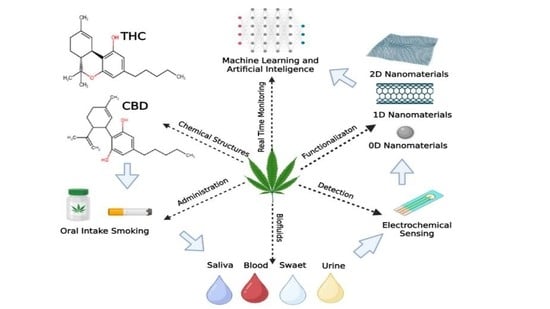
Nanomaterials-Based Electrochemical Δ9-THC and CBD Sensors for Chronic Pain
Abstract
:1. Introduction
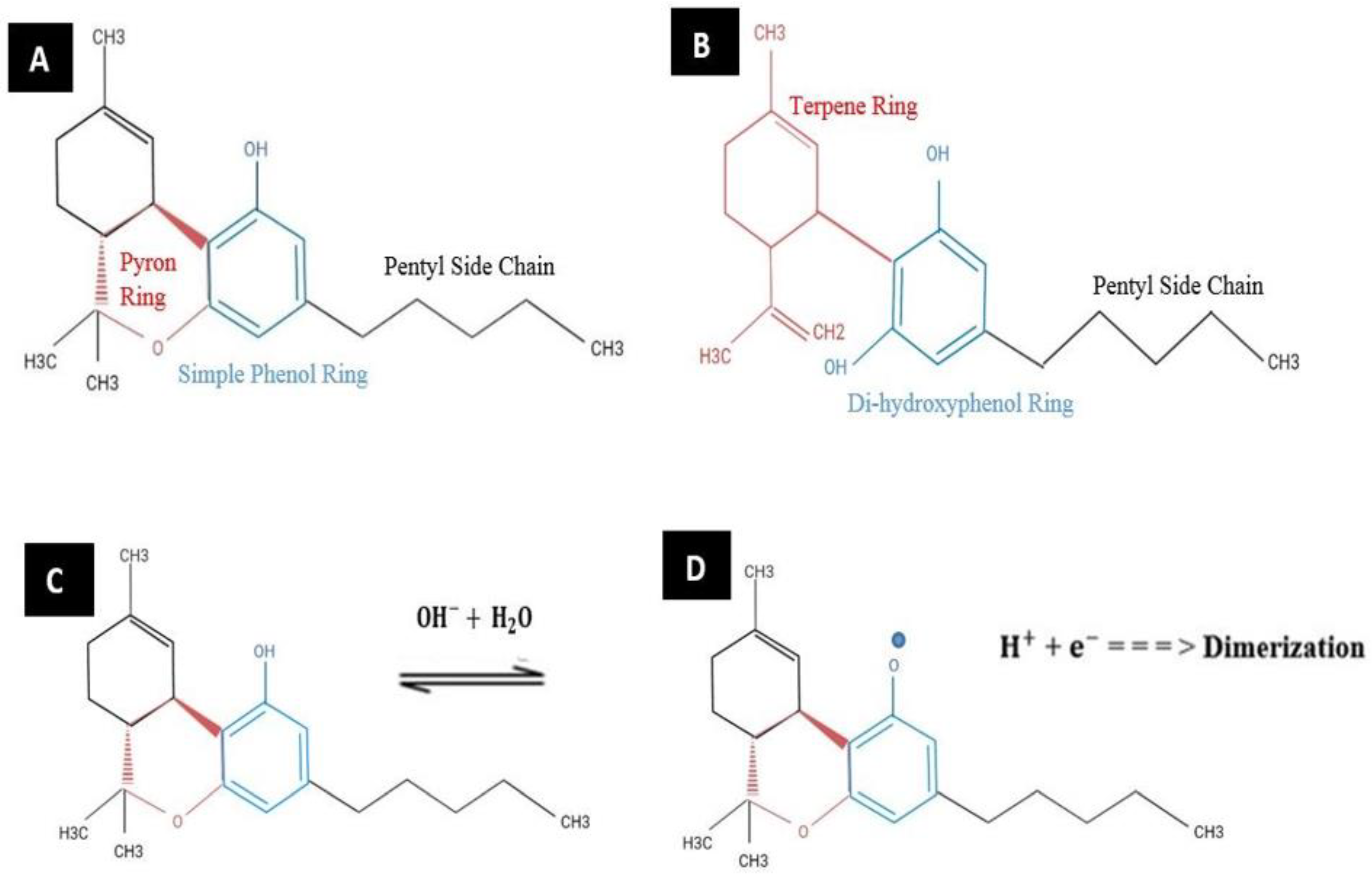
2. Structures, Oxidations, and Pharmacokinetics of -THC and CBD
3. Recent Advances
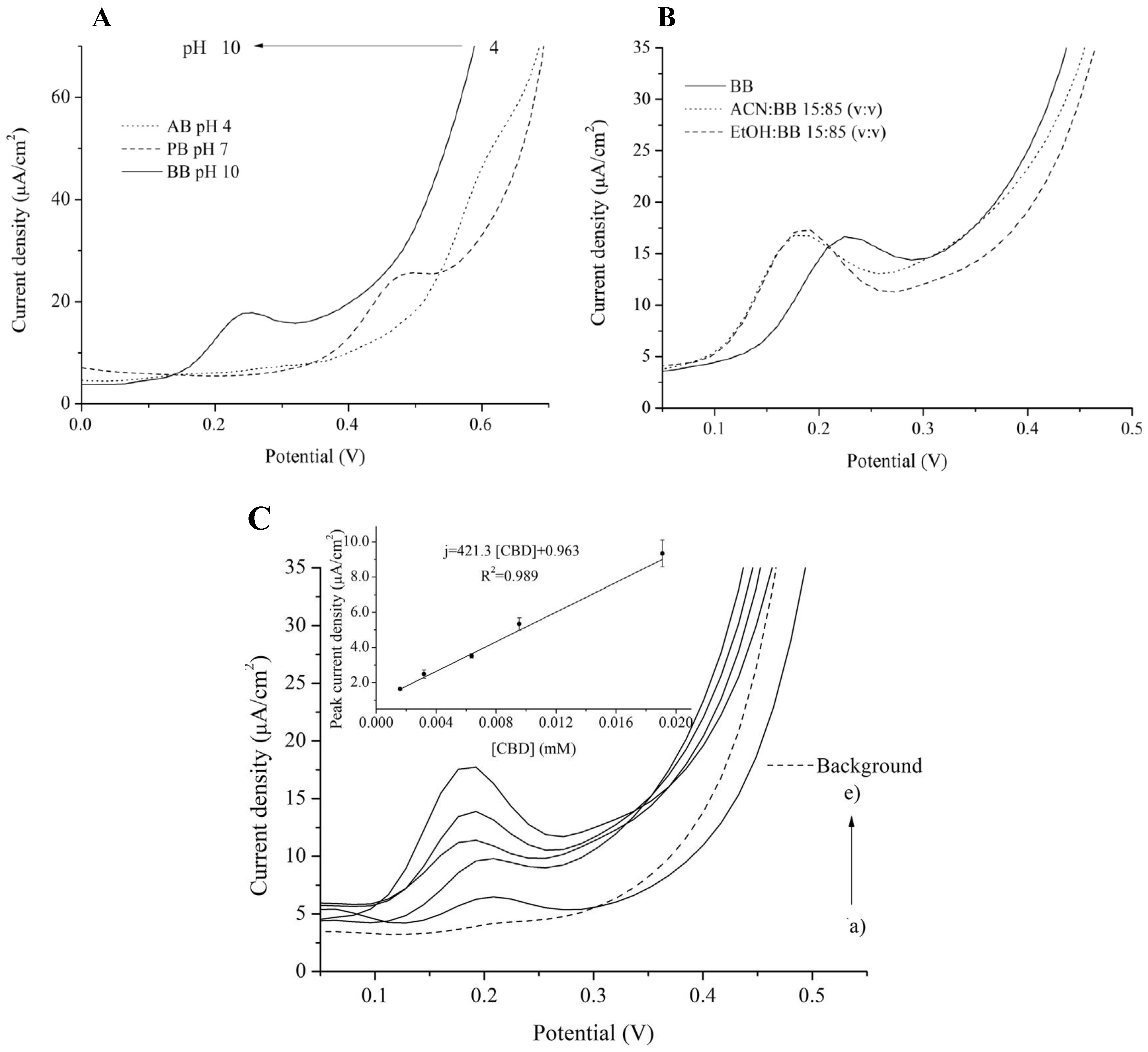
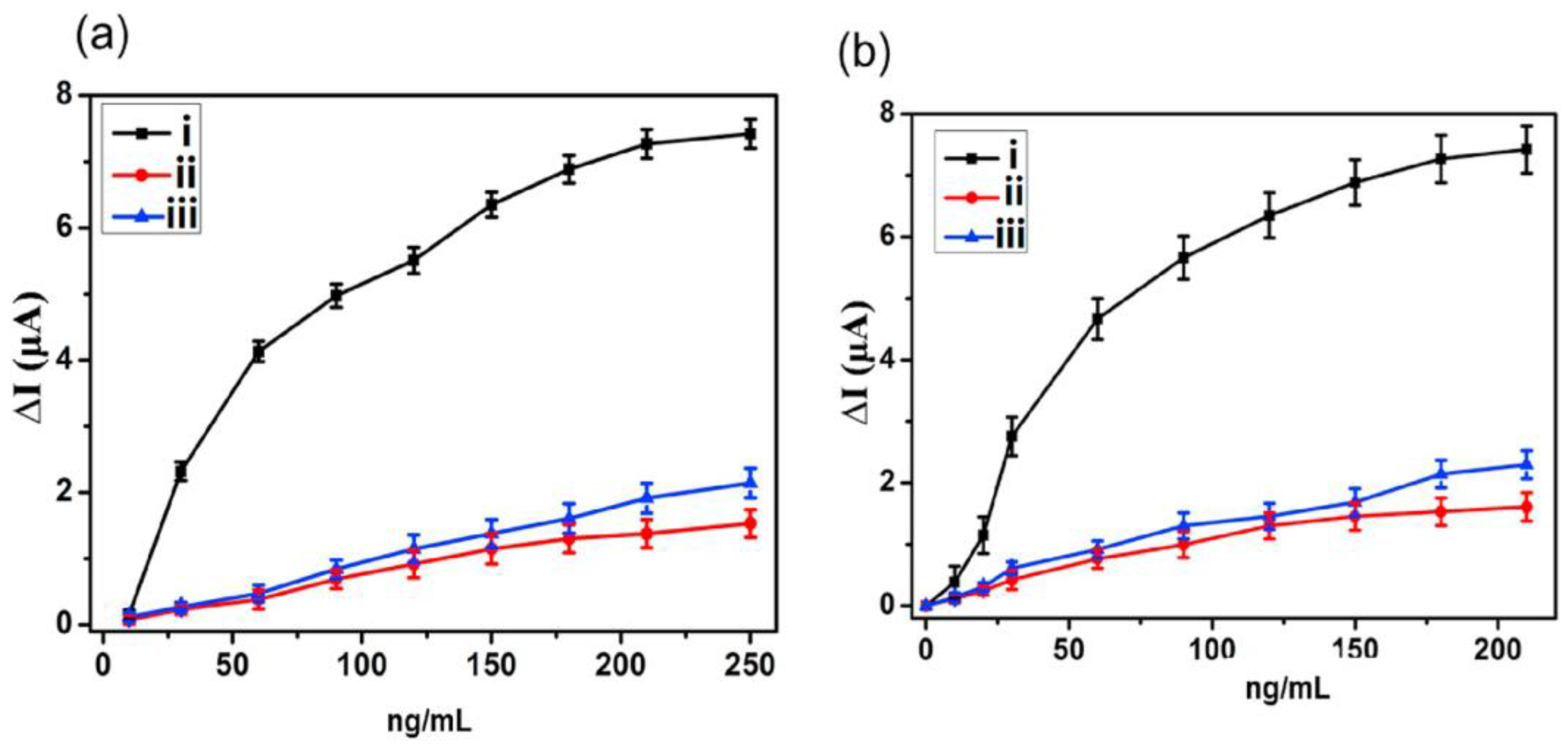
3.1. Direct Electrochemical Detection
3.1.1. Conventional Materials
| Electrode | Technique | Sensitivity | LOD (ng/mL) | LR (ng/mL) | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| Black Conductive PLA | DPV | --------- | 4717.03 (PBS) | -------- | -------- | [27] |
| Silicon Oxide/doped PEDOT/CPE | DPV | 421 ± 26.1 µA/mM.cm2 | 295.6 (ACN:BB) (15:85) (EtOH:BB) (15:85) | 185.52–6005.1 | --------- | [8] |
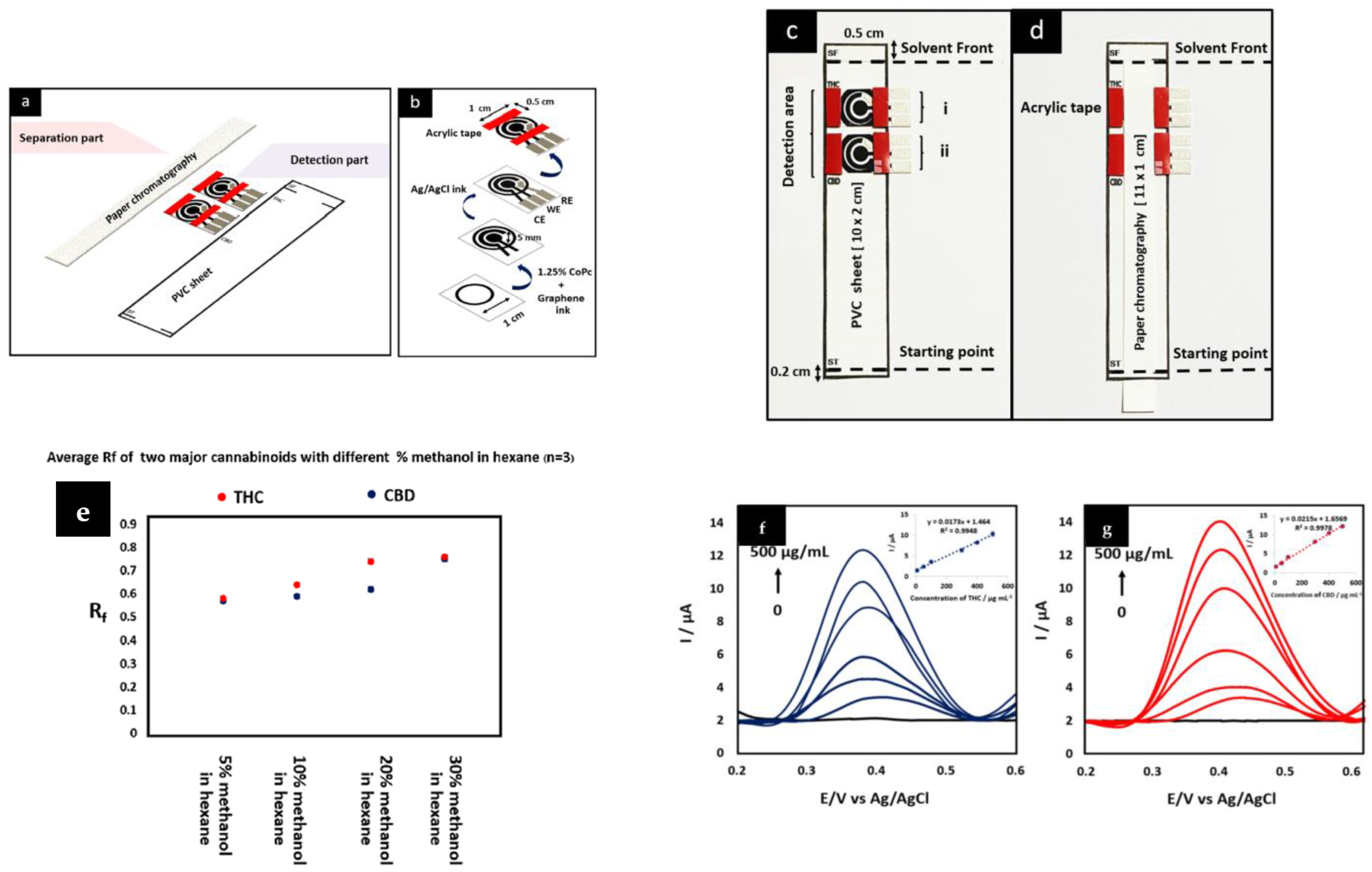
| CoPc/SPE | DPV | 173 × µA·mL/ng 215 × µA·mL/ng | 3270 2850 (PBS) | –5 × | Paper Chromotography | [7] |
| Aptamer/G-SPE | DPV | --------- | 0.314 (PBS) 3.14 (Saliva) | --------- | Aptamer | [35] |
| MIP/NSC/GCE | DPV | -------- | 0.91 (Cannabis oil) | 1.26–25.16 × | MIP | [36] |
3.1.2. 0D Nanomaterials
| Electrode | Technique | Sensitivity (µA·mL/ng) | LOD (ng/mL) | LR (ng/mL) | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| antibody/cys/glu/AuNPs/DEP | SWV | -------- | 0.007 (PBS) | 0.01–10,000.0 | Antibody | [28] |
| CBNPS/GCE | CV, and DPV | 0.206 × | 110 (cannabis oil) | 300.0–2000.0 | -------------- | [40] |
3.1.3. 1D Nanomaterials
3.1.4. 2D Nanomaterials and Nanocomposites
3.2. Alternative Sensors
| Electrode | Sensing Mechanism | Sensitivity | LOD (ng/mL) | LR-DR (ng/mL) | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| IrMn/CoFe/Ru/Cu/CoFe/Silicon Wafer/Antibody/BSA | Resistance Changes due to THC oxidation | ---------- | ---------- | 0–200 | Antibody | [53] |
| Si//Cr/Au/Zn/Etched Silicon Wafer/Cr/Au/Antibody | Shifts Frequency due to THC Oxidation | ----------- | 1.5625 (Urine) | 1.5625–50 | Antibody | [54] |
| Platinum | Changes in bias between source and drain due to THC oxidation | 0.0162 ± 0.003/dec(DI water) −0.126 ± 0.004/dec(DI water) −0.003/dec (saliva) −0.02/dec (saliva) | 0.031 (DI water) 0.31 (saliva) | 0.031–180.2 (DI water) 180.2–1572.35 (DI water) 0.31–133.65 (saliva) 133.65–1572.35 (saliva) | ------- | [56] |
| NanoMIP/Gold | Capacitance changes due to THC oxidation | ----------- | 0.31 × (PBS) | 0.31 × –3144.7 | MIP | [57] |
4. Research Challenges and Future Perspectives
4.1. Sensitivity
4.2. Selectivity
4.3. Surface Fouling
4.4. Real-Time Monitoring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernell, S.; Howard, S.W.; Paterson, B.A. Use Your Words Carefully: What is a Chronic Disease? Front. Public Health 2016, 4, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Strong, K.; Mathers, C.; Leeder, S.; Beaglehole, R. Preventing chronic diseases: How many lives can we save? Lancet 2005, 366, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Argueta, D.A.; Ventura, C.M.; Kiven, S.; Sagi, V. A Balanced Approach for Cannabidiol Use in Chronic Pain. Front. Pharmacol. 2020, 11, 561. [Google Scholar] [CrossRef]
- Labianca, R.; Sarzi-puttini, P.; Zuccaro, S.M.; Cherubino, P.; Vellucci, R.; Fornasari, D. Adverse Effects Associated with Non-opioid and Opioid Treatment in Patients with Chronic Pain. Clin. Drug Investig. 2012, 32, 53–63. [Google Scholar] [CrossRef]
- Klimuntowski, M.; Alam, M.M.; Singh, G.; Howlader, M.M.R. Electrochemical Sensing of Cannabinoids in Biofluids: A Noninvasive Tool for Drug Detection. ACS Sens. 2020, 5, 620–636. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Linher-melville, K.; Wu, J.; Zhu, K.L.; Singh, G. Bone cancer-induced pain is associated with glutamate signalling in peripheral sensory neurons. Mol. Pain 2020, 16, 1744806920911536. [Google Scholar] [CrossRef] [PubMed]
- Pholsiri, T.; Lomae, A.; Pungjunun, K.; Vimolmangkang, S.; Siangproh, W.; Chailapakul, O. A chromatographic paper-based electrochemical device to determine Δ9-tetrahydrocannabinol and cannabidiol in cannabis oil. Sens. Actuators B Chem. 2022, 355, 131353. [Google Scholar] [CrossRef]
- López-Iglesias, D.; García-Guzmán, J.J.; Zanardi, C.; Palacios-Santander, J.M.; Cubillana-Aguilera, L.; Pigani, L. Fast electroanalytical determination of Cannabidiol and Cannabinol in aqueous solution using Sonogel-Carbon-PEDOT devices. J. Electroanal. Chem. 2020, 878, 114591. [Google Scholar] [CrossRef]
- Zanfrognini, B.; Pigani, L.; Zanardi, C. Recent advances in the direct electrochemical detection of drugs of abuse. J. Solid State Electrochem. 2020, 24, 2603–2616. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, R.; Constantinescu, C.S. Expert Opinion on Drug Metabolism & Toxicology Pharmacokinetic evaluation of nabiximols for the treatment of multiple sclerosis pain Pharmacokinetic evaluation of nabiximols for the treatment of multiple sclerosis pain. Expert Opin. Drug Metab. Toxicol. 2013, 9, 5255. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Trends in Analytical Chemistry Recent biosensing advances in the rapid detection of illicit drugs. Trends Anal. Chem. 2020, 131, 116006. [Google Scholar] [CrossRef]
- Ramzy, V.; Priefer, R. Talanta THC detection in the breath. Talanta 2021, 222, 121528. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two- Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. InfoMat 2020, 2, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Qi, X.; Wan, X.; Liu, Y.; Chen, Y.; Xu, L. Molecularly imprinted polypyrrole film-coated poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate-functionalized black phosphorene for the selective and robust detection of norfloxacin. Mater. Today Chem. 2022, 26, 101043. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Qin, Y.; Kwon, H.J.; Howlader, M.M.R.; Deen, M.J. Microfabricated electrochemical pH and free chlorine sensors for water quality monitoring: Recent advances and research challenges. RSC Adv. 2015, 5, 69086–69109. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent developments in polymer nanocomposite-based electrochemical sensors for detecting environmental pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef] [PubMed]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, Y.; Zhang, Y.; Zhang, M.; Zhang, Y.; Qiao, L.; Yin, N.; Song, K.; Liu, M.; Wang, D. Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection. Microchem. J. 2021, 171, 106776. [Google Scholar] [CrossRef]
- Lam, L.H.; Lin, S.D.; Sun, J. Pharmacokinetics and Pharmacodynamics of Immunotherapy. Curr. Cancer Res. 2018, 42, 29–67. [Google Scholar] [CrossRef]
- Ashton, C.H. Pharmacology and effects of cannabis: A brief review. Br. J. Psychiatry J. Ment. Sci. 1991, 178, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R. Cannabidiol: An overview of some chemical and pharmacological aspects. Part I: Chemical aspects. Chem. Phys. Lipids 2002, 121, 35–43. [Google Scholar] [CrossRef]
- Oiye, É.N.; Ribeiro, M.F.M.; Ferreira, B.; Botelho, R.C.B.; Oliveira, M.F. Disposable 3D Printed Electrode for the Electrochemical Detection of Delta-9-Tetrahydrocannabinol in Aqueous Solution and 11-Nor-9-Carboxy-Tetrahydrocannabinol in Saliva. Braz. J. Forensic Sci. Med. Law Bioeth. 2020, 9, 521–533. [Google Scholar] [CrossRef]
- Eissa, S.; Almthen, R.A.; Zourob, M. Disposable electrochemical immunosensor array for the multiplexed detection of the drug metabolites morphine, tetrahydrocannabinol and benzoylecgonine. Microchim. Acta 2019, 186, 523. [Google Scholar] [CrossRef]
- Cubillana-Aguilera, L.M.; Palacios-Santander, J.M.; Naranjo-Rodríguez, I.; Hidalgo-Hidalgo-De-Cisneros, J.L. Study of the influence of the graphite powder particle size on the structure of the Sonogel-Carbon materials. J. Sol-Gel Sci. Technol. 2006, 40, 55–64. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; Sullivan, S.E.O. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Lanzalaco, S.; Bertran, O.; Alem, C. Electrochemical multi-sensors obtained by applying an electric discharge treatment to 3D-printed poly (lactic acid) Adria. Appl. Surf. Sci. 2022, 597, 153623. [Google Scholar] [CrossRef]
- Calatayud-mac, P.; David, L.; Sierra-padilla, A. Bulk Modification of Sonogel—Carbon with Polyaniline: A Suitable Redox Mediator for Chlorophenols Detection. Chemosensors 2023, 11, 63. [Google Scholar] [CrossRef]
- Kantize, K.; Booysen, I.N.; Mambanda, A. Electrochemical sensing of acetaminophen using nanocomposites comprised of cobalt phthalocyanines and multiwalled carbon nanotubes. J. Electroanal. Chem. 2019, 850, 113391. [Google Scholar] [CrossRef]
- Cinti, S.; Neagu, D.; Carbone, M.; Cacciotti, I.; Moscone, D.; Arduini, F. Novel carbon black-cobalt phthalocyanine nanocomposite as sensing platform to detect organophosphorus pollutants at screen-printed electrode. Electrochim. Acta 2016, 188, 574–581. [Google Scholar] [CrossRef]
- Perry, J.M.; Little, S.R.; Llorens, O.Y.; Shih, C. An electrochemical aptasensor for Δ9 -tetrahydrocannabinol detection in saliva on a microfluidic platform. Biosens. Bioelectron. 2023, 222. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Cao, J.; Zhao, F.; Zeng, B. A ratiometric electrochemical sensing platform based on multifunctional molecularly imprinted polymer with catalytic activity for the detection of psychoactive substances. Biosens. Bioelectron. 2023, 220, 114929. [Google Scholar] [CrossRef]
- Safavi, A.; Maleki, N.; Tajabadi, F.; Farjami, E. High electrocatalytic effect of palladium nanoparticle arrays electrodeposited on carbon ionic liquid electrode. Electrochem. Commun. 2007, 9, 1963–1968. [Google Scholar] [CrossRef]
- Crivianu-gaita, V.; Thompson, M. Aptamers, antibody scFv, and antibody Fab’ fragments: An overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens. Bioelectron. 2016, 85, 32–45. [Google Scholar] [CrossRef]
- Nodehi, M.; Baghayeri, M.; Behazin, R.; Veisi, H. Electrochemical aptasensor of bisphenol A constructed based on 3D mesoporous structural SBA-15-Met with a thin layer of gold nanoparticles. Microchem. J. 2021, 162, 105825. [Google Scholar] [CrossRef]
- Cirrincione, M.; Zanfrognini, B.; Pigani, L.; Protti, M.; Mercolini, L.; Zanardi, C. Development of an electrochemical sensor based on carbon black for the detection of cannabidiol in vegetable extracts. Analyst 2021, 146, 612–619. [Google Scholar] [CrossRef]
- Fu, S.; Ma, X.; Wang, S.; Zha, Q.; Wen, W.; Hu, B. Surfactant-assisted carbon black for the electrochemical detection of endocrine disruptors. Surf. Interfaces 2021, 24, 101128. [Google Scholar] [CrossRef]
- Ali, Y.; Ul, A.; Howlader, M.M.R. Fabrication of highly sensitive Bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B Chem. 2020, 320, 128319. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Hu, N.X.; Deen, M.J. Electrochemical sensing of lead in drinking water using Β-cyclodextrin-modified MWCNTs. Sens. Actuators B Chem. 2019, 296, 126632. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.M.B.F.; Morais, S. New generation of electrochemical sensors based on multi-walled carbon nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Sempionatto, J.R.; Li, Z.; Brown, C.; Galdino, N.M.; Shah, R.; Liu, S.; Hubble, L.J.; Bagot, K.; Tapert, S.; et al. Simultaneous detection of salivary Δ9-tetrahydrocannabinol and alcohol using a Wearable Electrochemical Ring Sensor. Talanta 2020, 211, 120757. [Google Scholar] [CrossRef]
- Ben, N.; Emilia, M.; Dridi, C.; Ben, M.; Brett, C.M.A. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Goulart, L.A.; Mascaro, L.H. GC electrode modified with carbon nanotubes and NiO for the simultaneous determination of bisphenol A, hydroquinone and catechol. Electrochim. Acta 2016, 196, 48–55. [Google Scholar] [CrossRef]
- Sinha, K.; Uddin, Z.; Kawsar, H.I.; Islam, S.; Deen, M.J.; Howlader, M.M.R. Trends in Analytical Chemistry Analyzing chronic disease biomarkers using electrochemical sensors and artificial neural networks. Trends Anal. Chem. 2023, 158, 116861. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Zhang, Q.; Berg, D.; Mugo, S.M. Molecularly imprinted carbon based electrodes for tetrahydrocannabinol sensing. Inorg. Chem. Commun. 2019, 107, 107459. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Z.; Hou, C.; Liu, L.; Xiao, A. An electrochemical sensor based on amino magnetic nanoparticle-decorated graphene for detection of cannabidiol. Nanomaterials 2021, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Gu, Y.; Tang, P.; Liu, L. Electrochemical Sensor Based on Magnetic Molecularly Imprinted Polymer and Graphene-UiO-66 Composite Modified Screen-printed Electrode for Cannabidiol Detection. Int. J. Electrochem. Sci. 2022, 17, 220562. [Google Scholar] [CrossRef]
- Lee, J.R.; Choi, J.; Shultz, T.O.; Wang, S.X. Small Molecule Detection in Saliva Facilitates Portable Tests of Marijuana Abuse. Anal. Chem. 2016, 88, 7457–7461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, J.W.; Hsieh, C.H.; Huang, I.Y.; Lin, Y.C.; Tsai, T.Y.; Wang, C.C. Highly sensitive FPW-based microsystem for rapid detection of tetrahydrocannabinol in human urine. Sensors 2017, 17, 2760. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh, M.; Shadjou, N.; De, M. Aptamer-based assay of biomolecules: Recent advances in electro-analytical approach. Trends Anal. Chem. 2017, 89, 119–132. [Google Scholar] [CrossRef]
- Majak, D. Delta-9-tetrahydrocannabinol (Δ9-THC) sensing using an aerosol jet printed organic electrochemical transistor (OECT). J. Mater. Chem. B 2021, 9, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Czulak, J.; Guerreiro, A.; Garcia, A.; Piletsky, S.; Ertürk, G.; Hedström, M.; Mattiasson, B. A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2018, 120, 108–114. [Google Scholar] [CrossRef]
- Nur, D.; Sercan, Y.; Kurbanoglu, S.; Uslu, B. Current trends and roles of surfactants for chromatographic and electrochemical sensing Limit of Detection Ultraviolet e Visible Detector. Trends Anal. Chem. 2021, 144, 116418. [Google Scholar] [CrossRef]
- Najafi, M.; Maleki, L.; Abbas, A. Novel surfactant selective electrochemical sensors based on single walled carbon nanotubes. J. Mol. Liq. 2011, 159, 226–229. [Google Scholar] [CrossRef]
- Yazdi, A.S. Surfactant-based extraction methods. Trends Anal. Chem. 2011, 30, 918–929. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Zhao, Q. Low temperature greatly enhancing responses of aptamer electrochemical sensor for aflatoxin B1 using aptamer with short stem. ACS Sens. 2020, 5, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Douaki, A.; Garoli, D.; Inam, A.K.M.S.; Angeli, M.A.C.; Cantarella, G.; Rocchia, W.; Wang, J.; Petti, L.; Lugli, P. Smart Approach for the Design of Highly Selective Aptamer-Based Biosensors. Biosensors 2022, 12, 574. [Google Scholar] [CrossRef]
- Alam, M.M.; Howlader, M.M.R. Nonenzymatic electrochemical sensors via Cu native oxides (CuNOx) for sweat glucose monitoring. Sens. Bio-Sens. Res. 2021, 34, 100453. [Google Scholar] [CrossRef]
- Howlader, M.M.R. Integration of Two-Dimensional Materials: Recent Advances and Challenges. In Proceedings of the 6th International Work-Shop on Low Temperature Bonding for 3D Integration (LTB-3D), Kanazawa, Japan, 21–25 May 2019; p. 35. [Google Scholar] [CrossRef]
- Howlader, M.M.R.; Suehara, S.; Takagi, H.; Kim, T.H.; Maeda, R.; Suga, T. Room-temperature microfluidics packaging using sequential plasma activation process. IEEE Trans. Adv. Packag. 2006, 29, 448–456. [Google Scholar] [CrossRef]
- Howlader, M.M.R.; Okada, H.; Kim, T.H.; Itoh, T.; Suga, T. Wafer Level Surface Activated Bonding Tool for MEMS Packaging. J. Electrochem. Soc. 2004, 151, G461. [Google Scholar] [CrossRef]
- Howlader, M.M.R.; Selvaganapathy, P.R.; Deen, M.J.; Suga, T. Nanobonding technology toward electronic, fluidic, and photonic systems integration. IEEE J. Sel. Top. Quantum Electron. 2011, 17, 689–703. [Google Scholar] [CrossRef]
- Haddara, Y.M.; Howlader, M.M.R. Integration of Heterogeneous Materials for Wearable Sensors. Polymers 2018, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.U.; Qin, Y.; Nambiar, S.; Yeow, J.T.W.; Howlader, M.M.R.; Hu, N.X.; Deen, M.J. Polymers and organic materials-based pH sensors for healthcare applications. Prog. Mater. Sci. 2018, 96, 174–216. [Google Scholar] [CrossRef]
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Elcin, A. Electrochemical sensors targeting salivary biomarkers: A comprehensive review. Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Foley, K.M. Advances in cancer pain management in 2005. Gynecol. Oncol. 2005, 99, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Uddin, Z.; Singh, G.; Howlader, M.M.R. Glutamate sensing in biofluids: Recent advances and research challenges of electrochemical sensors. Analyst 2020, 145, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Mccrae, L.E.; Ting, W.; Howlader, M.M.R. Advancing electrochemical biosensors for interleukin-6 detection. Biosens. Bioelectron. X 2023, 13, 100288. [Google Scholar] [CrossRef]


| Cannabinoid | Administration Route | Bioavailability in Plasma | Ref. | ||
|---|---|---|---|---|---|
| CBD | Oral | Little to none | 1.64–4.2 h | 2.05–3.3 ng/mL | [16] |
| -THC | Inhalation (smoking) | 10–35% | 3–10 min | 150 ng/mL | [21] |
| -THC | Oral | 10–20% | 1–2 h or even 6 h | 58 ng/mL | [26] |
| Electrode | Technique | Sensitivity (µA·mL/ng) | LOD (ng/mL) | LR (ng/mL) | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| WE1: 1% MWCNTs/Graphite Ink WE2:alcohol oxidase/CH/GLU/PB ink | SWV CA | -------- | 157.23 PBS | 314.47–18886.82 | Dual Working Electrode | [45] |
| Electrode | Technique | Sensitivity (µA·mL/ng) | LOD (ng/mL) | LR (ng/mL) | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| CNT/MIP | DPV | ------------ | 0.18 ± 0.02 Methanol: DIW (1:1) | ----------- | MIP | [50] |
| -NPs/GN/GCE | CV | 4.08 × 0.56 × 0.19 × | 12.58 (PBS) | 31.45–306.29 306.29–6130.28 6130.28–314,470.0 | --------- | [51] |
| MagMIP/graphene/UiO66/SPE | CV | 0.16 × | 15.72 (PBS) | 1572.35–314,470.0 | MIP | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazuki, D.; Ghosh, R.; Howlader, M.M.R. Nanomaterials-Based Electrochemical Δ9-THC and CBD Sensors for Chronic Pain. Biosensors 2023, 13, 384. https://doi.org/10.3390/bios13030384
Pazuki D, Ghosh R, Howlader MMR. Nanomaterials-Based Electrochemical Δ9-THC and CBD Sensors for Chronic Pain. Biosensors. 2023; 13(3):384. https://doi.org/10.3390/bios13030384
Chicago/Turabian StylePazuki, Dadbeh, Raja Ghosh, and Matiar M. R. Howlader. 2023. "Nanomaterials-Based Electrochemical Δ9-THC and CBD Sensors for Chronic Pain" Biosensors 13, no. 3: 384. https://doi.org/10.3390/bios13030384
APA StylePazuki, D., Ghosh, R., & Howlader, M. M. R. (2023). Nanomaterials-Based Electrochemical Δ9-THC and CBD Sensors for Chronic Pain. Biosensors, 13(3), 384. https://doi.org/10.3390/bios13030384