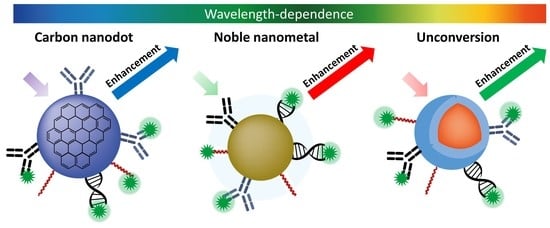
Wavelength-Dependent Metal-Enhanced Fluorescence Biosensors via Resonance Energy Transfer Modulation
Abstract
:1. Introduction
2. Carbon Nanodots in the Ultraviolet-Visible Region
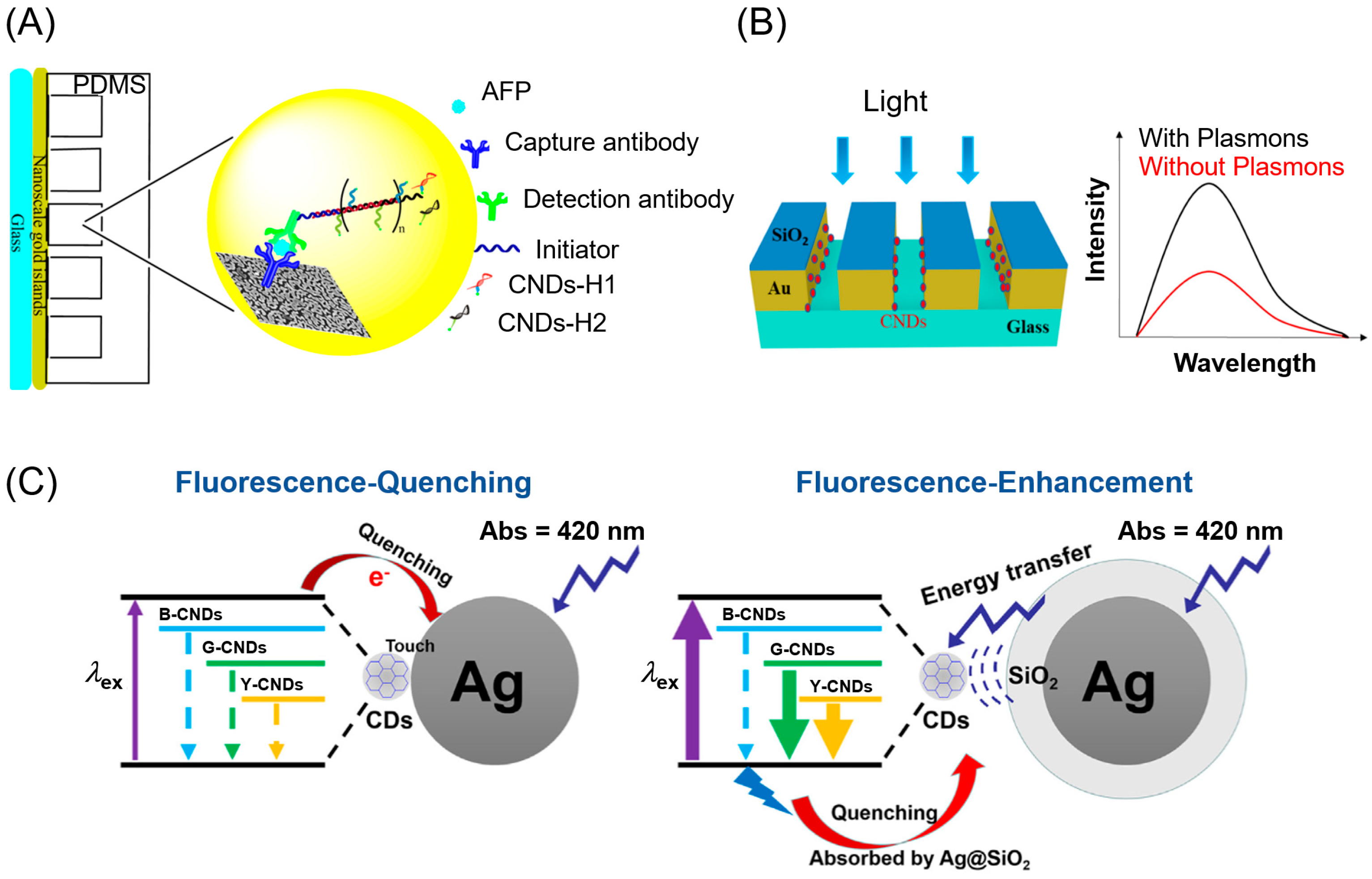
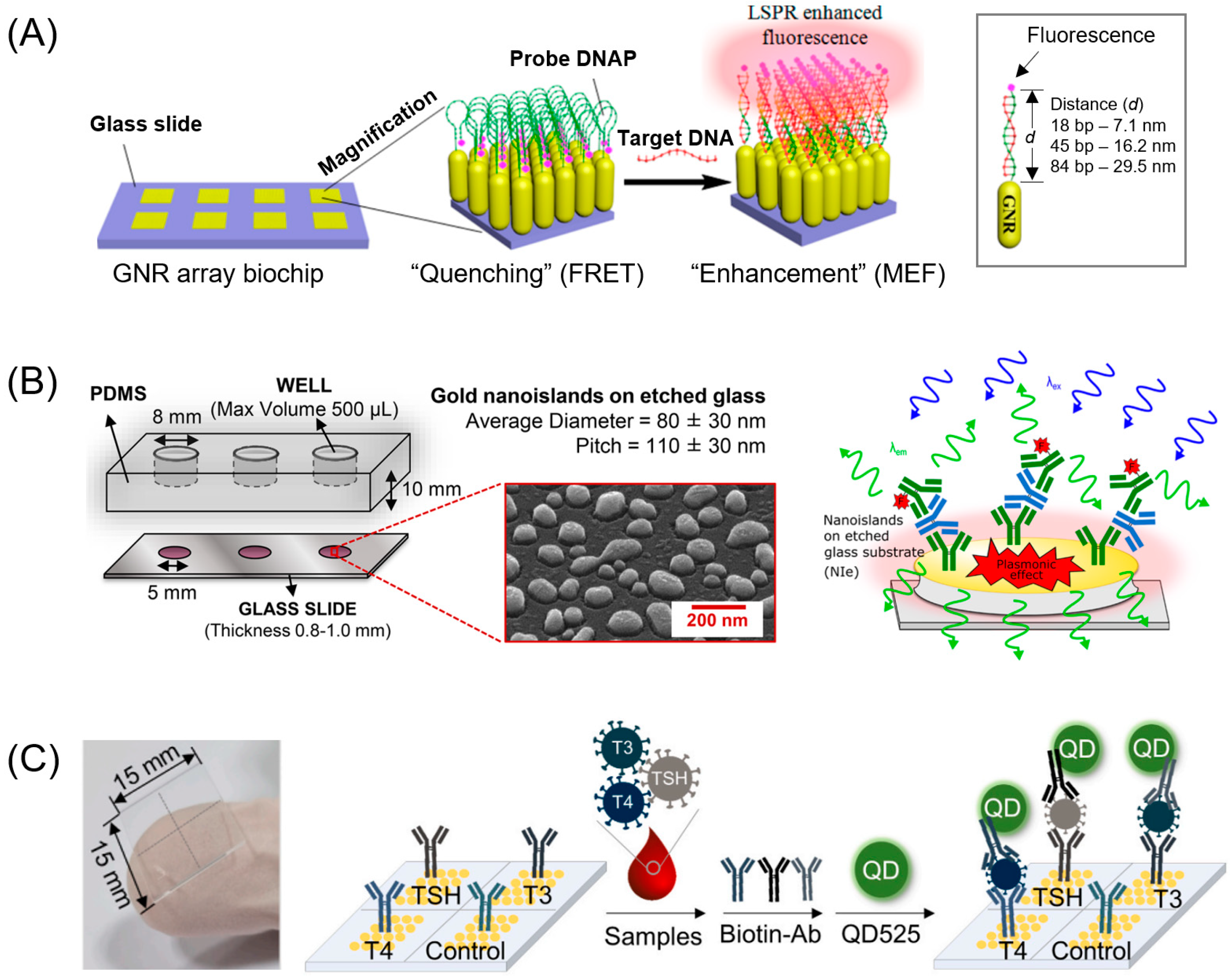
3. Noble Metals in the Visible Region
4. Upconversion Nanoparticles from Near-Infrared to Visible Region
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta Choudhury, S.; Badugu, R.; Ray, K.; Lakowicz, J.R. Silver–Gold Nanocomposite Substrates for Metal-Enhanced Fluorescence: Ensemble and Single-Molecule Spectroscopic Studies. J. Phys. Chem. C 2012, 116, 5042–5048. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Kook, Y.-M.; Lee, K.; Koh, W.-G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U. Analytical Protein Microarrays: Advancements Towards Clinical Applications. Sensors 2017, 17, 256. [Google Scholar] [CrossRef] [Green Version]
- Förster, T. Intermolecular Energy Migration and Fluorescence. Ann. Phys. 1948, 437, 55. [Google Scholar] [CrossRef]
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7, 100. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Yamamoto, Y.; Nicovich, P.R.; Goyette, J.; Rossy, J.; Gooding, J.J.; Gaus, K. A Fret Sensor Enables Quantitative Measurements of Membrane Charges in Live Cells. Nat. Biotechnol. 2017, 35, 363–370. [Google Scholar] [CrossRef]
- Ni, Y.; Arts, R.; Merkx, M. Ratiometric Bioluminescent Sensor Proteins Based on Intramolecular Split Luciferase Complementation. ACS Sens. 2019, 4, 20–25. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Xiao, H.; Zhang, W.; Wang, L.; Tang, B. A New Polymer Nanoprobe Based on Chemiluminescence Resonance Energy Transfer for Ultrasensitive Imaging of Intrinsic Superoxide Anion in Mice. J. Am. Chem. Soc. 2016, 138, 2893–2896. [Google Scholar] [CrossRef]
- Darbha, G.K.; Ray, A.; Ray, P.C. Gold Nanoparticle-Based Miniaturized Nanomaterial Surface Energy Transfer Probe for Rapid and Ultrasensitive Detection of Mercury in Soil, Water, and Fish. ACS Nano 2007, 1, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Long, Y.-T.; Choi, Y.; Kang, T.; Lee, L.P. Quantized Plasmon Quenching Dips Nanospectroscopy Via Plasmon Resonance Energy Transfer. Nat. Methods 2007, 4, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.F.; Li, Y.F.; Huang, C.Z. Plasmonics-Attended Nset and Pret for Analytical Applications. TrAC Trends Anal. Chem. 2020, 124, 115805. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2022, 2107986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-Induced Resonance Energy Transfer for Solar Energy Conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ha, T.; Shin, M.; Lee, S.-N.; Choi, J.-W. Nanomaterial-Based Fluorescence Resonance Energy Transfer (FRET) and Metal-Enhanced Fluorescence (MEF) to Detect Nucleic Acid in Cancer Diagnosis. Biomedicines 2021, 9, 928. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Shen, Y.; D’Auria, S.; Malicka, J.; Fang, J.; Gryczynski, Z.; Gryczynski, I. Radiative Decay Engineering: 2. Effects of Silver Island Films on Fluorescence Intensity, Lifetimes, and Resonance Energy Transfer. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-Controlled Fluorescence: A New Paradigm in Fluorescence Spectroscopy. Analyst 2008, 133, 1308–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.-C.; Zhang, Q.; Maier, S.A.; Lei, D. Plasmonic Particle-on-Film Nanocavities: A Versatile Platform for Plasmon-Enhanced Spectroscopy and Photochemistry. Nanophotonics 2018, 7, 1865–1889. [Google Scholar] [CrossRef]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal Enhanced Fluorescence Biosensing: From Ultra-Violet Towards Second near-Infrared Window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, C.D. Surface Plasmon Enhanced, Coupled and Controlled Fluorescence; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Akselrod, G.M.; Argyropoulos, C.; Hoang, T.B.; Ciracì, C.; Fang, C.; Huang, J.; Smith, D.R.; Mikkelsen, M.H. Probing the Mechanisms of Large Purcell Enhancement in Plasmonic Nanoantennas. Nat. Photonics 2014, 8, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-Enhanced Optical Sensors: A Review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent Advances in Carbon Nanodots: Synthesis, Properties and Biomedical Applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, W.; Arvapalli, D.M.; Bloom, B.; Sheardy, A.; Mabe, T.; Liu, Y.; Ji, Z.; Chevva, H.; Waldeck, D.H.; et al. A Fluorescence-Electrochemical Study of Carbon Nanodots (CNDs) in Bio- and Photoelectronic Applications and Energy Gap Investigation. Phys. Chem. Chem. Phys. 2017, 19, 20101–20109. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, P.; Pengpumkiat, S.; Schumacher, E.A.; Remcho, V.T. Development of a Carbon Dot (C-Dot)-Linked Immunosorbent Assay for the Detection of Human A-Fetoprotein. Anal. Chem. 2015, 87, 8510–8516. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-Dot-Based Ratiometric Fluorescent Sensor for Detecting Hydrogen Sulfide in Aqueous Media and inside Live Cells. Chem. Commun. 2013, 49, 403–405. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef]
- Zhu, A.; Qu, Q.; Shao, X.; Kong, B.; Tian, Y. Carbon-Dot-Based Dual-Emission Nanohybrid Produces a Ratiometric Fluorescent Sensor for in Vivo Imaging of Cellular Copper Ions. Angew. Chem. Int. Ed. 2012, 51, 7185–7189. [Google Scholar] [CrossRef] [PubMed]
- Bagra, B.; Zhang, W.; Zeng, Z.; Mabe, T.; Wei, J. Plasmon-Enhanced Fluorescence of Carbon Nanodots in Gold Nanoslit Cavities. Langmuir 2019, 35, 8903–8909. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.A.; Chen, A.; et al. Engineering Triangular Carbon Quantum Dots with Unprecedented Narrow Bandwidth Emission for Multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Miao, X.; Yan, X.; Qu, D.; Li, D.; Tao, F.F.; Sun, Z. Red Emissive Sulfur, Nitrogen Codoped Carbon Dots and Their Application in Ion Detection and Theraonostics. ACS Appl. Mater. Interfaces 2017, 9, 18549–18556. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.-S.; Zhong, N.; Gao, Q.-Y.; Xiong, H.-M. Highly Efficient Red-Emitting Carbon Dots with Gram-Scale Yield for Bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef]
- Ghosh, S.; Ali, H.; Jana, N.R. Water Dispersible Red Fluorescent Carbon Nanoparticles Via Carbonization of Resorcinol. ACS Sustain. Chem. Eng. 2019, 7, 12629–12637. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.; Li, W.; Li, N.; Chen, Y.; Cai, L.; et al. Red/Orange Dual-Emissive Carbon Dots for Ph Sensing and Cell Imaging. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Xu, D.-D.; Liu, C.; Li, C.-Y.; Song, C.-Y.; Kang, Y.-F.; Qi, C.-B.; Lin, Y.; Pang, D.-W.; Tang, H.-W. Dual Amplification Fluorescence Assay for Alpha Fetal Protein Utilizing Immunohybridization Chain Reaction and Metal-Enhanced Fluorescence of Carbon Nanodots. ACS Appl. Mater. Interfaces 2017, 9, 37606–37614. [Google Scholar] [CrossRef] [PubMed]
- Jana, J.; Aditya, T.; Ganguly, M.; Mehetor, S.K.; Pal, T. Fluorescence Enhancement Via Varied Long-Chain Thiol Stabilized Gold Nanoparticles: A Study of Far-Field Effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 551–560. [Google Scholar] [CrossRef]
- Emam, A.N.; Mostafa, A.A.; Mohamed, M.B.; Gadallah, A.S.; El-Kemary, M. Enhancement of the Collective Optical Properties of Plasmonic Hybrid Carbon Dots Via Localized Surface Plasmon. J. Lumin. 2018, 200, 287–297. [Google Scholar] [CrossRef]
- Yuan, K.; Qin, R.; Yu, J.; Li, X.; Li, L.; Yang, X.; Yu, X.; Lu, Z.; Zhang, X.; Liu, H. Effects of Localized Surface Plasmon Resonance of Ag Nanoparticles on Luminescence of Carbon Dots with Blue, Green and Yellow Emission. Appl. Surf. Sci. 2020, 502, 144277. [Google Scholar] [CrossRef]
- Sciortino, A.; Panniello, A.; Minervini, G.; Mauro, N.; Giammona, G.; Buscarino, G.; Cannas, M.; Striccoli, M.; Messina, F. Enhancing Carbon Dots Fluorescence Via Plasmonic Resonance Energy Transfer. Mater. Res. Bull. 2022, 149, 111746. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhang, H.; Zhao, Q. Visible–Ultraviolet Upconversion Carbon Quantum Dots for Enhancement of the Photocatalytic Activity of Titanium Dioxide. ACS Omega 2021, 6, 4247–4254. [Google Scholar] [CrossRef]
- Anuar, N.K.K.; Tan, H.L.; Lim, T.P.; So’aib, M.S.; Balar, N.F.A. A Review on multifunctional Carbon-Dots Synthesized From Biomass Waste: Design/Fabrication, Characterization and Application. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campion, A.; Gallo, A.R.; Harris, C.B.; Robota, H.J.; Whitmore, P.M. Electronic Energy Transfer to Metal Surfaces: A Test of Classical Image Dipole Theory at Short Distances. Chem. Phys. Lett. 1980, 73, 447–450. [Google Scholar] [CrossRef]
- Ranjan Gartia, M.; Eichorst, J.P.; Clegg, R.M.; Logan Liu, G. Lifetime Imaging of Radiative and Non-Radiative Fluorescence Decays on Nanoplasmonic Surface. Appl. Phys. Lett. 2012, 101, 023118. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, H.; Chen, Y.; Liu, F.; Huang, C.Z.; Li, N. A Distance-Dependent Metal-Enhanced Fluorescence Sensing Platform Based on Molecular Beacon Design. Biosens. Bioelectron. 2014, 52, 367–373. [Google Scholar] [CrossRef]
- Qin, H.; Ma, D.; Du, J. Distance Dependent Fluorescence Quenching and Enhancement of Gold Nanoclusters by Gold Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 161–166. [Google Scholar] [CrossRef]
- Jiang, L.; Hang, X.; Zhang, P.; Zhang, J.; Wang, Y.; Wang, W.; Ren, L. A Highly Sensitive Fluorescence-Enhanced Aptasensor Based on Polyan-Aptamer Nanostructure. Microchem. J. 2019, 148, 285–290. [Google Scholar] [CrossRef]
- Kim, K.-S.; Zakia, M.; Yoon, J.; Yoo, S.I. Metal-Enhanced Fluorescence in Polymer Composite Films with Au@Ag@SiO2 Nanoparticles and Inp@ZnS Quantum Dots. RSC Adv. 2019, 9, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, A.; Nikkhah, M.; Moshaii, A. Development of Metal-Enhanced Fluorescence-Based Aptasensor for Thrombin Detection Using Silver Dendritic Nanostructures. Plasmonics 2019, 14, 561–568. [Google Scholar] [CrossRef]
- Trotsiuk, L.; Muravitskaya, A.; Kulakovich, O.; Guzatov, D.; Ramanenka, A.; Kelestemur, Y.; Demir, H.V.; Gaponenko, S. Plasmon-Enhanced Fluorescence in Gold Nanorod-Quantum Dot Coupled Systems. Nanotechnology 2020, 31, 105201. [Google Scholar] [CrossRef]
- Shi, K.; Na, N.; Ouyang, J. Label- and Enzyme-Free Plasmon-Enhanced Single Molecule Fluorescence Detection of Hiv DNA Fragments Based on a Catalytic Hairpin Assembly. Analyst 2022, 147, 604–613. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lim, J.; Shin, M.; Paek, S.-H.; Choi, J.-W. CRISPR-Cas12a-Based Nucleic Acid Amplification-Free DNA Biosensor Via Au Nanoparticle-Assisted Metal-Enhanced Fluorescence and Colorimetric Analysis. Nano Lett. 2021, 21, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Chakkarapani, S.K.; Lee, S.; Park, B.; Seo, H.-Y.; Kang, S.H. Plasmon-Amplified Endogenous Fluorescence Nanospectroscopic Sensor Based on Inherent Elastic Scattering for Ultratrace Ratiometric Detection of Capsaicinoids. ACS Sens. 2019, 4, 953–960. [Google Scholar] [CrossRef]
- Chakkarapani, S.K.; Lee, S.; Kang, S.H. Ultrasensitive Capsaicin Sensor Based on Endogenous Single-Molecule Fluorophore Enhancement and Quenching Interface on Gold Nanoislands. Bull. Korean Chem. Soc. 2021, 42, 1319–1326. [Google Scholar] [CrossRef]
- Gartia, M.R.; Hsiao, A.; Pokhriyal, A.; Seo, S.; Kulsharova, G.; Cunningham, B.T.; Bond, T.C.; Liu, G.L. Colorimetric Plasmon Resonance Imaging Using Nano Lycurgus Cup Arrays. Adv. Opt. Mater. 2013, 1, 68–76. [Google Scholar] [CrossRef]
- Mei, Z.; Tang, L. Surface-Plasmon-Coupled Fluorescence Enhancement Based on Ordered Gold Nanorod Array Biochip for Ultrasensitive DNA Analysis. Anal. Chem. 2017, 89, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, L.; Wong, T.I.; Zhang, J.; Liu, X.; Zhou, X.; Bai, P.; Liedberg, B.; Wang, Y. Surface Plasmon-Enhanced Fluorescence on Au Nanohole Array for Prostate-Specific Antigen Detection. Int. J. Nanomed. 2017, 12, 2307–2314. [Google Scholar] [CrossRef] [Green Version]
- Miranda, B.; Chu, K.-Y.; Maffettone, P.L.; Shen, A.Q.; Funari, R. Metal-Enhanced Fluorescence Immunosensor Based on Plasmonic Arrays of Gold Nanoislands on an Etched Glass Substrate. ACS Appl. Nano Mater. 2020, 3, 10470–10478. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Batjikh, I.; Yu, H.; Kang, S.H. Ultrasensitive Hypoxia Sensing at the Single-Molecule Level Via Super-Resolution Quantum Dot-Linked Immunosandwich Assay. ACS Sens. 2022, 7, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Lee, S.; Lee, J.; Ha, J.-H.; Kang, S.H. Ultrasensitive Biogenic Amine Sensor Using an Enhanced Multiple Nanoarray Chip Based on Competitive Reactions in an Evanescent Field. Sens. Actuators B Chem. 2021, 345, 130354. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Lee, G.; Kang, S.H. Simultaneous Quantification of Thyroid Hormones Using an Ultrasensitive Single-Molecule Fourplex Nanoimmunosensor in an Evanescent Field. Biosens. Bioelectron. 2023, 220, 114894. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Kang, S.H. Supersensitive Detection of Single-Histamine Molecule on Nanoplates by Turn-On Small Molecule Fluorescence Sandwich Immunoassay. Analyst 2023, 148, 714–718. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Li, F. Upconversion Nanophosphors for Small-Animal Imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Recent Advances in the Chemistry of Lanthanide-Doped Upconversion Nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous Phase and Size Control of Upconversion Nanocrystals through Lanthanide Doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Haase, M.; Schäfer, H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Lin, M.; Zhao, Y.; Wang, S.; Liu, M.; Duan, Z.; Chen, Y.; Li, F.; Xu, F.; Lu, T. Recent Advances in Synthesis and Surface Modification of Lanthanide-Doped Upconversion Nanoparticles for Biomedical Applications. Biotechnol. Adv. 2012, 30, 1551–1561. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In Vivo Photodynamic Therapy Using Upconversion Nanoparticles as Remote-Controlled Nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Gnanasammandhan, M.K.; Zhang, Y. Small Upconverting Fluorescent Nanoparticles for Biomedical Applications. Small 2010, 6, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Kolesov, R.; Xia, K.; Reuter, R.; Stöhr, R.; Zappe, A.; Meijer, J.; Hemmer, P.R.; Wrachtrup, J. Optical Detection of a Single Rare-Earth Ion in a Crystal. Nat. Commun. 2012, 3, 1029. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tu, D.; Zhu, H.; Ma, E.; Chen, X. Lanthanide-Doped Luminescent Nano-Bioprobes: From Fundamentals to Biodetection. Nanoscale 2013, 5, 1369–1384. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Sun, L.-D.; Tian, H.; Liu, Y.; Wang, J.-F.; Yan, C.-H. Reversible Luminescence Switching of Nayf4:Yb,Er Nanoparticles with Controlled Assembly of Gold Nanoparticles. Chem. Commun. 2009, 2547–2549. [Google Scholar] [CrossRef]
- Das, A.; Bae, K.; Park, W. Enhancement of Upconversion Luminescence Using Photonic Nanostructures. Nanophotonics 2020, 9, 1359–1371. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with F and D Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Gamelin, D.R.; Gudel, H.U. Upconversion Processes in Transition Metal and Rare Earth Metal Systems. In Transition Metal and Rare Earth Compounds: Excited States, Transitions, Interactions Ii; Yersin, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–56. [Google Scholar]
- Zhang, H.; Xu, D.; Huang, Y.; Duan, X. Highly Spectral Dependent Enhancement of Upconversion Emission with Sputtered Gold Island Films. Chem. Commun. 2011, 47, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, G.B.; Shi, Y.; Zhang, Y.; Sun, X.; Reich, N.O.; Zhao, D.; Stucky, G. Fabrication of Ag@SiO2@Y2O3:Er Nanostructures for Bioimaging: Tuning of the Upconversion Fluorescence with Silver Nanoparticles. J. Am. Chem. Soc. 2010, 132, 2850–2851. [Google Scholar] [CrossRef]
- Murray, W.A.; Barnes, W.L. Plasmonic Materials. Adv. Mater. 2007, 19, 3771–3782. [Google Scholar] [CrossRef]
- Xia, Y.; Halas, N.J. Shape-Controlled Synthesis and Surface Plasmonic Properties of Metallic Nanostructures. MRS Bull. 2005, 30, 338–348. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Ni, W.; Ambjörnsson, T.; Apell, S.P.; Chen, H.; Wang, J. Observing Plasmonic−Molecular Resonance Coupling on Single Gold Nanorods. Nano Lett. 2010, 10, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Goodrich, G.P.; Tam, F.; Oubre, C.; Nordlander, P.; Halas, N.J. Controlled Texturing Modifies the Surface Topography and Plasmonic Properties of Au Nanoshells. J. Phys. Chem. B 2005, 109, 11083–11087. [Google Scholar] [CrossRef]
- Bai, J.; Huang, S.; Wang, L.; Chen, Y.; Huang, Y. Fluid Assisted Assembly of One-Dimensional Nanoparticle Array inside Inorganic Nanotubes. J. Mater. Chem. 2009, 19, 921–923. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Li, Y.; Huang, Y. Multifunctional Nanoparticles Displaying Magnetization and Near-IR Absorption. Angew. Chem. Int. Ed. 2008, 47, 2439–2442. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; He, J.; Sun, T.; Bao, Z.Y.; Wang, F.; Lei, D.Y. Plasmonic Dual-Enhancement and Precise Color Tuning of Gold Nanorod@SiO2 Coupled Core–Shell–Shell Upconversion Nanocrystals. Adv. Funct. Mater. 2017, 27, 1701842. [Google Scholar] [CrossRef]
- Du, P.; Zhang, P.; Kang, S.H.; Yu, J.S. Hydrothermal Synthesis and Application of Ho3+-Activated NaYbF4 Bifunctional Upconverting Nanoparticles for in Vitro Cell Imaging and Latent Fingerprint Detection. Sens. Actuators B Chem. 2017, 252, 584–591. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Yang, H.; Chen, J.; Yang, H. A Novel Arginine Bioprobe based on Up-Conversion Fluorescence Resonance Energy Transfer. Anal. Chim. Acta 2019, 1079, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Murali, A.; Giri, S. Upconversion Nanoplatform for FRET-Based Sensing of Dopamine and pH. ChemistrySelect 2019, 4, 5407–5414. [Google Scholar] [CrossRef]
- Wang, J.; Hua, G.; Li, L.; Li, D.; Wang, F.; Wu, J.; Ye, Z.; Zhou, X.; Ye, S.; Yang, J.; et al. Upconversion Nanoparticle and Gold Nanocage Satellite Assemblies for Sensitive ctDNA Detection in Serum. Analyst 2020, 145, 5553–5562. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, H.; Zhou, B.; Xu, S.; Shen, B.; Dong, B.; Yin, Z.; Xu, S.; Sun, L.; Lv, J.; et al. Double Stopband Bilayer Photonic Crystal Based Upconversion Fluorescence PSA Sensor. Sens. Actuators B Chem. 2021, 326, 128816. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Deng, H.; Xiong, X.; Zhang, H.; Liang, T.; Li, C. An Aptamer Biosensor for CA125 Quantification in Human Serum based on Upconversion Luminescence Resonance Energy Transfer. Microchem. J. 2021, 161, 105761. [Google Scholar] [CrossRef]
- Liu, J.; Yu, C.; Han, L.; Shen, Y.; Fang, Y.; Xia, Y.; Yao, X.; Wu, F.; Li, C.; Chen, J.; et al. Upconversion Luminescence–based Aptasensor for the Detection of Thyroid-Stimulating Hormone in Serum. Mikrochim. Acta 2022, 189, 179. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, F.; Chou, S.Y. Large Enhancement of Upconversion Luminescence of Nayf4:Yb3+/Er3+ Nanocrystal by 3d Plasmonic Nano-Antennas. Adv. Mater. 2012, 24, OP236–OP241. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Fujii, M.; Sowa, S.; Imakita, K.; Aoki, K. Upconversion Luminescence of Rare-Earth-Doped Y2O3 Nanoparticle with Metal Nano-Cap. J. Phys. Chem. C 2015, 119, 1175–1179. [Google Scholar] [CrossRef]
- Gao, Y.; Murai, S.; Zhang, F.; Tamura, S.; Tomita, K.; Tanaka, K. Enhancing Upconversion Photoluminescence by Plasmonic-Photonic Hybrid Mode. Opt. Express 2020, 28, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.L.; You, M.L.; Tian, L.; Singamaneni, S.; Liu, M.; Duan, Z.; Lu, T.J.; Xu, F.; Lin, M. Distance-Dependent Plasmon-Enhanced Fluorescence of Upconversion Nanoparticles Using Polyelectrolyte Multilayers as Tunable Spacers. Sci. Rep. 2015, 5, 7779. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, E.H.; Madsen, S.P.; Julsgaard, B.; Hofmann, C.L.M.; Goldschmidt, J.C.; Balling, P. Enhanced Upconversion Via Plasmonic Near-Field Effects: Role of the Particle Shape. J. Opt. 2019, 21, 035004. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, D.; Li, H.; Feng, X.; Li, F.; Liang, Q.; Ma, T.; Han, J.; Tang, J.; Chen, G.; et al. Bright Single-Nanocrystal Upconversion at Sub 0.5 W cm−2 Irradiance Via Coupling to Single Nanocavity Mode. Nat. Photonics 2023, 17, 73–81. [Google Scholar] [CrossRef]
- Purcell, E.M.; Torrey, H.C.; Pound, R.V. Resonance Absorption by Nuclear Magnetic Moments in a Solid. Phys. Rev. 1946, 69, 37–38. [Google Scholar] [CrossRef]
- Haroche, S.; Kleppner, D. Cavity Quantum Electrodynamics. Phys. Today 1989, 42, 24–30. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kang, S.H. Wavelength-Dependent Metal-Enhanced Fluorescence Biosensors via Resonance Energy Transfer Modulation. Biosensors 2023, 13, 376. https://doi.org/10.3390/bios13030376
Lee S, Kang SH. Wavelength-Dependent Metal-Enhanced Fluorescence Biosensors via Resonance Energy Transfer Modulation. Biosensors. 2023; 13(3):376. https://doi.org/10.3390/bios13030376
Chicago/Turabian StyleLee, Seungah, and Seong Ho Kang. 2023. "Wavelength-Dependent Metal-Enhanced Fluorescence Biosensors via Resonance Energy Transfer Modulation" Biosensors 13, no. 3: 376. https://doi.org/10.3390/bios13030376