Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19
Abstract
:1. Introduction
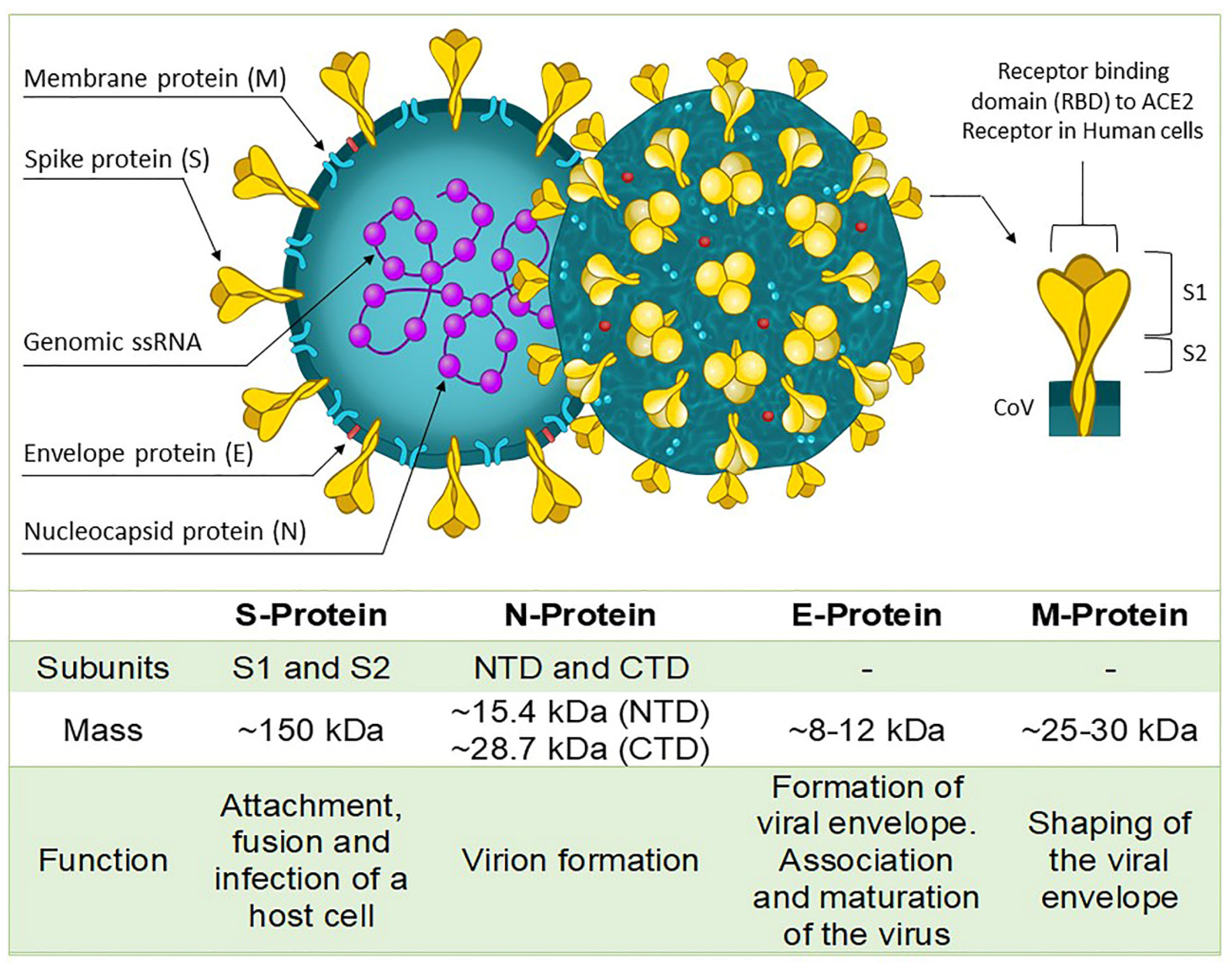
2. Structure of SARS-CoV-2 in Diagnostic Development and Sampling Methods
3. Electrochemical Techniques Used in Disease Diagnostics
4. Electrochemical Biosensors for Detection of SARS-CoV-2
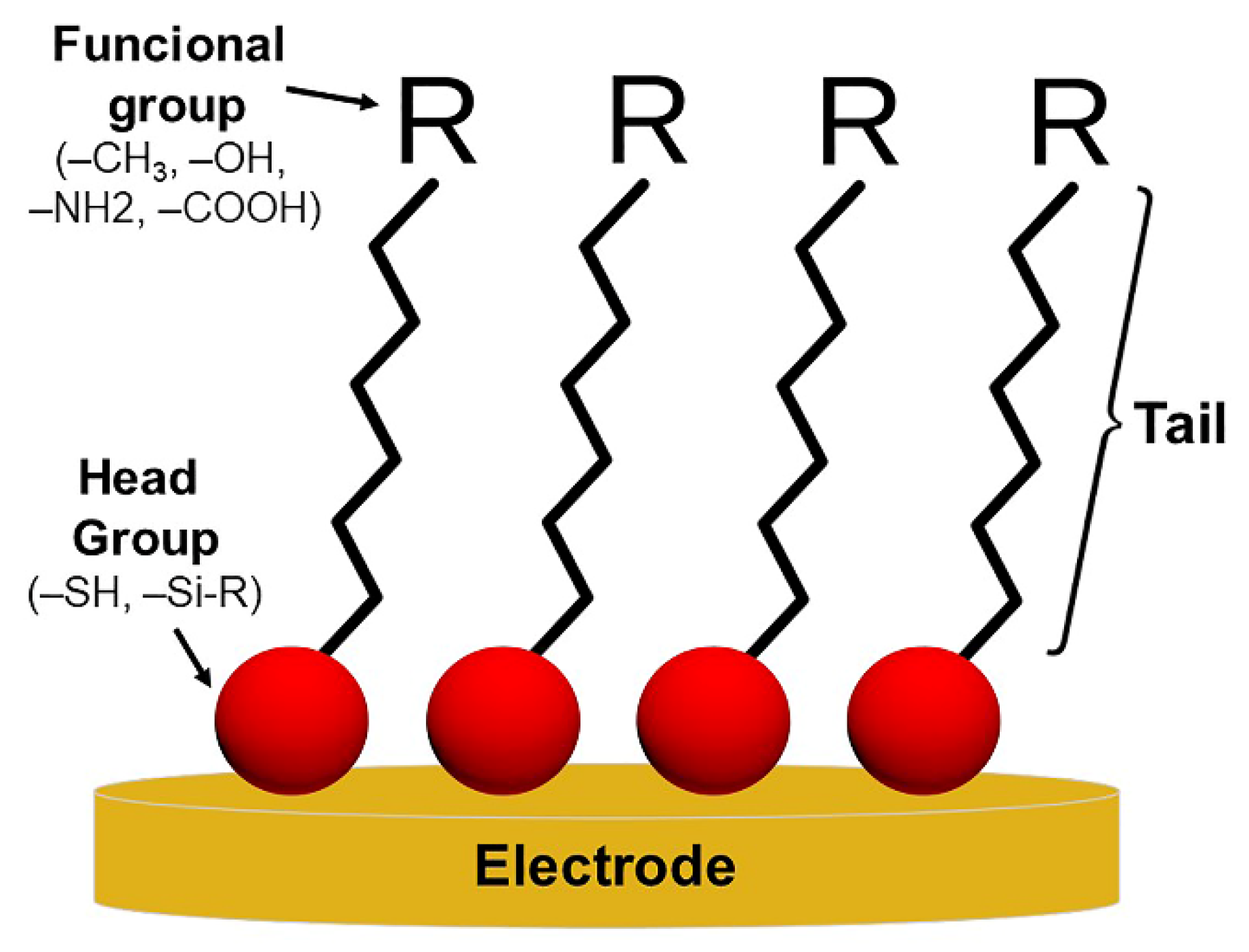
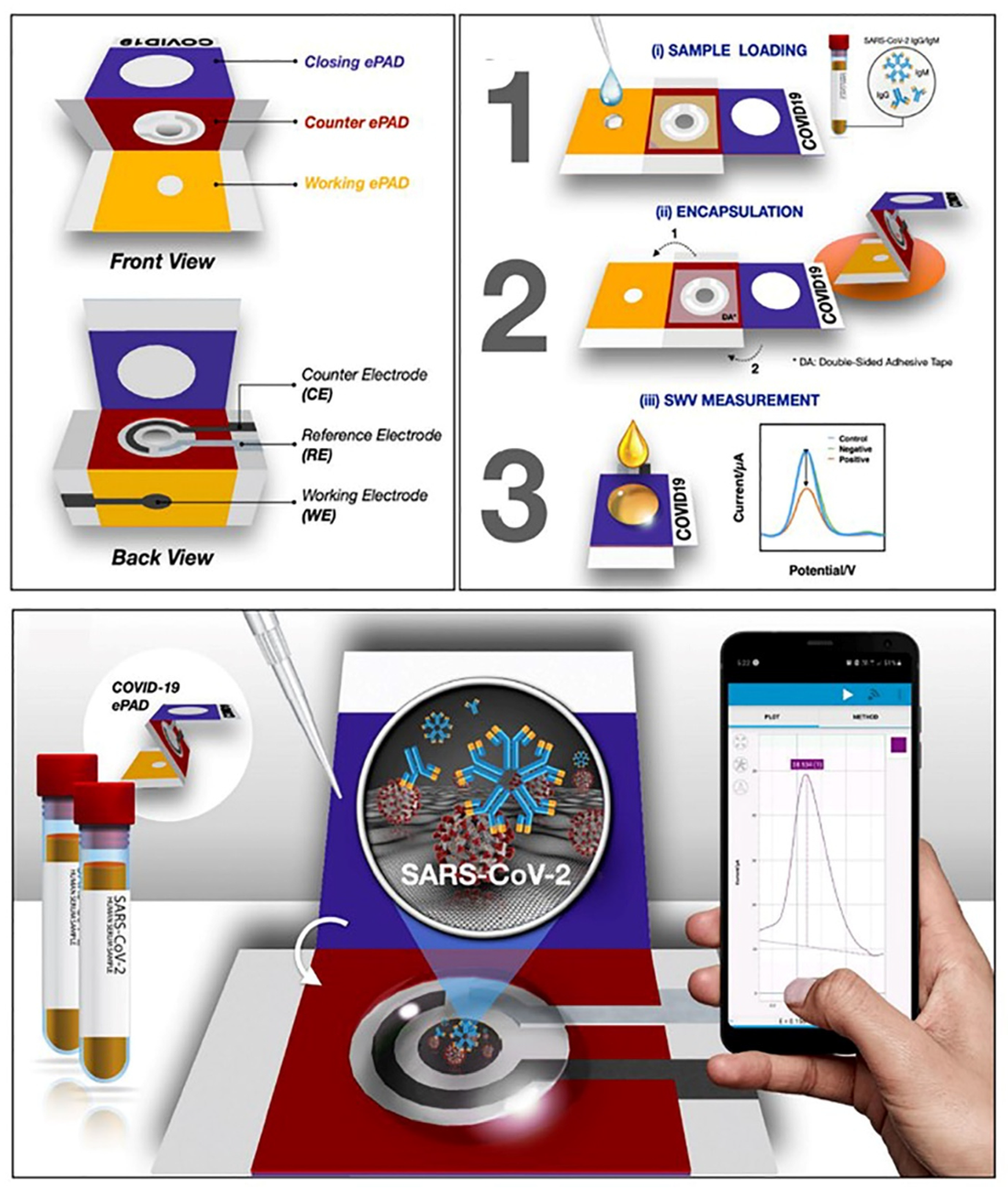
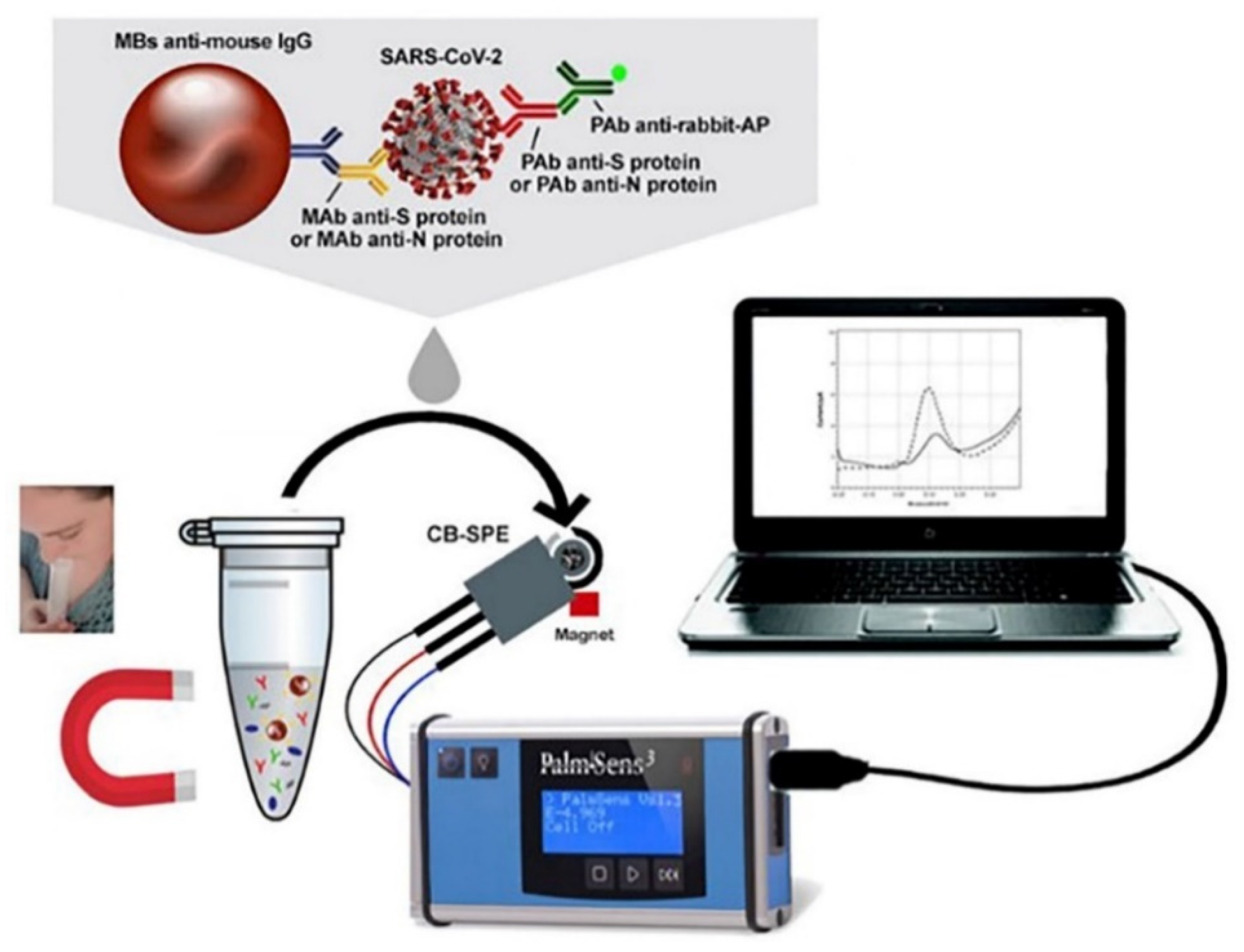
4.1. Electrochemical Immunosensors
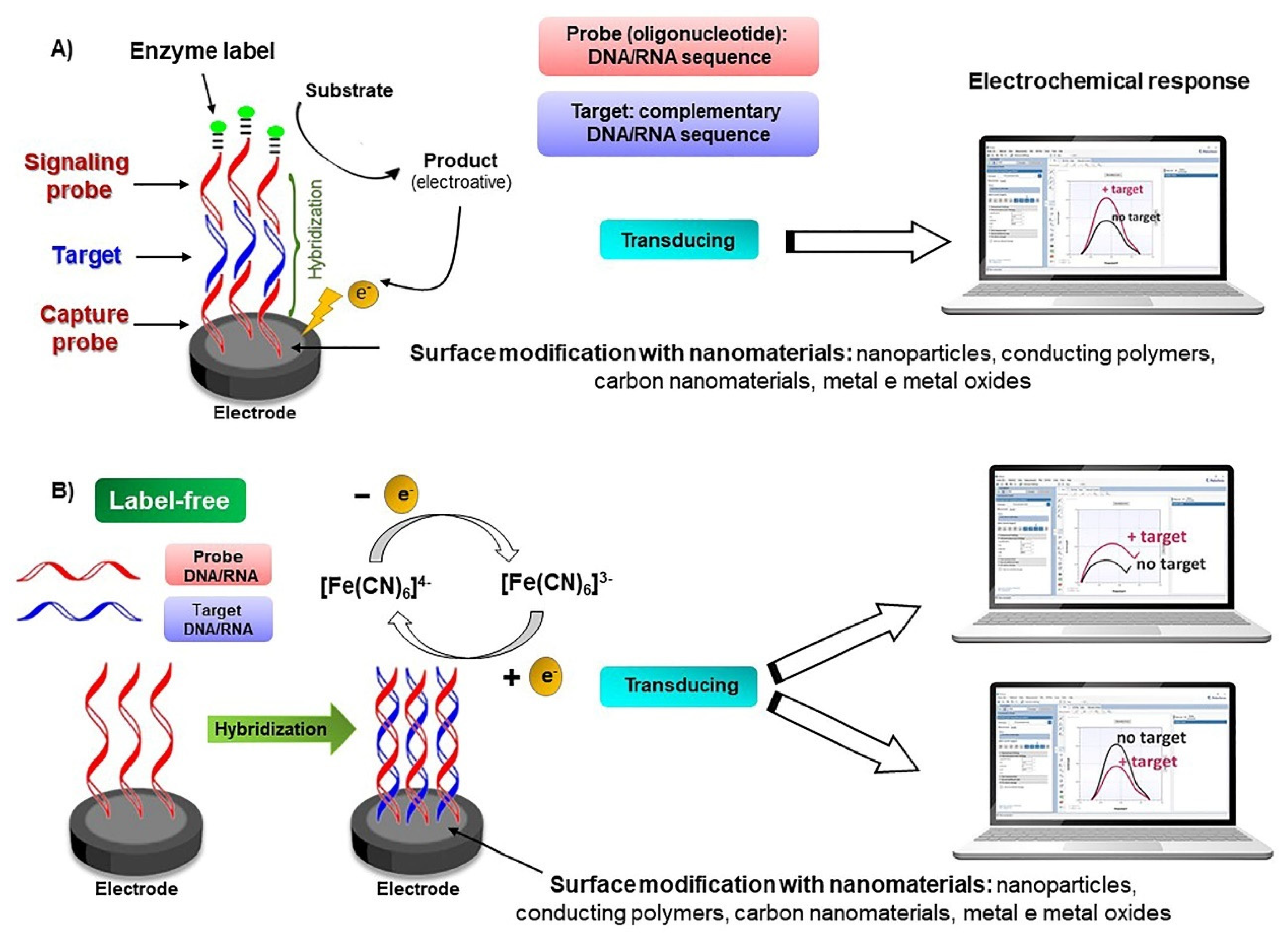
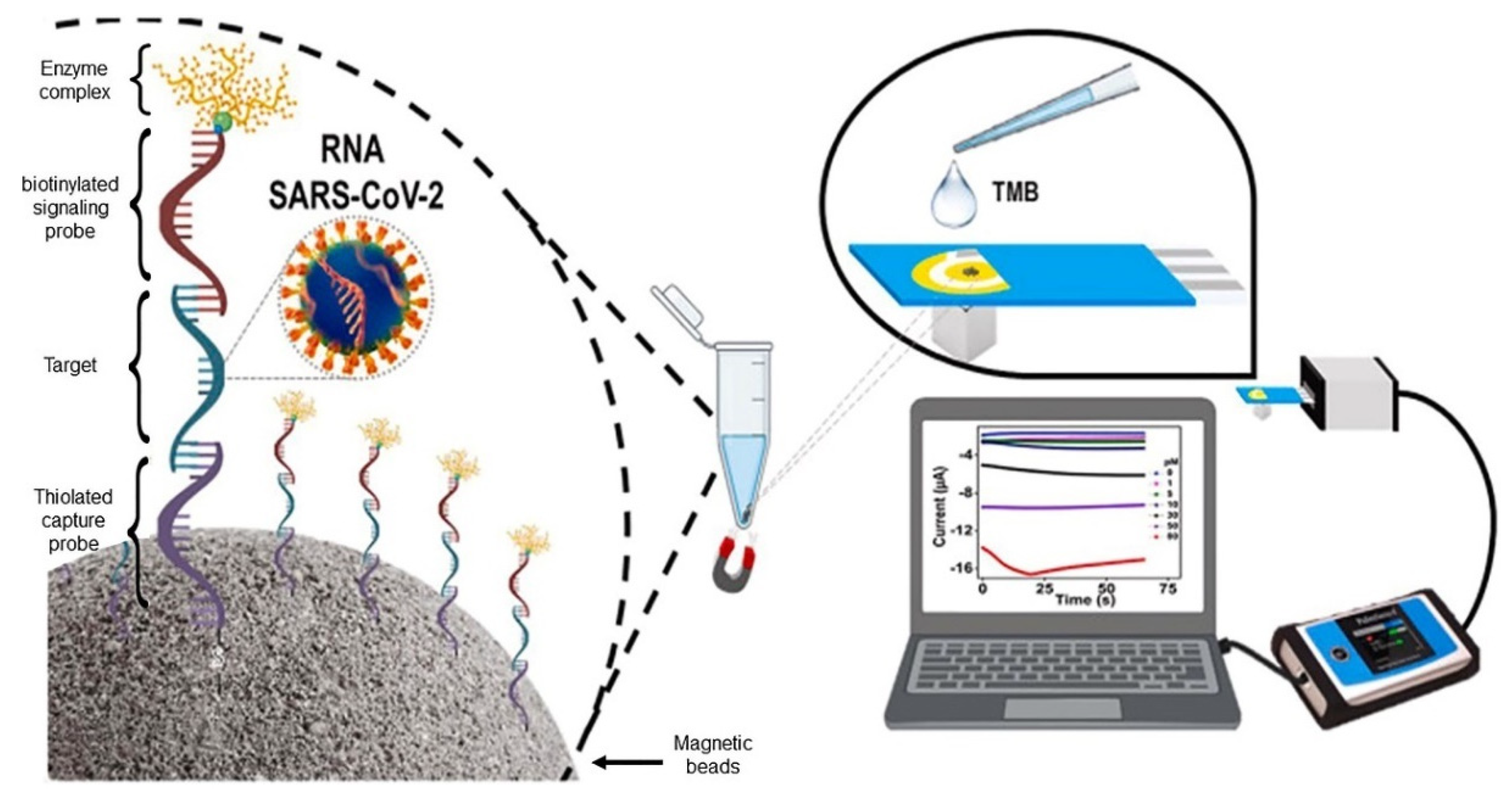
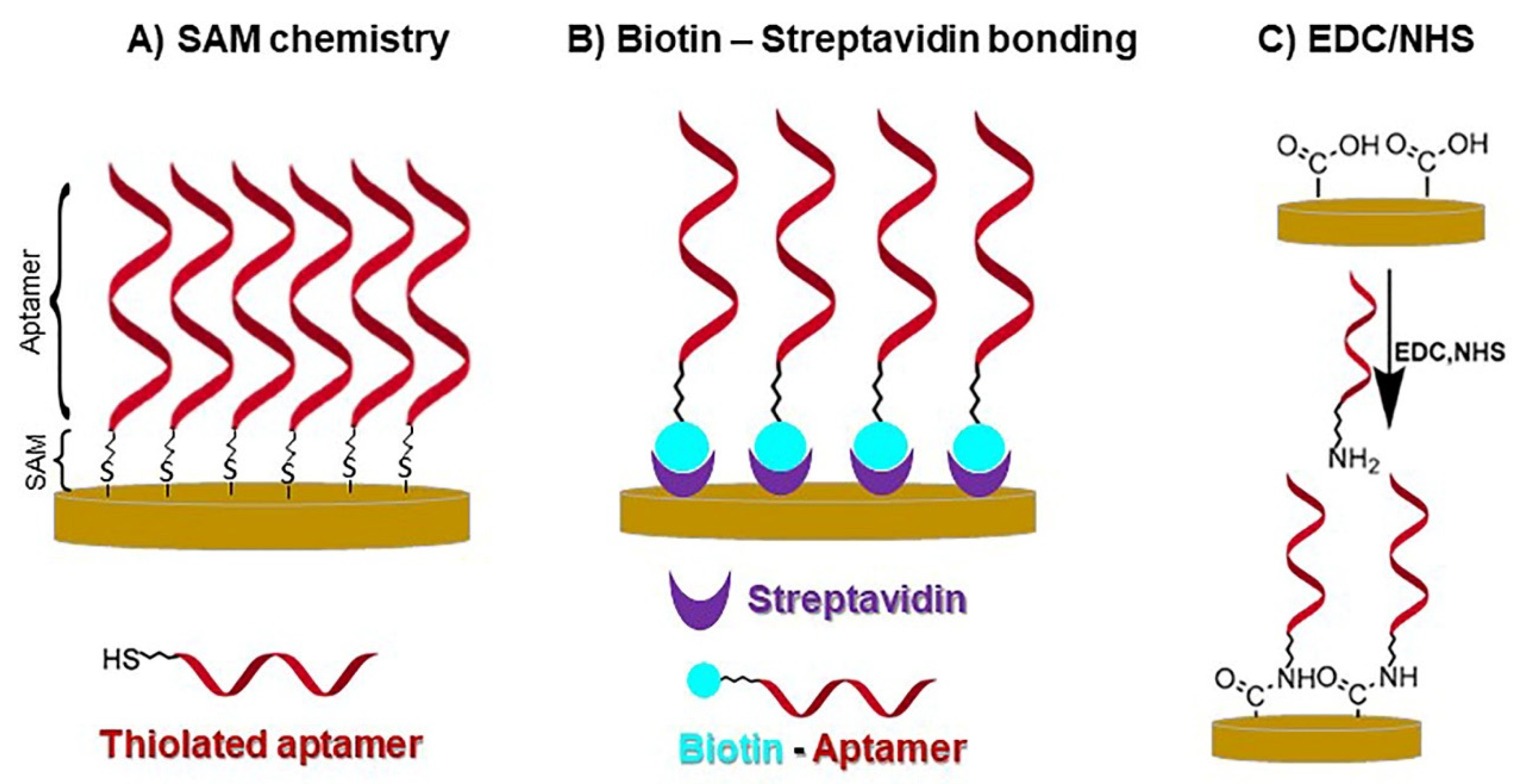
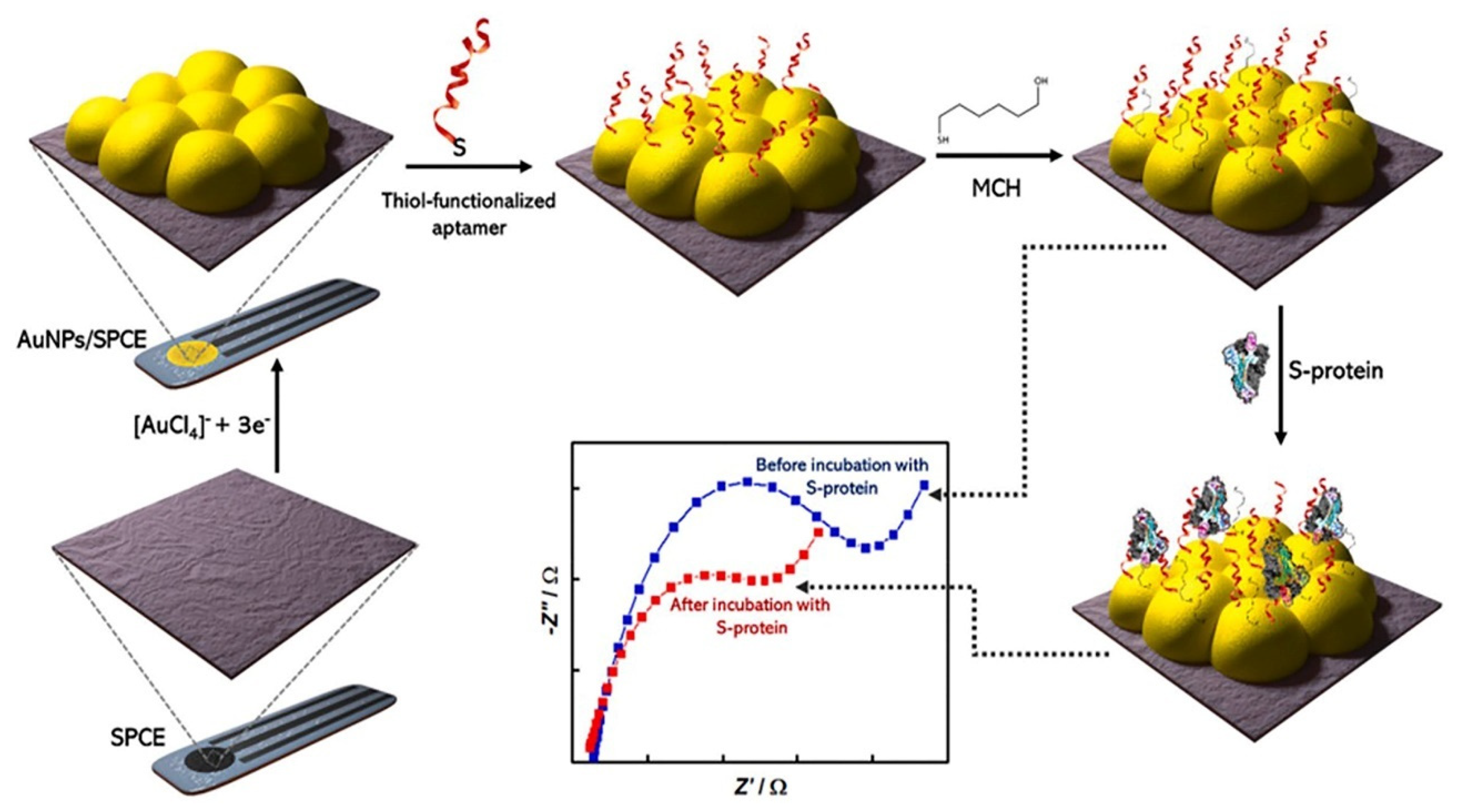
4.2. Electrochemical Genosensors and Aptasensors
5. Electrochemical Sensors for the Detection of SARS-CoV-2
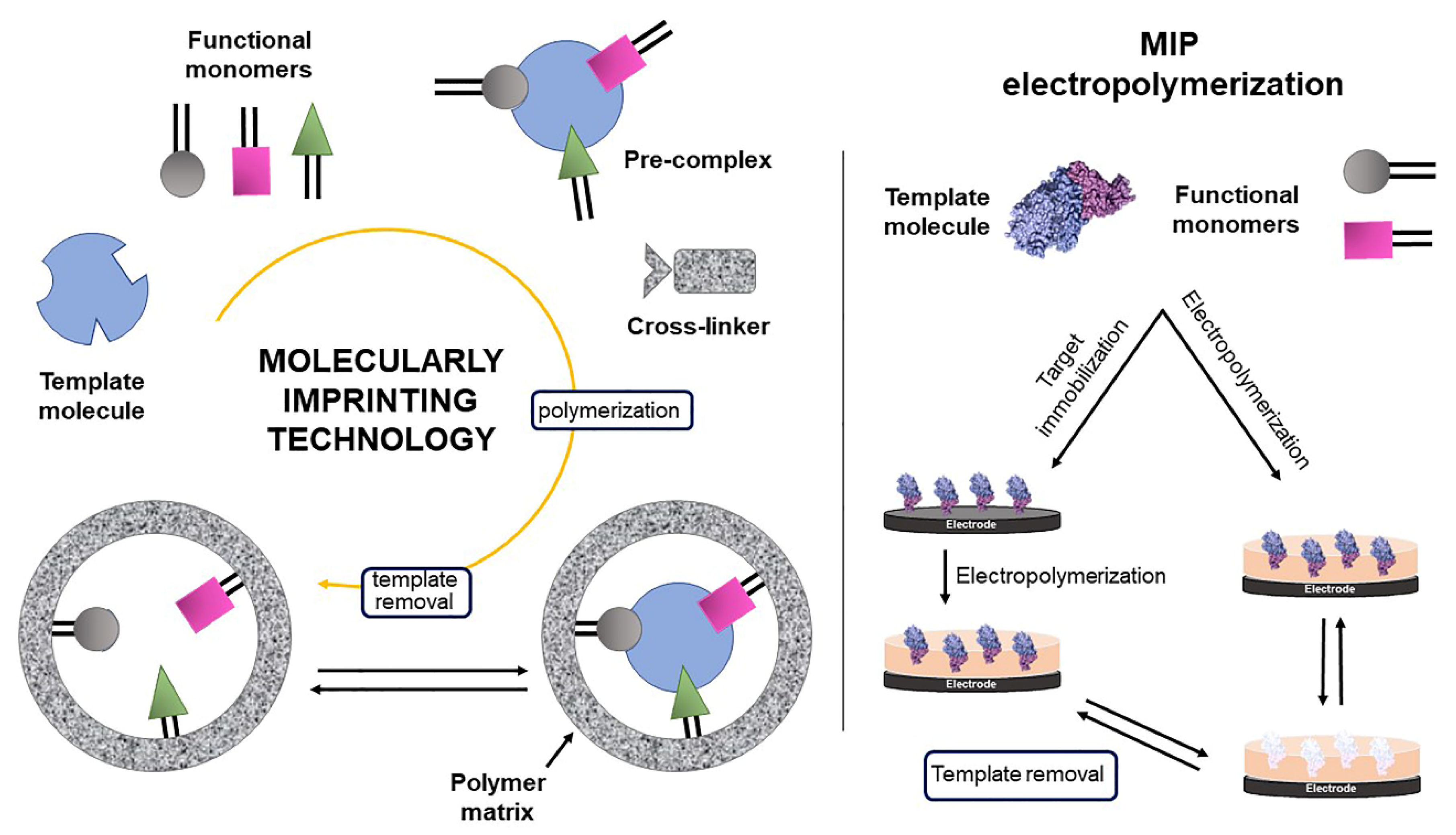
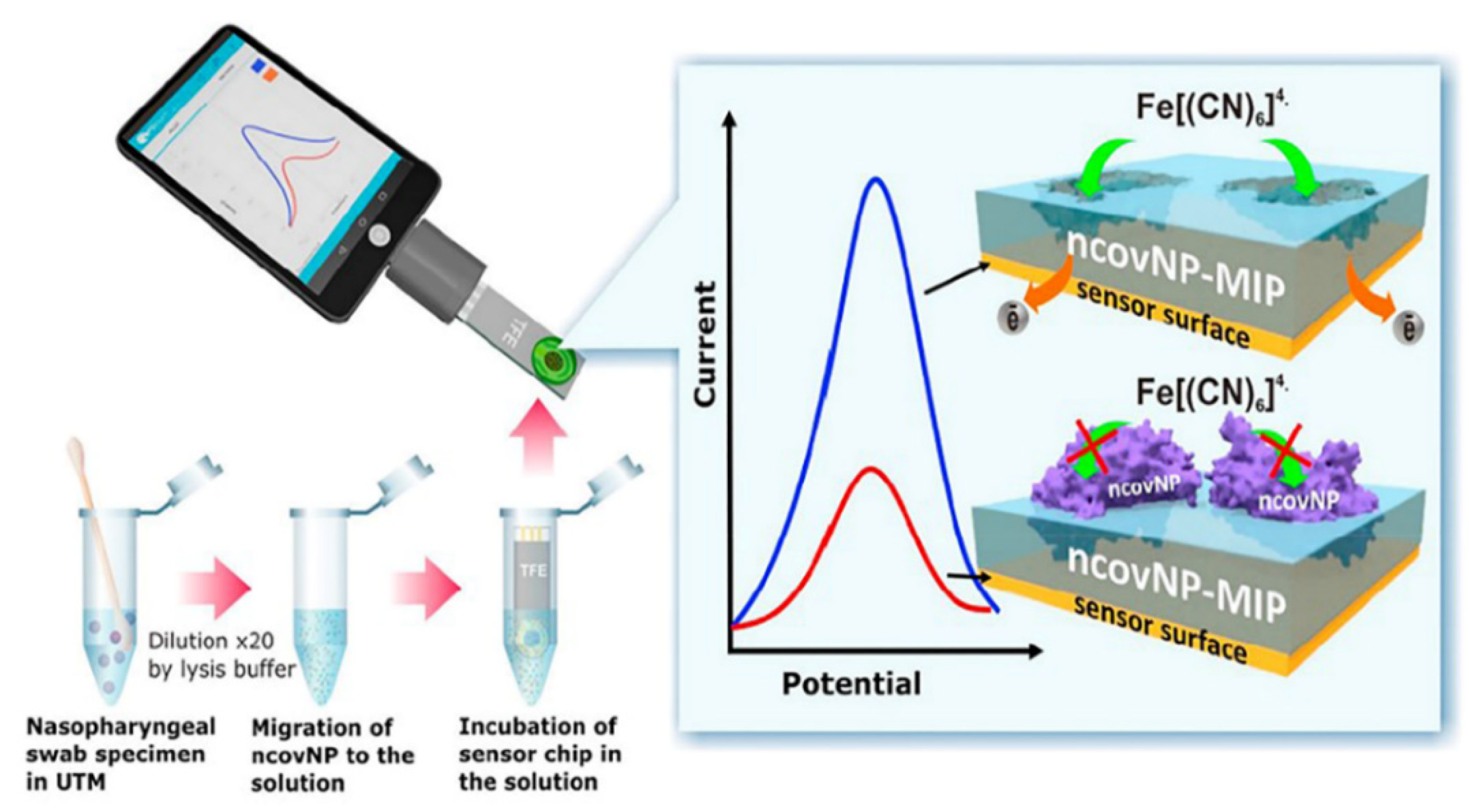
MIP-Based Electrochemical
6. Closing Remarks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OPAS—Organização Pan-Americana da Saúde. Available online: https://www.paho.org/pt/covid19/historico-da-pandemia-covid-19 (accessed on 14 October 2022).
- Coccia, M. Optimal levels of vaccination to reduce COVID-19 infected individuals and deaths: A global analysis. Environ. Res. 2022, 204, 112314. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Cao, W.; Liu, Z.; Lin, L.; Zhou, X.; Zeng, Y.; Wei, Y.; Chen, L.; Liu, X.; Han, Y.; et al. Prolonged presence of viral nucleic acid in clinically recovered COVID-19 patients was not associated with effective infectiousness. Emerg. Microbes Infect. 2020, 9, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, R.; Lee, R.A.; Lee, G.R.; Callahan, C.; Yen, D.F.; Smith, K.P.; Arora, R.; Kirby, J.E. SARS-CoV2 Testing: The Limit of Detection Matters. bioRxiv 2020, 131144. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Gumus, Z.P.; Soylak, M.; Erk, N. Electrochemical immunosensor for rapid and highly sensitive detection of SARS-CoV-2 antigen in the nasal sample. Talanta 2022, 240, 123211. [Google Scholar] [CrossRef]
- Sharif, H.E.; Dennison, S.R.; Tully, M.; Crossley, S.; Mwangi, W.; Bailey, D.; Graham, S.P.; Reddy, S.M. Evaluation of electropolymerized molecularly imprinted polymers (E-MIPs) on disposable electrodes for detection of SARS-CoV-2 in saliva. Anal. Chim. Acta 2022, 1206, 339777. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Byun, J.-H.; Cho, Y.; Rim, J.H. RT-PCR for SARS-CoV-2: Quantitative versus qualitative. Lancet Infect. Dis. 2021, 21, 165. [Google Scholar] [CrossRef]
- Ying, L.; Liu, Y.-p.; Bo, D.; Feifei, R.; Wang, Y.; Jinya, D.; Qianchuan, H. Diagnostic Indexes of a Rapid IgG/IgM Combined Antibody Test for SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Ayankojo, A.G.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Molecularly imprinted polymer based electrochemical sensor for quantitative detection of SARS-CoV-2 spike protein. Sens. Actuators B Chem. 2022, 353, 131160. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591. [Google Scholar] [CrossRef]
- Tarassishin, L. The Evolution of the Enzyme Immunoassay/Enzyme-Linked Immunosorbent Assay. J. Proteom. Genom. Res. 2021, 3, 13. [Google Scholar] [CrossRef]
- Lim, R.R.X.; Bonanni, A. The potential of electrochemistry for the detection of coronavirus-induced infections. TrAC Trends Anal. Chem. 2020, 133, 116081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Pan, W.; Arasthfer, A.; Fang, W.; Ling, L.; Fang, H.; Daneshnia, F.; Yu, J.; Liao, W.; Pei, H.; et al. Development and Validation of a Rapid, Single-Step Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) System Potentially to Be Used for Reliable and High-Throughput Screening of COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 331. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Ormond, M.; Keynan, Y. Extraction-free RT-LAMP to detect SARS-CoV-2 is less sensitive but highly specific compared to standard RT-PCR in 101 samples. J. Clin. Virol. 2021, 136, 104764. [Google Scholar] [CrossRef]
- Mahshid, S.S.; Flynn, S.E.; Mahshid, S. The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2. Biosens. Bioelectron. 2021, 176, 112905. [Google Scholar] [CrossRef]
- Bar-on, Y.M.; Flamholz, A.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. Elife 2020, 9, e57309. [Google Scholar] [CrossRef] [Green Version]
- Pizzato, M.; Baraldi, C.; Boscato Sopetto, G.; Finozzi, D.; Gentile, C.; Gentile, M.D.; Marconi, R.; Paladino, D.; Raoss, A.; Riedmiller, I.; et al. SARS-CoV-2 and the Host Cell: A Tale of Interactions. Front. Virol. 2022, 1, 815388. [Google Scholar] [CrossRef]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020, 54, 159. [Google Scholar] [CrossRef]
- Ning, B.; Yu, T.; Zhang, S.; Huang, Z.; Tian, D.; Lin, Z.; Niu, A.; Golden, N.; Hensley, K.; Threeton, B.; et al. A smartphone-read ultrasensitive and quantitative saliva test for COVID-19. Sci. Adv. 2021, 7, 19. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 843. [Google Scholar] [CrossRef] [Green Version]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 620, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergevin, M.A.; Freppel, W.; Robert, G.; Ambaraghassi, G.; Aubry, D.; Haeck, O.; Saint-Jean, M.; Carignan, A. Validation of saliva sampling as an alternative to oro-nasopharyngeal swab for detection of SARS-CoV-2 using unextracted rRT-PCR with the Allplex 2019-nCoV assay. J. Med. Microbiol. 2021, 70, 001404. [Google Scholar] [CrossRef]
- To, K.K.W.; Tsang, O.T.Y.; Yip, C.C.Y.; Chan, K.H.; Wu, T.C.; Chan, J.M.C.; Leung, W.S.; Chik, T.S.H.; Choi, C.Y.C.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020, 7, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasomsub, E.; Watcharananan, S.P.; Boonyawat, K.; Janchompoo, P.; Wongtabtim, G.; Suksuwan, W.; Sungkanuparph, S.; Phuphuakrat, A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease-2019 (COVID-19): A cross-sectional study. Clin. Microbiol. Infect. 2021, 27, 285.e1. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283. [Google Scholar] [CrossRef] [PubMed]
- Fatibello-Filho, O.; Silva, T.; Moraes, F.; Sitta, E. Eletroanálises: Aspectos Teóricos e Práticos; EDUFSCAR: São Carlos, Brazil, 2022; 504p. [Google Scholar]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Nascimento, E.D.; Fonseca, W.T.; de Oliveira, T.R.; de Correia, C.R.; Faça, V.M.; de Morais, B.P.; Silvestrini, V.C.; Pott-Junior, H.; Teixeira, F.R.; Faria, R.C. COVID-19 diagnosis by SARS-CoV-2 Spike protein detection in saliva using an ultrasensitive magneto-assay based on disposable electrochemical sensor. Sens. Actuators B Chem. 2022, 353, 131128. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.K.; Hartati, Y.W.; Gaffar, S.; Anshori, I.; Hidayat, D.; Wiraswati, H.L. The optimization of an electrochemical aptasensor to detect RBD protein S SARS-CoV-2 as a biomarker of COVID-19 using screen-printed carbon electrode/AuNP. J. Electrochem. Sci. Eng. 2022, 21, 219. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A.; Scheller, F.W. How Reliable Is the Electrochemical Readout of MIP Sensors? Sensors 2020, 20, 2677. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Adornetto, G.; Palleschi, G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta 2012, 84, 74. [Google Scholar] [CrossRef]
- Piro, B.; Shi, S.; Reisberg, S.; Noël, V.; Anquetin, G. Comparison of Electrochemical Immunosensors and Aptasensors for Detection of Small Organic Molecules in Environment, Food Safety, Clinical and Public Security. Biosensors 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Witt, S.; Rogien, A.; Werner, D.; Siegenthaler, J.; Lesiyon, R.; Kurien, N.; Rechenberg, R.; Baule, N.; Hardy, A.; Becker, M. Boron doped diamond thin films for the electrochemical detection of SARS-CoV-2 S1 protein. Diam. Relat. Mater. 2021, 118, 108542. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Volpe, J.; Bach-Toledo, J.; Kurpel, K.C.; Deller, A.E.; Soares, A.L.; Nardin, J.M.; Marchesi, L.F.; Simas, F.F.; Oliveira, C.C.; et al. Development of polypyrrole (nano)structures decorated with gold nanoparticles toward immunosensing for COVID-19 serological diagnosis. Mater. Today Chem. 2022, 24, 100817. [Google Scholar] [CrossRef]
- Arya, S.K.; Solanki, P.R.; Datta, M.; Malhotra, B.D. Recent advances in self-assembled monolayers based biomolecular electronic devices. Biosens. Bioelectron. 2009, 24, 2810. [Google Scholar] [CrossRef] [PubMed]
- Mandler, D.; Kraus-Ophir, S. Self-assembled monolayers (SAMs) for electrochemical sensing. J. Solid State Electrochem. 2011, 15, 1535. [Google Scholar] [CrossRef]
- Liv, L. Electrochemical immunosensor platform based on gold-clusters, cysteamine and glutaraldehyde modified electrode for diagnosing COVID-19. Microchem. J. 2021, 168, 106445. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojsoska, B.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A. Rapid SARS-CoV-2 Detection Using Electrochemical Immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Wang, Q.; Xiao, M.; Zhong, D.; Sun, W.; Zhang, G.; Zhang, Z. Ultrasensitive Monolayer MoS2 Field-Effect Transistor Based DNA Sensors for Screening of Down Syndrome. Nano Lett. 2019, 19, 1437. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Peng, Z.; Holl, N.J.; Hassan, M.R.; Pappas, J.M.; Wei, C.; Izadi, O.H.; Wang, Y.; Dong, X.; Wang, C.; et al. MXene—Graphene Field-Effect Transistor Sensing of Influenza Virus and SARS-CoV-2. ACS Omega 2021, 6, 6643. [Google Scholar] [CrossRef]
- Kaushik, M.; Khurana, S.; Mehra, K.; Yadav, N.; Mishra, S.; Kukreti, S. Emerging trends in Advanced Nanomaterials based electrochemical genosensors. Curr. Pharm. Des. 2018, 24, 3697. [Google Scholar] [CrossRef]
- Paleček, E.; Bartošík, M. Electrochemistry of Nucleic Acids. Chem. Rev. 2012, 112, 3427. [Google Scholar] [CrossRef] [PubMed]
- Paniel, N.; Baudart, J. Colorimetric and electrochemical genosensors for the detection of Escherichia coli DNA without amplification in seawater. Talanta 2013, 115, 133. [Google Scholar] [CrossRef] [PubMed]
- Labuda, J.; Brett, A.M.O.; Evtugyn, G.; Fojta, M.; Mascini, M.; Ozsoz, M.; Palchetti, I.; Paleček, E.; Wang, J. Electrochemical nucleic acid-based biosensors: Concepts, terms, and methodology (IUPAC Technical Report). Pure Appl. Chem. 2010, 82, 1161. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef] [PubMed]
- Cajigas, S.; Alzate, D.; Fernández, M.; Muskus, C.; Orozco, J. Electrochemical genosensor for the specific detection of SARS-CoV-2. Talanta 2022, 45, 123482. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ma, Y.; Chen, M.; Ambrosi, A.; Ding, C.; Luo, X. Electrochemical Biosensor with Enhanced Antifouling Capability for COVID-19 Nucleic Acid Detection in Complex Biological Media. Anal. Chem. 2021, 93, 5963. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal. Chem. 2020, 92, 9895. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91. [Google Scholar] [CrossRef]
- Radi, A.-E.; Abd-Ellatief, M.R. Electrochemical Aptasensors: Current Status and Future Perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef]
- Sari, A.K.; Gaffar, S.; Hartati, Y.W.A. Review on the Development of Aptamer Immobilization Techniques in Aptamer-Based Electrochemical Biosensors for Viruses Detection. Anal. Bioanal. Electrochem. 2022, 14, 127. [Google Scholar]
- Tabrizi, M.A.; Acedo, P. An Electrochemical Impedance Spectroscopy-Based Aptasensor for the Determination of SARS-CoV-2-RBD Using a Carbon Nanofiber–Gold Nanocomposite Modified Screen-Printed Electrode. Biosensors 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Martinez, J.C.; Jafari, M.; Chergui, S.; Pavel, C.; Che, D.; Siaj, M. Aptamer-based electrochemical biosensor for rapid detection of SARS-CoV-2: Nanoscale electrode-aptamer-SARS-CoV-2 imaging by photo-induced force microscopy. Biosens. Bioelectron. 2022, 195, 113595. [Google Scholar] [CrossRef] [PubMed]
- Idili, A.; Parolo, C.; Alvarez-Diduk, R.; Merkoçi, A. Rapid and Efficient Detection of the SARS-CoV-2 Spike Protein Using an Electrochemical Aptamer-Based Sensor. ACS Sens. 2021, 6, 3093. [Google Scholar] [CrossRef] [PubMed]
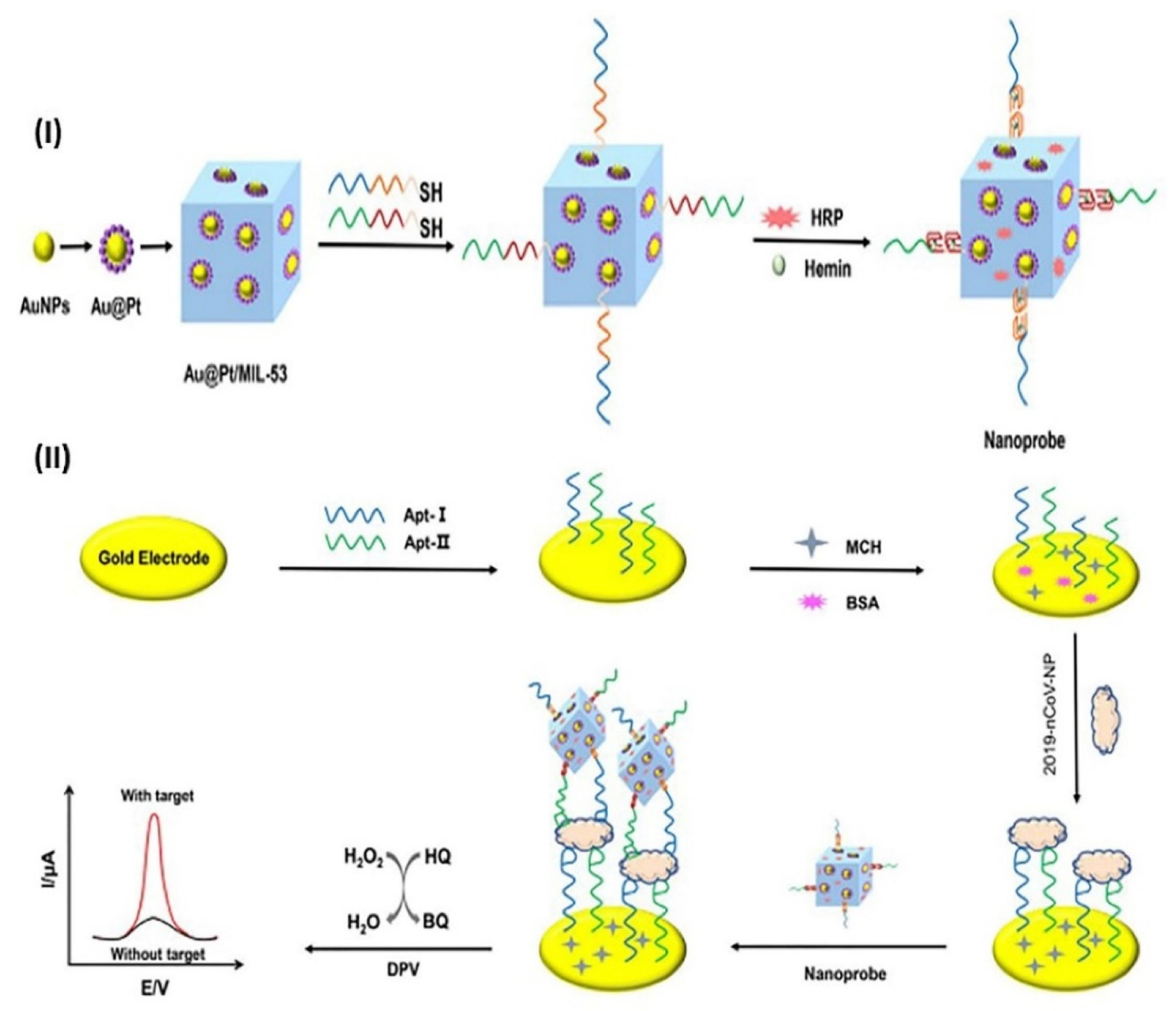
- Tian, J.; Liang, Z.; Hu, O.; He, Q.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Bahrani, S.; Mousavi, S.M.; Omidifar, N.; Behbahan, N.G.G.; Arjmand, M.; Ramakrishna, S.; Lankarani, K.B.; Moghadami, M.; Firoozsani, M. Graphene-Based Femtogram-Level Sensitive Molecularly Imprinted Polymer of SARS-CoV-2. Adv. Mater. Interfaces 2021, 8, 2101466. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Sotomayor, M.D.P.T.; Kubota, L.T. Polímeros biomiméticos em química analítica. Parte 2: Aplicações de MIP (“Molecularly Imprinted Polymers”) no desenvolvimento de sensores químicos. Química Nova 2005, 28, 1087. [Google Scholar] [CrossRef]
- Erdőssy, J.; Horváth, V.; Yarman, A.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polymers for protein recognition. TrAC Trends Anal. Chem. 2016, 79, 179. [Google Scholar] [CrossRef] [Green Version]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Hussein, H.A.; Kandeil, A.; Gomaa, M.; El Nashar, R.M.; El-Sherbiny, I.M.; Hassan, R.Y.A. SARS-CoV-2-Impedimetric Biosensor: Virus-Imprinted Chips for Early and Rapid Diagnosis. ACS Sens. 2021, 6, 4098. [Google Scholar] [CrossRef]
- Tabrizi, A.M.; Fernández-Blázquez, J.P.; Medina, D.M.; Acedo, P. An ultrasensitive molecularly imprinted polymer-based electrochemical sensor for the determination of SARS-CoV-2-RBD by using macroporous gold screen-printed electrode. Biosens. Bioelectron. 2022, 15, 113729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, L.; Zhang, Y. Highly sensitive electrochemical determination of the SARS-COV-2 antigen based on a gold/graphene imprinted poly-arginine sensor. Anal. Methods 2021, 13, 5772. [Google Scholar] [CrossRef] [PubMed]
- Sykora, S.; Cumbo, A.; Belliot, G.; Pothier, P.; Arnal, C.; Dudal, Y.; Corvini, P.F.X.; Shahgaldian, P. Virus-like particles as virus substitutes to design artificial virus-recognition nanomaterials. Chem. Commun. 2015, 51, 12–2256. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. An Overview of Bio-Inspired Intelligent Imprinted Polymers for Virus Determination. Biosensors 2021, 11, 89. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoglu, S. Molecularly Imprinted Polymer-Based Sensors for SARS-CoV-2: Where Are We Now? Biomimetics 2022, 7, 58. [Google Scholar] [CrossRef]
- Gast, M.; Sobek, H.; Mizaikoff, B. Advances in Imprinting Strategies for Selective Virus Recognition A Review. TrAC Trends Anal. Chem. 2019, 114, 218. [Google Scholar] [CrossRef]



| Electrochemical Techniques | Advantages | Disadvantages | |
|---|---|---|---|
| Voltammetric (time-dependent potential resulting in current as a function of applied potential) | DPV | High sensitivity; high peak-shaped resolution; minimization of background effects and double layers. | Slower than SWV. |
| SWV | Is slightly more sensitive than DPV; Is faster than DPV; Similar to DPV, it is able to discriminate the nonfaradaic currents; in the reverse cathodic process, the oxygen interference is avoided. | More evidence indicates a reversible redox process. | |
| Amperometric (fixed potential resulting in an initial transient current) | Chrono-amperometry | High sensitivity; minimized signal-to-noise; easy coupling of flow systems. | Selectivity is low when using high positive and negative potentials |
| Impedimetric (varied frequency, response obtained in impedance x imaginary impedance) | (EIS) | Sensitive; direct detection of biomolecular recognition. | Longer measurement time compared to other techniques. |
| Potentiometric (potential that develops in an electrochemical cell containing two electrodes, an indicator, and a reference. A high-impedance potential measuring device is necessary. | Low cost; easy coupling of flow systems; wide linear range and good sensitivity. | Less precise; very sensitive to changes in the ionic strength; longer time to stabilize the measurements | |
| Biomarker | Electrode | Modifiers | Technique | Linear Range | Limit of Detection | Analysis Time | Ref. |
|---|---|---|---|---|---|---|---|
| Antibodies IgG/IgM | SPCE | Ni(OH)2NPs/S-protein | DPV | 1 fg mL−1 to 1 µg mL−1 | 0.3 fg mL−1 | 20 min | [30] |
| Antibody anti-N protein | steel mesh | Ppy:PSS/AuNPs/3-MPA/EDC:NHS/N-protein | EIS | Ppy:PSS/AuNPs 10–60 ng mL−1 | Ppy:PSS/AuNPs 0.386 ng mL−1 | 30 min | [42] |
| Antibody anti-N protein | steel mesh | Ppy-NTs/AuNPs/MPA/EDC:NHS/N-protein | EIS | Ppy-NTs/AuNPs 0.4–8 ng mL−1 | Ppy-NTs/AuNPs 2.456 ng mL−1 | 30 min | [42] |
| Antibody anti-S protein | GCE | Au/Cysteamine/glutaraldehyde/S-protein | SWV | 0.1–1000 ag mL−1 | 0.01 ag mL−1 in sample | 30 min | [45] |
| Antibodies IgG/IgM | GO-ePAD | S-Protein RBD via EDC:NHS | SWV | 1–1.000 ng mL−1 | IgG—0.96 ng mL−1 IgM—0.14 ng mL−1 | 30 min | [32] |
| Protein S | GO-ePAD | IgM antibody via EDC/NHS | SWV | 1–1.000 ng mL−1 | 0.11 ng mL−1 | 45 min | [32] |
| Protein S | SPCE | MOFs/Cysteamine/glutaraldehyde/ACE2 | EIS | 100 fg mL−1 to 10 ng mL−1 | 100 fg mL−1 | 5 min | [5] |
| Protein S | SPCE | MBs-ACE2/ S-protein/ACE2-AuNPs | DPV | 0.0009–360 fg mL−1 | 0.35 ag mL−1 | 1 h | [33] |
| Protein S | BDD | APTMS/ biotin-straptavidin/anti-S antibody | EIS | 1–7 fg mL−1 | 0.9–1 fg mL−1 | 5 min | [41] |
| Protein S | Graphene-FET | Graphene/Mxene/ APTES/anti-S antibody | potentiometry | 1 fg mL−1 to 10 pg mL−1 | 1 fg mL−1 (PBS) | - | [51] |
| Protein S | SPGE | PBASE/anti-S antibody | SWV | 260–1040 nmol L−1 | - | 45 min | [47] |
| Protein S | Graphene-FET | Graphene/PBASE/anti-S antibody | potentiometry | 1 fg to 10 pg mL−1 (PBS) 100 fg to 100 pg mL−1 (sample) | 1 fg mL−1 (PBS) 100 fg mL−1 (sample) | - | [50] |
| Protein S | SPCE | MBs/Mab anti-S protein/SARS-CoV-2/Pab anti-S protein/Pab anti-rabbit-AP | DPV | 0.04–10 μg mL−1 | 14 ng mL−1 (PBS); 19 ng mL−1 (sample) | 32 min | [40] |
| Protein N | SPCE | MBs/Mab anti-N protein/SARS-CoV-2/Pab anti-N protein/Pab anti-rabbit-AP | DPV | 0.01–0.6 μg mL−1 | 4 ng mL−1 (PBS); 8 ng mL−1 (sample) | 32 min | [40] |
| Biomarker | Electrode | Modifiers | Technique | Linear Range | Limit of Detection | Analysis Time | Ref. |
|---|---|---|---|---|---|---|---|
| RNA | SPCE | Au@Fe3O4/CP/RNA/ Au@SCX8-RGO-TB | DPV | 10 amol L−1 to 1 pmol L−1 | 3 amol L−1 | 3 h incubation | [56] |
| RNA | SPAuE | MBs/maleimide/thiolated capture probe/RNA/ biotinylated signaling probe/streptavidin-horseradish peroxidase | Chron. | 0–80 pmol L−1 | 0.8 pmol L−1 | 1 min | [57] |
| RNA | GCE | PANI/biotin-peptide/SA/biotin-probe | DPV | 10 fmol L−1 to 1 nmol L−1 | 3.5 fmol L−1 | 1 h incubation | [58] |
| Biomarker | Electrode | Modifiers | Technique | Linear Range | Limit of Detection | Analysis Time | Ref. |
|---|---|---|---|---|---|---|---|
| RBD | SPCE | AuNPs/MPA/EDC:NHS/ streptavidin/biotinylated aptamer | DPV | 10.0 to 50.0 ng mL−1 | 2.63 ng mL−1 | 1 h | [34] |
| RBD | SPCE | CNF@AuNPs/thiolated aptamer | EIS | 0.01 to 64 nmol L−1 (0.35 to 2240 ng mL−1) | 7.0 pmol L−1 (0.24 ng mL−1) | 40 min | [64] |
| RBD | SPCE | AuNPs/thiolated aptamer | EIS | 10.0 pmol L−1 to 25.0 nmol L−1 | 1.30 pmol L−1 (66 pg mL−1). | 40 min | [65] |
| RBD | Gold wire electrode | Thiolated aptamer-Atto MB2 | SWV | 760 pg mL−1 to 76 ng mL−1 | - | 5 min | [66] |
| protein N | GE | Thiolated aptamer/N-protein/thiolated aptamer/Au@Pt/MOF | DPV | 0.025 to 50 ng mL−1 | 8.33 pg mL−1 | 2 h | [67] |
| Transducer | Template/Monomer | Technique | Linear Range | Limit of Detection | Analysis Time | Ref. |
|---|---|---|---|---|---|---|
| SPCE | SARS-CoV-2 pps/NHMA; MBAm (crosslinker) | EIS | 3.0 to 7.0 log10 pfu mL−1 | 4.9 log10 pfu mL−1 | <10 min | [6] |
| CNTs/WO3-SPCE | SARSCoV-2 whole virus/m-AP | EIS | 7.0 to 320 pg mL−1 | 57 pg mL−1 | 5 min | [72] |
| GCE | SARS-CoV-2 whole virus/(GO) Pyrrole; PBA (crosslinker) | DPV | 0.74 to 9.03 fg mL−1 | 0.33 fg mL−1 | 1 min | [68] |
| GCE | SARS-CoV-2 whole virus/(GO) Pyrrole; APBA (crosslinker) | Amp. | 13.14 to 118.9 fg mL−1 | 1.32 fg mL−1 | 20 s | [68] |
| MP-Au-SPE | SARS-CoV-2 RBD/ o-PD | DPV | 2 to 40 pg mL−1 | 0.7 pg mL−1 (20 fmol L−1) | 20 min | [73] |
| Pt electrode | S-protein/Pyrrole | Chron. in pulsed Amp. (PAD) | 5 μg mL−1 to 25 μg mL−1 | - | - | [71] |
| 4-ATP/Au-TFME | S-protein/APBA | SWV | PBS—26.7 to 194 fmol L−1 Sample—0 to 400 fmol L−1 | 15 fmol L−1 (PBS) 64 fmol L−1 (sample) | 15 min | [9] |
| 4-ATP/Au-TFE | N-protein/m-PD | DPV | 2 to 111 f mol L−1 | 15 fmol L−1 | - | [31] |
| Au/Gr/SPCE | N-protein/Arginine | DPV | 10 to 200 f mol L−1 | 3.0 fmol L−1 | - | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.d.P.; Yamada-Ogatta, S.F.; Teixeira Tarley, C.R. Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19. Biosensors 2023, 13, 336. https://doi.org/10.3390/bios13030336
Ferreira MdP, Yamada-Ogatta SF, Teixeira Tarley CR. Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19. Biosensors. 2023; 13(3):336. https://doi.org/10.3390/bios13030336
Chicago/Turabian StyleFerreira, Milena do Prado, Sueli Fumie Yamada-Ogatta, and César Ricardo Teixeira Tarley. 2023. "Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19" Biosensors 13, no. 3: 336. https://doi.org/10.3390/bios13030336
APA StyleFerreira, M. d. P., Yamada-Ogatta, S. F., & Teixeira Tarley, C. R. (2023). Electrochemical and Bioelectrochemical Sensing Platforms for Diagnostics of COVID-19. Biosensors, 13(3), 336. https://doi.org/10.3390/bios13030336






