Photoelectrochemical Determination of Cardiac Troponin I as a Biomarker of Myocardial Infarction Using a Bi2S3 Film Electrodeposited on a BiVO4-Coated Fluorine-Doped Tin Oxide Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Experimental Apparatus
2.3. Synthesis of BiVO4
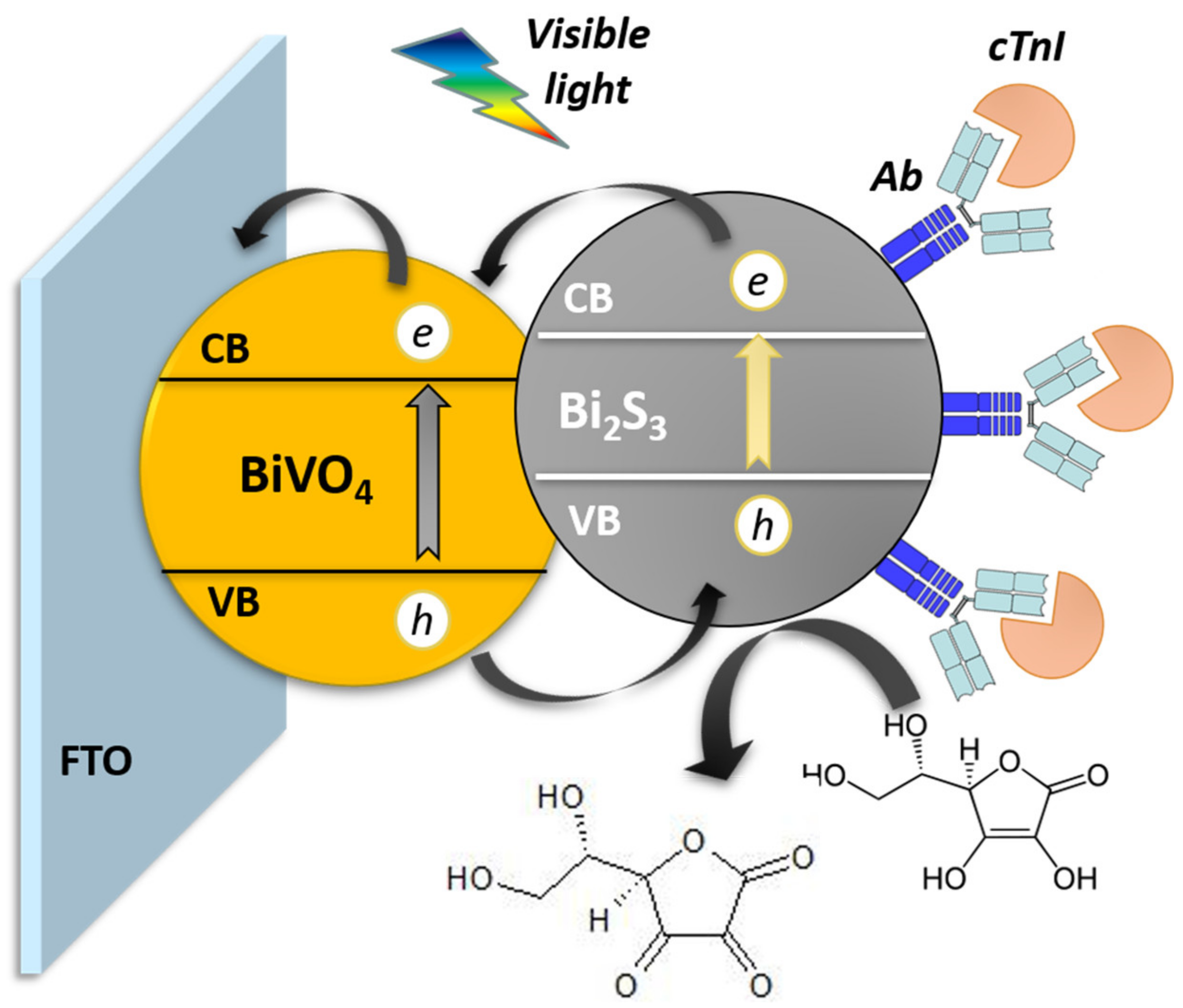
2.4. Construction of Bi2S3/BiVO4/FTO Photoelectrochemical Sensor
2.5. Optimization of the Platform Response for the AA before and after the Immobilization of the Biological Materials
2.6. Preparation and Analysis of Artificial Blood Plasma Samples
2.7. X-ray Diffraction and Scanning Electron Microscopy
3. Results and Discussion
3.1. Characterization of the Materials by X-Ray Diffraction and Scanning Electron Microscopy
3.2. EIS and Photoelectrochemical Characterization of Bi2S3/BiVO4/FTO Platform
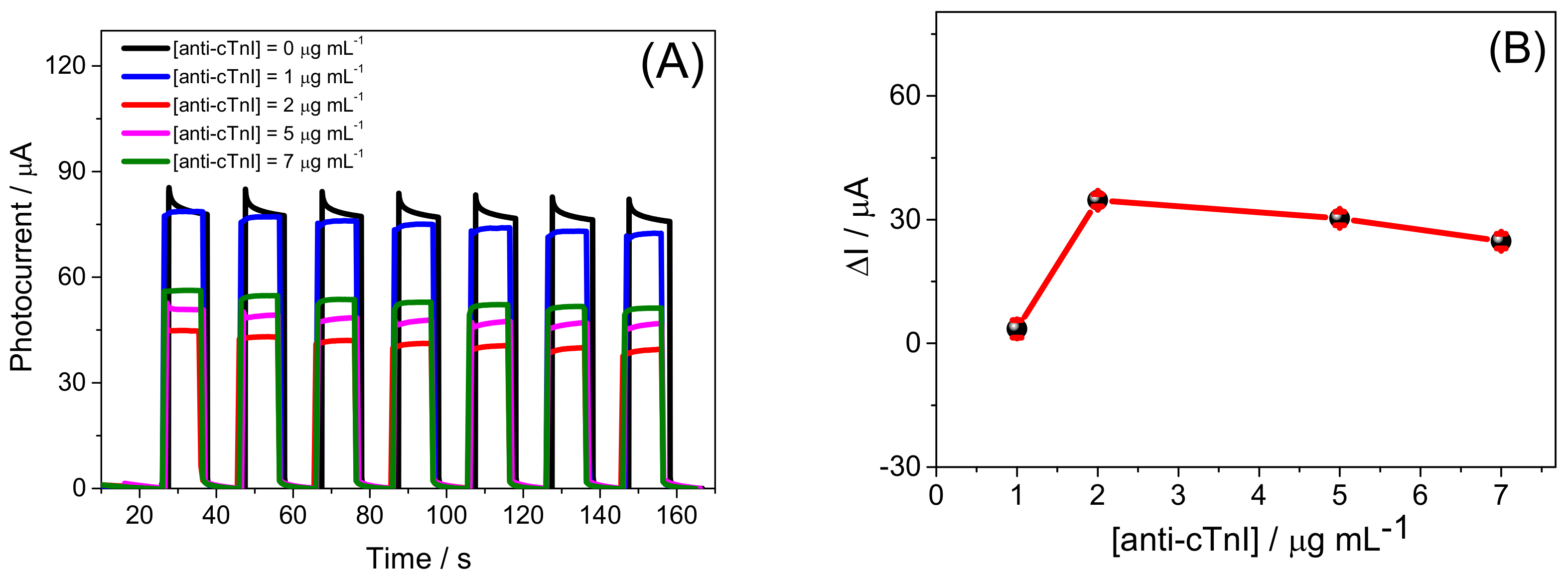
3.3. Evaluation of Experimental Parameters on the Bi2S3/BiVO4/FTO PEC Platform Response
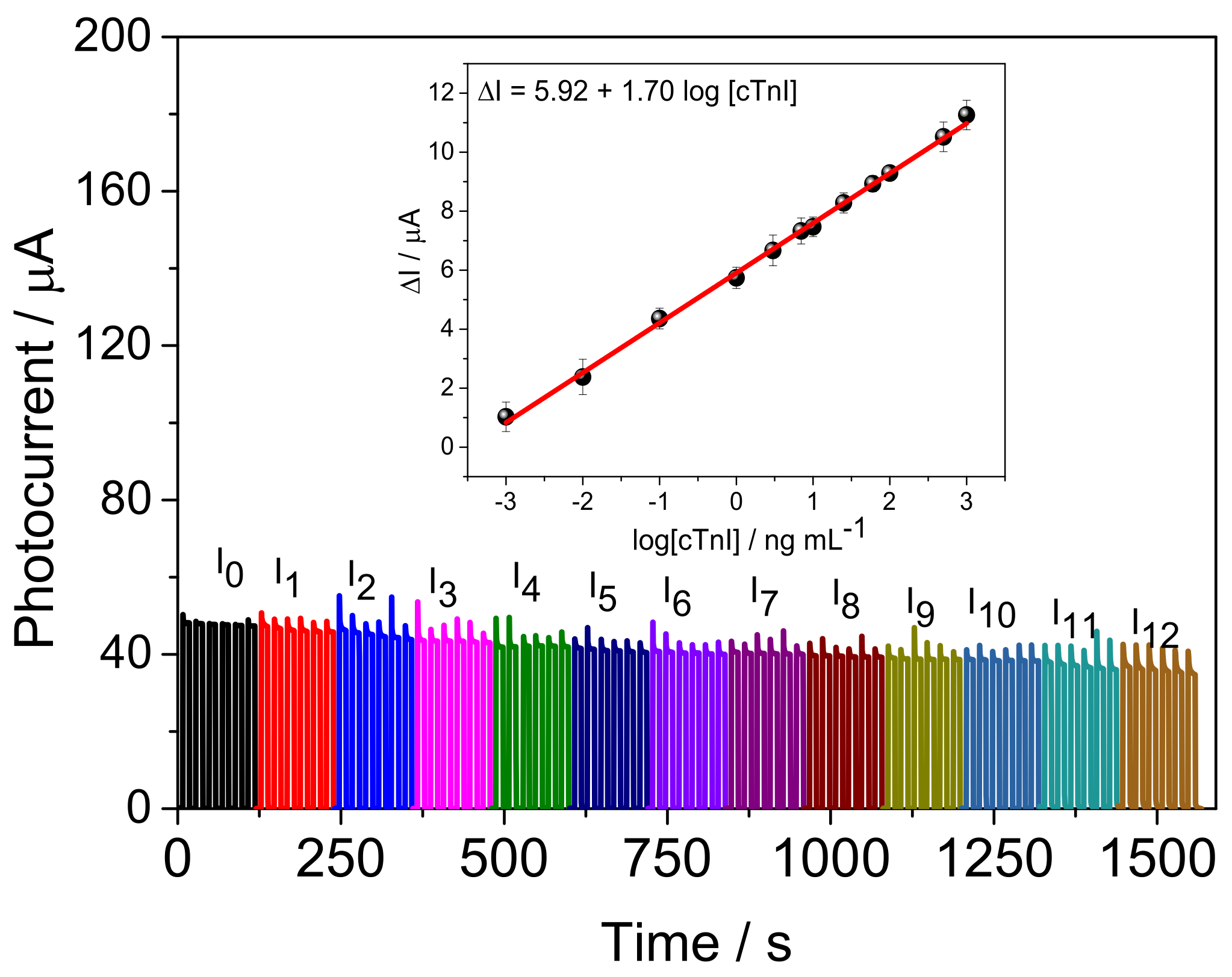
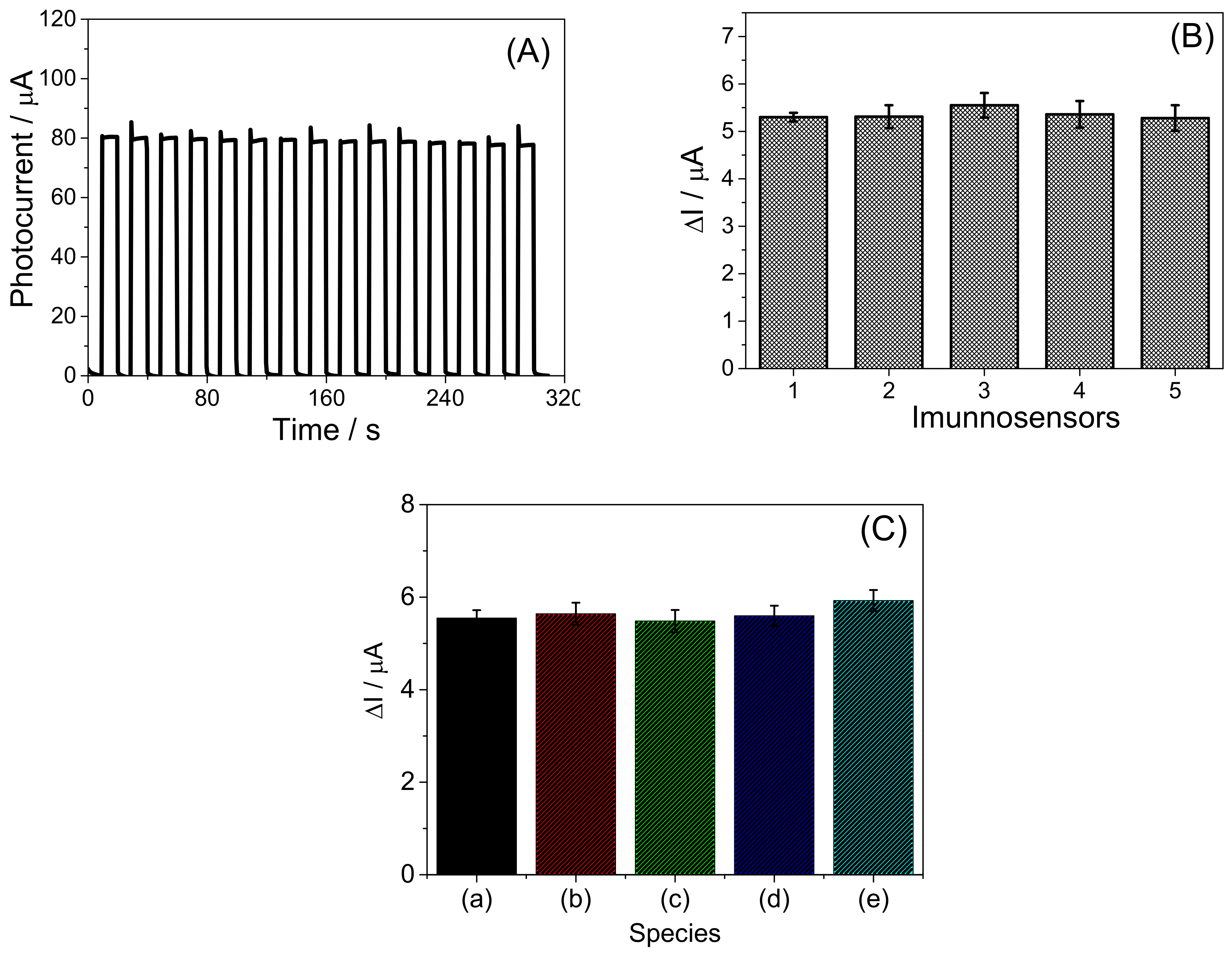
3.4. Analytical Performance of Bi2S3/BiVO4/FTO PEC Immunosensor
3.5. Detection of cTnI in the Artificial Blood Plasma Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fathil, M.F.M.; Md Arshad, M.K.; Gopinath, S.C.B.; Hashim, U.; Adzhri, R.; Ayub, R.M.; Ruslinda, A.R.; Nuzaihan, M.N.M.; Azman, A.H.; Zaki, M.; et al. Diagnostics on Acute Myocardial Infarction: Cardiac Troponin Biomarkers. Biosens. Bioelectron. 2015, 70, 209–220. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases. Available online: http://www.who.int/cardiovascular_diseases/en/ (accessed on 26 November 2022).
- Tan, Y.; Wang, Y.; Li, M.; Ye, X.; Wu, T.; Li, C. Enhanced Photoelectrochemical Immunosensing of Cardiac Troponin I Based on Energy Transfer between N-Acetyl-L-Cysteine Capped CdAgTe Quantum Dots and Dodecahedral Au Nanoparticles. Biosens. Bioelectron. 2017, 91, 741–746. [Google Scholar] [CrossRef]
- Campu, A.; Muresan, I.; Craciun, A.M.; Cainap, S.; Astilean, S.; Focsan, M. Cardiac Troponin Biosensor Designs: Current Developments and Remaining Challenges. Int. J. Mol. Sci. 2022, 23, 7728. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of Electrochemical Immunosensors for Early Clinical Diagnostics. Talanta 2015, 132, 162–174. [Google Scholar] [CrossRef]
- Song, S.Y.; Han, Y.D.; Kim, K.; Yang, S.S.; Yoon, H.C. A Fluoro-Microbead Guiding Chip for Simple and Quantifiable Immunoassay of Cardiac Troponin I (CTnI). Biosens. Bioelectron. 2011, 26, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-M.; Kim, S.-W.; Park, J.-N.; Cho, J.-H.; Kim, H.-S.; Paek, S.-H. A Fluorescent Immunosensor for High-Sensitivity Cardiac Troponin I Using a Spatially-Controlled Polymeric, Nano-Scale Tracer to Prevent Quenching. Biosens. Bioelectron. 2016, 83, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Jiao, L.; Tang, Q.; Li, H.; Zhang, L.; Wei, Q. A Nanozyme-Linked Immunosorbent Assay for Dual-Modal Colorimetric and Ratiometric Fluorescent Detection of Cardiac Troponin I. Sens. Actuators B Chem. 2019, 288, 60–64. [Google Scholar] [CrossRef]
- Chen, F.; Wu, Q.; Song, D.; Wang, X.; Ma, P.; Sun, Y. Fe3O4@PDA Immune Probe-Based Signal Amplification in Surface Plasmon Resonance (SPR) Biosensing of Human Cardiac Troponin I. Colloids Surf. B Biointerfaces 2019, 177, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K. Wavelength Modulation Based Surface Plasmon Resonance Sensor for Detection of Cardiac Marker Proteins Troponin I and Troponin T. Sens. Actuators A Phys. 2021, 332, 113104. [Google Scholar] [CrossRef]
- Schneck, N.A.; Phinney, K.W.; Lee, S.B.; Lowenthal, M.S. Quantification of Cardiac Troponin I in Human Plasma by Immunoaffinity Enrichment and Targeted Mass Spectrometry. Anal. Bioanal. Chem. 2018, 410, 2805–2813. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Bratashov, D.N.; Byzova, N.A.; Dzantiev, B.B.; Khlebtsov, N.G. SERS-Based Lateral Flow Immunoassay of Troponin I by Using Gap-Enhanced Raman Tags. Nano Res. 2019, 12, 413–420. [Google Scholar] [CrossRef]
- Li, H.; Qiao, Y.; Li, J.; Fang, H.; Fan, D.; Wang, W. A Sensitive and Label-Free Photoelectrochemical Aptasensor Using Co-Doped ZnO Diluted Magnetic Semiconductor Nanoparticles. Biosens. Bioelectron. 2016, 77, 378–384. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Z.-Y.; Ruan, Y.-F.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Simultaneous Photoelectrochemical Immunoassay of Dual Cardiac Markers Using Specific Enzyme Tags: A Proof of Principle for Multiplexed Bioanalysis. Anal. Chem. 2016, 88, 1990–1994. [Google Scholar] [CrossRef]
- Chi, H.; Han, Q.; Chi, T.; Xing, B.; Ma, N.; Wu, D.; Wei, Q. Manganese Doped CdS Sensitized Graphene/Cu2MoS4 Composite for the Photoelectrochemical Immunoassay of Cardiac Troponin I. Biosens. Bioelectron. 2019, 132, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arunpandian, M.; Kaviyarasu, K.; Asath Bahadur, S.; Swaminathan, M. Visible Active Reduced Graphene Oxide-BiVO4-ZnO Ternary Photocatalyst for Efficient Removal of Ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Liu, Y.; Zhang, J. Graphene-Doped Bi2S3 Nanorods as Visible-Light Photoelectrochemical Aptasensing Platform for Sulfadimethoxine Detection. Biosens. Bioelectron. 2016, 86, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Y.; Li, W.; Li, J.; Li, Y.; Chen, Q. In Situ Synthesis of Bi2S3 Sensitized WO3 Nanoplate Arrays with Less Interfacial Defects and Enhanced Photoelectrochemical Performance. Sci. Rep. 2016, 6, 23451. [Google Scholar] [CrossRef]
- Yin, H.; Sun, B.; Zhou, Y.; Wang, M.; Xu, Z.; Fu, Z.; Ai, S. A New Strategy for Methylated DNA Detection Based on Photoelectrochemical Immunosensor Using Bi2S3 Nanorods, Methyl Bonding Domain Protein and Anti-His Tag Antibody. Biosens. Bioelectron. 2014, 51, 103–108. [Google Scholar] [CrossRef]
- Sun, B.; Qiao, F.; Chen, L.; Zhao, Z.; Yin, H.; Ai, S. Effective Signal-on Photoelectrochemical Immunoassay of Subgroup J Avian Leukosis Virus Based on Bi2S3 Nanorods as Photosensitizer and in Situ Generated Ascorbic Acid for Electron Donating. Biosens. Bioelectron. 2014, 54, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, T.O.; dos Santos, C.C.; do Prado, T.M.; Damos, F.S.; de Luz, R.C.S.; Fatibello-Filho, O. Highly Sensitive Photoelectrochemical Immunosensor Based on Anatase/Rutile TiO2 and Bi2S3 for the Zero-Biased Detection of PSA. J. Solid State Electrochem. 2020, 24, 1801–1809. [Google Scholar] [CrossRef]
- Prado, T.M.; Carrico, A.; Cincotto, F.H.; Fatibello-Filho, O.; Moraes, F.C. Bismuth Vanadate/Graphene Quantum Dot: A New Nanocomposite for Photoelectrochemical Determination of Dopamine. Sens. Actuators B Chem. 2019, 285, 248–253. [Google Scholar] [CrossRef]
- Saitou, M.; Yamaguchi, R.; Oshikawa, W. Novel Process for Electrodeposition of Bi2S3 Thin Films. Mater. Chem. Phys. 2002, 73, 306–309. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Chen, Q.; Zhang, S.M. Corrosion Behavior of Zr-Based Bulk Metallic Glasses in Different Artificial Body Fluids. J. Alloy. Compd. 2006, 425, 268–273. [Google Scholar] [CrossRef]
- Han, Q.; Wang, R.; Xing, B.; Zhang, T.; Khan, M.S.; Wu, D.; Wei, Q. Label-Free Photoelectrochemical Immunoassay for CEA Detection Based on CdS Sensitized WO3@BiOI Heterostructure Nanocomposite. Biosens. Bioelectron. 2018, 99, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Shaislamov, U.; Krishnamoorthy, K.; Kim, S.J.; Chun, W.; Lee, H.-J. Facile Fabrication and Photoelectrochemical Properties of a CuO Nanorod Photocathode with a ZnO Nanobranch Protective Layer. RSC Adv. 2016, 6, 103049–103056. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, L.; Yue, Q.; Wang, T.; Huang, Q.; Du, P. Efficient Suppression of Surface Charge Recombination by CoP-Modified Nanoporous BiVO4 for Photoelectrochemical Water Splitting. Int. J. Hydrog. Energy 2021, 46, 15517–15525. [Google Scholar] [CrossRef]
- Liu, D.; Qian, Y.; Xu, R.; Zhang, Y.; Ren, X.; Ma, H.; Wei, Q. A Dual-Signal Amplification Photoelectrochemical Immunosensor for Ultrasensitive Detection of CYFRA 21-1 Based on the Synergistic Effect of SnS2/SnS/Bi2S3 and ZnCdS@NPC-ZnO. Sens. Actuators B Chem. 2021, 346, 130456. [Google Scholar] [CrossRef]
- Chekin, F.; Vasilescu, A.; Jijie, R.; Singh, S.K.; Kurungot, S.; Iancu, M.; Badea, G.; Boukherroub, R.; Szunerits, S. Sensitive Electrochemical Detection of Cardiac Troponin I in Serum and Saliva by Nitrogen-Doped Porous Reduced Graphene Oxide Electrode. Sens. Actuators B Chem. 2018, 262, 180–187. [Google Scholar] [CrossRef]
- Zhu, S.-R.; Qi, Q.; Zhao, W.-N.; Fang, Y.; Han, L. Enhanced Photocatalytic Activity in Hybrid Composite Combined BiOBr Nanosheets and Bi2S3 Nanoparticles. J. Phys. Chem. Solids 2018, 121, 163–171. [Google Scholar] [CrossRef]
- Guo, W.; Wang, J.; Guo, W.; Kang, Q.; Zhou, F. Correction to: Interference-Free Photoelectrochemical Immunoassays Using Carboxymethylated Dextran-Coated and Gold-Modified TiO2 Nanotube Arrays. Anal. Bioanal. Chem. 2021, 413, 5921–5922. [Google Scholar] [CrossRef]
- Fan, D.; Liu, X.; Shao, X.; Zhang, Y.; Zhang, N.; Wang, X.; Wei, Q.; Ju, H. A Cardiac Troponin I Photoelectrochemical Immunosensor: Nitrogen-Doped Carbon Quantum Dots–Bismuth Oxyiodide–Flower-like SnO2. Microchim. Acta 2020, 187, 332. [Google Scholar] [CrossRef]
- Dong, W.; Mo, X.; Wang, Y.; Lei, Q.; Li, H. Photoelectrochemical Immunosensor Based on ZnIn2S4/Bi2Se3 Nanocomposite for the Determination of Cardiac Troponin I. Anal. Lett. 2020, 53, 1888–1901. [Google Scholar] [CrossRef]
- Fan, D.; Bao, C.; Khan, M.S.; Wang, C.; Zhang, Y.; Liu, Q.; Zhang, X.; Wei, Q. A Novel Label-Free Photoelectrochemical Sensor Based on N,S-GQDs and CdS Co-Sensitized Hierarchical Zn2SnO4 Cube for Detection of Cardiac Troponin I. Biosens. Bioelectron. 2018, 106, 14–20. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Zeng, Y.; Liu, X.; Tang, D. Tunable Competitive Absorption-Induced Signal-On Photoelectrochemical Immunoassay for Cardiac Troponin I Based on Z-Scheme Metal–Organic Framework Heterojunctions. Anal. Chem. 2022, 94, 13582–13589. [Google Scholar] [CrossRef]
- Feng, J.; Li, N.; Du, Y.; Ren, X.; Wang, X.; Liu, X.; Ma, H.; Wei, Q. Ultrasensitive Double-Channel Microfluidic Biosensor-Based Cathodic Photo-Electrochemical Analysis via Signal Amplification of SOD-Au@PANI for Cardiac Troponin I Detection. Anal. Chem. 2021, 93, 14196–14203. [Google Scholar] [CrossRef]
- Liao, X.-J.; Xiao, H.-J.; Cao, J.-T.; Ren, S.-W.; Liu, Y.-M. A Novel Split-Type Photoelectrochemical Immunosensor Based on Chemical Redox Cycling Amplification for Sensitive Detection of Cardiac Troponin I. Talanta 2021, 233, 122564. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Zhi, S.; Zhang, S.; Guo, X.; Huang, Y.; Xu, L.; Wang, X.; Wang, D.; Zhu, M.; He, B. A Novel Photoelectrochemical Sensor Based on SiNWs@PDA for Efficient Detection of Myocardial Infarction. Biomater. Sci. 2022, 10, 4627–4634. [Google Scholar] [CrossRef]
- Chen, J.; Kong, L.; Sun, X.; Feng, J.; Chen, Z.; Fan, D.; Wei, Q. Ultrasensitive Photoelectrochemical Immunosensor of Cardiac Troponin I Detection Based on Dual Inhibition Effect of Ag@Cu2O Core-Shell Submicron-Particles on CdS QDs Sensitized TiO2 Nanosheets. Biosens. Bioelectron. 2018, 117, 340–346. [Google Scholar] [CrossRef] [PubMed]



| Modified Electrode | Technique | Linear Range (ng mL−1) | LOD (pg mL−1) | Reference |
|---|---|---|---|---|
| Bi2S3/BiVO4/FTO | PEC | 0.0010–1000 | 1.0 | This work |
| a NAC-CdAgTe QDs/AuNPs/GCE | PEC | 0.0050–20 | 1.70 | [3] |
| b Mn:CdS@Cu2MoS4/G/ITO | PEC | 0.0050–1000 | 0.18 | [15] |
| c CM-dextran/Au/TiO2 NTA/Ti | PEC | 0.00484–0.484 | 2.20 | [31] |
| d SnO2/NCQDs/BiOI/ITO | PEC | 0.00100–100 | 0.30 | [32] |
| e Au NPs/ZIS/Bi2Se3/ITO-PET | PEC | 0.080–40 | 26.00 | [33] |
| f Zn2SnO4/N,S-GQDs/CdS/ITO | PEC | 0.0010–50 | 0.30 | [34] |
| g Cu2+@Zr-MOF@TiO2 NRs | PEC | 0.01–10 | 8.60 | [35] |
| h Pd/I:BiOBr-OVs/SOD-Au@PANI | PEC | 0.0001–100 | 0.042 | [36] |
| i Ag2S/ZnO/ITO | PEC | 0.00001–1 | 0.003 | [37] |
| j SiNWs@PDA | PEC | 0.005–10 | 1.47 | [38] |
| k Ag@Cu2O core-shell SPs/TiO2/CdS | PEC | 0.00002–50 | 0.0067 | [39] |
| Sample | Additioned/ng mL−1 | Found/ng mL−1 | Recovery/% | RSD/% |
|---|---|---|---|---|
| 1 | 0.05 | 0.049 (±0.001) | 98.0 | 2.04 |
| 2 | 2.0 | 1.96 (±0.01) | 98.0 | 0.51 |
| 3 | 50 | 49.24 (± 0.05) | 98.5 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, T.O.; Neto, A.G.d.S.; de Menezes, A.S.; Damos, F.S.; Luz, R.d.C.S.; Fatibello-Filho, O. Photoelectrochemical Determination of Cardiac Troponin I as a Biomarker of Myocardial Infarction Using a Bi2S3 Film Electrodeposited on a BiVO4-Coated Fluorine-Doped Tin Oxide Electrode. Biosensors 2023, 13, 379. https://doi.org/10.3390/bios13030379
Monteiro TO, Neto AGdS, de Menezes AS, Damos FS, Luz RdCS, Fatibello-Filho O. Photoelectrochemical Determination of Cardiac Troponin I as a Biomarker of Myocardial Infarction Using a Bi2S3 Film Electrodeposited on a BiVO4-Coated Fluorine-Doped Tin Oxide Electrode. Biosensors. 2023; 13(3):379. https://doi.org/10.3390/bios13030379
Chicago/Turabian StyleMonteiro, Thatyara Oliveira, Antônio Gomes dos Santos Neto, Alan Silva de Menezes, Flávio Santos Damos, Rita de Cássia Silva Luz, and Orlando Fatibello-Filho. 2023. "Photoelectrochemical Determination of Cardiac Troponin I as a Biomarker of Myocardial Infarction Using a Bi2S3 Film Electrodeposited on a BiVO4-Coated Fluorine-Doped Tin Oxide Electrode" Biosensors 13, no. 3: 379. https://doi.org/10.3390/bios13030379
APA StyleMonteiro, T. O., Neto, A. G. d. S., de Menezes, A. S., Damos, F. S., Luz, R. d. C. S., & Fatibello-Filho, O. (2023). Photoelectrochemical Determination of Cardiac Troponin I as a Biomarker of Myocardial Infarction Using a Bi2S3 Film Electrodeposited on a BiVO4-Coated Fluorine-Doped Tin Oxide Electrode. Biosensors, 13(3), 379. https://doi.org/10.3390/bios13030379





