochemical field [75]. One example was reported by P. Arul et al. [66]. A COF was prepared with p-Phenylenediamine and terephthalaldehyde, and then Ag nanoparticles were embedded into the COF. Finally, the Ag NPs-COF/GCE was obtained after the composite was deposited on the GCE surface. Due to the synergistic effect of the COF and AgNPs, the AgNPs-COF/GCE showed 1.7 and 1.5 times higher electrical conductivity than the bare GCE, COF/GCE, and AgNPs/GCE alone. A similar approach, described by Wu Yang et al. [67], consisted of using Pt nanoparticles together with COFs as the electrode materials. In this example, the obtained electrode showed excellent electrocatalytic properties compared with those modified by individual materials. These studies confirm that the prepared COF/metal nanoparticle material is an effective strategy to obtain efficient electrochemical sensors, but the high cost of metal nanoparticles limits their large-scale application.
2.2.3. COF/Metallic Oxides
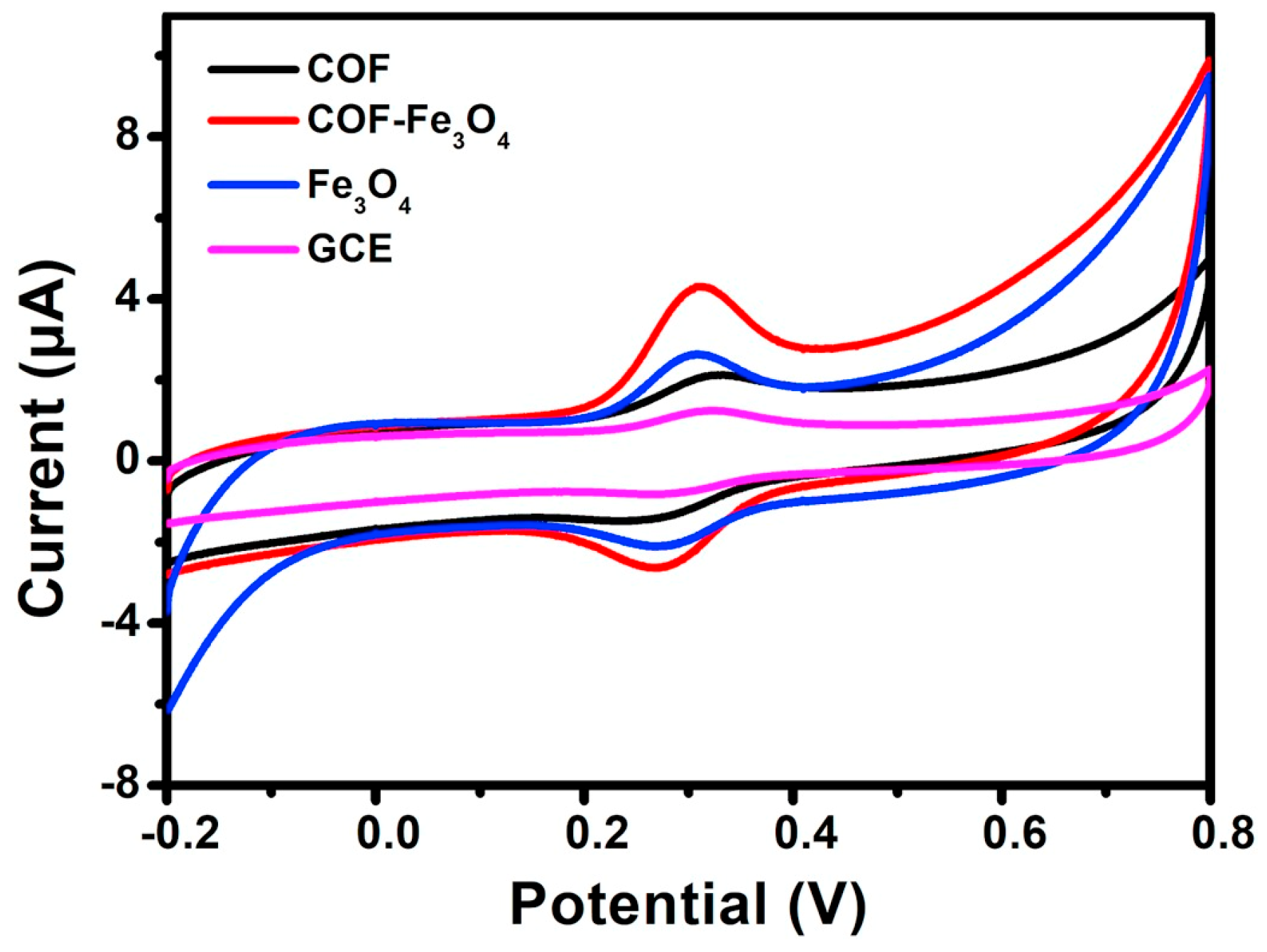
It is also an attractive approach to combine COFs with metal oxide nanomaterials to obtain hybrid materials for the development of electrochemical sensors. Until now, Fe3O4 [68], CuO [69], ZnO/ZnNi2O4 [70] and La2O3 [71] have been combined with COFs to finally construct electrochemical sensors. For example, Yang Wang et al. [68] prepared a core-shell structured magnetic COF (Fe3O4@TAPB-DMTP-COFs) by a step-by-step assembly method under facile conditions. The biggest peak current of luteolin on the TAPB-DMTP-COFs/GCE was observed on the Fe3O4@TAPB-DMTP-COFs/GCE (Figure 3), due to the synergistic effects of the high surface area of the TAPB-DMTP-COFs and the excellent catalytic activity of Fe3O4. Another example of the synthesis of COF/metal oxide composites was reported by Yang Wang et al. [69]. They prepared a CuO@TAPB-DMTP-COF composite with a core-shell structure by incorporating CuO nanorods into the TAPB-DMTP-COF framework. The electrochemical parameters showed that the electrical analytical performance of the TAPB-DMTP-COF/GCE was improved significantly owing to the synergistic effects of TAPB-DMTP-COF and CuO. These examples demonstrate that COF/metallic oxides are good candidates for preparing electrochemical sensors in various applications.
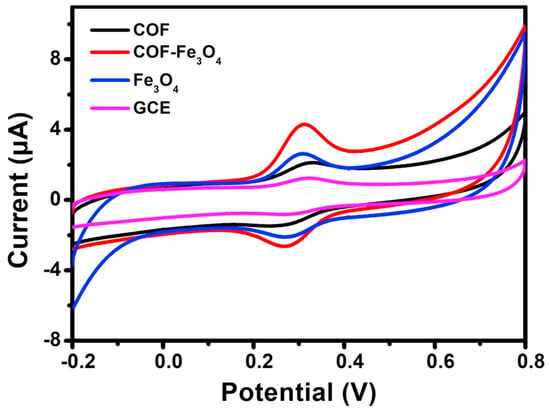
Figure 3.
Cyclic voltammograms of Fe3O4 @TAPB-DMTP-COFs/GCE, bare GCE, TAPB-DMTP-COFs/GCE, and Fe3O4/GCE from Ref. [68]. Copyright 2020 Elsevier.
2.2.4. COF/Conducting Polymers
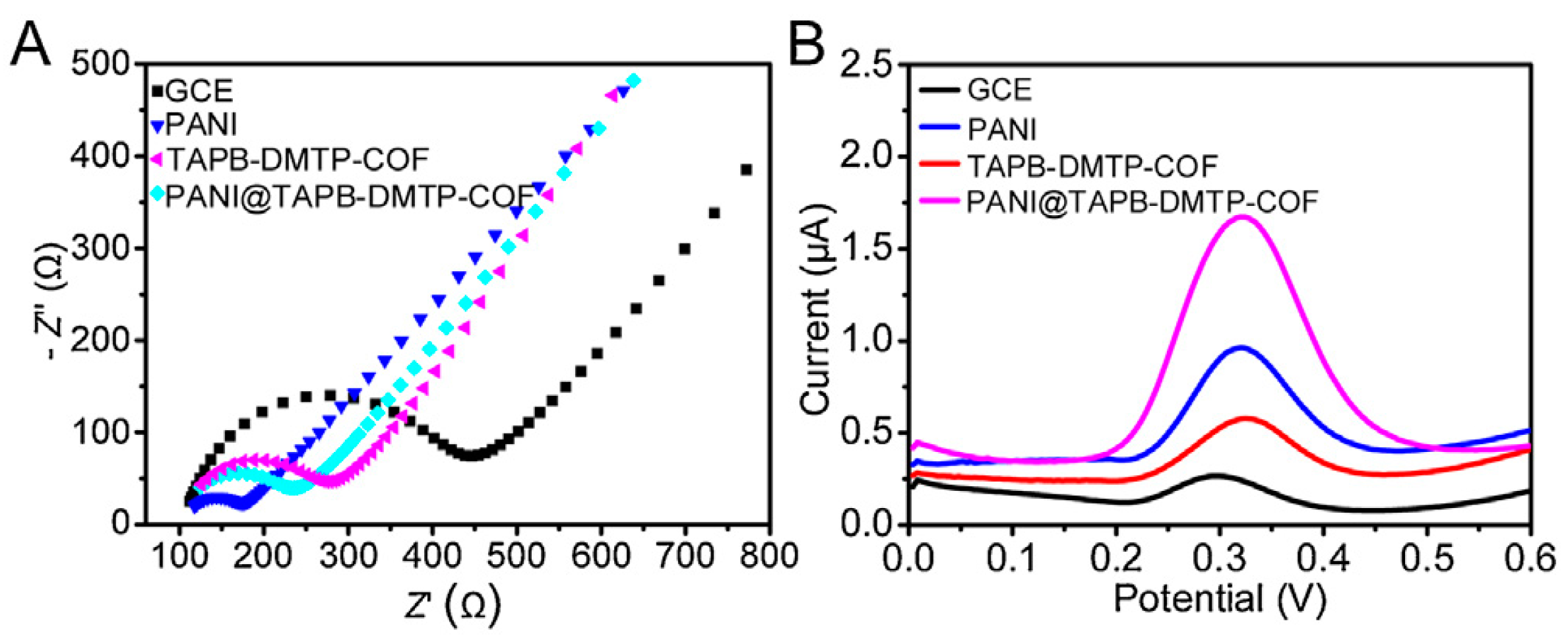
Another effective approach to improve the conductivity of COFs is to integrate the COFs and a conducting polymer. In the example described by William R. Dichtel et al. [72], in order to enhance the conductivity of a COF film, they encapsulated 3,4-ethylenedioxythiophene into COF pores by a facile electropolymerization method. The composite possessed significant improvement in involumetric energy and power densities relative to COF film alone. Moreover, the composite also had excellent cyclic stability. Another example for the preparation of a COF/conducting polymer composite was reported by Yi Wang et al. [73]. In that work, a core-shell structured COF/conducting polymer composite (TAPB-DMTP-COF@PANI) was synthesized using a simple polymerization approach. The electrochemical responses experiments showed that the TAPB-DMTP-COF@PANI/GCE displayed a stronger electrochemical signal compared to bare GCE, PANI/GCE, TAPB-DMTP-COF/GCE alone (Figure 4). Although only a few studies have been reported, it is still proved that COF/conducting polymer composites are promising functional materials to fabricate electrochemical sensors. Thus, more studies are necessary to expand the variety of these composites and further validate these findings.
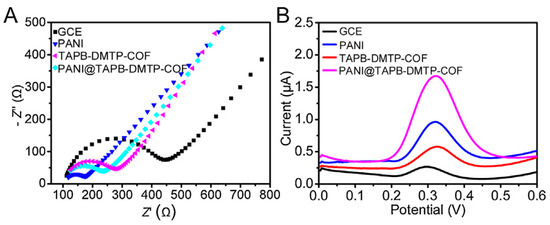
Figure 4.
(A) EIS Nyquist plots and (B) Differential pulse voltammetry voltammograms of bare GCE, PANI/GCE, TAPB-DMTP-COF/GCE, and TAPB-DMTP-COF@PANI/GCE from Ref. [73]. Copyright 2021 Elsevier.
Although the above strategies to improve the electrochemical properties of COFs have excellent performance, the types of conductive materials are relatively few. In the future, a variety of conductive composite materials need to be developed to meet the broad application of COF in the electrochemical field.
3. Applications in Food Safety Analysis
Based on their fascinating structures and properties, COFs have been successfully applied in the field of electrochemical sensing [76]. Nowadays, numerous food contaminants have been detected by COF-based electrochemical sensors, which have excellent selectivity, high sensitivity and quick response speed. An overview of these sensors for the determination of food contaminants, such as bisphenols, antibiotics, pesticides, heavy metal ions, fungal toxin and bacterium, is shown below in Table 3.

Table 3.
COF-based sensors for food safety analysis.
3.1. Bisphenols
Bisphenol compounds widely exist in plastic food packaging materials, and they have various adverse health effects on organisms, which will lead to serious diseases [99]. Therefore, it is necessary to develop a simple and sensitive method for bisphenol compound determination.
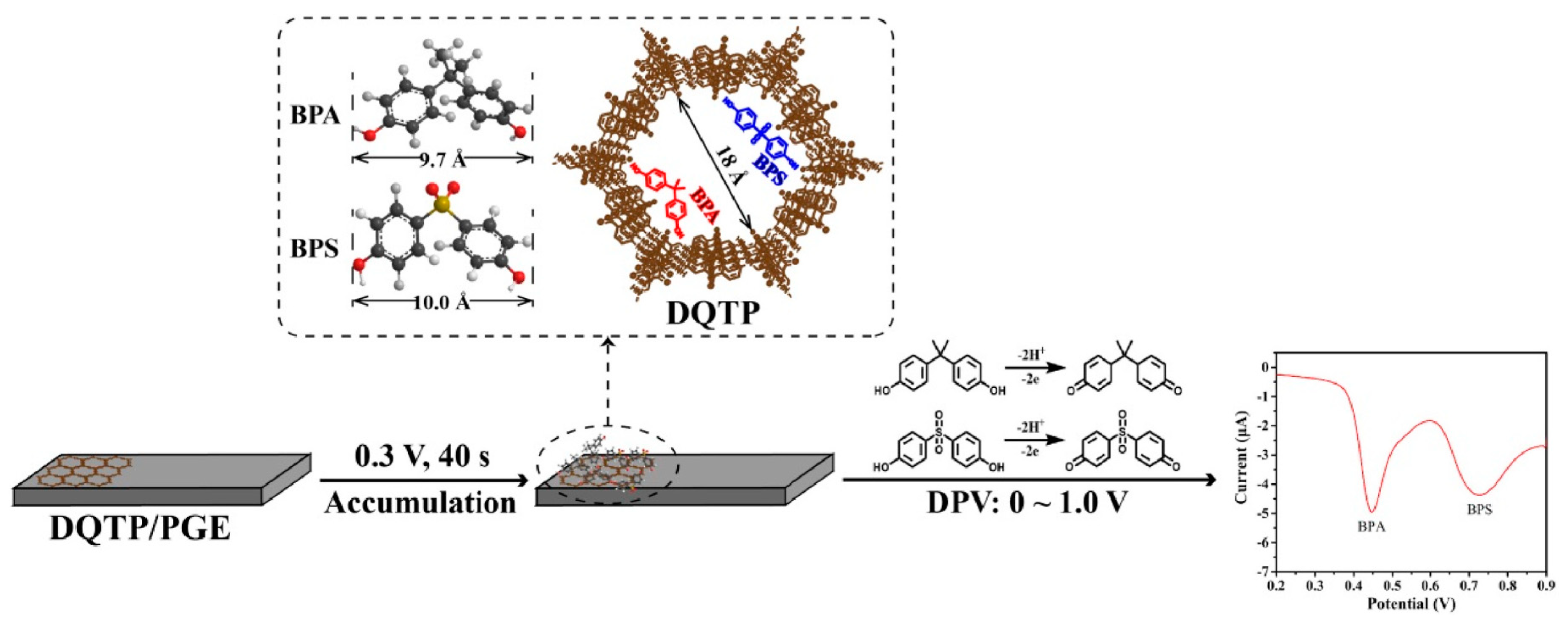
JinYu Qiao et al. [77] proposed a ratiometric electrochemical sensor for the simultaneous determination of BPA and BPS. The ratiometric sensor was prepared based on COF-LZU1 and silver nanoparticles modified with carbon cloth electrode (COF/AgNPs/CC). This electrode displayed excellent electrocatalytic activity on BPA and BPS due to its high electrocatalytic surface area and good conductivity. The LODs (S/N = 3) of the determination for BPA and BPS were both 0.15 μmol/L. Moreover, other electrochemical sensors based on COF (DQTP) modified with pencil graphite electrode (DQTP/PGE) (Figure 5) [78] and COF (CTpPa-2)-modified GCE [79] have also been established, and they have been successfully applied to BPA and BPS determination. Tert-butylhydroquinone (TBHQ) as one of a strong phenolic antioxidant is widely used in food products due to its excellent chemical stability and anti-lipid peroxidation property [80]. However, health studies indicated that excess intake of TBHQ would induce serious health issues towards humans. Recently, Yi Wang et al. [80] developed an electrochemical sensor based on core-shell Co3O4@TAPB-DMTP-COF composite for the sensitive and selective determination of TBHQ. The Co3O4@TAPB-DMTP-COF was synthesized using a monomer-mediated in situ growth strategy. In the electrochemical sensor, the COFs acted as a protective layer to accelerate current mobilization and the Co3O4 nanoparticles as the electrocatalytic active center. The LOD for the TBHQ determination was 0.002 μmol/L (S/N = 3).
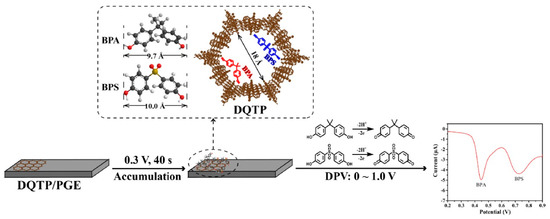
Figure 5.
Schematic illustration of a DQTP-based electrochemical sensor for the simultaneous determination of BPA and BPS from Ref. [78]. Copyright 2022 Elsevier.
The above studies prove that a COF-based electrochemical sensor can achieve the determination of bisphenol compounds. However, the current reports mainly involve bisphenol A and bisphenol S determination. Hence, it is necessary to design a novel electrochemical sensor based on COFs to determine more kinds of bisphenol compounds.
3.2. Antibiotics
Antibiotics, as a class of pharmaceuticals that can inhibit or kill pathogens, are widely used to prevent and treat infectious diseases caused by bacteria, fungi, molds and other microorganisms [100]. However, the excessive consumption of antibiotics can result in many health problems including blood dyscrasias, liver toxicity and allergic reactions [101].
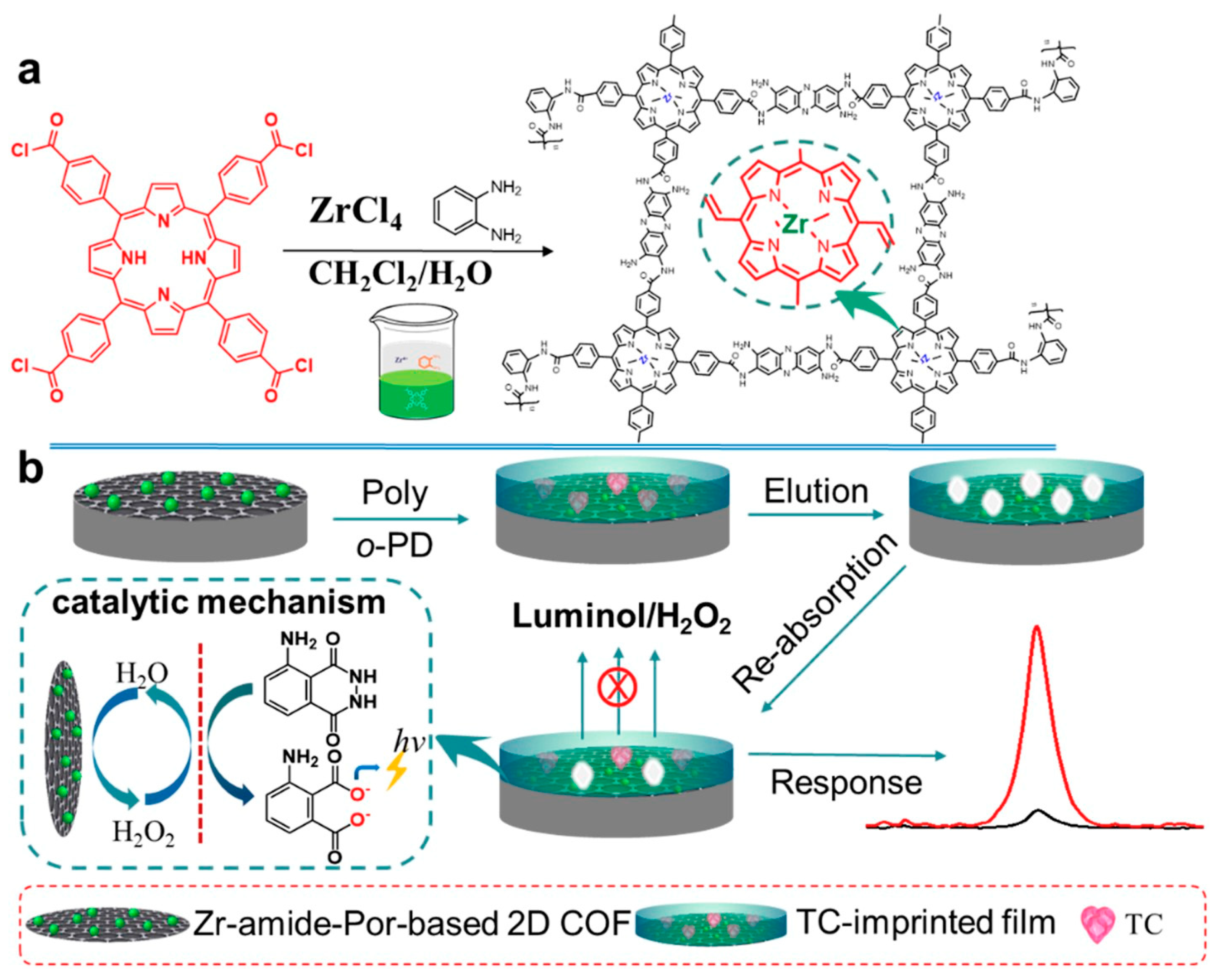
Tetracycline (TC) is a typical antibiotic that can cause resistance and other side effects such as allergic reactions, nephrotoxicity and liver impairment. At present, some electrochemical sensors based on COFs have been used for tetracycline antibiotic detection. For example, Yukun Yang et al. [81] proposed a portable and on-site electrochemical sensor based on surface molecularly imprinted polymer (MIP)-modified magnetic COF (Fe3O4@COFs@MIPs) for the sensitive and rapid determination of TC. The Fe3O4@COFs@MIPs was synthesized according to a layer-by-layer modification method, which showed outstanding adsorption properties and good magnetism. The liner range of the electrochemical sensor for TC determination was 1 × 10−10–1 × 10−4 g/mL and the LOD was 2.4 × 10−11 g/mL (S/N = 3). The prepared sensor has also been successfully applied to milk and chicken samples. Xionghui Ma et al. [82] constructed an MIP electrochemiluminescence sensor based on a novel Zr- amide porphyrin-based 2D COF for TC determination in milk samples (Figure 6). The COF was obtained by a facile liquid–liquid interface method and showed remarkable electrocatalytic performance due to its inherently-ordered structure and abundant Zr catalytic active center. The LOD of the sensor for TC determination was 2.3 × 10−6 μmol/L. In another example, Hongming He et al. [83] synthesized a ferriporphyrin-based COF (Fe-PPOF) by a sonogashira coupling reaction. Then, an electrochemical aptasensor was constructed using aptamer-immobilized Fe-PPOF for oxytetracycline determination with an LOD of 2.05 fg/mL. Moreover, the electrochemical aptasensor has also been applied to the analysis of oxytetracycline in milk samples. Apart from TC, some other electrochemical sensors based on COFs have been reported for the determination of sulfamerazine [84], sulfadiazine [61], furazolidone [85], penicillin [86] and chloramphenicol [87].
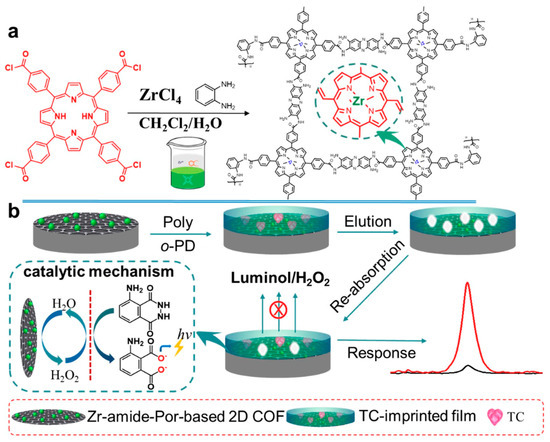
Figure 6.
Schematic illustration of the synthesis of a Zr-amide-Por-based 2D COF and its application for the determination of TC from Ref. [82]. Copyright 2019 Elsevier.
3.3. Pesticides
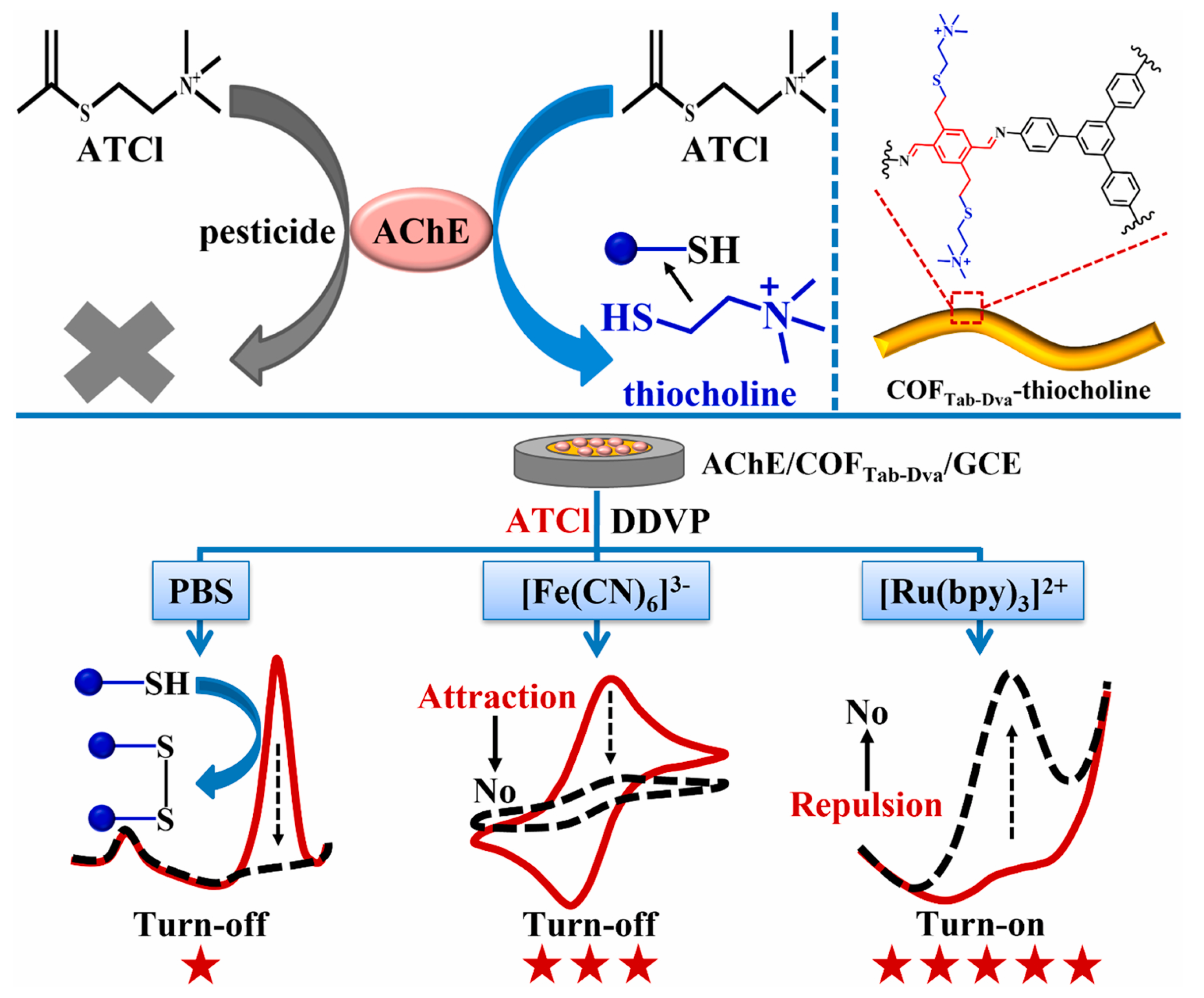
Pesticide residues in food samples have become a great threat to human life. Thus, it is urgent to detect pesticide residues in a sensitive, selective, simple and rapid manner. Carbaryl is a carbamate pesticide that can cause damage to the nervous system and brain [102]. Lili Chen et al. [88] proposed a N, O-rich COF paper constructed-based electrochemical biosensor with repeatability, selectivity and stability for carbaryl detection. The COF was prepared through an aminealdehyde condensation reaction, which had abundant C=O, NH and OH groups. Then, the COF was fixed on a paper electrode to load acetylcholinesterase, which could greatly enhance the bioactivity of acetylcholinesterase. The COF was able to capture thiocholine by hydrogen bonding. The linear range of the biosensor was 0.39–35 μmol/L with an LOD of 0.13 μmol/L. The electrochemical biosensor has also been used for the determination of carbaryl in lettuce juice samples. In a similar example, Shuo Wang et al. [89] designed an MIP electrochemiluminescence sensor, based on COF and carbon nitride nanosheets, which can detect carbaryl sensitively and accurately. Organophosphorus pesticides are organic compound pesticides containing the phosphorus element; they are widely used in agricultural production, resulting in various degrees of residues in crops [103]. Guangran Ma et al. [90] reported a ratiometric electrochemical biosensor based on a COFTab-Dva nanofiber with excellent electroactivity for O, O-dimethyl-O-2, 2-dichlorovinylphosphate (DDVP) determination (Figure 7). COFTab-Dva nanofibers can effectively immobilize acetylcholine and enrich thiocholine due to their large surface area and abundant vinyl. The LOD of the electrochemical biosensors was 0.11 μmol/L. More interestingly, the ratiometric electrochemical biosensor was successfully used to detect the DDVP residual in lettuce juice samples. Xianbo Lu et al. [91] also synthesized a COF with abundant carbonyl groups and used the same to construct an electrochemical sensor for paraoxon determination in cucumber samples. In addition, Xue Wang [92] and Yonggui Song [93] have constructed COF-based electrochemical sensors for the detection of malathion and trichlorfon, respectively.
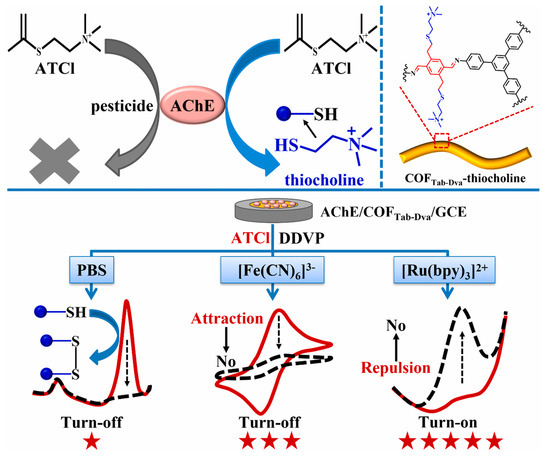
Figure 7.
Schematic illustration of enzyme inhibition and DDVP determination from Ref. [90]. Copy right 2022 Elsevier.
Although there are few reports on the application of COF-based electrochemical sensors in pesticide residue determination, they still prove that this sensor is a promising tool for pesticide determination. Therefore, more research is urgently needed to expand the variety of COF composites and pesticides to validate these findings.
3.4. Heavy Metal Ions
Heavy metals, such as Pb2+, Hg2+, Cu2+, and Cd2+, can accumulate in the food chain at different stages. These heavy metals have serious effects on human health even at ultra-trace levels [104]. Therefore, it is very important to detect heavy metals in food samples.
Tayyebeh Madrakian et al. [94] developed a novel simple, sensitive and rapid electrochemical sensor based on melamine-COF-modified glassy carbon electrode for the simultaneous measurement of Pb2+ and Hg2+. The linear range of the sensor was 0.01–0.3 and 0.05–0.3 μmol/L for Pb2+ and Hg2+, respectively, with LODs of 0.72 × 10−3 and 1.2 × 10−2 μmol/L. Moreover, the electrochemical sensor was also used to detect Pb2+ and Hg2+ in edible samples. Madrakian et al. [95] also proposed a glass carbon electrode modified by an electroplated bismuth film and a nanocomposite of melamine-COF and Fe3O4 nanoparticles. The glass carbon electrode successfully realized the selective detection of Pb2+ with a LOD of 0.95 nnmol/L. In order to achieve the simultaneous detection of multiple heavy metal ions, Yanyan Zhang et al. [96] reported a COF with calcium lignosulfonate-modified multiwalled CNTs and nafion (COF/MWCNTs/CLS) was used as an electrocatalytic material to modify GCE. The modified electrode has been used for the simultaneous detection of Cu2+, Pb2+ and Cd2+ due to the high enrichment capacity of the COF and the good conductivity of the MWCNTs. The LODs of the electrochemical sensor for Cu2+, Pb2+ and Cd2+ determination were 0.2, 0.7 and 0.4 μg/L, respectively.
3.5. Fungal Toxin and Bacterium
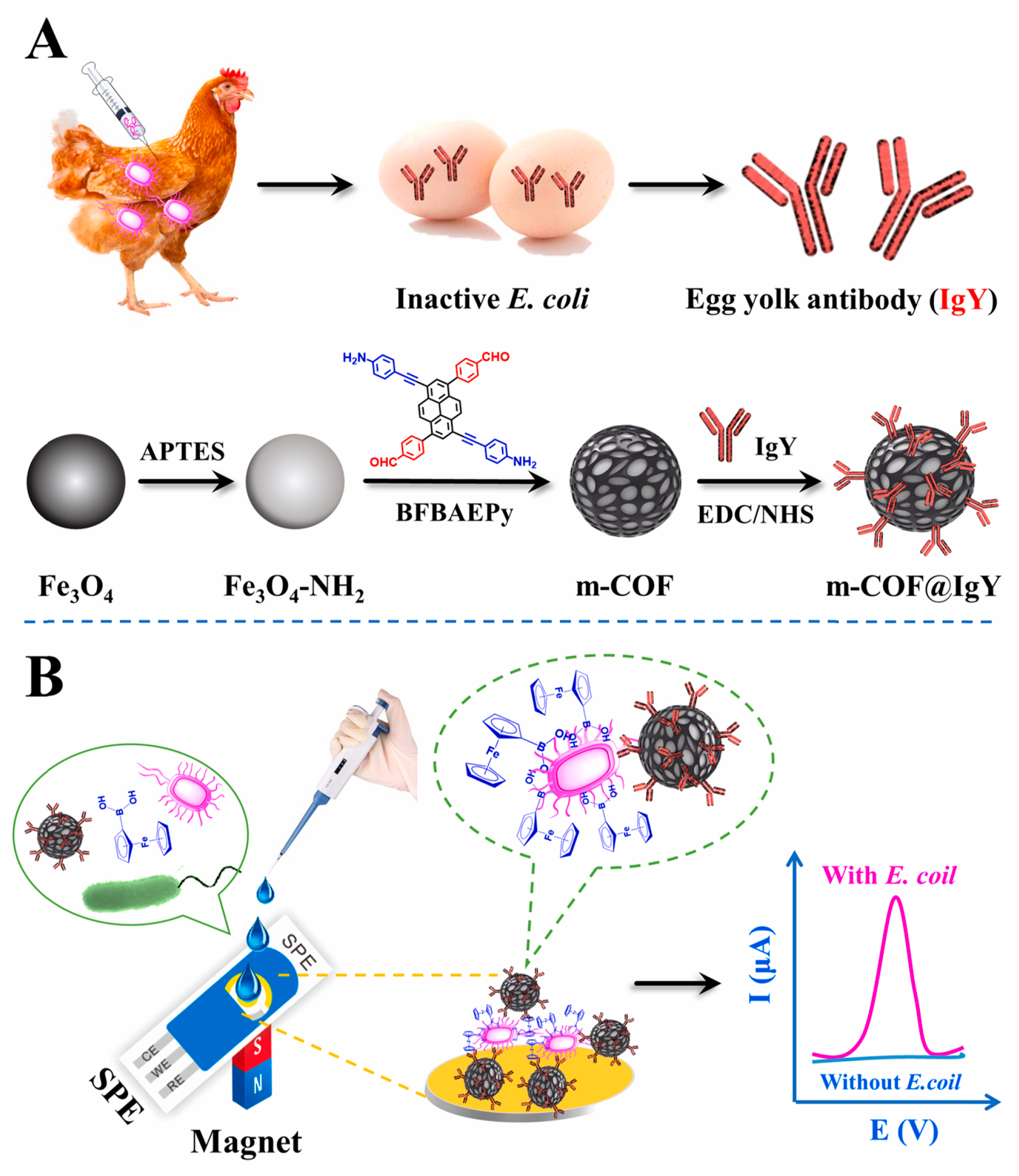
Aflatoxin is a metabolite of aspergillus flavus, aspergillus parasiticus and aspergillus norvegicus, which mainly exists in moldy peanuts, cereals and other foods [105]. Due to the high toxicity and carcinogenicity of aflatoxin, it is necessary to determine it in food. Recently, Yuehong Pang et al. [97] prepared a COF (TpBD) -modified GCE by a reliable in situ growth method. Then, the modified GCE was used as a working electrode for the electrochemical analysis of aflatoxin M1 in milk samples with magnetic separation technology. The proposed electrochemical sensor had a lower LOD of 0.15 ng/mL (S/N = 3) due to the large surface area of the COF. Bacterial contamination has attracted increasing attention because it can cause foodborne diseases. Thus, it is important for human health to use simple and rapid methods to monitor bacterial contamination. Ning Gan et al. [98] developed an electrochemical biosensor based on egg yolk antibody-labeled magnetic COF for the determination of Escherichia coli in milk, beef and shrimp samples (Figure 8). Magnetic COF, due to its large surface area and abundant holes, was used to label egg yolk antibodies so as to achieve the efficient enrichment and detection of E. coli. The linear range of the electrochemical biosensor was 10–108 CFU/mL with the LOD of 3 CFU/mL.
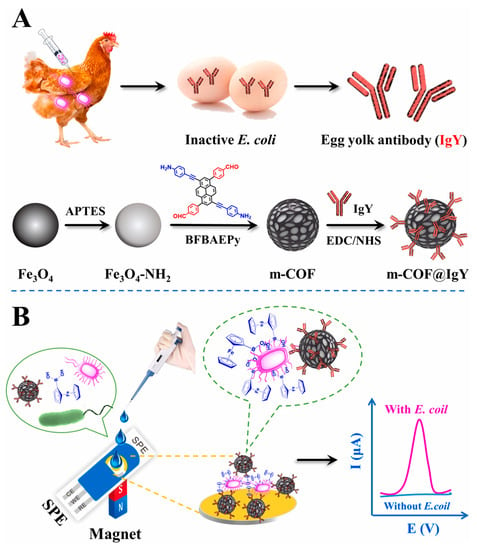
Figure 8.
(A) Schematic illustration of the preparation process of m-COF@IgY and (B) application for determination of E. coli from Ref. [98]. Copyright 2022 Elsevier.
Although only a few COF-based electrochemical sensors have been designed and applied to fungal toxin and bacterium analysis, it has still been proven that the COFs are a propagable material to construct an electrochemical sensor for fungal toxin and bacterium determination.
4. Conclusions
Food safety is one of the critically important issues for human health with the continuous growth of food consumption and more reports of food contamination incidents. To ensure food safety, electrochemical sensors have been employed for food quality and safety testing due to their accurate, simple, cost-effective and fast response. Herein, we have reviewed the reported COF-based electrochemical sensors in the context of food safety analysis. Specifically, the design and synthesis methods of COFs, namely solvothermal synthesis, mechanochemical synthesis, solvent-free synthesis, microwave-assisted synthesis and sonochemical synthesis, have been summarized. Moreover, these reports have been discussed in terms of the strategies to improve the performance of COF based electrochemical sensors as well as their use in the determination of food contaminants, including bisphenols, antibiotics, pesticides, heavy metal ions, fungal toxins and bacterium. Thus, COFs provide an emerging platform for researchers to develop electrochemical sensors for food safety applications. Although COF-based electrochemical sensors have demonstrated excellent performance and promising prospects, there are still some challenges, such as the need to strengthen the stability and repeatability of the sensors and to realize the miniaturization and field operation of the sensors. Although challenges still exist, the exciting promise of these sensors in the electrical analysis of food will encourage researchers to conduct further research, combining new COFs with advanced electrochemical techniques to give electrochemical sensors perfect analytical performance.
In general, the application of COF-based electrochemical sensors in the field of food analysis is still at an early stage, and there is still much room for research in this innovative field. Taking a future outlook, the rational design and controlled synthesis of multifunctional electrode materials based on COFs, while enhancing their selectivity to food contaminants, are very desirable for expanding their applications in the field of food analysis. In addition, the poor biocompatibility of COFs limits their potential application in contaminant detection in biological foods. In the future, the feasibility study of biocompatibility of electrochemical sensors based on COFs should be strengthened. The synthesis of new compatible/biocompatible COFs is very important for the analysis of contaminants in biological food. Furthermore, when detecting targets in complex systems, one of the main challenges faced by electrochemical sensors is the fouling of the electrode surface, which leads to the limited life of the sensors. This problem should be solved by designing and synthesizing COF electrode materials with antifouling performance.
Author Contributions
Z.L.: Investigation, writing—original draft preparation, writing—review and editing; Y.W.: Investigation, data curation; G.L.: Visualization, resources, funding acquisition, supervision, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the State Key Program of National Natural Science of China (No. 22134007), the National Natural Science Foundation of China (No. 21976213), the Research and Development Plan for Key Areas of Food Safety in Guangdong Province of China (No. 2019B020211001) and the National Key Research and Development Program of China (No. 2019YFC1606101), respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elfadil, D.; Lamaoui, A.; Pelle, F.D.; Amine, A.; Compagnone, D. Molecularly imprinted polymers combined with electrochemical sensors for food contaminants analysis. Molecules 2021, 26, 4607. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Senthil Kumar, P.; Hong, T.K.A.; Soto-Moscoso, M.; Jalil, A.A. Recent developments on graphene and its derivatives based electrochemical sensors for determinations of food contaminants. Food. Chem. Toxicol. 2022, 165, 113169. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537. [Google Scholar] [CrossRef]
- Hou, X.D.; Xu, H.; Zhen, T.Y.; Wu, W. Recent developments in three-dimensional graphene-based electrochemical sensors for food analysis. Trends. Food. Sci. Tech. 2020, 105, 76–92. [Google Scholar] [CrossRef]
- Maculewicz, J.; Świacka, K.; Stepnowski, P.; Dołżonek, J.; Bielińska, B. Ionic liquids as potentially hazardous pollutants: Evidences of their presence in the environment and recent analytical developments. J. Hazard. Mater. 2022, 437, 129353. [Google Scholar] [CrossRef]
- Steiner, D.; Malachová, A.; Sulyok, M.; Krska, R. Challenges and future directions in LC-MS-based multiclass method development for the quantification of food contaminants. Anal. Bioanal. Chem. 2021, 413, 25–34. [Google Scholar] [CrossRef]
- Invanova, A.S.; Merkuleva, A.D.; Andreev, S.V.; Sakharov, K.A. Method for determination of hydrogen peroxide in adulterated milk using high performance liquid chromatography. Food. Chem. 2019, 283, 431–436. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Q.; Qiu, X.L.; Jin, Y.; Ji, J.Y.; Lin, Y.J.; Le, S.Y.; Wang, G.Q.; Lu, D.S. Comprehensive strategy for analysis of pesticide multi-residues in food by GC–MS/MS and UPLC-Q-Orbitrap. Food. Chem. 2020, 320, 126576. [Google Scholar] [CrossRef]
- Wen, X.; Neethirajan, S. Ensuring food safety: Quality monitoring using microfluidics. Trends. Food. Sci. Tech. 2017, 65, 10–22. [Google Scholar]
- Sun, Z.C.; Lv, J.W.; Liu, X.; Tang, Z.W.; Wang, X.R.; Xu, Y.; Hammock, B.D. Development of a nanobody-aviTag fusion protein and its application in a streptavidin-biotin-amplified enzyme-linked immunosorbent assay for ochratoxin a in cereal. Anal. Chem. 2018, 90, 10628–10634. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Zhong, Y.H.; Li, G.K.; Hu, Y.F. Rapid analysis of quinoxalines in feeds by a fluorescence sensor based ontetraphenylmethane porous organic framework membrane. Sensor. Actuat. B. Chem. 2022, 356, 131350. [Google Scholar] [CrossRef]
- Santos, A.D.C.; Fonseca, F.A.; Lião, L.M.; Alcantara, G.B.; Barison, A. High-resolution magic angle spinning nuclear magnetic resonance in foodstuff analysis. Trac. Trend. Anal. Chem. 2015, 73, 10–18. [Google Scholar] [CrossRef]
- Ferrari, A.G.; Crapnell, R.D.; Banks, C.E. Electroanalytical overview: Electrochemical sensing platforms for food and drink safety. Biosensors 2021, 11, 291. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.W.; Gao, S.W.; Wang, B.; Kong, H.; Yang, G.Z.; Shu, W.D.; Xu, P.L.; Wei, G. Two-Dimensional material-based electrochemical sensors/biosensors for food safety and biomolecular detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Gan, T.; Wang, Z.K.; Shi, Z.X.; Zheng, D.Y.; Sun, J.Y.; Liu, Y.M. Graphene oxide reinforced core-shell structured Ag@Cu2O with tunable hierarchical morphologies and their morphology-dependent electrocatalytic properties for bio-sensing applications. Biosens. Bioelectron. 2018, 112, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.J.; Wang, Q.W.; Xue, S.J.; Wang, G.L.; Zou, Z.Q.; Yu, N.F.; Huang, Q.H.; Fu, L.J.; Wu, Y.P. A sensor of liquid methanol for direct methanol fuel cells. Anal. Chim. Acta. 2021, 1177, 338785. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, M.M.; Shetti, N.P.; Kulkarni, R.M.; Chandra, P. Nanostructured Ba/ZnO modified electrode as a sensor material for detection of organosulfur thiosalicylic acid. Microchem. J. 2020, 159, 105409. [Google Scholar] [CrossRef]
- Li, F.Z.; Ni, B.B.; Zheng, Y.R.; Huang, Y.X.; Li, G.L. A simple and efficient voltammetric sensor for dopamine determination based on ZnO nanorods/electro-reduced graphene oxide composite. Surf. Interfaces 2021, 26, 101375. [Google Scholar] [CrossRef]
- Murugan, P.; Nagarajan, R.D.; Shetty, B.H.; Govindasamy, M.; Sundramoothy, A.K. Recent trends in the applications of thermally expanded graphite for energy storage and sensors–A review. Nanoscale Adv. 2021, 3, 6294–6309. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.P.; Zhang, L.N.; Zhang, Y.C.; Shen, L. Phosphorus-doped graphene-based electrochemical sensor for sensitive detection of acetaminophen. Anal. Chim. Acta. 2018, 1036, 26–32. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.C.; Sun, X.; Zha, X.Q.; Khan, S.U.; Wang, Y. Ultrasensitive electrochemical detection of butylated hydroxy anisole via metalloporphyrin covalent organic frameworks possessing variable catalytic active sites. Biosensors 2022, 12, 975. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Nie, B.B.; Qu, X.Y.; Wang, M.H.; Yang, J.; Li, G.X. Histostar-functionalized covalent organic framework for electrochemical detection of exosomes. Biosensors 2022, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, N.; Wang, L.Y.; Song, Y.H.; Du, Y.; Ma, G.R. Biosensor based on covalent organic framework immobilized acetylcholinesterase for ratiometric detection of carbaryl. Biosensors 2022, 12, 625. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Martínez-Fernández, M.; Segura, J.L.; Lorenzo, E. Electrochemical (bio)sensors based on covalent organic frameworks (COFs). Sensors 2022, 22, 4758. [Google Scholar] [CrossRef]
- Zha, X.Q.; Sun, X.; Chu, H.C.; Wang, Y. Synthesis of bimetallic covalent organic framework nanocomposite for enhanced electrochemical detection of gallic acid. Colloid. Surf. A 2022, 651, 129748. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, G.L.; Wu, D.; Zhng, B.; Hu, N.; Wang, H.L.; Liu, J.H.; Wu, Y.N. Recent advances in the construction of functionalized covalent organic frameworks and their applications to sensing. Biosens. Bioelectron. 2019, 145, 111699. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.L.; Wu, Z.; Wen, W.; Zhang, X.H.; Wang, S.F. Electrochemical sensor based on confined synthesis of gold nanoparticles @covalent organic frameworks for the detection of bisphenol A. Anal. Chim. Acta 2023, 1239, 340743. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hu, H.Y.; Lu, S.L.; Hu, J.D.; Zhu, H.; Duan, F.; Du, M.L. Conductive metal and covalent organic frameworks for electrocatalysis: Design principles, recent progress and perspective. Nanoscale 2022, 14, 277–288. [Google Scholar] [CrossRef]
- Cȏté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keefe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Dong, J.Q.; Li, X.; Peh, S.B.; Yuan, Y.D.; Wang, Y.X.; Ji, D.X.; Peng, S.J.; Liu, G.L.; Ying, S.M.; Yuan, D.Q.; et al. Restriction of molecular rotors in ultrathin two-dimensional covalent organic framework nanosheets for sensing signal amplification. Chem. Mater. 2019, 31, 146–160. [Google Scholar] [CrossRef]
- Lyu, H.; Diercks, C.S.; Zhu, C.H.; Yaghi, O.M. Porous crystalline olefin-linked covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 6848–6852. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Gan, S.X.; Zhang, X.; Li, H.; Qi, Q.Y.; Cui, F.Z.; Lu, J.; Zhao, X. Aminal-linked covalent organic frameworks through condensation of secondary amine with aldehyde. J. Am. Chem. Soc. 2019, 141, 14981–14986. [Google Scholar] [CrossRef]
- Wang, K.W.; Jia, Z.F.; Bai, Y.; Wang, X.; Hodgkiss, S.E.; Chen, L.J.; Chong, S.Y.; Wang, X.Y.; Yang, H.F.; Xu, Y.J.; et al. Synthesis of stable thiazole-linked covalent organic frameworks via a multicomponent reaction. J. Am. Chem. Soc. 2020, 142, 11131–11138. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.R.; Li, F.F.; Xu, R.H.; Zhang, C.R.; Chen, X.R.; Yan, R.H.; Liang, R.P.; Qiu, J.D. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew. Chem. Int. Ed. 2020, 59, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jagt, R.V.D.; Vasileiadis, A.; Veldhuizen, H.; Shao, P.P.; Feng, X.; Ganapathy, S.; Habisreutinger, N.C.; Veen, M.A.V.D.; Wang, C.; Wagemaker, M.; et al. Synthesis and structure-property relationships of polyimide covalent organic frameworks for carbon dioxide capture and (aqueous) sodium-ion batteries. Chem. Mater. 2021, 33, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Preet, K.; Gupta, G.; Kotal, M.; Kansal, S.K.; Salunke, D.B.; Sharma, H.K.; Sahoo, S.C.; Voort, P.V.D.; Roy, S. Mechanochemial synthesis of a new triptycene-based imine- linked covalent organic polymer for degradation of organic dye. Cryst. Growth. Des. 2019, 19, 2525–2530. [Google Scholar] [CrossRef]
- Lv, H.Z.; Zhao, X.L.; Niu, H.Y.; He, S.J.; Tang, Z.; Wu, F.C.; Giesy, J.P. Ball milling synthesis of covalent organic framework as a highly active photocatalyst for degradation of organic contaminants. J. Hazard. Mater. 2019, 369, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhao, W.J.; Niu, H.Y.; Cai, Y.Q. Mechanochemical construction 2D/2D covalent organic nanosheets heterojunctions based on substoichiometric covalent organic frameworks. ACS Appl. Mater. Interfaces 2021, 13, 42035–42043. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yang, Y.; Zhao, Z.F.; Zhang, P.H.; Zhang, Y.S.; Liu, J.J.; Ma, S.Q.; Cheng, P.; Chen, Y.; Zhang, Z.J. Green synthesis of olefin-linked covalent organic frameworks for hydrogen fuel cell applications. Nat. Commun. 2021, 12, 1982. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Meng, F.C.; Wu, D.Q.; Zhang, F. Synthesis of vinylene-linked covalent organic frameworks by monomer self-catalyzed sctivation of knoevenagel condensation. J. Am. Chem. Soc. 2022, 144, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Ma, R.; Xu, S.Q.; Zheng, T.Y.; Fu, G.G.; Wu, Y.L.; Liao, Z.Q.; Kuang, Y.B.; Hou, Y.; Wang, D.S.; et al. Direct construction of isomeric benzobisoxazole-vinylene-linked covalent organic frameworks with distinct photocatalytic properties. J. Am. Chem. Soc. 2022, 144, 13953–13960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.H.; Wang, Z.F.; Yang, Y.; Wang, S.; Wang, T.; Liu, J.J.; Cheng, P.; Chen, Y.; Zhang, Z.J. Melt polymerization synthesis of a class of robust self-shaped olefin-linked COF foams as high-efficiency separators. Sci. China. Chem. 2022, 6, 65. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Chen, X.P.; Li, B.Y.; Zhao, S.; Niu, L.W.; Zhang, Z.J.; Chen, Y. Spatial regulation of acceptor units in olefin-linked COFs toward highly efficient photocatalytic H2 evolution. Adv. Sci. 2022, 9, 2203832. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Mao, T.H.; Hao, L.Q.; Sun, T.K.; Wang, T.H.; Cheng, P.; Chen, Y.; Wang, Z.F.; Zhang, Z.J. Solvent-free synthesis of C=N linked two-dimensional covalent organic frameworks. Macromol. Rapid. Commun. 2022, 2200722, 1–8. [Google Scholar] [CrossRef]
- Campbell, N.L.; Clowes, R.; Ritchie, L.K.; Cooper, A.I. Rapid microwave synthesis and purification of porous covalent organic frameworks. Chem. Mater. 2009, 21, 204–206. [Google Scholar] [CrossRef]
- Ren, S.J.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, L.G.; Yuan, Y.P.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Microwave-assisted synthesis of highly fluorescent nanoparticles of a melamine-based porous covalent organic framework for trace-level detection of nitroaromatic explosives. J. Hazard. Mater. 2012, 221–222, 147–154. [Google Scholar] [CrossRef]
- Wei, H.; Chai, S.Z.; Hu, N.T.; Yang, Z.; Wei, L.M.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178. [Google Scholar] [CrossRef]
- Ding, Y.S.; Wang, Y.; Su, Y.J.; Yang, Z.; Liu, J.Q.; Hua, X.L.; Wei, H. A novel channel-wall engineering strategy for two-dimensional cationic covalent organic frameworks: Microwave-assisted anion exchange and enhanced carbon dioxide capture. Chin. Chem. Lett. 2020, 31, 193–196. [Google Scholar] [CrossRef]
- Chen, L.; Du, J.C.; Zhou, W.; Shen, H.Z.; Tan, L.X.; Zhou, C.L.; Dong, L.C. Microwave-Assisted solvothermal synthesis of covalent organic frameworks (COFs) with stable superhydrophobicity for oil/water separation. Chem-Asian. J. 2020, 15, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Kim, J.; Cho, H.Y.; Kim, S.; Ahn, W.S. Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method. RSC Adv. 2021, 2, 10179–10181. [Google Scholar] [CrossRef]
- Yoo, J.T.; Lee, S.H.; Hirata, S.; Kim, C.R.; Lee, C.K.; Shiraki, T.; Nakashima, N.; Shim, J.K. In situ synthesis of covalent organic frameworks (COFs) on carbon nanotubes and graphenes by sonochemical reaction for CO2 adsorbents. Chem. Lett. 2015, 44, 560–562. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, P.Y.; Yang, H.F.; Bahri, M.; James, A.M.; Chen, H.M.; Liu, L.J.; Li, B.Y.; Pang, Z.F.; Clowes, R.; et al. Using sound to synthesize covalent organic frameworks in water. Nat. Synth. 2022, 1, 87–95. [Google Scholar] [CrossRef]
- Kumar, S.; Lgnacz, G.; Szekely, G. Synthesis of covalent organic frameworks using sustainable solvents and machine learning. Green Chem. 2021, 23, 8932–8939. [Google Scholar] [CrossRef]
- Aykol, M.; Montoya, J.H.; Hummelshøj, J. Rational solid-state synthesis routes for inorganic materials. J. Am. Chem. Soc. 2021, 143, 9244–9259. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X.M. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef]
- Sun, Y.F.; He, J.B.; Waterhouse, G.I.N.; Xu, L.H.; Zhang, H.Y.; Qiao, X.G.; Xu, Z.X. A selective molecularly imprinted electrochemical sensor with GO@COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sensor. Actuat. B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Pan, F.; Tong, C.Y.; Wang, Z.Y.; Han, H.T.; Liu, P.; Pan, D.W.; Zhu, R.L. Nanocomposite based on graphene and intercalated covalent organic frameworks with hydrosulphonyl groups for electrochemical determination of heavy metal ions. Microchim. Acta 2021, 188, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Xie, Y.; Yang, Y.X.; Liang, H.H.; Wang, L.; Song, Y.H. Electroactive covalent organic frameworks/carbon nanotubes composites for electrochemical sensing. ACS Appl. Nano Mater. 2020, 3, 1412–1419. [Google Scholar] [CrossRef]
- Yuan, R.R.; Yan, Z.J.; He, H.M. Crystal engineering of C60 fullerenes trapped in covalent organic frameworks for enhanced electrochemical impedimetric aptasensing performance. Appl. Surf. Sci. 2022, 573, 151556. [Google Scholar] [CrossRef]
- Wu, N.; Wang, L.Y.; Xie, Y.; Du, Y.; Song, Y.H.; Wang, L. Double signal ratiometric electrochemical riboflavin sensor based on macroporous carbon/electroactive thionine-contained covalent organic framework. J. Colloid Interfaces Sci. 2022, 608, 219–226. [Google Scholar] [CrossRef]
- Zhu, P.H.; Li, S.S.; Zhou, S.; Ren, N.; Ge, S.G.; Zhang, Y.; Wang, Y.F.; Yu, J.H. In situ grown COFs on 3D strutted graphene aerogel for electrochemical detection of NO released from living cells. Chem. Eng. J. 2021, 420, 127559. [Google Scholar] [CrossRef]
- Arul, P.; Huang, S.T.; Gowthaman, N.S.K.; Shankar, S. Simultaneous electrochemical determination of DNA nucleobases using AgNPs embedded covalent organic framework. Microchim. Acta 2021, 188, 358. [Google Scholar] [CrossRef]
- Sun, L.; Guo, H.; Pan, Z.L.; Liu, B.Q.; Zhang, T.T.; Yang, M.; Wu, N.; Zhang, J.Y.; Yang, F.; Yang, W. In-situ reducing platinum nanoparticles on covalent organic framework as a sensitive electrochemical sensor for simultaneous detection of catechol, hydroquinone and resorcinol. Colloids Surf. A 2022, 635, 128114. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, T.; Chen, Y.L.; Wang, Y.; Wang, L. Fabrication of core-shell magnetic covalent organic frameworks composites and their application for highly sensitive detection of luteolin. Talanta 2020, 213, 120843. [Google Scholar] [CrossRef]
- Chu, H.C.; Sun, X.; Zha, X.Q.; Zhang, Y.; Wang, Y. Synthesis of core-shell structured metal oxide@covalent organic framework composites as a novel electrochemical platform for dopamine sensing. Colloids Surf. A 2022, 648, 129238. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.X.; Wang, L.Y.; Wang, L.; Chen, S.H. An ultrafine ZnO/ZnNi2O4@porous carbon@covalent-organic framework for electrochemical detection of paracetamol and tert-butyl hydroquinone. J. Alloy. Compd. 2022, 906, 164369. [Google Scholar] [CrossRef]
- Pan, Z.L.; Guo, H.; Sun, L.; Liu, B.Q.; Chen, Y.; Zhang, T.T.; Wang, M.Y.; Peng, L.P.; Yang, W. A novel electrochemical platform based on COF/La2O3/MWCNTS for simultaneous detection of dopamine and uric acid. Colloids Surf. A 2022, 635, 128083. [Google Scholar] [CrossRef]
- Mulzer, C.R.; Shen, L.X.; Bisbey, R.P.; McKone, J.R.; Zhang, N.; Abruña, H.D.; Dichtel, W.R. Superior charge storage and power density of a conducting polymer-modified covalent organic framework. ACS Central Sci. 2016, 2, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Y.L.; Sun, X.; Wang, Y.; Wang, Y. Conducting polymer engineered covalent organic framework as a novel electrochemical amplifier for ultrasensitive detection of acetaminophen. Chin. Chem. Lett. 2021, 32, 2061–2065. [Google Scholar] [CrossRef]
- Xie, F.; Yang, M.; Jiang, M.; Huang, X.J.; Liu, W.Q.; Xie, P.H. Carbon-based nanomaterials–A promising electrochemical sensor toward persistent toxic substance. Trac-Trend. Anal. Chem. 2019, 119, 115624. [Google Scholar] [CrossRef]
- Lu, S.Y.; Wang, S.L.; Wu, P.; Wang, D.Q.; Yi, J.C.; Li, L.; Ding, P.; Pan, H.Z. A composite prepared from covalent organic framework and gold nanoparticles for the electrochemical determination of enrofloxacin. Adv. Powder Technol. 2021, 32, 2106–2115. [Google Scholar] [CrossRef]
- Wang, L.J.; Gao, W.L.; Ng, S.; Pumera, M. Chiral protein-covalent organic framework 3D-Printed structures as chiral biosensors. Anal. Chem. 2021, 93, 5277–5283. [Google Scholar] [CrossRef]
- Pang, Y.H.; Wang, Y.Y.; Shen, X.F.; Qiao, J.Y. Covalent organic framework modified carbon cloth for ratiometric electrochemical sensing of bisphenol A and S. Microchim. Acta 2022, 189, 189. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Pang, Y.H.; Shen, X.F.; Jiang, R.; Wang, Y.Y. Covalent organic framework DQTP modified pencil graphite electrode for simultaneous determination of bisphenol A and bisphenol S. Talanta 2022, 236, 122859. [Google Scholar] [CrossRef]
- Goulart, L.A.; Teixeira, A.R.L.; Ramalho, D.A.; Terezo, A.J.; Castilho, M. Development of an analytical method for the determination of tert-butylhydroquinone in soybean biodiesel. Fuel 2014, 115, 126–131. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xie, Y.; Sun, X.; Wang, Y.; Wang, Y. Tunable construction of crystalline and shape-tailored Co3O4@TAPB-DMTP-COF composites for the enhancement of tert-butylhydroquinone electrocatalysis. Sensor. Actuat. B Chem. 2021, 331, 129438. [Google Scholar] [CrossRef]
- Yang, Y.K.; Shi, Z.; Wang, X.M.; Bai, B.Q.; Qin, S.; Li, J.D.; Jing, X.; Tian, Y.; Fang, G.Z. Portable and on-site electrochemical sensor based on surface molecularly imprinted magnetic covalent organic framework for the rapid detection of tetracycline in food. Food Chem. 2022, 395, 133532. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Pang, C.H.; Li, S.H.; Xiong, Y.H.; Li, J.P.; Luo, J.H.; Yang, Y. Synthesis of Zr-coordinated amide porphyrin-based two-dimensional covalent organic framework at liquid-liquid interface for electrochemical sensing of tetracycline. Biosens. Bioelectron. 2019, 146, 111734. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.R.; Liu, Z.B.; Sun, H.; He, H.M. Porphyrin-based porous organic frameworks for the ultrasensitive electrochemical impedimetric aptasensing of oxytetracycline. Appl. Surf. Sci. 2021, 569, 151038. [Google Scholar] [CrossRef]
- Sun, Y.F.; Xu, L.H.; Waterhouse, G.I.N.; Wang, M.L.; Qiao, X.G.; Xu, Z.X. Novel three-dimensional electrochemical sensor with dual signal amplification based on MoS2 nanosheets and high-conductive NH2-MWCNT@COF for sulfamerazine determination. Sensor. Actuat. B Chem. 2019, 281, 107–114. [Google Scholar] [CrossRef]
- Sun, Y.F.; Waterhouse, G.I.N.; Xu, L.H.; Qiao, X.G.; Xu, Z.X. Three-dimensional electrochemical sensor with covalent organic framework decorated carbon nanotubes signal amplification for the detection of furazolidone. Sensor. Actuat. B Chem. 2020, 321, 128501. [Google Scholar] [CrossRef]
- Yuan, R.R.; He, H.M. Construction of an electrochemical aptasensor based on a carbazole-bearing porous organic polymer for rapid and ultrasensitive detection of penicillin. Appl. Surf. Sci. 2021, 563, 150307. [Google Scholar] [CrossRef]
- Han, Z.Y.; Zhang, H.; Li, H.K.; Zhu, Q.Q.; He, H.M. Ingenious construction of an electrochemical aptasensor based on a Au@COF/GO-NH2 composite with excellent detection performance. J. Mater. Chem. C 2021, 9, 4576. [Google Scholar] [CrossRef]
- Xiao, Y.W.; Wu, N.; Wang, L.; Chen, L.L. A novel paper-based electrochemical biosensor based on N, O-rich covalent organic frameworks for carbaryl detection. Biosensors 2022, 12, 899. [Google Scholar] [CrossRef]
- Liu, C.; Cai, L.; Wang, X.H.; Guo, Y.; Fang, G.Z.; Wang, S. Construction of molecularly imprinted sensor based on covalent organic frameworks DAFB-DCTP-doped carbon nitride nanosheets with high electrochemiluminescence activity for sensitive detection of carbaryl. Microchem. J. 2022, 178, 107416. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wu, N.; Wang, L.; Song, Y.H.; Ma, G.R. Accurate detection of organophosphorus pesticides based on covalent organic framework nanofiber with a turn-on strategy. Sensor. Actuat. B Chem. 2022, 372, 132608. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, Y.Y.; Chen, J.P.; Lu, X.B. 2D conductive covalent organic frameworks with abundant carbonyl groups for electrochemical sensing. ACS Sens. 2022, 7, 3551–3559. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Shan, J.J.; Bai, X.T. Novel electrochemical acetylcholinesterase biosensor based on core-shell covalent organic Framework@Multi-Walled carbon nanotubes (COF@MWCNTs) composite for detection of malathion. Int. J. Electrochem. Sci. 2022, 17, 220543. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, M.Y.; Jin, C.; Zeng, J.X.; Huang, C.; Song, Q.Y.; Song, Y.G. Preparation of a sensor based on biomass porous carbon/covalent-organic frame composites for pesticide residues detection. Front. Chem. 2020, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, M.R.J.; Madrakian, T.; Afkhami, A. Simultaneous determination of Pb2+ and Hg2+ at food specimens by a melamine-based covalent organic framework modified glassy carbon electrode. Food Chem. 2023, 402, 134246. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, M.R.J.; Madrakian, T.; Afkhami, A. Ultra-trace levels voltammetric determination of Pb2+ in the presence of Bi3+ at food samples by a Fe3O4@Schiff base Network1 modified glassy carbon electrode. Talanta 2022, 250, 123716. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhai, H.G.; Wang, Y.; Wang, B.; Chu, G.L.; Guo, Q.; Zhang, Y.H.; Sun, X.; Guo, Y.M.; Zhang, Y.Y. COF/MWCNTs/CLS Based electrochemical sensor for simultaneous and sensitive detection of multiple heavy metal ions. Food Anal. Method. 2022, 15, 3244–3256. [Google Scholar] [CrossRef]
- Guo, L.L.; Wang, Y.Y.; Pang, Y.H.; Shen, X.F.; Yang, N.C.; Ma, Y.; Zhang, Y. In situ growth of covalent organic frameworks TpBD on electrode for electrochemical determination of aflatoxin M1. J. Electroanal. Chem. 2021, 881, 114931. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, X.; Wu, J.Y.; Liu, Q.L.; Li, D.F.; Huang, S.F.; Xie, H.Z.; Yu, Z.Z.; Gan, N. Reusable electrochemical biosensing platform based on egg yolk antibody-labeled magnetic covalent organic framework for on-site detection of Escherichia coli in foods. Sensor. Actuat. B Chem. 2022, 369, 132320. [Google Scholar] [CrossRef]
- Deceuninck, Y.; Bichon, E.; Gény, T.; Veyrand, B.; Grandin, F.; Viguié, C.; Marchand, P.; Bizec, B.L. Quantitative method for conjugated metabolites of bisphenol A and bisphenol S determination in food of animal origin by ultra high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1601, 232–242. [Google Scholar] [CrossRef]
- Fernandes, P.; Martens, E. Antibiotics in late clinical development. Biochem. Pharmacol. 2017, 133, 152–163. [Google Scholar] [CrossRef]
- Magalhães, D.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Ramos, F. The use of ultra-high-pressure-liquid-chromatography tandem time-of-flight mass spectrometry as a confirmatory method in drug residue analysis: Application to the determination of antibiotics in piglet liver. J. Chromatogr. B 2017, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.X.; Lai, K.Q.; Rasco, B.A.; Huang, Y.Q. Determination of carbaryl pesticide in Fuji apples using surface-enhanced raman spectroscopy coupled with multivariate analysis. LWT Food Sci. Technol. 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Harshit, D.; Charmy, K.; Nrupesh, P. Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chem. 2017, 230, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Fathabad, A.E.; Shariatifar, N.; Moazzen, M.; Nazmara, S.; Fakhri, Y.; Alimohammadi, M.; Azari, A.; Khaneghah, A.M. Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: A risk assessment study. Food Chem. Toxicol. 2018, 115, 436–446. [Google Scholar] [CrossRef]
- Jia, Y.M.; Zhou, G.H.; Wang, X.D.; Zhang, Y.Z.; Li, Z.G.; Liu, P.L.; Yu, B.; Zhang, J. A metal-organic framework/aptamer system as a fluorescent biosensor for determination of aflatoxin B1 in food samples. Talanta 2020, 219, 121342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).