Abstract
Over the past two decades, lanthanide-based upconversion nanoparticles (UCNPs) have been fascinating scientists due to their ability to offer unprecedented prospects to upconvert tissue-penetrating near-infrared light into color-tailorable optical illumination inside biological matter. In particular, luminescent behavior UCNPs have been widely utilized for background-free biorecognition and biosensing. Currently, a paramount challenge exists on how to maximize NIR light harvesting and upconversion efficiencies for achieving faster response and better sensitivity without damaging the biological tissue upon laser assisted photoactivation. In this review, we offer the reader an overview of the recent updates about exciting achievements and challenges in the development of plasmon-modulated upconversion nanoformulations for biosensing application.
1. Introduction
Lanthanide-based upconversion nanoparticles (UCNPs) have become the engine of numerous ground-breaking inventions in a wide variety of research areas. By disobeying the Stokes Law, UCNPs are capable of producing higher energy output photons out of multiple (>2) lower energy input photons. For example, one can obtain ultraviolet (UV) or visible emission by exposing UCNPs to near infrared (NIR) laser. Contrary to other light upconverting analogues, such as organic luminophores and quantum dots, lanthanide-based UCNPs indeed possess overwhelming advantages, which include a diversity of emission colors, long lifetime luminescence, large anti-Stokes spectral shifts, weak background autofluorescence, narrow emission bands, nonphotobleaching nature, blinking-free continuous emission capability, and relatively low toxicity [1,2,3]. So far, UCNPs with tailored multicolor emissions have underpinned a vast array of applications in energy conversion photovoltaics [4], fingerprint detection and anticounterfeiting barcoding [5,6], biosensing [7], super resolution nanoscopy [8], photodetectors [9], drug and gene delivery [10], photodynamic therapy [11], light-triggered on–off tattoo systems [12], photochemical tissue bonding [13] and so on.
In particular, UCNPs have garnered increased interest in the quantitative detection of various biologically relevant targets, such as biomolecules, pH, ions, viruses, bacteria, reactive oxygen species and temperature [14]. However, the development of upconversion biosensing has been seriously hampered by poor NIR harvesting ability and a long-standing issue of quenching, which has multiple origin sources causing a great deal for scientists in finding the straightforward solution. At the edge of the UCNP surface, the excited state electrons undergo parasitic interaction with the surface defects (e.g., stacking faults, vacancies, dislocation, dangling bonds) which deplete the excitation energy leading to a partial decrease or complete “turn off” of the UCNP luminescence.
Composition tailoring strategies by architecting the core with single or multiple shell structures are at the forefront to combat quenching issue. However, luminescence producing dopants are still kept at low doping levels to support quenching stability at the expense of upconversion brightness. In fact, quenching issue and composition limits can be addressed by extremely high NIR laser excitation powers (~106 W/cm2 [15,16]), of which the main concern is associated with harmfulness and inapplicability for biomedical applications [17]. NIR laser power densities should be compatible with the tolerances of the skin tissue and kept below the maximum permissible exposure (MPE) values calculated according to ANSI Z136.1-2007 American National Standard for the Safe Use of Lasers [18]. The calculated MPE values for the corresponding wavelength of the NIR lasers are presented in Table 1.

Table 1.
Calculated safe power densities of common NIR lasers [19] (MPE—maximum permissible exposure, NIR—near-infrared).
The Förster (or fluorescence) resonance energy transfer (FRET) process has vast implications in quantitative and sensitive biosensing applications. In principle, an efficient FRET scenario occurs under specified conditions. First, there should a large degree of overlap in the emission-excitation profiles of the donor (excited state) and acceptor (ground state). Second, the long-range nonradiative dipole-dipole interaction between the donor-acceptor pair should be within the effective range of 10 nm [20]. The FRET process can be utilized for tuning optical properties of UCNPs by sizeable enhancement or depletion of their excitation or emission energy. Therefore, the FRET-based upconversion mechanism has been extensively exploited for bioanalytical sensors and assays, which enlivened modern bioimaging and biosensor research [21,22,23]. To date, extensive efforts have been focused on developing various energy acceptors, such as plasmonic nanostructures and organic dyes, which can aid in modulation photoluminescence efficiency of energy donating UCNPs via the FRET process. Compared to organic dyes which are prone to photodegradation, plasmon-enhanced upconversion has been considered as a breakthrough engineering strategy to minimize quenching issue, maximize upconversion efficiency and stimulate NIR harvesting offering unprecedented biosensing precision. In particular, the plasmon resonances could serve to achieve control over the luminescence properties of UCNPs by intentional amplification or quenching of the specific emission band [24], which could be beneficial for designing sensing platforms with specificity, low detection limits and linear response to the presence of the target bioanalyte. A recent literature survey has shown that several reviews related to the biosensing applications of UCNPs were published recently [7,14,25,26]. On the other hand, a focused overview of plasmon-modulated upconversion biosensing is still not available in the literature. Therefore, the objective of this review is to offer the reader a brief overview of the up-to-date strategies to incorporate plasmonic nanoparticles to lanthanide UCNPs for potential biosensing applications.
2. Characteristics of UCNPs
2.1. Lanthanide Upconversion Basics: Mechanism and Origin
The upconversion is a nonlinear optical effect which gained widespread scientific interest with the advent of lasers. It is generally acknowledged that the concept of upconversion was proposed in the 1950s by Bloembergen and further developed by Auzel in the 1960s who introduced groundbreaking theoretical studies on the energy transfer upconversion process [27]. Since the 1990s, the emergence of nanoscience has brought a huge diversification of nanofabrication technologies leading to efficient downscaling upconversion phosphors to nanometer regime making them tremendously attractive across a range of biomedical applications [28].
Even now, the field of upconversion keeps evolving. Over time, it is believed to practically benefit health care and different industrial sectors. Ongoing research in the upconversion field continues to uncover and explain the complicated mechanisms behind photon upconversion. Meanwhile, there are several known mechanisms responsible for upconversion origins, such as excited state absorption, energy transfer upconversion, cooperative upconversion, photon avalanche, and energy migration upconversion [2]. This review deals with energy transfer based upconversion, therefore another deep-in depth description of upconversion mechanisms is beyond the scope of this review.
Upconversion in lanthanides originates from their ladder-like arrangement of energy levels which impart effective absorption and subsequent retaining of incident photons from external laser until a sufficient quantity is reached to proceed with upconverted emission. In principle, the upconversion process relies on electronic transitions within partially filled 4f orbitals. Notably, 4f shells in lanthanides are well protected by 5s and 5p shells (secondary electrons) rendering significant advantages, such as high resistance to (environmental influences) photobleaching, photochemical stability and sharp emission bands from 4f-4f transitions. However, the electron shield impedes 4f transitions and imposes limitations on light harvesting properties leading to poor absorption cross sections of lanthanides [29]. For a single and isolated lanthanide ion, these transitions are forbidden by selection rules of quantum mechanics, but this situation drastically changes when lanthanides are embedded as dopants inside the host matrix enhancing the probability of 4f-4f transitions. Selection rules on the spin are relaxed by a larger spin-orbit coupling. In particular, doping distorts site symmetry, which forms a stronger crystal field around lanthanide ions. Such absence of inversion symmetry (noncentrosymmetric system) distorts the electron cloud leading to intermixing with f states. As a result, Coulombic interactions and spin-orbit coupling of 4f subshell electrons lead to abundant energy sublevels and a large number of emitting levels [30].
2.2. Lanthanide Upconversion Composition
The most common configuration of lanthanide based UCNPs is composed of lanthanide ion dopants, such as sensitizers, emissive activators, and sometimes energy migration ions, that reside in the crystal host lattice. The concentration and distance between the sensitizer and emitter ions are critical parameters for tailoring the optical properties of UCNPs. In particular, higher concentration and greater proximity are essential to increase luminosity, but also invoke non-radiative cross-relaxations, known as the "concentration quenching effect". Presently, as the main strategy for overcoming the concentration quenching hurdle, the doping levels of sensitizer (~20%) and emitter ions (<2%) are kept at low percentages to optimize the luminescence brightness [31].
Host and dopants interaction occur in the form of energy exchange. Thus, some energy is absorbed by the host through vibrational coupling, which explains the choice of host lattices with the lowest phonon energy. Decades of research in the upconversion field have spawned an extensive array of host nanomaterials, such as various metal oxides, oxysulfides, vanadates, halides, phosphates and so on [32]. Among them, the most popular host is hexagonal phase β-NaYF4, which meets the requirements for ideal host materials, such as the lowest phonon frequency, high optical transparency, less hygroscopic and brighter emission than lanthanide fluoride (LnF3) host [33,34,35]. Sodium ion (Na+) has been widely introduced into the lanthanide fluoride lattice because it has nearly the same ionic radius as lanthanide ions [36]. For instance, Ayadi et al. reported that introducing Na+ ions into GdF3 led to lattice expansion and imparted stabilization of its hexagonal crystal structure [37]. For simplicity, this review specifically concentrates on plasmon modulated upconversion systems constructed using metal lanthanide fluoride hosts.
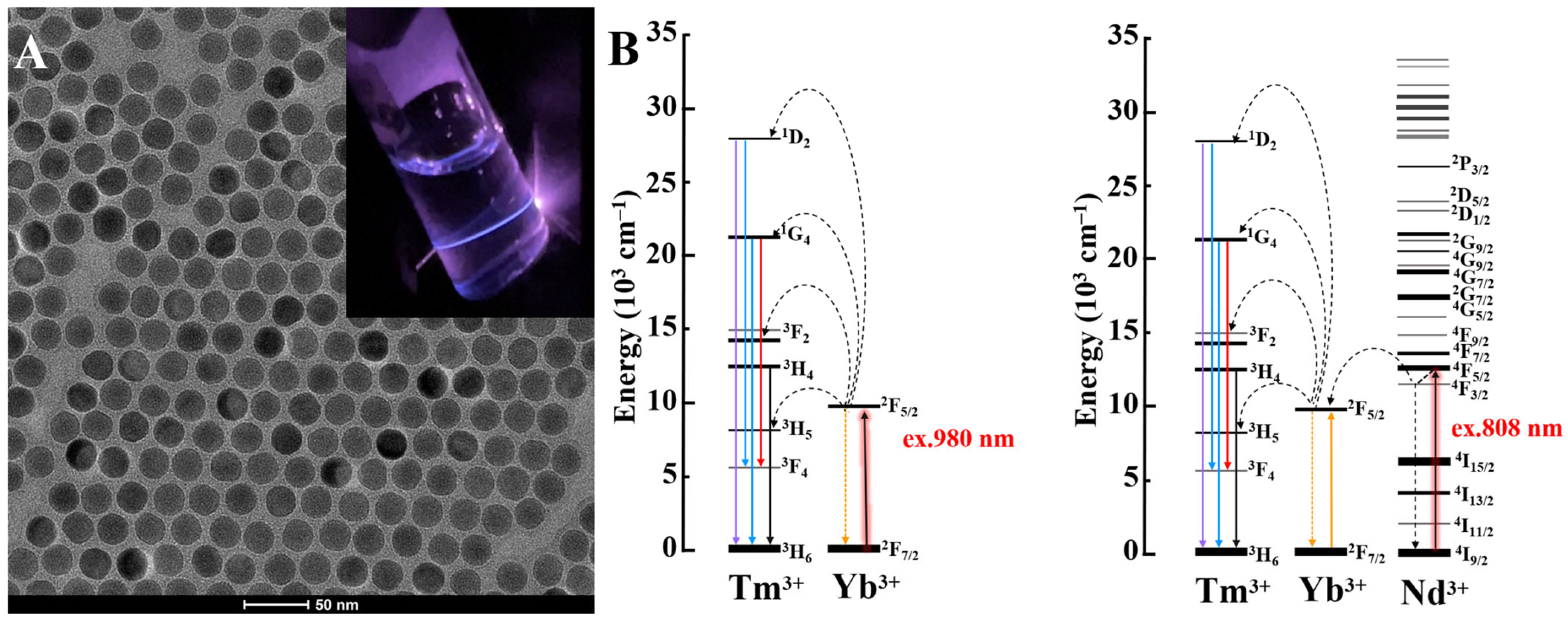
Activator ions, as their name suggests, receive the excitation energy from the sensitizer or energy migration ion to activate emission. The choice of activator ion plays a decisive role in finely tuning emission profiles of UCNPs, for instance, erbium (Er3+) ion is recruited to produce red/green emissions, while thulium (Tm3+) is broadly exploited for obtaining UV/blue emissions from UCNPs (Figure 1A) [5]. Figure 1A exemplifies a typical transmission electron micrograph of NaYF4:Yb,Tm UCNPs. Digital inset illustrates visible to naked-eye blue emission of NaYF4:Yb,Tm UCNPs under NIR laser illumination. Popular activators, such as erbium (Er3+), thulium (Tm3+) and holmium (Ho3+) ions, possess narrow energy gap of 2000 cm−1. Thereby, they are more efficient for the energy transfer process compared to other luminescent ions with a wide energy gap of 7000 cm−1, e.g., terbium (Tb3+), europium (Eu3+) and dysprosium (Dy3+) which are prone to detrimental nonradiative energy losses. Activators, in general, have a complicated electronic structure and extremely small absorption cross-sections of ~10−21 cm2 responsible for a low quantum yield (<1%) and necessary in photoactivation using high laser power densities [38].
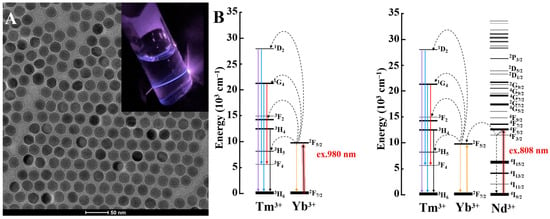
Figure 1.
(A) Representative TEM image of NaYF4:Yb,Tm UCNPs (scale 50 nm) with digital inset of their UC emission under NIR laser. (B) Upconversion process of Tm3+ activator under 980 nm and 808 nm excitation.
Sensitizers serve to accumulate incoming NIR photons and further transfer these photons to the activator ions directly or indirectly through energy migration ions. For example, ytterbium (Yb) is utilized as a ~980 nm NIR sensitizer with a single transition 2F7/2–2F5/2 levels, which showcases efficient energy transfer when paired with Tm3+ activator. Moreover, co-doping Yb ion with neodymium ion (Nd3+, <1 mol.%) allows tailoring 808 nm NIR harvesting upconversion system properties in UCNPs, where Yb3+ act as a migration ion facilitating the energy extraction from Nd3+ ions and its further transfer to activator ions [39]. Figure 1B displays the schematic energy diagrams of Tm3+ ion upconverted emission photoactivation under 980 nm and 808 nm NIR stimulation. Table 2 shows common lanthanide sensitizers and their corresponding absorption cross sections.

Table 2.
Common lanthanide sensitizers and their absorption cross-sections [40,41,42].
Importantly, 808 nm NIR harvesting UCNPs have arisen as a feasible platform for biomedical application to overcome the safety shortcomings of those excitable under conventional 980 nm lasers, since water molecules absorption of 808 nm photons is 20 times weaker than 980 nm ones [31].
Over the past few decades, explosive growth in the development of nanotechnology offered numerous synthetic protocols and optimized designs of UCNPs. By now, several routes to synthesize UCNPs have been proposed and widely utilized, such as co-precipitation [28], thermal decomposition [43], hydrothermal method [44], microwave-assisted synthesis [45], microemulsion method [46] and the liquid–solid solution (LSS) process [47]. Among these synthesis methods, co-precipitation and thermal decomposition methods have found remarkably broad utility in the scientific community for the fabrication of highly monodisperse and bright upconversion nanocrystals. However, excitement over developments in the controlled synthesis of small UCNPs is moderated by luminescence intensity decrease as the size shrinks the quenching issue becomes more prominent. Especially, the enhanced surface-to-volume ratio leads to greater exposure of the UCNPs surface to external quenchers, such as OH impurities, and organic ligands C-H and C-C bonds with a high vibrational energy that present in the dispersion solvent or capping ligands [48,49]. The main strategy so far to remove surface quenching relies on adopting surface passivation with single or multiple outermost shell layers, which mitigate surface defects and preclude the interaction with the external quenchers. However, the core-shell structure fabrication process usually requires prolonged and multi-step synthetic procedures. Therefore, researchers in the upconversion field are faced with the need to accelerate (e.g., by continuous process [50]) or even automate the synthesis process [51].
3. Principles and Applications of Photon Modulation in Upconversion-Based Biosensors
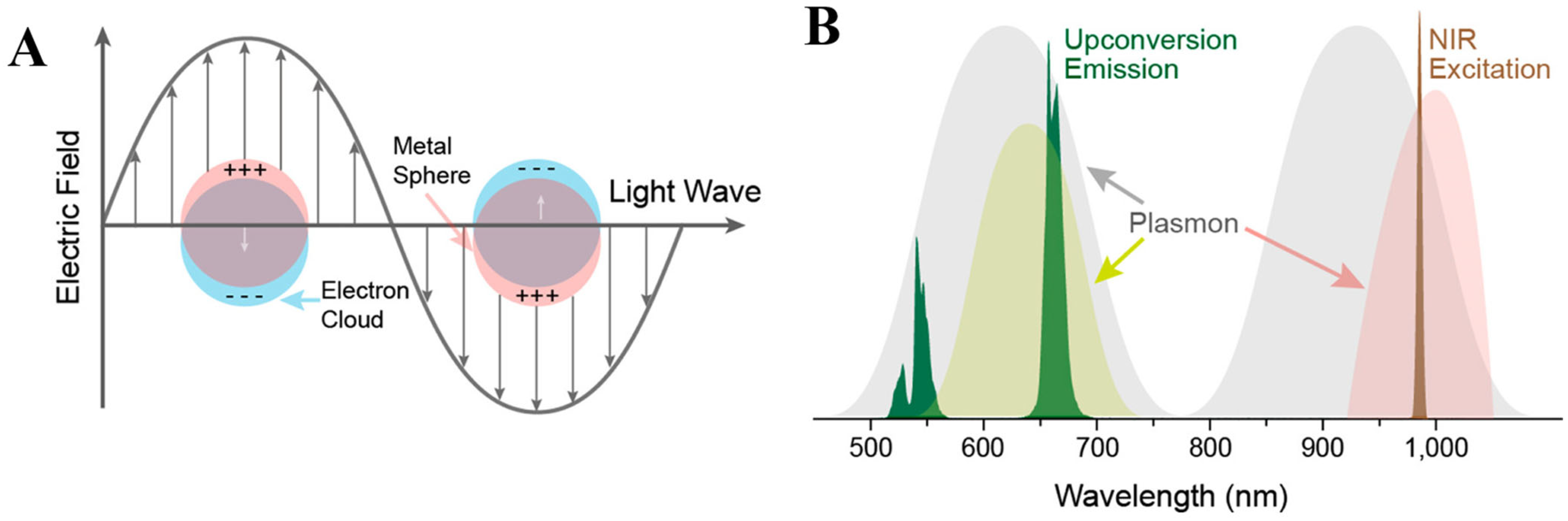
Surface plasmon resonance (SPR) is a light-matter interaction phenomenon inherent to materials with a negative real and small positive imaginary dielectric constant. When incident light interacts with the electron cloud of these materials, electrons start collectively oscillating inducing resonant effect (Figure 2A). Accordingly, surface plasmons could be localized or propagating. In the case of the localized surface plasmons, the excitation wavelength is larger than the nanoparticle causing its electron oscillation in a localized manner. Usually, the evanescent field forms the propagating surface plasmon (also known as surface plasmon polariton), in particular, when the incident light is polarized leading to surface charge oscillation along the metal/dielectric interface in the longitudinal direction and subsequently decay [52].
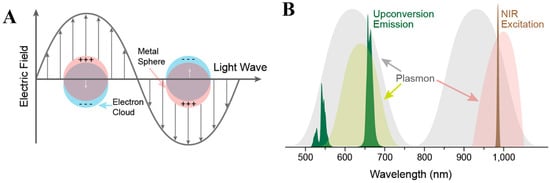
Figure 2.
(A) Schematics of a metal nanoparticle’s electron cloud oscillation. (B) Spectral overlap between metal nanoparticle’s SPR and upconversion nanoparticles emission or excitation profile. Reprinted with permission from reference [53].
3.1. A Brief Theoretical Principles of Plasmon Modulated Upconversion
SPR extension in UCNPs using plasmonic nanoparticles (PNPs) can serve to augment the functionality of upconversion sensing platforms by resonating its excitation or emission (Figure 2B) [53]. For example, upon plasmon-light coupling energy confinement leads to the amplification of the electromagnetic field benefiting NIR light harvesting abilities and radiative decay rates of UCNPs. Theoretically, plasmonic modulation can afford an enhancement of the upconversion emission intensity E2n time without energy transfer alteration. So far, 100-fold enhancements were verified experimentally [54].
Thus, PNPs are exploited as optical nanoantennae that could also provide modulation over the radiation features of UCNPs through excitation energy redistribution leading to luminescence enhancement (Purcell effect) or intentional quenching [24,55].
The design of plasmon-mode upconversion nanoprobes is commonly based on the matched SPR band of plasmonic nanoparticles and emission/excitation profiles of UCNPs. For example, the aspect ratio of gold nanorods governs the SPR band location and hence could be easily adjusted to modulate upconversion process [56]. Noteworthy, the proximity and isolation between UCNPs and PNPs are critically important for efficient energy transfer and sustaining undesired quenching [57]. For example, the use of a silica shell as a spacer between UCNPs and PNPs enables to prevent of their direct contact and unintentional quenching [58]. Meanwhile, the plasmon-upconversion interaction considerably depends on the geometrical morphology of PNPs. For instance, a variety of plasmonic nanostructures, such as nanoshells, nanofilms, nanowires, nanorods and nanoparticle arrays/cavity, were coupled to UCNPs to achieve plasmon modulated upconversion [59].
Gonzalez et al. investigated the upconversion quenching ability of different sizes of Au NPs ranging from 3.9 nm to 66 nm. Their findings suggest that size range Au NPs between 15 and 20 nm is optimal to initiate intentional quenching in silica coated UCNPs. Conversely, larger Au NPs with the size >50 nm tend to amplify the upconversion emission. In addition, their study also elucidated that an optimal shell thickness for upconversion enhancement is ca. 12 nm, while efficient quenching demands the thinnest possible shell [60].
By exploiting plasmon modulated upconversion strategy, a range of nanoprobes was constructed and proposed for examining a broad variety of analytes in biological environments. We analyzed recent literature published on the practical implications of plasmonic modulation of upconversion biosensors to detect diverse analytes and compiled our review in Table 3, which serves to provide insights into key parameters for designing plasmon modulated upconversion nanoprobes, such as (1) the size and corresponding absorption (or SPR) band of PNPs; (2) the physicochemical and optical characteristic of UCNPs, such as the size, surface coating (spacer), NIR activation laser wavelength, emission profiles; (3) target analytes and achieved the limit of detection (LOD). In the following sections, we will highlight trending topics through discussion of some representative nanoprobes that are expected to provide a brief overview of the current research status in the plasmon-modulated upconversion biosensing field.

Table 3.
Photon-modulated upconversion nanoparticles (PNPs—plasmonic nanoparticles, —upconversion nanoparticles, λ abs.—emission wavelength, NIR—near-infrared, λ em.—emission wavelength, LOD—limit of detection).
3.2. Plasmon Modulated Upconversion Sensing of Ions and Small Biomolecules
Anion and cation recognition plays a crucial role to evaluate the operation of vital biological processes and garner information about overall cell health. There is a wealth of literature reporting the ion sensing platforms that utilize plasmon modulated upconversion, while capable of simultaneous measuring changes in the concentration of different small biomolecules. Therefore, we decided to combine the discussion on ions and small biomolecules sensing in one section. The detection principle is based on the ability of PNPs to modulate the radiation properties of UCNPs by disabling them in closer proximity and retrieving “turn on” them upon segregation. For example, Chen et al. constructed an assay for chromium (Cr3+) ion detection, which was composed of electrostatically coupled lysine-capped UCNPs and dimercaptosuccinic acid-capped gold nanoparticles (Au NPs). The presence of Cr3+ ions induces a drift away of Au NPs from UCNPs surface recovering the emission profiles in a linear response [61].
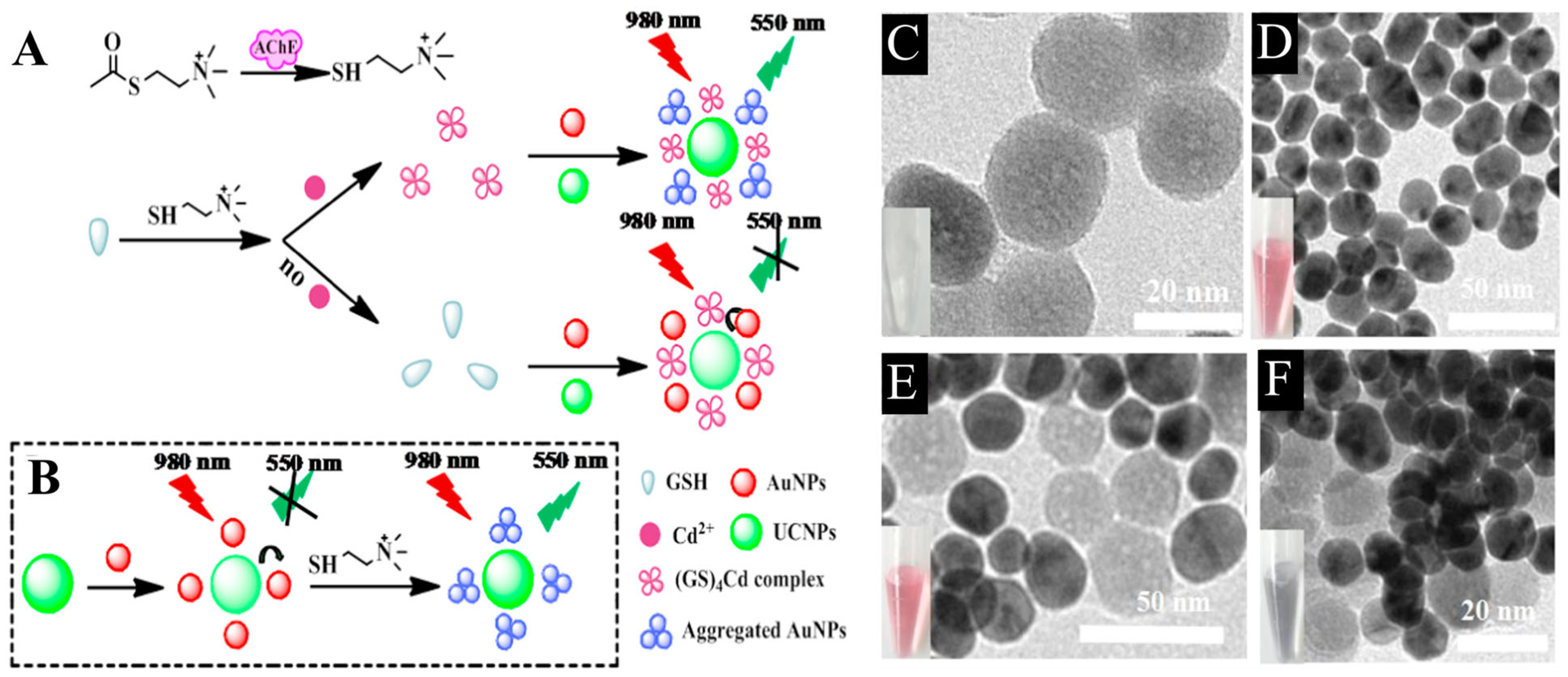
Fang et al. developed a bifunctional biosensing platform using plasmon modulated upconversion effect of quantitative detection of acetylcholinesterase (AChE) and cadmium (Cd2+) ions with the help of glutathione (GSH). Two mechanisms account for the tendency of Au NPs to aggregate and drift away from UCNPs to recover the emission profile (Figure 3A,B). The first is that the presence of AChE promotes the hydrolysis of acetylthiocholine (ATC) which tend to destabilize the surface chemistry of Au NPs and lead to their aggregation (Figure 3C–F). The second mechanism relies on the ability of Cd2+ ions to detach GSH from the surface of Au NPs to form spherical shaped complex, which disrupts the stability of Au NPs and effectively triggers their gradual isolation from UCNPs. The designed biosensor exhibited LODs of 0.015 mU/mL and 0.2 µM for AchE and Cd2+ ions, respectively [62].
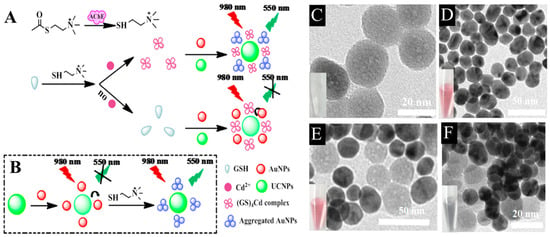
Figure 3.
Sensing principle of the bifunctional UCNPs/AuNPs based nanoprobe for detection of (A) AchE and (B) Cd2+ ions with GSH regulation. Representative TEM images of (C) UCNPs, (D) AuNPs, (E) UCNPs/AuNPs, and (F) aggregation in UCNPs/AuNPs caused by post-addition of AChE and ATC. Reprinted with permission from reference [62].
Hu et al. reported a bifunctional Au-UCNPs nanoprobe which exhibits a dose dependent response to Cd2+ ions and GSH with LODs 0.059 μM and 0.016 μM, respectively. In this study, the presence of GSH restrained the Au NPs aggregation, while co-existence Cd2+ ions impaired their stability leading to the gradual weakening of UCNPs red emission [63].
Sun and Gradzielski employed plasmon-modulated upconversion for the detection of poisonous cyanide ions. The sensor operation was based on the redox consumption of Au NPs by CN− ions which enabled the emancipation of UCNPs to regain their luminescence and produce detection signal. Moreover, the nanoprobe demonstrated excellent selectivity and sensitivity by distinguishing cyanide ions from different interfering ions with a detection limit of 1.53 μM [66].
Remarkably, gold nanorods have gained great momentum from researchers around the world owing to their versatility in SPR band control via aspect ratio adjustment. For example, Kim et al. demonstrated 27-fold upconversion enhancement by altering the aspect ratio of Au NRs to match their SPR band with the emission of UCNPs at 805 nm. The UCNPs have been preliminarily encapsulated in polyamidoamine generation 1 (PAMAM G1) dendrimer, which served as a spacer to prevent undesired quenching upon coupling with Au NRs. The obtained nanoprobe was post-functionalized with 2-thiouracil for sensitive and selective detection of uric acid, which is an important small biomolecule. Because its elevated content may indicate renal or cardiovascular disorders in the human body [68]. Interestingly, Zhu et al. obtained 50-fold SPR based enhancement of the UCNPs brightness, which is associated with a ~24 nm thick silica layer on the surface Au NRs. Thereby proposing a nanoprobe for potential microRNA-21 detection in human serum samples and human breast cancer cell (MCF-7) lysates [67].
Noteworthy, the use of silver nanoparticles for the modulation upconversion biosensors has been relatively limited compared to gold nanoparticles, even though theoretically Ag NPs exhibit stronger SPR [85]. Among recent reports, Liu et al. employed ultrasmall (1.9 nm) silver nanoclusters as acceptors for the construction of plasmon modulated NIR upconversion nanoprobe for intracellular biothiols detection. The authors demonstrated the biosensor performance in the liver tissue of mice to highlight its potential for in vivo sensing [69].
3.3. Plasmon Modulated Upconversion Sensing of Biomacromolecules
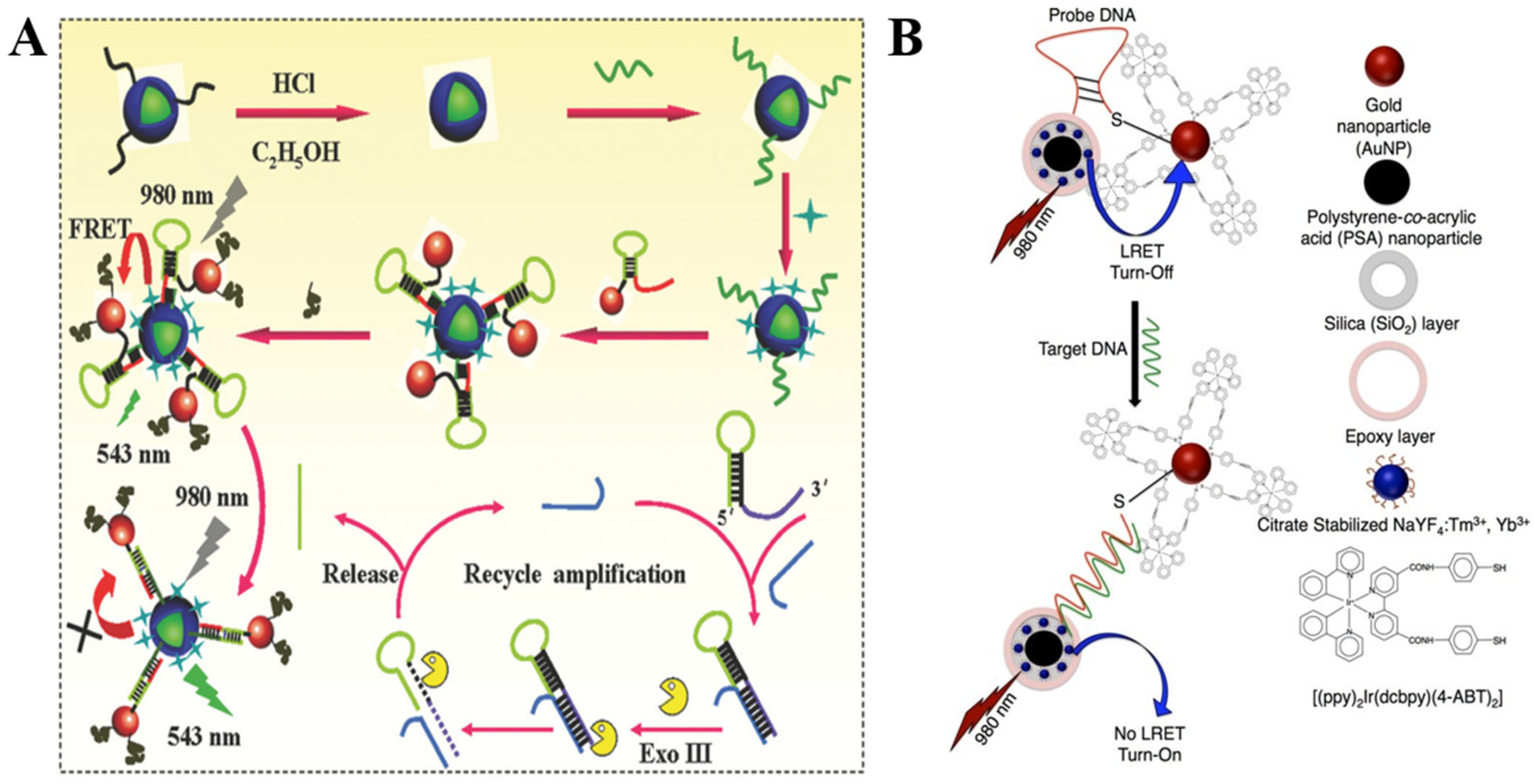
Over the past decade, our understanding of how to implement recognition of biomacromolecules, such as RNA or DNA nucleic acids, using plasmon modulated upconversion biosensing systems has significantly advanced. For example, recently, Zhu et al. reported a universal pathway for tumor related noncoding RNA (ncRNA) recognition (Figure 4A). The upconversion recovery principle in the designed biosensor is based on the uncoupling of DNA encapsulated UCNPs from Au NPs bearing a single molecule hairpin DNA (Hp) molecule via exonuclease III (Exo III)-assisted cycling amplification strategy. Notably, the biosensor exhibited a great sensitivity towards the expression level of miR-21 in human breast cancer cell (MCF-7) lysate with LOD of 0.54 fM [75].
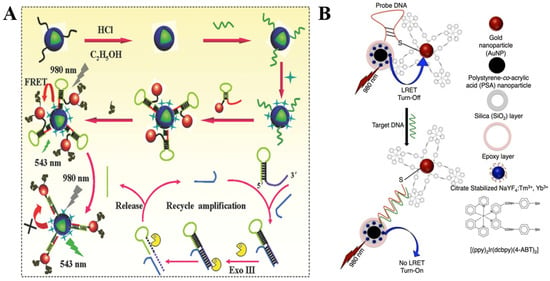
Figure 4.
(A) Schematics of the UCNPs–AuNPs plasmon-modulated biosensing platform for highly sensitive detection of tumor-related ncRNA via the Exo III-assisted cycling amplification strategy. Reprinted with permission from reference [75]. (B) Schematics of the composition and energy transfer mechanism of ssDNA optical sensor composed of PSA/SiO2 coated UCNP and cyclometalated Ir(III)-AuNPs. Reprinted with permission from reference [73].
Zourob et al. proposed an ssDNA target sequence sensing pathway containing blue-emitting UCNPs for incorporation into silica coated polystyrene-co-acrylic acid nanoparticles (PSA/SiO2) (donor) and Au NPs with immobilized Ir(III) complex (quencher), as shown in Figure 4B. In response to the sequential addition of the target DNA, the quencher could be effectively separated from the donor reactivating the upconversion signal under 975 nm NIR irradiation in a linear manner vs. DNA concentration. The developed sensor sensitivity was indicated at LOD as low as 1 pM. Furthermore, the selectivity of the DNA sensor was confirmed through the titration of the nanoprobe using the DNA conjugated nanohybrids with single base mismatch [73].
Conversely, some researchers employ the fluorescence quenching as a signal to recognize the analyte. For instance, Chen et al. employed a simple paper-supported aptasensor to construct Au NR/UCNPs based plasmon modulated nanoprobe for cancer biomarker exosome. The designed nanoprobes operation is activated in the presence of exosome. Upon conjugation with CD63 protein, Au NRs and UCNPs parts are brought to proximity enough to initiate linear quenching which is correlated with exosome concentration. The LOD of exosomes was estimated to be 1.1 × 103 particles/μL [74].
3.4. Plasmon Modulated Upconversion Sensing of Viruses
Despite encouraging progress in the development of safe disease diagnostic tools, such as reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), rapid and ultrasensitive bioassays for pathogenic viruses detection are still very demanding. So far UCNPs were utilized for the detection of H5N1 Influenza [86], oligonucleotide markers of the SARS-CoV-2 virus [87,88], hepatitis B Virus surface antigen (HBsAg) [89], anti-human immunodeficiency virus (HIV) antibodies [90], thrombocytopenia syndrome virus (SFTSV) total antibodies [91].
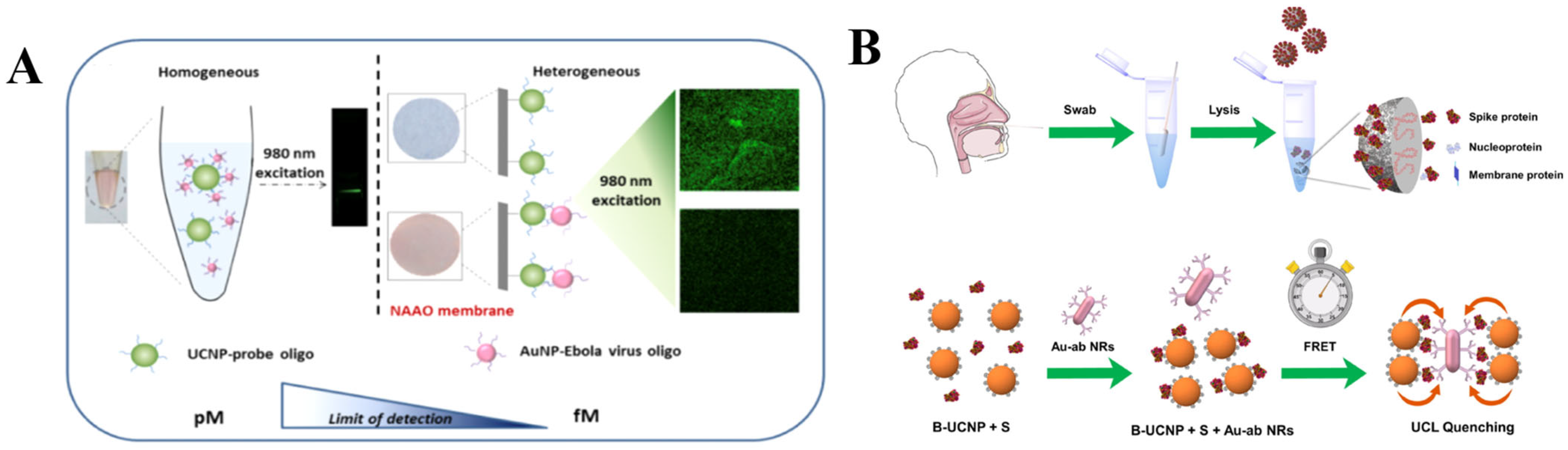
There are several reports on deploying plasmon modulated upconversion for construction of novel rapid biosensors for virus recognition. For instance, in 2016, Hao et al. employed plasmon modulated upconversion for ultrasensitive detection of Ebola virus, which outbreak threatened the world significantly between 2014 and 2016 (Figure 5A,B). In their research, they proposed a bioassay composed of BaGdF5:Yb,Er UCNPs and Au NPs that were immobilized on the 3D structured nanoporous alumina (NAAO) substrate. The spectral overlap between nanoparticles allowed to observe effective quenching of naked-eye observable green UC emission upon the combination of probe oligoconjugated with UCNPs and Ebola virus oligoconjugated with AuNPs. Moreover, the authors demonstrated that their sensor could effectively detect Ebola viral RNA in clinical samples with LOD of 500 fM, which is comparable with other conventional detection methods [80].
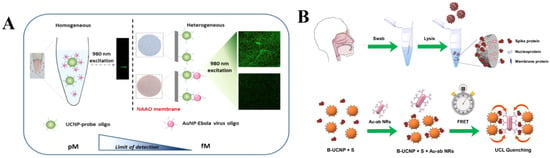
Figure 5.
(A) Comparison illustration of homogenous and heterogeneous sensor for Ebola virus detection. Reprinted with permission from reference [80]. (B) Schematics of COVID S protein detection using plasmon modulated upconversion biosensing system. Reprinted with permission from reference [81].
In another investigation by Hao et al., they introduced plasmon modulated biosensing of SARS-CoV-2 spike protein for COVID-19 point-of-care diagnostics (Figure 5C,D). In this study, the NaYF4:Yb/Tm UCNPs nanoprobe was complemented with Au NRs, which effectively captured and detected S protein endowing LOD of 1.06 fg mL−1 [81].
Hepatitis B (commonly abbreviated as HBV) is a DNA virus that causes a serious damage to the human liver. It has been estimated that more than 300 million people around the globe are infected with HBV [92]. Researchers have devoted substantial efforts to the development of sensitive biosensing tools for the prevention of HBV infection spread. For example, Zhu et al. proposed to employ Au-UCNPs-based nanoprobes for HBV DNA detection. In this study, Au NPs were bound to UCNPs via DNA hybridization keeping upconversion emission quenched. The situation drastically changed upon introducing target DNA, which initiated the departure of Au NPs from the UCNPs surface with subsequent restoration of the emission intensity. The proposed nanoprobe exhibited a LOD of 250 pM [79].
3.5. Plasmon Modulated Upconversion Sensing of Temperature
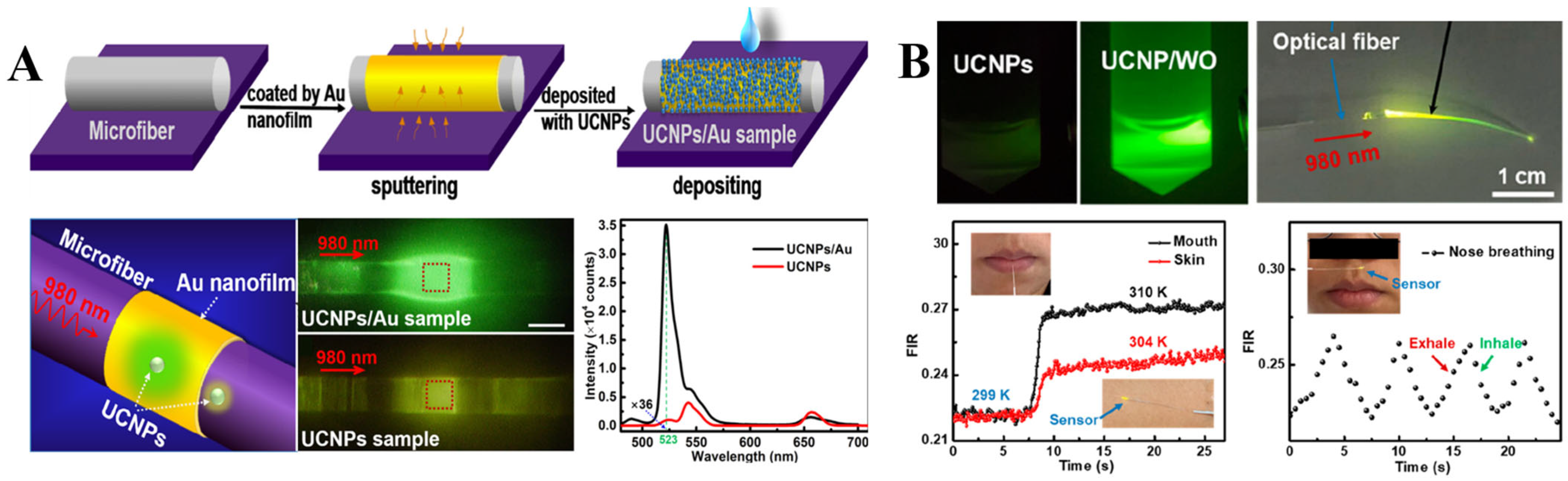
Temperature-dependent properties of UCNPs arising from complex thermally coupled energy levels are suitable for plasmon modulated thermometric biosensing. For example, Li et al. fabricated an optical microfiber (~3 μm) coated with an 18 nm thin gold film and decorated with UCNPs for temperature sensing (Figure 6A). The work principle of the constructed temperature sensor is based on the plasmon enhanced upconversion luminescence, which facilitated temperature dependent upconversion emission enhancement. In particular, plasmonic properties of Au nanofilm were activated by 980 nm laser which selectively increased the green emission intensity of UCNPs at 523 nm by 36 times, which is ascribed to the Au nanofilm-assisted local field enhancement of the incident light. Upon precise control of the UCNPs’ amount and laser dosage, the designed sensor can respond to the temperature range of 325–811 K with a resolution of 0.034–0.046 K [83].
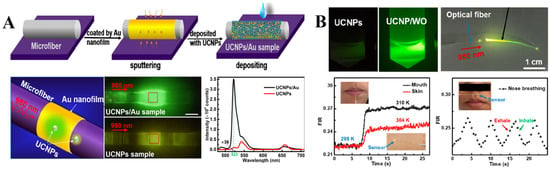
Figure 6.
(A) Illustration of the selective plasmon-enhanced green emission upconversion nanoprobe for temperature sensing, which include: schematics of the fabrication process, optical images of the sensor upon laser excitation and comparative illustration of the upconversion photoluminescence enhancement induced by Au nanofilm. Reprinted with permission from reference [83]. (B) Optical images of UCNPs and UCNPs/WO solutions under a 980 nm laser illumination. Fluorescence intensity ratio changes from the air to the mouth (black line), hand skin (red line) and time-dependent breath sensor response (dotted line). Reprinted with permission from reference [84].
Recently, Li et al. developed a flexible temperature sensor, consisting of polyacrylic acid (PLA) fiber and UCNPs/W18O49 upconversion/plasmonic semiconductor hybrid optical system for potential application in wearable health monitoring (Figure 6B). Co-doping W18O49 nanowires with UCNPs into PLA fiber with a high refractive index of 1.46 facilitated the plasmon enhanced upconversion green emission at 540 nm, which is suitable for ratiometric reading of the temperature changes under a 980 nm NIR laser excitation (5.5 mW). The hybrid sensor exhibited high sensitivity of 1.53% with 0.4 K LOD. In general, the ratiometric response could be measured based on the fluorescence intensity ratio of upconversion emission peaks produced by UCNPs under NIR laser excitation. In this study the intensity ratio between green emissions at 520 nm and 540 nm was used as a temperature change indicator, because these emissions originate from the thermally coupled energy levels 2H11/2 and 4S3/2 of Er3+ activator [84].
4. Future Directions
Continuing advances in the upconversion field opened the emerging frontiers of plasmon modulated biosensing technology, which affords background-free, rapid, well-timed and more sensitive quantitative data acquisition regarding bioanalytes compared to traditional sensing approaches. In this review, we covered the basic concepts of lanthanide upconversion and brief theoretical principles plasmon modulated upconversion, we systematically analyzed cutting edge advances in construction biosensing tools through the integration of plasmonic nanoparticles (predominantly gold nanoparticles) with upconversion nanoparticles for nanoprobing various biological analytes.
Herein, we have reviewed ongoing research progress made on the development of plasmon modulated upconversion biosensors. Thus, from the most recent experimental findings we derived the future trends and perspectives to better understand how to improve UCNPs luminescence features using plasmonic nanoparticles. In this regard, to fully realize their potential in practical plasmon modulated upconversion biosensing, the following criteria still need to be fulfilled to meet exceedingly high standards of in vivo models and their further clinical translation:
(1) The size of nanoparticles should be monodisperse and in the renal clearable range (<10 nm); however, the size should not be too small (<5 nm) to prevent too rapid excretion from the body. Another important consideration is that as the size of UCNPs decreases the brightness degrades dramatically because the quenching issue becomes more profound;
(2) the stabilization of UCNPs in biological environments with minimized toxicity, since the dissolution in aqueous media has been a general drawback of NaYF4 host materials which leads to the release of cytotoxic lanthanide and fluorine ions. Moreover, plasmon modulation efficiency is sensitive to the nature and thickness of the spacer coating on UCNPs or PNPs. This aspect makes it more demanding to understand the nanoparticle functionalization process and seek alternative coatings which impart more efficient plasmon-enabled control of upconversion properties;
(3) high photoluminescence quantum yields are generally desirable for sufficient brightness under safe NIR excitation laser powers;
(4) the use of 980 nm NIR laser based photoactivation remains a subject of debate, which is unlikely to be increasingly used in the future due to tissue overheating issues. We anticipate that the focus of the upconversion biosensing research will gradually shift toward safer and shorter NIR laser wavelengths, such as an 808 nm laser-stimulated biosensing, yet it has been restricted by a low permissible power dosage;
(5) from the perspective of temperature-sensitive luminescence of UCNPs, there is little doubt about the rationality of their complementary use, since PNPs tend to generate heat under NIR laser irradiation. It is widely acknowledged that elevated temperatures accelerate the decay rates of thermally coupled energy levels in UCNPs leading to their poor upconversion luminescence or even undesired quenching. Though from our literature survey, it is clear that plasmon enhancement does improve the upconversion efficiency and benefit its biosensing performance.
5. Conclusions
The progress in plasmon-modulated upconversion biosensing will continue to advance, which may enable to explore alternatives to gold plasmonic nanostructure that can provide better plasmonic resonances. Yet, a more profound understanding of the exact key parameters (size, geometry, thickness of the spacer, etc.) governing the efficient plasmon enhancement is therefore essential. Overall, plasmon-modulated upconversion is envisioned as exciting and very promising avenue for the future development of intelligent biosensors that could possibly benefit the healthcare system. We hope this literature survey will motivate the reader to explore solutions to tackle the remaining problems and possibly confront the conceptual challenges to overcome the current bottleneck.
Author Contributions
Conceptualization, J.-H.L. and K.S.K.; investigation, A.M., H.E.C., J.M.P. and K.S.K.; writing and original draft preparation, A.M., J.-H.L. and K.S.K.; visualization, A.M.; supervision, J.-H.L. and K.S.K.; funding acquisition, J.-H.L. and K.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the ‘2022 Post-Doc. Development Program’ of Pusan National University. This work was implemented within the National Research Foundation of Korea (NRF) grant supported by the Ministry of Science and ICT (No. 2022R1C1C1009533, 2021M3H4A4079509, 2022R1A5A2027161), and the Korea Health Industry Development Institute (KHIDI) grant funded by the Ministry of Health & Welfare (No. HF21C0156), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AChE | acetylcholinesterase |
| Con-A | concanavalin A |
| CTAB | cetyl trimethyl ammonium bromide |
| COVID | coronavirus disease |
| DNA | deoxyribonucleic acid |
| FRET | fluoresecence resonance energy transfer |
| GSH | gluthathione |
| HBV | Hepatitis B virus |
| Ln | Lanthanide |
| LOD | limit of detection |
| NAAO | nanoporous alumina |
| NCs | nanoclusters |
| NDs | nanodots |
| NPs | nanoparticle |
| NIR | near-infrared |
| NSs | nanosheets |
| PAA | polyacrylic acid |
| PAMAM | polyamidoamine |
| PEI | polyethylene imine |
| PLA | polyacrylic acid |
| PNPs | plasmonic nanoparticles |
| PSA | polystyrene-co-acrylic acid |
| RNA | ribonucleic acid |
| SPR | surface plasmon resonance |
| TEM | transmission electron microscopy |
| UCNPs | upconversion nanoparticles |
References
- Zhu, X.; Zhang, J.; Liu, J.; Zhang, Y. Recent Progress of Rare-Earth Doped Upconversion Nanoparticles: Synthesis, Optimization, and Applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Sun, L.; Wei, R.; Feng, J.; Zhang, H. Tailored Lanthanide-Doped Upconversion Nanoparticles and Their Promising Bioapplication Prospects. Coord. Chem. Rev. 2018, 364, 10–32. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Recent Advances in the Chemistry of Lanthanide-Doped Upconversion Nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, A.; Safdar, M.; Lastusaari, M.; Savin, H.; Karppinen, M. Advances in Upconversion Enhanced Solar Cell Performance. Sol. Energy Mater. Sol. Cells 2021, 230, 111234. [Google Scholar] [CrossRef]
- Yang, X.; Jin, X.; Zheng, A.; Duan, P. Dual Band-Edge Enhancing Overall Performance of Upconverted Near-Infrared Circularly Polarized Luminescence for Anticounterfeiting. ACS Nano 2023, 17, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Aldajani, K.M.; AlHazaa, A.N.; Albrithen, H.A. Recent Progress of Fluorescent Materials for Fingermarks Detection in Forensic Science and Anti-Counterfeiting. Coord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- Peltomaa, R.; Benito-Peña, E.; Gorris, H.H.; Moreno-Bondi, M.C. Biosensing Based on Upconversion Nanoparticles for Food Quality and Safety Applications. Analyst 2021, 146, 13–32. [Google Scholar] [CrossRef]
- Xu, R.; Cao, H.; Lin, D.; Yu, B.; Qu, J. Lanthanide-Doped Upconversion Nanoparticles for Biological Super-Resolution Fluorescence Imaging. Cell Rep. Phys. Sci. 2022, 3, 100922. [Google Scholar] [CrossRef]
- Xie, L.; Hong, Z.; Zan, J.; Wu, Q.; Yang, Z.; Chen, X.; Ou, X.; Song, X.; He, Y.; Li, J.; et al. Broadband Detection of X-Ray, Ultraviolet, and Near-Infrared Photons Using Solution-Processed Perovskite–Lanthanide Nanotransducers. Adv. Mater. 2021, 33, 1–7. [Google Scholar] [CrossRef]
- Lee, G.; Park, Y. Il Lanthanide-Doped Upconversion Nanocarriers for Drug and Gene Delivery. Nanomaterials 2018, 8, 511. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Han, S.; Beack, S.; Jeong, S.; Hwang, B.W.; Shin, M.H.; Kim, H.; Kim, S.; Hahn, S.K. Hyaluronate Modified Upconversion Nanoparticles for near Infrared Light-Triggered on-off Tattoo Systems. RSC Adv. 2017, 7, 14805–14808. [Google Scholar] [CrossRef]
- Han, S.; Hwang, B.W.; Jeon, E.Y.; Jung, D.; Lee, G.H.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Cha, H.J.; Hahn, S.K. Upconversion Nanoparticles/Hyaluronate-Rose Bengal Conjugate Complex for Noninvasive Photochemical Tissue Bonding. ACS Nano 2017, 11, 9979–9988. [Google Scholar] [CrossRef]
- Sun, C.; Gradzielski, M. Advances in Fluorescence Sensing Enabled by Lanthanide-Doped Upconversion Nanophosphors. Adv. Colloid Interface Sci. 2022, 300, 102579. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, D.; Schartner, E.P.; Lu, Y.; Liu, Y.; Zvyagin, A.V.; Zhang, L.; Dawes, J.M.; Xi, P.; Piper, J.A.; et al. Single-Nanocrystal Sensitivity Achieved by Enhanced Upconversion Luminescence. Nat. Nanotechnol. 2013, 8, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gargas, D.J.; Chan, E.M.; Ostrowski, A.D.; Aloni, S.; Altoe, M.V.P.; Barnard, E.S.; Sanii, B.; Urban, J.J.; Milliron, D.J.; Cohen, B.E.; et al. Engineering Bright Sub-10-Nm Upconverting Nanocrystals for Single-Molecule Imaging. Nat. Nanotechnol. 2014, 9, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Qin, X.; Zheng, K.; Liu, X. Energy Flux Manipulation in Upconversion Nanosystems. Acc. Chem. Res. 2019, 52, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Maini, A.K. Lasers and Optoelectronics: Fundamentals, Devices and Applications; Maini, A.K., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 2013; ISBN 9781118688977. [Google Scholar]
- Molkenova, A.; Atabaev, T.S.; Hong, S.W.; Mao, C.; Han, D.-W.; Kim, K.S. Designing Inorganic Nanoparticles into Computed Tomography and Magnetic Resonance (CT/MR) Imaging-Guidable Photomedicines. Mater. Today Nano 2022, 18, 100187. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Jovin, T.M. FRET Imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef]
- Francés-Soriano, L.; Estebanez, N.; Pérez-Prieto, J.; Hildebrandt, N. DNA-Coated Upconversion Nanoparticles for Sensitive Nucleic Acid FRET Biosensing. Adv. Funct. Mater. 2022, 32, 2201541. [Google Scholar] [CrossRef]
- Kotulska, A.M.; Pilch-Wróbel, A.; Lahtinen, S.; Soukka, T.; Bednarkiewicz, A. Upconversion FRET Quantitation: The Role of Donor Photoexcitation Mode and Compositional Architecture on the Decay and Intensity Based Responses. Light Sci. Appl. 2022, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Corbella Bagot, C.; Rappeport, E.; Ba Tis, T.; Park, W. Quantitative Modeling and Experimental Verification of Förster Resonant Energy Transfer in Upconversion Nanoparticle Biosensors. J. Appl. Phys. 2021, 130, 023102. [Google Scholar] [CrossRef]
- Wu, D.M.; García-Etxarri, A.; Salleo, A.; Dionne, J.A. Plasmon-Enhanced Upconversion. J. Phys. Chem. Lett. 2014, 5, 4020–4031. [Google Scholar] [CrossRef]
- Jiang, W.; Yi, J.; Li, X.; He, F.; Niu, N.; Chen, L. A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications. Biosensors 2022, 12, 1036. [Google Scholar] [CrossRef]
- Jin, H.; Yang, M.; Gui, R. Ratiometric Upconversion Luminescence Nanoprobes from Construction to Sensing, Imaging, and Phototherapeutics. Nanoscale 2022, 15, 859–906. [Google Scholar] [CrossRef]
- Auzel, F. History of Upconversion Discovery and Its Evolution. J. Lumin. 2020, 223, 116900. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. An Efficient and User-Friendly Method for the Synthesis of Hexagonal-Phase NaYF4:Yb, Er/Tm Nanocrystals with Controllable Shape and Upconversion Fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef]
- Liu, G. Advances in the Theoretical Understanding of Photon Upconversion in Rare-Earth Activated Nanophosphors. Chem. Soc. Rev. 2015, 44, 1635–1652. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z. Tuning the Photoluminescence Properties of β-NaYF4:Yb,Er by Bi3+ Doping Strategy. Cryst. Res. Technol. 2022, 2100162, 3–7. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in Highly Doped Upconversion Nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Du, S.; Wu, J.; Wang, Y. Application of Upconversion-Luminescent Materials in Temperature Sensors. In Upconversion Nanophosphors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 291–310. [Google Scholar]
- Wang, L.; Li, P.; Li, Y. Down- and up-Conversion Luminescent Nanorods. Adv. Mater. 2007, 19, 3304–3307. [Google Scholar] [CrossRef]
- Naccache, R.; Yu, Q.; Capobianco, J.A. The Fluoride Host: Nucleation, Growth, and Upconversion of Lanthanide-Doped Nanoparticles. Adv. Opt. Mater. 2015, 3, 482–509. [Google Scholar] [CrossRef]
- Ansari, A.A.; Muthumareeswaran, M.R.; Lv, R. Coordination Chemistry of the Host Matrices with Dopant Luminescent Ln3+ Ion and Their Impact on Luminescent Properties. Coord. Chem. Rev. 2022, 466, 214584. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Aharen, T.; Murugesu, M. Paramagnetic Nanocrystals: Remarkable Lanthanide-Doped Nanoparticles with Varied Shape, Size, and Composition. J. Phys. Chem. Lett. 2012, 3, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, H.; Fang, W.; Mishra, S.; Jeanneau, E.; Ledoux, G.; Zhang, J.; Daniele, S. Influence of Na+ Ion Doping on the Phase Change and Upconversion Emissions of the GdF3: Yb3+, Tm3+ Nanocrystals Obtained from the Designed Molecular Precursors. RSC Adv. 2015, 5, 100535–100545. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, R.; Xie, X.; Huang, L.; Liu, X. Nonlinear Spectral and Lifetime Management in Upconversion Nanoparticles by Controlling Energy Distribution. Nanoscale 2016, 8, 6666–6673. [Google Scholar] [CrossRef]
- del Rosal, B.; Rocha, U.; Ximendes, E.C.; Martín Rodríguez, E.; Jaque, D.; Solé, J.G. Nd3+ Ions in Nanomedicine: Perspectives and Applications. Opt. Mater. Amst. 2017, 63, 185–196. [Google Scholar] [CrossRef]
- Chen, G.; Damasco, J.; Qiu, H.; Shao, W.; Ohulchanskyy, T.Y.; Valiev, R.R.; Wu, X.; Han, G.; Wang, Y.; Yang, C.; et al. Energy-Cascaded Upconversion in an Organic Dye-Sensitized Core/Shell Fluoride Nanocrystal. Nano Lett. 2015, 15, 7400–7407. [Google Scholar] [CrossRef]
- Slooff, L.H.; Polman, A.; Oude Wolbers, M.P.; Van Veggel, F.C.J.M.; Reinhoudt, D.N.; Hofstraat, J.W. Optical Properties of Erbium-Doped Organic Polydentate Cage Complexes. J. Appl. Phys. 1998, 83, 497–503. [Google Scholar] [CrossRef]
- Wiesholler, L.M.; Frenzel, F.; Grauel, B.; Würth, C.; Resch-Genger, U.; Hirsch, T. Yb,Nd,Er-Doped Upconversion Nanoparticles: 980 Nm: Versus 808 Nm Excitation. Nanoscale 2019, 11, 13440–13449. [Google Scholar] [CrossRef]
- Boyer, J.C.; Vetrone, F.; Cuccia, L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4 Nanocrystals Doped with Er3+, Yb3+ and Tm3+, Yb3+ via Thermal Decomposition of Lanthanide Trifluoroacetate Precursors. J. Am. Chem. Soc. 2006, 128, 7444–7445. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Cao, T.; Peng, J.; Liu, Y.; Wu, Y.; Feng, W.; Zhang, Y.; Li, F. Hydrothermal Synthesis of NaLuF 4: 153Sm,Yb,Tm Nanoparticles and Their Application in Dual-Modality Upconversion Luminescence and SPECT Bioimaging. Biomaterials 2013, 34, 774–783. [Google Scholar] [CrossRef]
- Marin, R.; Halimi, I.; Errulat, D.; Mazouzi, Y.; Lucchini, G.; Speghini, A.; Murugesu, M.; Hemmer, E. Harnessing the Synergy between Upconverting Nanoparticles and Lanthanide Complexes in a Multiwavelength-Responsive Hybrid System. ACS Photonics 2019, 6, 436–445. [Google Scholar] [CrossRef]
- Huang, Y.; You, H.; Song, Y.; Jia, G.; Yang, M.; Zheng, Y.; Zhang, L.; Liu, K. Half Opened Microtubes of NaYF4:Yb,Er Synthesized in Reverse Microemulsion under Solvothermal Condition. J. Cryst. Growth 2010, 312, 3214–3218. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A General Strategy for Nanocrystal Synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Bergstrand, J.; Duan, S.; Zhan, Q.; Widengren, J.; Ågren, H.; Liu, H. Overtone Vibrational Transition-Induced Lanthanide Excited-State Quenching in Yb3+/Er3+-Doped Upconversion Nanocrystals. ACS Nano 2018, 12, 10572–10575. [Google Scholar] [CrossRef]
- Rabouw, F.T.; Prins, P.T.; Villanueva-Delgado, P.; Castelijns, M.; Geitenbeek, R.G.; Meijerink, A. Quenching Pathways in NaYF4:Er3+,Yb3+ Upconversion Nanocrystals. ACS Nano 2018, 12, 4812–4823. [Google Scholar] [CrossRef]
- Li, X.; Shen, D.; Yang, J.; Yao, C.; Che, R.; Zhang, F.; Zhao, D. Successive Layer-by-Layer Strategy for Multi-Shell Epitaxial Growth: Shell Thickness and Doping Position Dependence in Upconverting Optical Properties. Chem. Mater. 2013, 25, 1, 106–112. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, Y.; Li, D.; Jia, H.; Qin, W. Growth Regularity and Phase Diagrams of NaLu0.795−xYxF4 Upconversion Nanocrystals Synthesized by Automatic Nanomaterial Synthesizer. Nano Res. 2021, 14, 4760–4767. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Qin, X.; Carneiro Neto, A.N.; Longo, R.L.; Wu, Y.; Malta, O.L.; Liu, X. Surface Plasmon-Photon Coupling in Lanthanide-Doped Nanoparticles. J. Phys. Chem. Lett. 2021, 12, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, D.; Xu, W.; Cui, S.; Chen, X.; Wang, H.; Xu, S.; Song, H. Plasmon-Enhanced Upconversion Luminescence on Vertically Aligned Gold Nanorod Monolayer Supercrystals. ACS Appl. Mater. Interfaces 2016, 8, 11667–11674. [Google Scholar] [CrossRef] [PubMed]
- Saboktakin, M.; Ye, X.; Oh, S.J.; Hong, S.H.; Fafarman, A.T.; Chettiar, U.K.; Engheta, N.; Murray, C.B.; Kagan, C.R. Metal-Enhanced Upconversion Luminescence Tunable through Metal Nanoparticle-Nanophosphor Separation. ACS Nano 2012, 6, 8758–8766. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, X.; Wang, K.; Guo, Z. Sequence-Specific Detection of Cytosine Methylation in DNA: Via the FRET Mechanism between Upconversion Nanoparticles and Gold Nanorods. Chem. Commun. 2016, 52, 8377–8380. [Google Scholar] [CrossRef]
- Dong, J.; Gao, W.; Han, Q.; Wang, Y.; Qi, J.; Yan, X.; Sun, M. Plasmon-Enhanced Upconversion Photoluminescence: Mechanism and Application. Rev. Phys. 2019, 4, 100026. [Google Scholar] [CrossRef]
- Wang, L.; Guo, S.; Liu, D.; He, J.; Zhou, J.; Zhang, K.; Wei, Y.; Pan, Y.; Gao, C.; Yuan, Z.; et al. Plasmon-Enhanced Blue Upconversion Luminescence by Indium Nanocrystals. Adv. Funct. Mater. 2019, 29, 1901242. [Google Scholar] [CrossRef]
- Zong, H.; Mu, X.; Sun, M. Physical Principle and Advances in Plasmon-Enhanced Upconversion Luminescence. Appl. Mater. Today 2019, 15, 43–57. [Google Scholar] [CrossRef]
- Mendez-Gonzalez, D.; Melle, S.; Calderón, O.G.; Laurenti, M.; Cabrera-Granado, E.; Egatz-Gómez, A.; López-Cabarcos, E.; Rubio-Retama, J.; Díaz, E. Control of Upconversion Luminescence by Gold Nanoparticle Size: From Quenching to Enhancement. Nanoscale 2019, 11, 13832–13844. [Google Scholar] [CrossRef]
- Liu, B.; Tan, H.; Chen, Y. Upconversion Nanoparticle-Based Fluorescence Resonance Energy Transfer Assay for Cr(III) Ions in Urine. Anal. Chim. Acta 2013, 761, 178–185. [Google Scholar] [CrossRef]
- Fang, A.; Chen, H.; Li, H.; Liu, M.; Zhang, Y.; Yao, S. Glutathione Regulation-Based Dual-Functional Upconversion Sensing-Platform for Acetylcholinesterase Activity and Cadmium Ions. Biosens. Bioelectron. 2017, 87, 545–551. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.; Sun, Y.; Li, Z.; Song, H.; Zhang, B.; Zhou, G.; Zhou, H.; Hu, J. Fluorescence Resonance Energy Transfer between NH2–NaYF4:Yb,Er/NaYF4@SiO2 Upconversion Nanoparticles and Gold Nanoparticles for the Detection of Glutathione and Cadmium Ions. Talanta 2020, 207, 120294. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Shi, Z.; Fang, C.; Wang, Z. Dual Fluorescence Resonance Energy Transfer Assay between Tunable Upconversion Nanoparticles and Controlled Gold Nanoparticles for the Simultaneous Detection of Pb2+ and Hg2+. Talanta 2014, 128, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, L.; Chen, M.; Chen, Q. Amine Functionalized NaY/GdF4:Yb,Er Upconversion-Silver Nanoparticles System as Fluorescent Turn-off Probe for Sensitive Detection of Cr(III). J. Photochem. Photobiol. A Chem. 2020, 388, 112203. [Google Scholar] [CrossRef]
- Sun, C.; Gradzielski, M. Fluorescence Sensing of Cyanide Anions Based on Au-Modified Upconversion Nanoassemblies. Analyst 2021, 146, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lu, F.; Cai, Z.; Song, S.; Jiang, L.; Min, Q.; Wu, X.; Zhu, J.J. Plasmonic Modulation of the Upconversion Luminescence Based on Gold Nanorods for Designing a New Strategy of Sensing MicroRNAs. Anal. Chem. 2020, 92, 11795–11801. [Google Scholar] [CrossRef]
- Kannan, P.; Abdul Rahim, F.; Chen, R.; Teng, X.; Huang, L.; Sun, H.; Kim, D.H. Au Nanorod Decoration on NaYF4:Yb/Tm Nanoparticles for Enhanced Emission and Wavelength-Dependent Biomolecular Sensing. ACS Appl. Mater. Interfaces 2013, 5, 3508–3513. [Google Scholar] [CrossRef]
- Xiao, Y.; Zeng, L.; Xia, T.; Wu, Z.; Liu, Z. Construction of an Upconversion Nanoprobe with Few-Atom Silver Nanoclusters as the Energy Acceptor. Angew. Chem. Int. Ed. 2015, 54, 5323–5327. [Google Scholar] [CrossRef]
- Wiesholler, L.M.; Genslein, C.; Schroter, A.; Hirsch, T. Plasmonic Enhancement of NIR to UV Upconversion by a Nanoengineered Interface Consisting of NaYF4:Yb,Tm Nanoparticles and a Gold Nanotriangle Array for Optical Detection of Vitamin B12 in Serum. Anal. Chem. 2018, 90, 14247–14254. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Q.; Li, H.; Ouyang, Q.; Zhao, J. Fabricating a Novel Label-Free Aptasensor for Acetamiprid by Fluorescence Resonance Energy Transfer between NH2-NaYF4: Yb, Ho@SiO2 and Au Nanoparticles. Biosens. Bioelectron. 2016, 80, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, Y.; Wang, S.; Ye, Z.; Wei, L.; Xiao, L. Single-Particle LRET Aptasensor for the Sensitive Detection of Aflatoxin B1 with Upconversion Nanoparticles. Anal. Chem. 2019, 91, 11856–11863. [Google Scholar] [CrossRef]
- Jesu Raj, J.G.; Quintanilla, M.; Mahmoud, K.A.; Ng, A.; Vetrone, F.; Zourob, M. Sensitive Detection of SsDNA Using an LRET-Based Upconverting Nanohybrid Material. ACS Appl. Mater. Interfaces 2015, 7, 18257–18265. [Google Scholar] [CrossRef]
- Chen, X.; Lan, J.; Liu, Y.; Li, L.; Yan, L.; Xia, Y.; Wu, F.; Li, C.; Li, S.; Chen, J. A Paper-Supported Aptasensor Based on Upconversion Luminescence Resonance Energy Transfer for the Accessible Determination of Exosomes. Biosens. Bioelectron. 2018, 102, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, L.; Lu, F.; Wu, X.; Zhu, J.J. A Universal Upconversion Sensing Platform for the Sensitive Detection of Tumour-Related NcRNA through an Exo III-Assisted Cycling Amplification Strategy. Small 2018, 14, 1703858. [Google Scholar] [CrossRef]
- Li, X.; Wei, L.; Pan, L.; Yi, Z.; Wang, X.; Ye, Z.; Xiao, L.; Li, H.W.; Wang, J. Homogeneous Immunosorbent Assay Based on Single-Particle Enumeration Using Upconversion Nanoparticles for the Sensitive Detection of Cancer Biomarkers. Anal. Chem. 2018, 90, 4807–4814. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Yang, C.; Ge, Z.; Yang, H. Fluorescence Resonance Energy Transfer from NaYF4:Yb,Er to Nano Gold and Its Application for Glucose Determination. Sens. Actuators B Chem. 2018, 255, 1316–1324. [Google Scholar] [CrossRef]
- Wu, S.; Kong, X.J.; Cen, Y.; Yuan, J.; Yu, R.Q.; Chu, X. Fabrication of a LRET-Based Upconverting Hybrid Nanocomposite for Turn-on Sensing of H2O2 and Glucose. Nanoscale 2016, 8, 8939–8946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lu, F.; Wu, X.C.; Zhu, J.J. An Upconversion Fluorescent Resonant Energy Transfer Biosensor for Hepatitis B Virus (HBV) DNA Hybridization Detection. Analyst 2015, 140, 7622–7628. [Google Scholar] [CrossRef]
- Tsang, M.K.; Ye, W.; Wang, G.; Li, J.; Yang, M.; Hao, J. Ultrasensitive Detection of Ebola Virus Oligonucleotide Based on Upconversion Nanoprobe/Nanoporous Membrane System. ACS Nano 2016, 10, 598–605. [Google Scholar] [CrossRef]
- Li, L.; Song, M.; Lao, X.; Pang, S.Y.; Liu, Y.; Wong, M.C.; Ma, Y.; Yang, M.; Hao, J. Rapid and Ultrasensitive Detection of SARS-CoV-2 Spike Protein Based on Upconversion Luminescence Biosensor for COVID-19 Point-of-Care Diagnostics. Mater. Des. 2022, 223, 111263. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, Z.; Han, L.; Zhou, D.; Wang, Y.; Pan, L.; Tu, K. Upconversion Fluorescence Nanoprobe-Based FRET for the Sensitive Determination of Shigella. Biosensors 2022, 12, 795. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Lei, H.; Li, B. Plasmon-Induced Selective Enhancement of Green Emission in Lanthanide-Doped Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 42935–42942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, X.; Liu, W.; Gao, Z.; Zhong, L.; Qin, Y.; Li, B.; Li, J. Semiconductor Plasmon Enhanced Upconversion toward a Flexible Temperature Sensor. ACS Appl. Mater. Interfaces 2023, 15, 4469–4476. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.; Almemar, Z.; Jiang, K.; Culhane, K.; Machado, R.; Hagen, G.; Kotko, A.; Dmytruk, I.; Spendier, K.; Pinchuk, A. Imaging of Biological Cells Using Luminescent Silver Nanoparticles. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Du, P.; Wang, X.; Huang, M.; Sun, L.D.; Wang, T.; Wang, Z. Upconversion Fluorescence Resonance Energy Transfer Aptasensors for H5N1 Influenza Virus Detection. ACS Omega 2021, 6, 15236–15245. [Google Scholar] [CrossRef]
- Alexaki, K.; Kyriazi, M.E.; Greening, J.; Taemaitree, L.; El-Sagheer, A.H.; Brown, T.; Zhang, X.; Muskens, O.L.; Kanaras, A.G. A SARS-Cov-2 Sensor Based on Upconversion Nanoparticles and Graphene Oxide. RSC Adv. 2022, 12, 18445–18449. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Tian, S.; Liu, K.; Ni, J.; Zhao, M.; Kang, Y.; Ma, X.; Guo, J. 5G-Enabled Ultra-Sensitive Fluorescence Sensor for Proactive Prognosis of COVID-19. Biosens. Bioelectron. 2021, 181, 113160. [Google Scholar] [CrossRef]
- Martiskainen, I.; Talha, S.M.; Vuorenpää, K.; Salminen, T.; Juntunen, E.; Chattopadhyay, S.; Kumar, D.; Vuorinen, T.; Pettersson, K.; Khanna, N.; et al. Upconverting Nanoparticle Reporter–Based Highly Sensitive Rapid Lateral Flow Immunoassay for Hepatitis B Virus Surface Antigen. Anal. Bioanal. Chem. 2021, 413, 967–978. [Google Scholar] [CrossRef]
- Martiskainen, I.; Juntunen, E.; Salminen, T.; Vuorenpää, K.; Bayoumy, S.; Vuorinen, T.; Khanna, N.; Pettersson, K.; Batra, G.; Talha, S.M. Double-Antigen Lateral Flow Immunoassay for the Detection of Anti-HIV-1 and -2 Antibodies Using Upconverting Nanoparticle Reporters. Sensors 2021, 21, 330. [Google Scholar] [CrossRef]
- Huang, C.; Wei, Q.; Hu, Q.; Wen, T.; Xue, L.; Li, S.; Zeng, X.; Shi, F.; Jiao, Y.; Zhou, L. Rapid Detection of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) Total Antibodies by up-Converting Phosphor Technology-Based Lateral-Flow Assay. Luminescence 2019, 34, 162–167. [Google Scholar] [CrossRef]
- Sheena, B.S.; Hiebert, L.; Han, H.; Ippolito, H.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abbastabar, H.; Abdoli, A.; Abubaker Ali, H.; Adane, M.M.; et al. Global, Regional, and National Burden of Hepatitis B, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).