Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Obtention of AETA Hydrogels
2.3. Preparation of Fluorescent Nanoparticles: PFO_CNPs and F8BT_CNPs
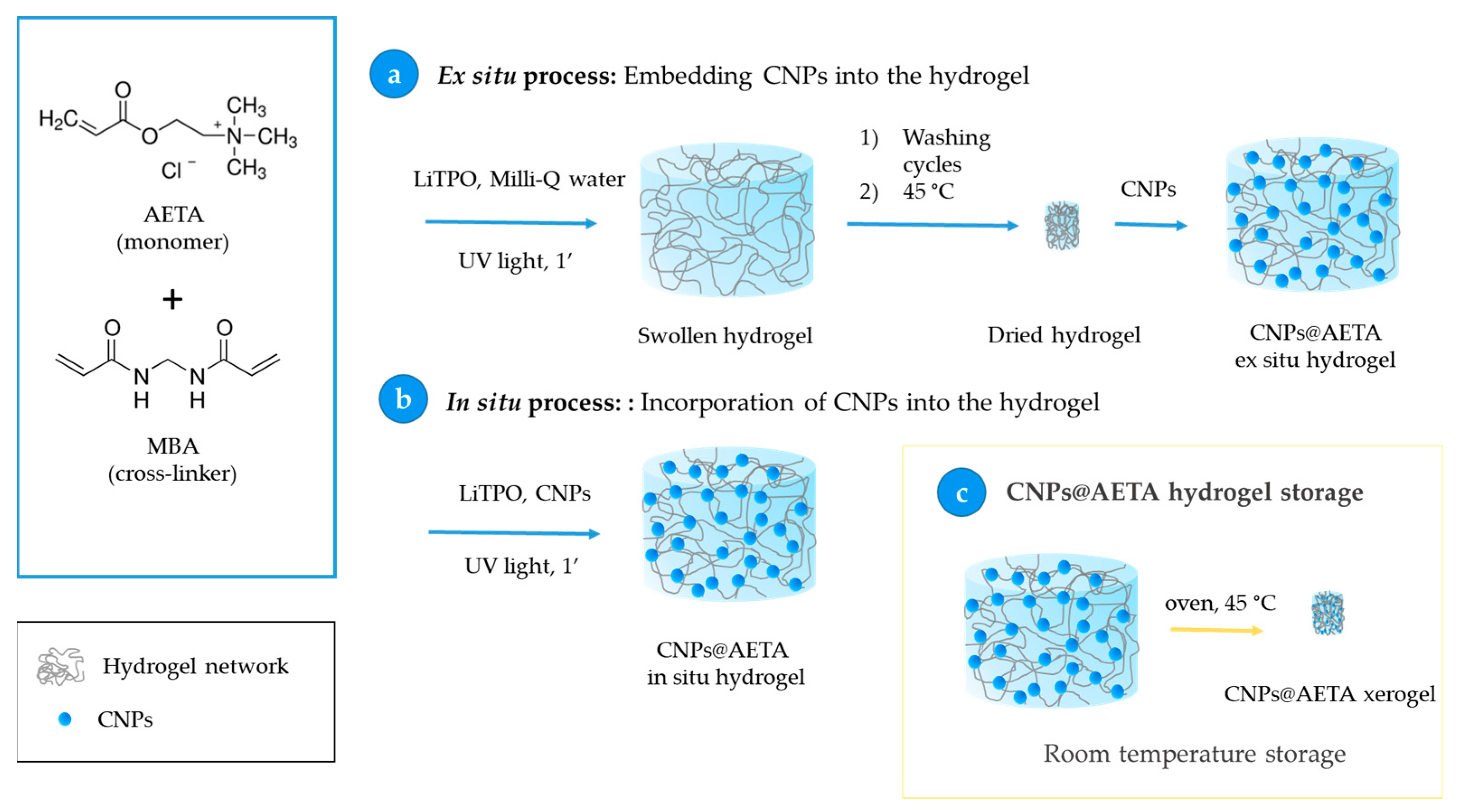
2.4. Obtention of PFO@AETA and F8BT@AETA Hydrogels
- Ex situ process: Embedment of the CNPs into the hydrogel. In this process, 0.07 g of hydrogel prepared by the above method was immersed in 1.8 mL of a 6 µM solution of already prepared CNPs for 24 h, to ensure that all the solution was completely absorbed, and then stored at 4 °C. This step allows the total loading of the fluorescent nanoparticles from the CNPs solution into the hydrogel network (Scheme 2a).
- In situ process: Incorporation of CNPs into the hydrogel. Nanoparticles of PFO and F8BT were incorporated before the polymerization process by adding 5 mL of a solution of CNPs 6 µM to the AETA-MBA-LiTPO mixture. After the irradiation with UV light, fluorescent nanocomposite hydrogels were obtained (Scheme 2b).
2.5. Absorbance Measurements
2.6. Steady-State Fluorescence Measurements
2.7. Time-Resolved Fluorescence Measurements
2.8. Swelling Measurements
2.9. Particle Size and Zeta Potential
2.10. Scanning Electron Microscope
3. Results and Discussion
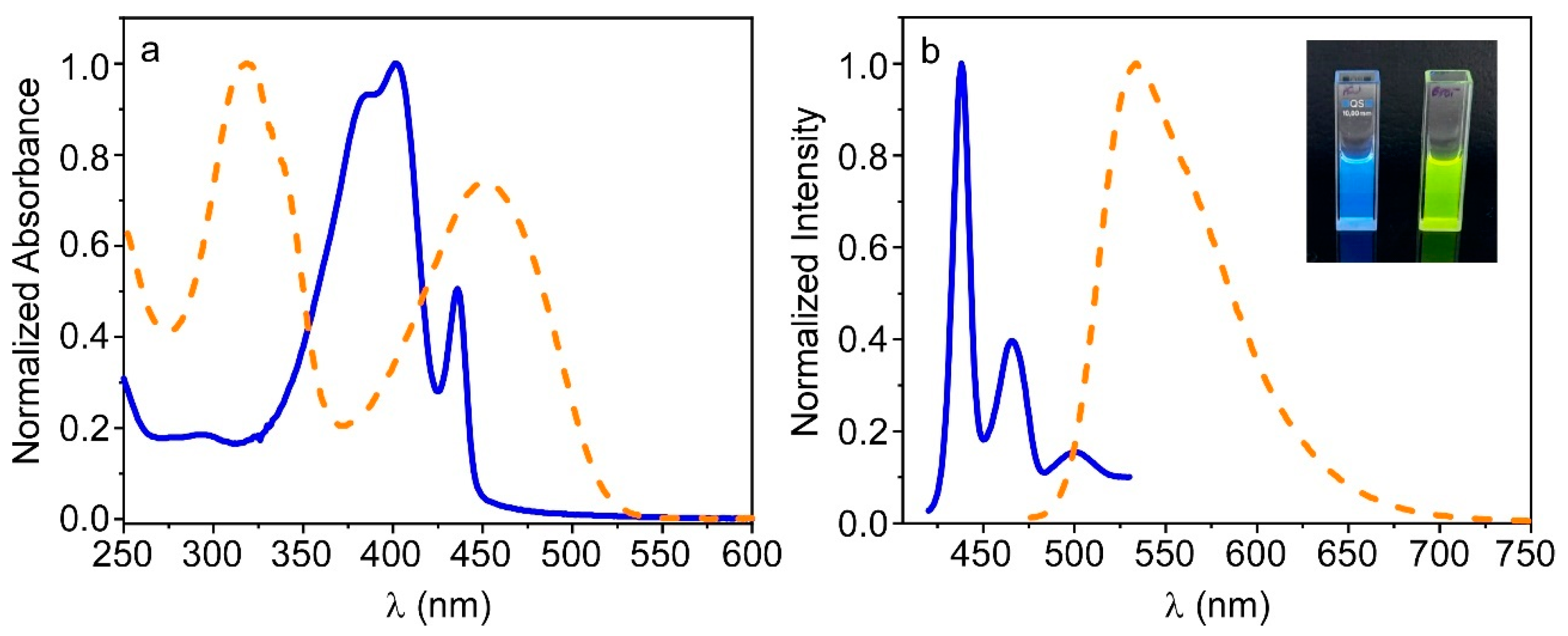
3.1. Preparation and Characterization of PFO_CNPs and F8BT_CNPs
3.2. Preparation and Swelling Behavior of PFO@AETA and F8BT@AETA Hydrogels
3.3. Fluorescence Properties of PFO@AETA and F8BT@AETA Hydrogels
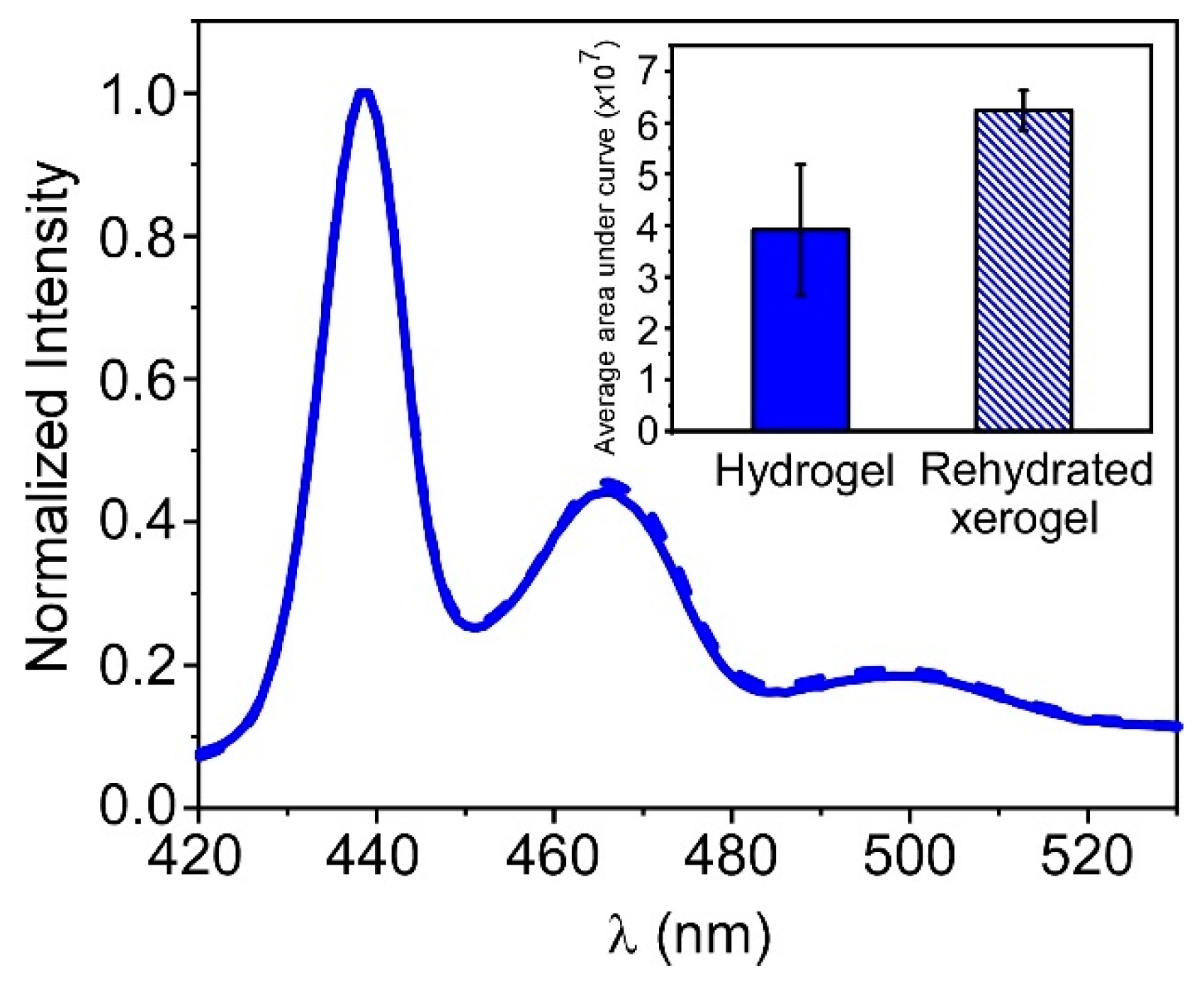
3.4. Storage of PFO@AETA as Xerogels
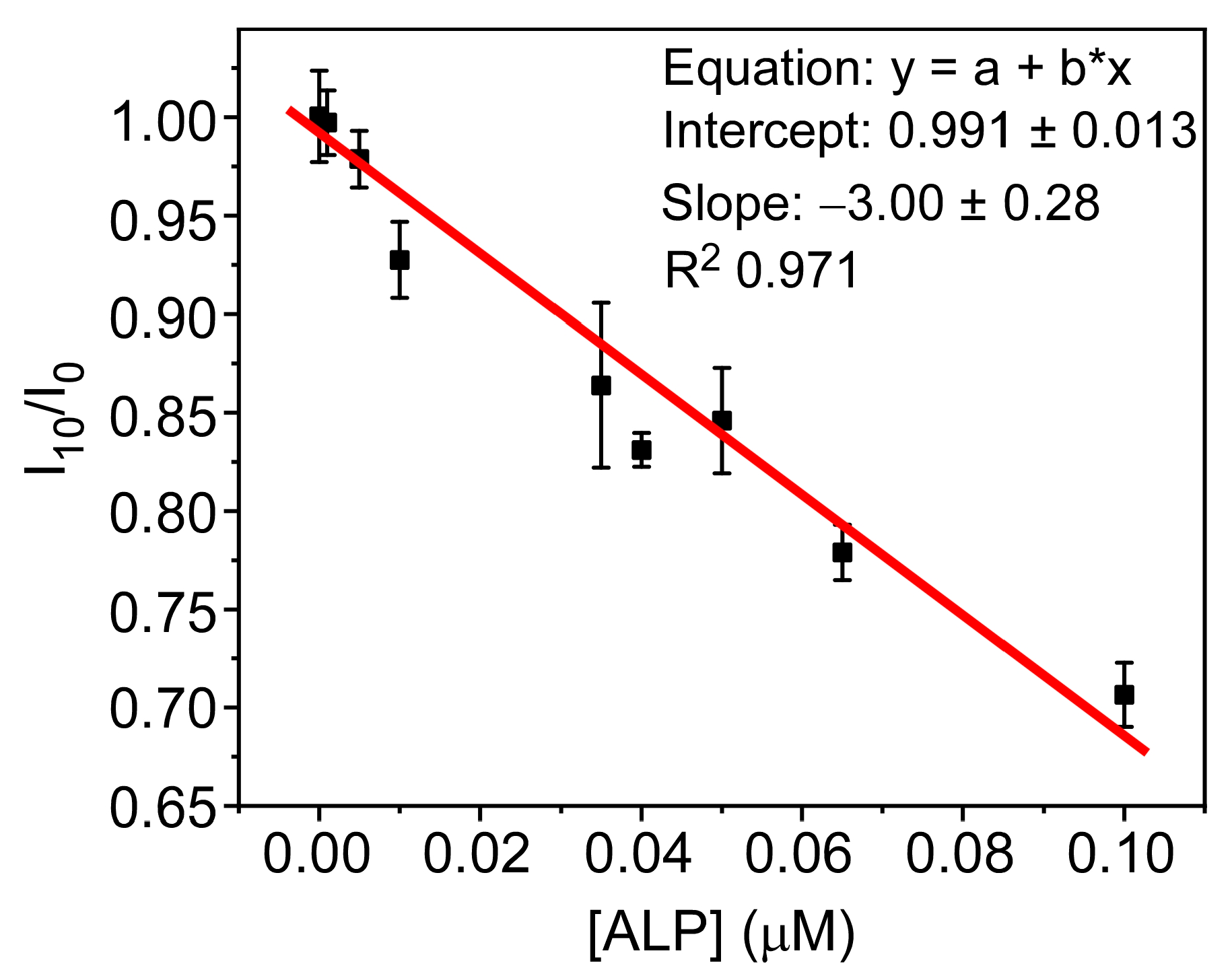
3.5. PFO@AETA as a Sensing Platform
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Barbero, C.A.; Martínez, M.V.; Acevedo, D.F.; Molina, M.A.; Rivarola, C.R. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol 2022, 2, 440–475. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Azady, M.A.R.; Ahmed, S.; Islam, M.S. A review on polymer nanocomposite hydrogel preparation, characterization, and applications. Eur. J. Chem. 2021, 12, 11. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent gels: A review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, e2100062. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. Engl. 2021, 60, 8608–8624. [Google Scholar] [CrossRef]
- Chang, C.; Peng, J.; Zhang, L.; Pang, D.-W. Strongly fluorescent hydrogels with quantum dots embedded in cellulose matrices. J. Mater. Chem. 2009, 19, 7771–7776. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Highly sensitive and strongly fluorescent gum tragacanth based superabsorbent hydrogel as a new biosensor for glucose optical detection. J. Mater. Chem. C 2020, 8, 4148–4156. [Google Scholar] [CrossRef]
- Chen, M.; Grazon, C.; Sensharma, P.; Nguyen, T.T.; Feng, Y.; Chern, M.; Baer, R.C.; Varongchayakul, N.; Cook, K.; Lecommandoux, S.; et al. Hydrogel-Embedded Quantum Dot-Transcription Factor Sensors for Quantitative Progesterone Detection. ACS Appl. Mater. Interfaces 2020, 12, 43513–43521. [Google Scholar] [CrossRef]
- Martín, C.; Martín-Pacheco, A.; Naranjo, A.; Criado, A.; Merino, S.; Díez-Barra, E.; Herrero, M.A.; Vázquez, E. Graphene hybrid materials? The role of graphene materials in the final structure of hydrogels. Nanoscale 2019, 11, 4822–4830. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Rahman, S.; Rostami, M.; Tabasi, Z.A.; Khan, F.; Alodhayb, A.; Zhang, Y. Carbon Quantum Dot-Incorporated Chitosan Hydrogel for Selective Sensing of Hg2+ Ions: Synthesis, Characterization, and Density Functional Theory Calculation. ACS Omega 2021, 6, 23504–23514. [Google Scholar] [CrossRef]
- Shao, J.; Yu, Q.; Wang, S.; Hu, Y.; Guo, Z.; Kang, K.; Ji, X. Poly(vinyl alcohol)–Carbon Nanodots Fluorescent Hydrogel with Superior Mechanical Properties and Sensitive to Detection of Iron(III) Ions. Macromol. Mater. Eng. 2019, 304, 1900326. [Google Scholar] [CrossRef]
- Martín-Pacheco, A.; Del Río Castillo, A.E.; Martín, C.; Herrero, M.A.; Merino, S.; García Fierro, J.L.; Díez-Barra, E.; Vázquez, E. Graphene Quantum Dot–Aerogel: From Nanoscopic to Macroscopic Fluorescent Materials. Sensing Polyaromatic Compounds in Water. ACS Appl. Mater. Interfaces 2018, 10, 18192–18201. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Hu, D.; Liu, X.; Zhang, X.; Yuan, Z.; Sheng, Z.; Zheng, H. Recent Advances in Conjugated Polymer Nanoparticles for NIR-II Imaging and Therapy. ACS Appl. Polym. Mater. 2020, 2, 4241–4257. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Koralli, P.; Nega, A.; Vagiaki, L.; Pavlou, A.; Siskos, M.; Dimitrakopoulou-Strauss, A.; Gregoriou, V.; Chochos, C. New Conjugated Polymer Nanoparticles with High Photoluminescence Quantum Yields for Far-red and Near Infrared Fluorescence Bioimaging. Mater. Chem. Front. 2020, 4, 2357–2369. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Abbas, A.; Hao, Y.; Malik, A.H.; Tian, X.; Song, H.; Gao, R. Conjugated polymer nanoparticles and their nanohybrids as smart photoluminescent and photoresponsive material for biosensing, imaging, and theranostics. Mikrochim. Acta 2022, 189, 83. [Google Scholar] [CrossRef]
- Alacid, Y.; Martínez-Tomé, M.J.; Mateo, C.R. Reusable Fluorescent Nanobiosensor Integrated in a Multiwell Plate for Screening and Quantification of Antidiabetic Drugs. ACS Appl. Mater. Interfaces 2021, 13, 25624–25634. [Google Scholar] [CrossRef]
- Aguado, R.; Santos, A.; Vallejos, S.; Valente, A.J.M. Paper-Based Probes with Visual Response to Vapors from Nitroaromatic Explosives: Polyfluorenes and Tertiary Amines. Molecules 2022, 27, 2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hussain, S.; Chen, X.; Hao, Y.; Zhang, P.; Gao, R. Fabrication of conjugated polymer encapsulated fluorescent hybrid micelles for augmented, highly selective and step-wise detection of nitroaromatic pollutants and hepatobiliary biomarker. Sens. Actuators B Chem. 2023, 377, 133081. [Google Scholar] [CrossRef]
- Ji, X.; Yao, Y.; Li, J.; Yan, X.; Huang, F. A Supramolecular Cross-Linked Conjugated Polymer Network for Multiple Fluorescent Sensing. J. Am. Chem. Soc. 2013, 135, 74–77. [Google Scholar] [CrossRef]
- Namgung, H.; Jo, S.; Lee, T.S. Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel. Polymers 2021, 13, 4315. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Camacho, M.; Martínez-Tomé, M.J.; Mira, A.; Mallavia, R.; Mateo, C.R. Formation of Multicolor Nanogels Based on Cationic Polyfluorenes and Poly(methyl vinyl ether-alt-maleic monoethyl ester): Potential Use as pH-Responsive Fluorescent Drug Carriers. Int. J. Mol. Sci. 2021, 22, 9607. [Google Scholar] [CrossRef] [PubMed]
- Alacid, Y.; Martínez-Tomé, M.J.; Esquembre, R.; Herrero, M.A.; Mateo, C.R. Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing. Int. J. Mol. Sci. 2023, 24, 2672. [Google Scholar] [PubMed]
- Alacid, Y.; Quintero Jaime, A.F.; Martínez-Tomé, M.J.; Mateo, C.R.; Montilla, F. Disposable Electrochemical Biosensor Based on the Inhibition of Alkaline Phosphatase Encapsulated in Acrylamide Hydrogels. Biosensors 2022, 12, 698. [Google Scholar] [CrossRef]
- Qu, F.; Meng, L.; Zi, Y.; You, J. Ratiometric detection of alkaline phosphatase based on aggregation-induced emission enhancement. Anal. Bioanal. Chem. 2019, 411, 7431–7440. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Guo, W.; Wang, Y.; Hua, Q.; Tang, F.; Luan, F.; Tian, C.; Zhuang, X.; Zhao, L. Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors 2022, 12, 372. [Google Scholar] [CrossRef]
- He, J.; Jiang, X.; Ling, P.; Sun, J.; Gao, F. Ratiometric Sensing for Alkaline Phosphatase Based on Two Independent Signals from in Situ Formed Nanohybrids of Semiconducting Polymer Nanoparticles and MnO2 Nanosheets. ACS Omega 2019, 4, 8282–8289. [Google Scholar] [CrossRef] [Green Version]
- Kahveci, Z.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. Fluorescent Biosensor for Phosphate Determination Based on Immobilized Polyfluorene-Liposomal Nanoparticles Coupled with Alkaline Phosphatase. ACS Appl. Mater. Interfaces 2017, 9, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pecher, J.; Mecking, S. Nanoparticles of Conjugated Polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef] [PubMed]
- Urbano, L.; Clifton, L.; Ku, H.K.; Kendall-Troughton, H.; Vandera, K.-K.A.; Matarese, B.F.E.; Abelha, T.; Li, P.; Desai, T.; Dreiss, C.A.; et al. Influence of the Surfactant Structure on Photoluminescent π-Conjugated Polymer Nanoparticles: Interfacial Properties and Protein Binding. Langmuir 2018, 34, 6125–6137. [Google Scholar] [CrossRef]
- Eggimann, H.J.; Le Roux, F.; Herz, L.M. How β-Phase Content Moderates Chain Conjugation and Energy Transfer in Polyfluorene Films. J. Phys. Chem. 2019, 10, 1729–1736. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; McNeill, J. Swelling-Controlled Polymer Phase and Fluorescence Properties of Polyfluorene Nanoparticles. Langmuir 2008, 24, 5855–5861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Li, R.; Jia, Y. Tuning the formation of β-phase poly(9,9-di-n-octylfluorenyl-2,7-diyl) via nano-confinement and polystyrene blending for improved photocatalysis. Chem. Phys. Mater. 2022, 1, 219–226. [Google Scholar] [CrossRef]
- Cao, A.; Tang, Y.; Liu, Y. Novel Fluorescent Biosensor for α-Glucosidase Inhibitor Screening Based on Cationic Conjugated Polymers. ACS Appl. Mater. Interfaces 2012, 4, 3773–3778. [Google Scholar] [CrossRef]
- Astier, Y.; Bartlett, P.N. The measurement of alkaline phosphatase at nanomolar concentration within 70 s using a disposable microelectrochemical transistor. Bioelectrochemistry 2004, 64, 53–59. [Google Scholar] [CrossRef]
- National Library of Medicine. ALP—Blood Test. Available online: https://medlineplus.gov/ency/article/003470.htm (accessed on 14 February 2023).
- Shaban, S.M.; Jo, S.B.; Hafez, E.; Cho, J.H.; Kim, D.-H. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coord. Chem. Rev. 2022, 465, 214567. [Google Scholar] [CrossRef]
- Martínez-Crego, B.; Romero, J.; Alcoverro, T. The use of surface alkaline phosphatase activity in the seagrass Posidonia oceanica as a biomarker of eutrophication. Mar. Ecol. 2006, 27, 381–387. [Google Scholar] [CrossRef]
- Bañeras, L.; Ros-Ponsatí, M.; Cristina, X.P.; Garcia-Gil, J.L.; Borrego, C.M. Phosphorus deficiency and kinetics of alkaline phosphatase in isolates and natural populations of phototrophic sulphur bacteria. FEMS Microbiol. Ecol. 2010, 73, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sample | d ± SD (nm) | PDI ± SD | ZP ± SD (mV) |
|---|---|---|---|
| PFO_CNPs | 76.6 ± 0,5 | 0.203 ± 0.005 | −45 ± 1 |
| F8BT_CNPs | 65.7 ± 0,9 | 0.155 ± 0.010 | −62 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alacid, Y.; Esquembre, R.; Montilla, F.; Martínez-Tomé, M.J.; Mateo, C.R. Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection. Biosensors 2023, 13, 408. https://doi.org/10.3390/bios13030408
Alacid Y, Esquembre R, Montilla F, Martínez-Tomé MJ, Mateo CR. Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection. Biosensors. 2023; 13(3):408. https://doi.org/10.3390/bios13030408
Chicago/Turabian StyleAlacid, Yolanda, Rocío Esquembre, Francisco Montilla, María José Martínez-Tomé, and C. Reyes Mateo. 2023. "Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection" Biosensors 13, no. 3: 408. https://doi.org/10.3390/bios13030408
APA StyleAlacid, Y., Esquembre, R., Montilla, F., Martínez-Tomé, M. J., & Mateo, C. R. (2023). Fluorescent Nanocomposite Hydrogels Based on Conjugated Polymer Nanoparticles as Platforms for Alkaline Phosphatase Detection. Biosensors, 13(3), 408. https://doi.org/10.3390/bios13030408





