Recent Advances in Nanoparticle-Based Optical Sensors for Detection of Pesticide Residues in Soil
Abstract
:1. Introduction
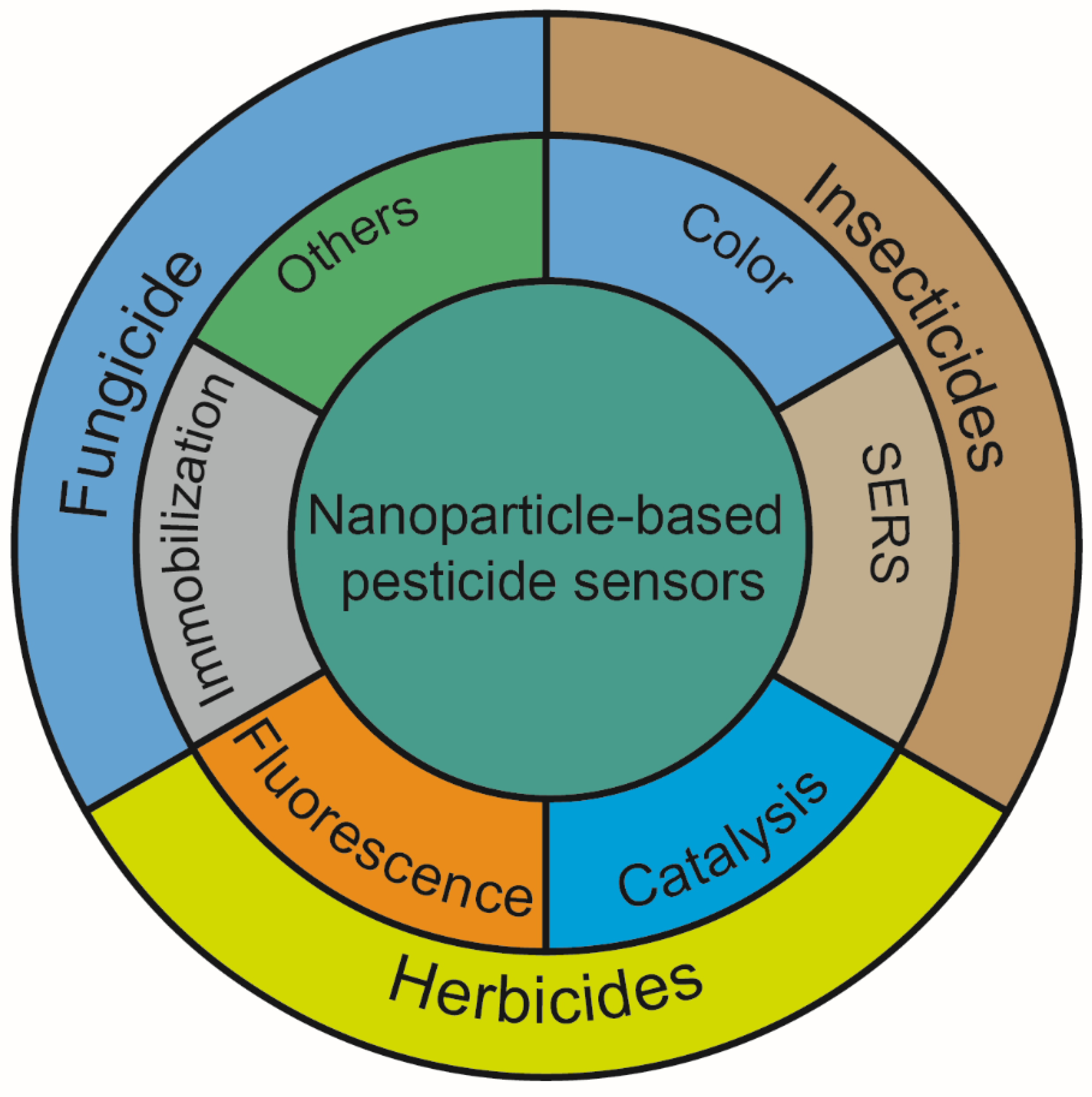
2. The Role of Nanoparticles in the Detection of Pesticides
2.1. Unique and Variable Color
2.1.1. The Original Color of the Nanoparticles
2.1.2. The Color Change Caused by the Aggregation of Nanoparticles
2.1.3. The Color Caused by Morphology Change of Nanoparticles

2.2. SERS
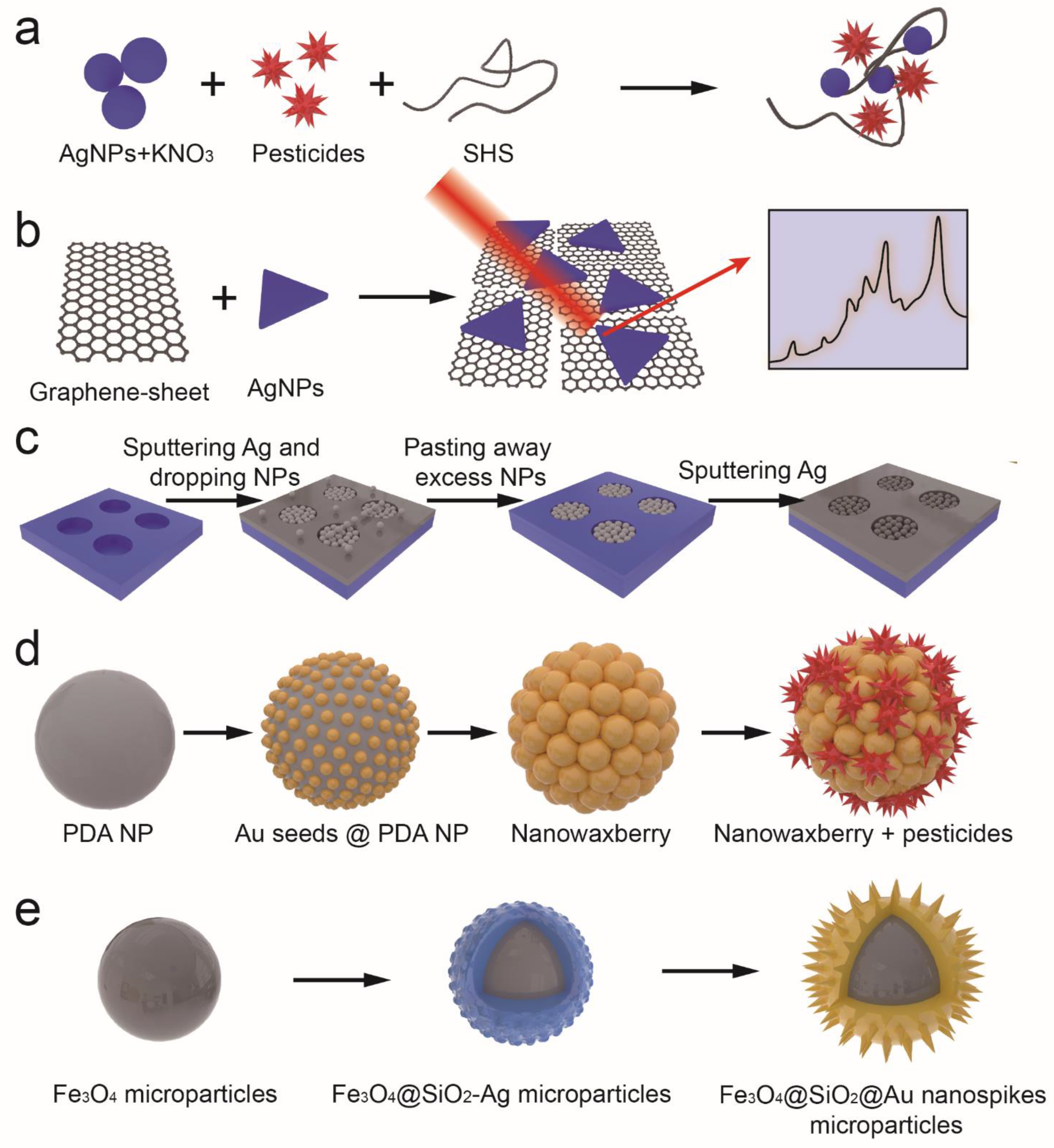
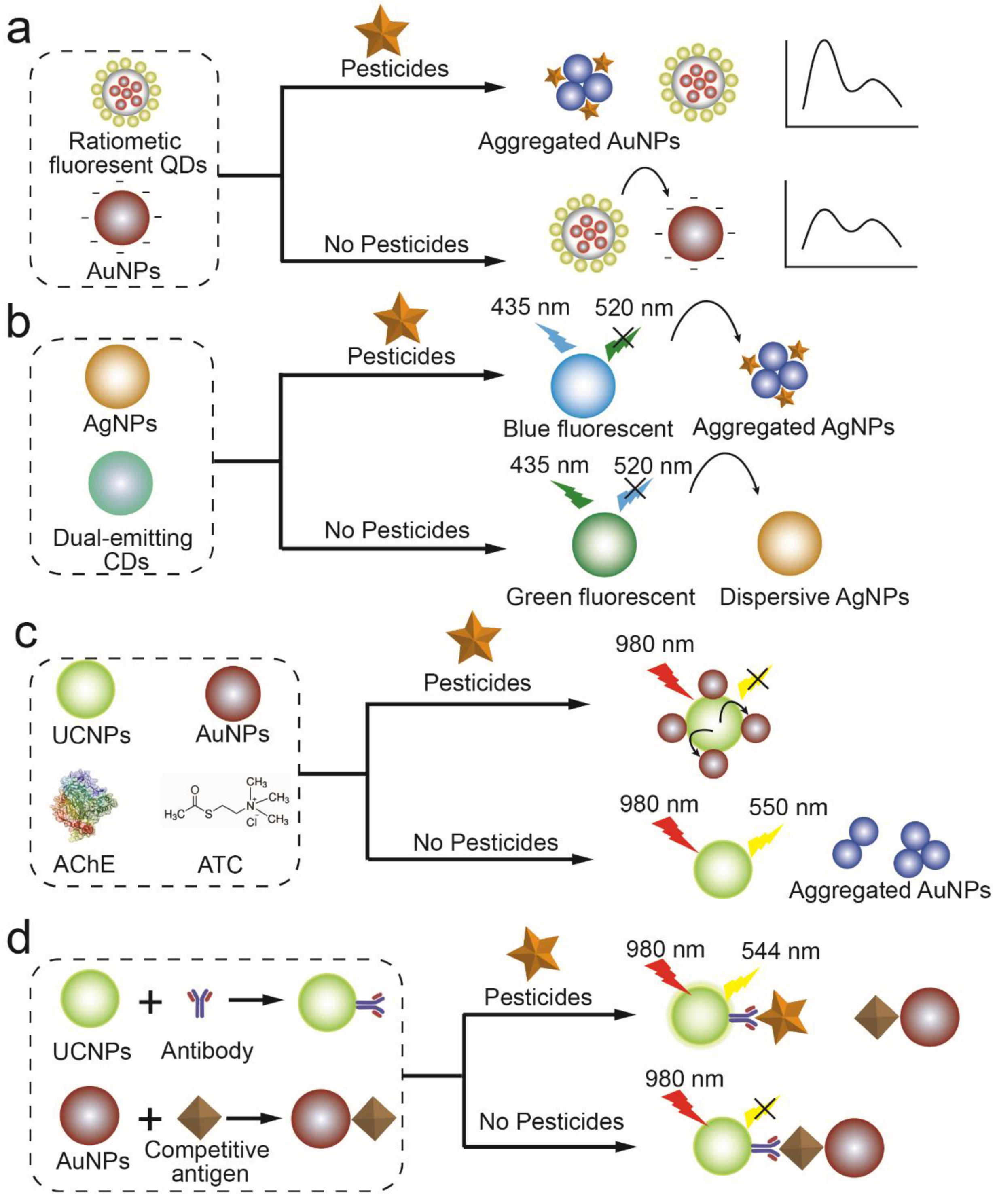
2.3. Fluorescence Enhancement or Quenching Characteristics
2.4. Catalytic Characteristics
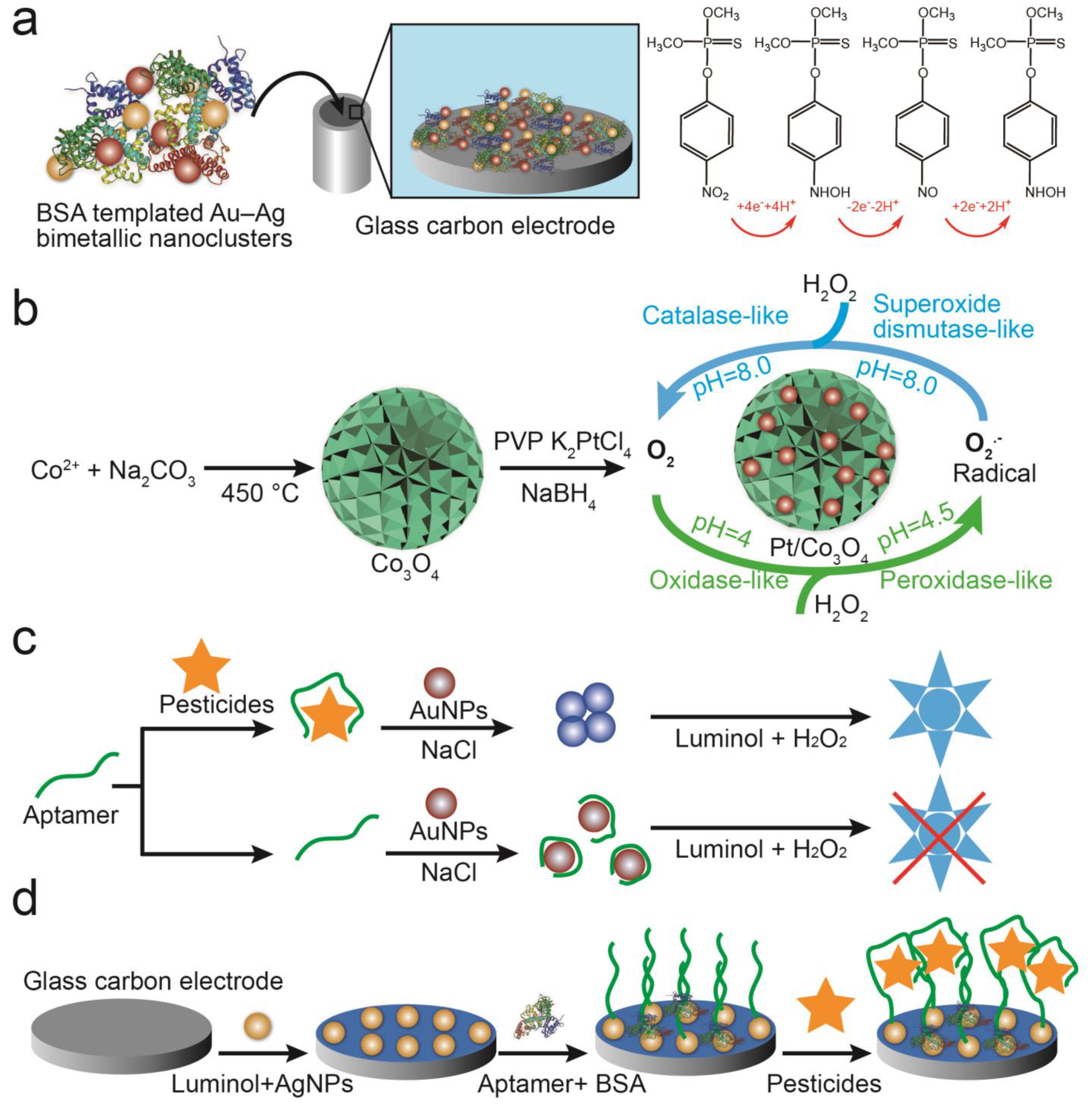
2.5. Immobile Substrate
2.6. Other Special Functions
3. Application of Nanoparticle-Based Sensors for Different Pesticides
3.1. Insecticides
3.1.1. Organophosphorus Insecticides
3.1.2. Neonicotinoid Insecticides
3.1.3. Pyrethroids and Organochlorine Insecticides
| Pesticides | Signals | Used Nanoparticles | Identification Method | Linear Range | LOD | Recovery |
|---|---|---|---|---|---|---|
| Methyl parathion [81] | DPV | Graphene AuNPs | Physisorption | 0.95–151.97 μM 1.90–227.95 μM | 0.86 μM 2.93 μM | 92–113% |
| Methyl parathion [82] | DPV | MWCNTs-PAAM nanocomposite | Electrocatalysis | 0.005–10 μM | 2.0 nM | NG |
| Methyl parathion [83] | DPV | MWCNTs | MIP | 0.2–10 μM | 67 nM | 94.9–106.2% |
| Methyl parathion [51] | DPV | CuO NPs | Affinity between the Cu and P=S or P=O groups | 0.19–5.7 μM | 10 nM | 80.18–105.48% |
| Methyl parathion [84] | SWV | AuNPs | Methyl parathion hydrolase | 0.075 nM–0.38 μM | 0.26 nM | 93–107% |
| Methyl parathion [46] | SWASV | Au–Ag nanoclusters | Electrocatalysis | 0.02–8.0 μM 8.0–200 μM | 8.2 nM | 102.9–104.0% |
| Methyl parathion [60] | PSA | Carbon NPs and halloysite nanoclay | Electrocatalysis | 0.00155–3.67 μM | 0.47 nM | NG |
| Methyl parathion [61] | SERS | Silver/polydopamine/calcium-oxide nanocomposites | SERS | 0.01 M–0.9 nM | 0.9 nM | NG |
| Methyl parathion [18] | Colorimetric | AuNPs | Lanthanum | 0.5–500 nM | 0.1 nM | 95.3–107.4% |
| Methyl parathion [85] | Fluorescence | N-doped CDs | Methyl parathion hydrolase | 2.38–73.78 μM | 338 nM | 95.1–108% |
| Methyl parathion [29] | SERS | Ag-nanoplate decorated GNS | SERS | 1–500 μM | 570 nM | NG |
| Ethyl parathion [60] | PSA | Carbon NPs and halloysite nanoclay | Electrocatalysis | 1.21 nM–4.92 μM | 0.367 nM | NG |
| Ethyl parathion [86] | DPV | Carbon nanotube | Intermolecular interactions | 0.02–6.50 μM | 5.3 nM | 97.2–104.6% |
| Chlorpyrifos [63] | Voltametric and ISFET | Flower shaped ZnO NPs | Alkaline phosphatase | 1 nM–0.1 M 0.1 nM–1 mM | 1 nM 0.1 nM | 96.6–108.9% |
| Chlorpyrifos [87] | DPAdSV | Ag/Cu alloy NPs | Electrocatalysis | 0.01–100 nM | 4 pM | 85.6–93.4% |
| Chlorpyrifos [56] | CV | Carboxylated MWCNTs | AChE acetylthiocholine | 0.1–50 nM | 0.1 nM | NG |
| Chlorpyrifos [64] | SFI | Pd-doped CdTe QDs MWCNTs | tryptophan residue | 0.5 pM–500 nM | 0.16 pM | 98.5–105.9% |
| Chlorpyrifos [25] | SERS | AuNPs | SERS | 0.01–10 mg/L | 10 μg/L | 97.5–103.3% |
| Chlorpyrifos [65] | Colorimetric | AuNPs | Interaction between−P=S group and Au NPs | 10–50 ppb | NG | NG |
| Chlorpyrifos [50] | Absorption spectra | Ag3PO4 NPs | Oxidase-mimicking | 20–80 ppm | 9.97 ppm | 112.2–164.0% |
| Chlorpyrifos-methyl [17] | Test paper | Gold NPs | Monoclonal antibodies | NG | 0.29 μM | NG |
| Malathion [66] | DPV | Chitosan-iron oxide nanocomposite | DNA aptamer | 0.001–10 ng/mL | 1 ng/L | 80–92% |
| Malathion [56] | CV | Iron oxide NPs Carboxylated-MWCNTs | AChE, ATC | 0.1–70 nM | 0.1 nM | NG |
| Malathion [39] | FRET | Au/Fe3O4 NPs | Au−S bond | 27.24–99.89 μM | 0.59 μM | NG |
| Fenthion [67] | DPV | Graphene QDs | Pralidoxime | 10 pM–0.5 μM | 6.8 pM | 95.4–104.8% |
| Fenitrothion [68] | CV | MWCNT | Electrocatalysis | 0.01–5.0 mM | 6.4 nM | 88.0–93.3% |
| Diazinon [69] | SWASVs | Au–Pt bimetallic nanoclusters | NPs catalyzes | 0.01–10.0 μM 10.0–170 μM | 2 nM | 95.3–105.0% |
| Monocrotophos [56] | CV | Iron oxide NPs Carboxylated-MWCNTs | AChE ATC | 0.1–70 nM | 0.1 nM | NG |
| Triazophos [55] | Test paper | AuNPs | Antibodies | NG | 5 ng/mL | NG |
| Isocarbophos [17] | Test paper | AuNPs | Antibodies | NG | 100 μg/L | NG |
| Methidathion [57] | Fluorescence | CdTe QDs | AChE ATC | 0.1–50 ng/mL | 0.027 ng/mL | 96–105% |
| Paraoxon-ethyl [71] | Paper strip | Carbonaceous nanoaggregates | Cholinesterase | NG | 1.3 ng/mL | 90–110% |
| Imidacloprid [74] | Fluorescence | MNPs UCNPs | Antibody | 0.32–299.21 ng/mL | 0.32 ng/mL | 82.5–102.3% |
| Imidacloprid [75] | SWSV fluorescence | Iron oxide NPs | MIP | 0.059–0.791 μg /L 0.039–0.942 μg/ L | 0.0125 μg/ L 0.0108 μg/ L | 5.9–100.4% |
| Imidacloprid [88] | Amperometric current responses | Cu-rGO nanofiber | Electrocatalysis | 100–500 nM | 2.511 nM | NG |
| Imidacloprid [89] | CV | MWCNTs | Electrocatalysis | 0.2–1.77 μM | 0.0374 nM | 94–97% |
| Imidacloprid [73] | SERS | AuNPs | SERS | NG | 30 µg/L | NG |
| Acetamiprid [53] | CL | AuNPs | Aptamer | 0.8–630 nM | 62 pM | 90.4–105.3% |
| Acetamiprid [21] | Colorimetric | AuNPs | Aptamer | 8.7–920 nM | 0.56 nM | 95.2–104.0% |
| Acetamiprid [20] | Colorimetric | AuNPs | Aptamer | 75 nM–7.5 μM | 5 nM | NG |
| Acetamiprid [77] | Fluorescence | MNPs UCNPs | Aptamer | 0.89–114.18 μg/L | 0.65 μg/L | 78.2–103.5% |
| Acetamiprid [41] | Fluorescence | UCNPs AuNPs | Antibody | 0.002–0.58 µg/L | 0.04 µg/L | 75.1–104.7% |
| Acetamiprid [76] | CL | GO/AuNPs | Aptamer | 0.221–9 nM | 8.9 pM | 90.4–108.3% |
| Acetamiprid [37] | Fluorescence | AuNPs QDs | Cyano group | 0.025–5.0 μg/mL | 16.8 μg/L | 96–105% |
| Acetamiprid [90] | Chronocoulometry Chronoamperometry | Polypyrrole nanowires | NG | 1 ng/L–0.1 g/L 1 pg/L–0.1 ug/L | 0.347 pg/mL 0.065 fg/mL | NG |
| Thiacloprid [74] | Fluorescence | MNPs UCNPs | Antibody | 0.61–169.82 ng/mL | 0.61 ng/mL | 78.4–105.9% |
| Clothianidin [78] | Test strips | AuNPs | Antibody | 3.8–372 ng/mL | 3.8 ng/mL 8 ng/mL | 78.0–114.5% |
| Deltamethrin [26] | SERS | AuNPs | SERS | 0.01–10 mg/L | 0.056 mg/kg | 76.0–106.0%, |
| Alpha-cypermethrin [79] | Fluorescence | UCNPs | MIP | 0.10–12 mg/L | 0.03 mg/L | 83.90–93.15% |
| Hexachlorobenzene [30] | SERS | Rough ferro-NPs | SERS | NG | ~10 pM | NG |
| Dicofol [80] | DPV | Ni nanowire | Electrocatalysis | 0.83–30.7 μM | 0.08 μM | 95–104.9% |
| Endosulfan [56] | CV | Iron oxide NPs MWCNTs | AChE | 0.1–100 nM | 0.1 nM | NG |
| Carbofuran [91] | DPV | PDDA and GO | Hydrophobic and van der Waals interactions | NG | 0.407 μM | 101.09 and 96.74% |
| Carbofuran [26] | SERS | AuNPs | SERS | 0.01–10 mg/L | 0.01 mg/L | 80.0–102.6%, |
3.2. Herbicides
3.2.1. Glycine Derivative Herbicides
3.2.2. Bipyridyliums Herbicides
3.2.3. Dinitroanilines Herbicides
3.2.4. Triazines Herbicides
3.2.5. Ureas Herbicides
3.2.6. Diphenyl Ether Herbicides
| Pesticides | Signals | Used Nanoparticles | Identification Method | Linear Range | LOD | Recovery |
|---|---|---|---|---|---|---|
| Glyphosate [93] | CA | AgNPs | Acid phosphatase inhibition | 0.05–0.5 μg/mL 0.5–22.0 μg/mL | 0.015 μg/mL 2 mg/kg | 95.6–104.7% |
| Glyphosate [94] | DPAnSV | AgNPs | MIP | 3.98–176.23 ng/mL | 0.35 ng/mL | 97.8–102.3% |
| Glyphosate [99] | Electropherograms | CdTe/CdS QDs | Electrophoretic mobility | 77.1–700 mg/kg | 25.7 mg/kg | 92.0–98.0% |
| Glyphosate [95] | Fluorescence | CDs; Magnetic NPs | Glyphosate antibody | 0.01–80 μg/mL | 8 ng/mL | 87.4–103.7% |
| Glyphosate [119] | Fluorescence | CdTe QDs; Au NPs | Electrostatic interactions, | 0.02–2.0 μg/kg | 9.8 ng/kg | 88.5–102.6% |
| Glyphosate [98] | SERS | Au NPs | SERS | 0.003–0.07 nM | 0.002 nM | 92.3–105.3% |
| Glyphosate [120] | SERS | rGO, AgNPs, TiO2 nanotube | SERS | 10−2–10−12 M | 3 µg/L | 100.2–103.1% |
| Glyphosate [96] | Bio-barcode immuno-PCR | AuNPs | Glyphosate antibody | 61.1 pg/g–31.3 ng/g | 4.5 pg/g | 99.8–103.7% |
| Glyphosate [121] | CL | ZnO NPs | [Ru(bpy)3]2+ | 1–10 μM | 300 nM | 92% |
| Glyphosate [97] | Resonance Rayleigh scattering | Gold-doped polystyrene nanoenzyme | Aptamer | 0.5–20 nM | 0.24 nM | NG |
| Paraquat [101] | Ad-DPCSV | AgNPs | Redox activity of paraquat | 19–1000 nM | 0.23 nM | 99–102% |
| Paraquat [100] | SQW | AuNP-MWCNT | Electrocatalysis | 1.0–2.0 μM | 32 nM | 93.5–101.6% |
| Paraquat [102] | DPV | AuNPs | Electrostatic interactions | 7.0–1500 nM | 0.2 nM | 95.0% |
| Paraquat [103] | SERS | AuNPs | SERS | NG | 10 nM | NG |
| Paraquat [104] | Colorimetric | AgNPs | Forming charge transfer complexes | 20–180 µM | 6.27 µM | NG |
| Paraquat [105] | Colorimetric | AgNPs | Coulombic attraction | 0. 194–194 µM | 0.05 mg/L | 89.5% and 86.6% |
| Trifluralin [109] | Fluorescence | CDs | Fluorescence Quenching | NG | 7.89 μM | NG |
| Trifluralin [108] | Fluorescence | CDs | Fluorescence Quenching | 0.050–200 μM | 0.5 nM | 94.6–103.2% |
| Trifluralin [107] | CL | BNQDs | Nanocatalysts | 0.02–90 µM | 6.0 nM | 94–104% |
| Trifluralin [122] | CV | MWNTs | Electrocatalysis | 5–6000 nM | 2.0 nM | 96.7–101.0% |
| Trifluralin [110] | FFT-SWV | Copper nanowire | Electrocatalysis | 100–0.02 nM | 0.008 nM | 99.3–101.5% |
| Trifluralin [111] | SWV | MWCNT Fe3O4/SiO2 NPs | Electrocatalysis | 0.01–8 μM | 3 nM | NG |
| DEHA [28] | SERS | AgNPs | SERS | 2.04–163 μM | 34.2 nM | NG |
| Prometryn [27] | SERS | AgNPs | SERS | NG | 5.6 nM | NG |
| Atrazine [27] | SERS | AgNPs | SERS | NG | 0.1 nM | NG |
| Atrazine [54] | ECL | AgNPs | Aptamer | 1 pg/mL–10 μg/mL | 0.33 pg/mL | 89.13–123.03% |
| Tribenuron-methyl [112] | ECL | AgNPs BNQDs | Cooperation effect | 5.0 pM–0.60 μM | 1.2 pM | 98.2–100.8% |
| Simazine [113] | CV | AuNPs | MIP | NG | 0.013 μM | 91.4–96.8% |
| Diuron [114] | SWV | NC | Electrocatalysis | 4.2–47 µM | 0.35 µM | 96% |
| Linuron [115] | DPV | PtNPs | Electrocatalysis | 0.61–26.0 μM | 0.18 μM | 90.9–104% |
| Linuron [116] | SWV | MWCNTs/ ZnO NPs | Electrocatalysis | 0.02–0.34 μM | 5.83 nM | 96.2–99.42% |
| Aclonifen [117] | DPV | GdNbO4 NPs | Electrocatalytic | 0.02–78 μM | 1.15 nM | 80–92.5% |
| Aclonifen [118] | SWV | g−C3N4 | Electrocatalytic | 0.01–1.2 μM | 1.28 nM | 97.4–98.7% |
3.3. Fungicide
3.3.1. Carbamates
3.3.2. Triazole Fungicides
3.3.3. Others
| Pesticides | Signals | Used Nanoparticles | Identification Method | Linear Range | LOD | Recovery |
|---|---|---|---|---|---|---|
| Thiram [33] | SERS | Cu2O nano-octahedrons | SERS | 10−3–10−7 M | 0.48 ng/g | NG |
| Thiram [31] | SERS | Rough Au NRs | SERS | 0.0192–0.96 µg/g | 0.0005 ppm | NG |
| Thiram [124] | SERS | Au@Ag nanocube | SERS | 0.24–4.8 mg/kg | 0.148 mg/kg | NG |
| Thiram [32] | SERS | PDA@Au nanowaxberry | SERS | NG | 0.31 μg/g | NG |
| Thiram [34] | SERS | Au nanospikes on magnetic microparticles | SERS | 10−5–10−8 M | 10 pM | NG |
| Thiram [35] | SERS | AuNPs | SERS | 0.1–12 μg/g | 50 ng/g | 91.76–112.3% |
| Thiram [13] | SERS | TSNP | SERS | 0.12–4.8 μg/g | 90 ng/g | 93–111.75% |
| Thiram [22] | Colorimetric | AuNPs | Competitive reaction between thiram and Ag+ | 0.05–2.0 µM | 0.04 μM | 80–90% |
| Thiram [23] | Colorimetric | TSNPs | Ag–S bonds | 0.025–0.35 μM | 19.7 nM | 94.7–97.5% |
| Thiram [52] | Absorption spectra | Pt/Co3O4 nanoflowers | Oxidase-like activity | 0.6–250 µM | 0.065 µM | 95.33–101.60% |
| Ziram [125] | Absorption spectra | AuNPs | Ziram influenced the formation of AuNPs | 0.12–2.52 ng/mL | 0.06 ng/mL | 95.1–103.9% |
| Carbendazim [127] | CV | AuNPs | Electrocatalytic | 0.05–25 μM | 2.9 nM | 100.1–103.3% |
| Carbendazim [47] | Amperometric response | Pd NPs | Electrocatalytic | 0.02–35 μM | 3 nM | 99.7–108.1% |
| Carbendazim [129] | Adsorptive Stripping DPV | GNs | Electrocatalytic | 8.36 nM–4.13 μM | 3.14 nM | 98.33–99.70% |
| Carbendazim [126] | CVs | Carbon nanofiber Cu NPs | Electrocatalytic | 0.8–277.0 µM | 28 nM | 97–99.5% |
| Carbendazim [128] | SWV | GO/g-C3N4 nanohybrids | Electrocatalytic | 1.0 × 10−8–2.5 × 10−4 M | 2.82 nM | 97.85–98.2% |
| Cymoxanil [38] | Ratiometric colorimetry | AgNPs | Electrostatic attraction hydrogen bonding | 0.01–0.8 μΜ | 3 nM | NG |
| Cymoxanil [38] | Ratiometric FL | AgNPs | Electrostatic attraction hydrogen bonding | 0–0.15 μg/mL | 2 nM | 97–105% |
| Chlorothalonil [133] | Ratiometric fluorescent | AuNPs CdTe QDs | Electrostatic attraction | 0.34–2320 ng/mL | 0.34–2320 ng/mL | 91.8–104.4% |
| Tebuconazole [130] | Colorimetric assay | AgNPs | Aptamers | 25–250 nM | 10 nM | 89.90–110.86% |
| Diniconazole [131] | Fluorescence | CdTe/CdS QDs | MIP | 20–160 µg/L | 6.4 µg/L | 95.6–105.5% |
| Chloroneb [132] | DPV | CoS NPs attached ZnS rods | MIP | 0.003–0.2 μM 0.2–3.2 μM | 0.87 nM | 95.7–101.2% |
| Chlorantraniliprole [134] | DPV | Carbon nanotube with thiophene-ferrocene moieties. | NG | 0.01–7.00 μM | 8.1 nM | 102.4–104.8% |
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetycholine |
| AChE | Acetylcholinesterase |
| AgNPs | Silver/Ag nanoparticles |
| AP-algae | Alkaline phosphatase |
| ATC | Acetylthiocholine |
| AuNRs | Gold/Au nanorods |
| AuNPs | Gold/Au nanoparticles |
| BNQDs | Boron nitride quantum dots |
| BSA | Bovine Serum Albumin |
| CA | Chronoamperometry |
| CDs | Carbon dots |
| CL | Chemiluminescence |
| DEHA | Deethylhydroxyatrazine |
| CNPs | Carbon nanoparticles |
| DPAdSV | Differential pulse adsorptive stripping voltammetric |
| DPAnSV | Differential Pulse Anodic Stripping Voltammetry |
| DPV | Differential pulse voltammetry |
| ECL | Electrochemiluminescence |
| Fe3O4NPs | iron oxide nanoparticles |
| FRET | Fluorescence resonance energy transfer |
| g-C3N4 | Graphitic carbon nitride |
| GdNbO4 | Gadolinium niobate |
| GNS | Graphene nanosheets |
| GO | Graphene oxide |
| HAS | Human serum albumin |
| HNC | Halloysite nanoclay |
| IFE | Inner filter effect |
| IPM | Integrated pest management |
| ISFET | Ion sensitive field effect transistor |
| LOD | Limit of detection |
| LSPR | Localized surface plasmon resonance |
| mAb | Monoclonal antibody |
| MIPs | Molecularly imprinted polymers |
| MNPs | Magnetic nanoparticles |
| MPS | 3-(methacryloxyl) propyl trimethoxysilane |
| MWCNTs | Multiwalled carbon nanotubes |
| nAChRs | Nicotinic acetylcholine receptors |
| NC | Nanocrystalline cellulose |
| NPs | Nanoparticles |
| NVs | Nanovines |
| Ops | Organophosphorus pesticides |
| PAAM | Poly(acrylamide) |
| Pd NPs | Palladium nanoparticles |
| PDA | Polydopamine |
| PLA | Polylactic acid |
| PRM | Prometryn |
| PSA | Potentiometric stripping analysis |
| QDs | Quantum dots |
| RF-QDs | Ratiometric fluorescent quantum dots |
| RhB | Rhodamine B |
| SEF | Surface enhanced fluorescence |
| SERS | Surface enhanced Raman spectroscopy |
| SFI | Single frequency impedance |
| ssDNA | Single-strand DNA |
| SWASV | Square wave anodic stripping voltammetry |
| SWSV | Square wave stripping voltammetry |
| SWV | Square wave voltammetry |
| TMB | 3,3,5,5-Tetramethylcyclohexanone |
| TSNPs | Triangular silver nanoplates |
| UCNPs | Upconversion nanoparticles |
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/rp (accessed on 11 October 2022).
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A short history of insecticides. J. Plant Prot. Res. 2015, 55, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Tien, C.; Chen, C.S. Assessing the toxicity of organophosphorous pesticides to indigenous algae with implication for their ecotoxicological impact to aquatic ecosystems. J. Environ. Sci. Health Part B 2012, 47, 901–912. [Google Scholar] [CrossRef]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-De Córdova, M.L.; Ruiz-Medina, A. Trends in flow-based analytical methods applied to pesticide detection: A review. Anal. Chim. Acta 2011, 684, 30–39. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, M.; Sun, X.; Cai, D.; Wu, Z. Controlling pesticide loss through nanonetworks. ACS Sustain. Chem. Eng. 2014, 2, 918–924. [Google Scholar] [CrossRef]
- Budd, R.; Wang, D.; Ensminger, M.; Phillips, B. An evaluation of temporal and spatial trends of pyrethroid concentrations in california surface waters. Sci. Total Environ. 2020, 718, 137402. [Google Scholar] [CrossRef]
- Nir, S.; Undabeytia, T.; Yaron-Marcovich, D.; El-Nahhal, Y.; Polubesova, T.; Serban, C.; Rytwo, G.; Lagaly, G.; Rubin, B. Optimization of adsorption of hydrophobic herbicides on montmorillonite preadsorbed by monovalent organic cations: Interaction between phenyl rings. Environ. Sci. Technol. 2000, 34, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide pollution in agricultural soils and sustainable remediation methods: A review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, J.; Li, J.; Zhao, J. Small and sharp triangular silver nanoplates synthesized utilizing tiny triangular nuclei and their excellent sers activity for selective detection of thiram residue in soil. ACS Appl. Mater. Interfaces 2017, 9, 17387–17398. [Google Scholar] [CrossRef] [PubMed]
- Pestovsky, Y.S.; Martínez-Antonio, A. The use of nanoparticles and nanoformulations in agriculture. J. Nanosci. Nanotechno. 2017, 17, 8699–8730. [Google Scholar] [CrossRef]
- Cho, W.J.; Kim, Y.; Kim, J.K. Ultrahigh-density array of silver nanoclusters for sers substrate with high sensitivity and excellent reproducibility. ACS Nano 2012, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Li, H.; Vuki, M.; Xu, D.; Chen, H. A novel aptasensor based on silver nanoparticle enhanced fluorescence. Biosens. Bioelectron. 2012, 32, 76–81. [Google Scholar] [CrossRef]
- Wang, L.; Cai, J.; Wang, Y.; Fang, Q.; Wang, S.; Cheng, Q.; Du, D.; Lin, Y.; Liu, F. A bare-eye-based lateral flow immunoassay based on the use of gold nanoparticles for simultaneous detection of three pesticides. Microchim. Acta 2014, 181, 1565–1572. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Dong, J.; Bei, F.; Ai, S. Lanthanum-functionalized gold nanoparticles for coordination-bonding recognition and colorimetric detection of methyl parathion with high sensitivity. Sens. Actuators B Chem. 2014, 204, 119–124. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, G.; Liu, M.; Fan, L.; Cao, T. Aptamer-based colorimetric sensing of acetamiprid in soil samples: Sensitivity, selectivity and mechanism. J. Hazard. Mater. 2013, 260, 754–761. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, Y.; Xiu, F.; Hou, J. An aptamer-based colorimetric sensing of acetamiprid in environmental samples: Convenience, sensitivity and practicability. Sens. Actuators B Chem. 2020, 304, 127359. [Google Scholar] [CrossRef]
- Liu, K.; Jin, Y.; Wu, Y.; Liang, J. Simple and rapid colorimetric visualization of tetramethylthiuram disulfide (thiram) sensing based on anti-aggregation of gold nanoparticles. Food Chem. 2022, 384, 132223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, X.; Yu, F.; Liu, Y.; Yue, Q.; Yang, P.; Liu, Y. Antagonistic action regulated anti-etching colorimetric detection of thiram residue in soil based on triangular silver nanoplates. Sens. Actuators B Chem. 2021, 344, 130304. [Google Scholar] [CrossRef]
- Perez-Mayen, L.; Oliva, J.; Salas, P.; De la Rosa, E. Nanomolar detection of glucose using SERS substrates fabricated with albumin coated gold nanoparticles. Nanoscale 2016, 8, 11862–11869. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiao, S.; Dong, T.; Nie, P. Gold nanoparticles with different particle sizes for the quantitative determination of chlorpyrifos residues in soil by SERS. Int. J. Mol. Sci. 2019, 20, 2817. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Xiao, S.; Dong, T.; Nie, P. Gold nanoparticles for qualitative detection of deltamethrin and carbofuran residues in soil by surface enhanced Raman scattering (SERS). Int. J. Mol. Sci. 2019, 20, 1731. [Google Scholar] [CrossRef] [Green Version]
- Rubira, R.J.G.; Camacho, S.A.; Constantino, C.J.L.; Sanchez Cortes, S. Increasing the sensitivity of surface-enhanced Raman scattering detection for s-triazine pesticides by taking advantage of interactions with soil humic substances. J. Raman Spectrosc. 2022, 53, 40–48. [Google Scholar] [CrossRef]
- Zanasi, G.; Rubira, R.J.G.; Francioso, O.; Cañamares, M.V.; Constantino, C.J.L.; Sanchez-Cortes, S. Sensing atrazine herbicide degradation products through their interactions with humic substances by surface-enhanced Raman scattering. Chemosensors 2021, 9, 148. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, C.; Hu, X.; Xu, Q.; Zhao, H.; Meng, G.; Lei, Y. Highly sensitive surface-enhanced Raman scattering detection of organic pesticides based on Ag-nanoplate decorated graphene-sheets. Appl. Surf. Sci. 2019, 486, 405–410. [Google Scholar] [CrossRef]
- Gong, T.; Huang, Y.; Wei, Z.; Huang, W.; Wei, X.; Zhang, X. Magnetic assembled 3d SERS substrate for sensitive detection of pesticide residue in soil. Nanotechnology 2020, 31, 205501. [Google Scholar] [CrossRef]
- Li, X.; Lin, X.; Lin, S.; Zhou, S.; Fang, G.; Zhao, H.; Wang, L.; Cong, S. From dilute to multiple layers: Bottom-up self-assembly of rough gold nanorods as SERS platform for quantitative detection of thiram in soil. Adv. Mater. Interfaces 2021, 8, 2100412. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, X.; Huang, J.; Wang, G.; Zhao, Y.; Chen, F.; Wei, J.; Song, Z.; Zhao, Y. Polydopamine@gold nanowaxberry enabling improved SERS sensing of pesticides, pollutants, and explosives in complex samples. Anal. Chem. 2018, 90, 9048–9054. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Cui, Q.; Li, S.; Tian, Y.; Ma, H.; Wang, C.; Zhang, M.; Chen, M.; Li, G.; Liu, X. Double profound enhancements of Cu2O nano-octahedrons connected by intertwined Ag nanovines for elevating SERS activity toward ultrasensitive pesticide detection. Opt. Express 2022, 30, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Wang, Y.; Zhou, S.; Yang, S.; Wang, Y. Seed/ligand-cooperative growth of dense Au nanospikes on magnetic microparticles for SERS applications. J. Mater. Chem. C 2022, 10, 3368–3374. [Google Scholar] [CrossRef]
- Chen, M.; Luo, W.; Liu, Q.; Hao, N.; Zhu, Y.; Liu, M.; Wang, L.; Yang, H.; Chen, X. Simultaneous in situ extraction and fabrication of surface-enhanced Rman scattering substrate for reliable detection of thiram residue. Anal. Chem. 2018, 90, 13647–13654. [Google Scholar] [CrossRef]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Li, Y.; Su, X. Visual and fluorescent detection of acetamiprid based on the inner filter effect of gold nanoparticles on ratiometric fluorescence quantum dots. Anal. Chim. Acta 2014, 852, 189–195. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, H.; Sun, Y.; Gui, R. Colorimetric and fluorometric dual-channel ratiometric determination of fungicide cymoxanil based on analyte-induced aggregation of silver nanoparticles and dually emitting carbon dots. Microchim. Acta 2019, 186, 580. [Google Scholar] [CrossRef]
- Jia, D.; Ma, D.; Du, X.; An, L. Highly sensitive detection of malathion based on fret between Au/Fe3O4 and rhodamine B. Bull. Korean Chem. Soc. 2019, 40, 812–818. [Google Scholar] [CrossRef]
- Dou, X.; Chu, X.; Kong, W.; Luo, J.; Yang, M. A gold-based nanobeacon probe for fluorescence sensing of organophosphorus pesticides. Anal. Chim. Acta 2015, 891, 291–297. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Qin, Y.; Cui, P.; Song, G.; Hua, X.; Wang, L.; Wang, M. Inner filter effect-based immunoassay for the detection of acetamiprid using upconversion nanoparticles and gold nanoparticles. Food Agric. Immunol. 2021, 32, 740–753. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Prasad, S.N.; Bansal, V.; Ramanathan, R. Detection of pesticides using nanozymes: Trends, challenges and outlook. TrAC Trends Anal. Chem. 2021, 144, 116429. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly sensitive electrochemical stripping analysis of methyl parathion at MWCNTs-CeO2-au nanocomposite modified electrode. Sens. Actuators B Chem. 2013, 186, 774–780. [Google Scholar] [CrossRef]
- Rahmani, T.; Hajian, A.; Afkhami, A.; Bagheri, H. A novel and high performance enzyme-less sensing layer for electrochemical detection of methyl parathion based on BSA templated Au–Ag bimetallic nanoclusters. New J. Chem. 2018, 42, 7213–7222. [Google Scholar] [CrossRef]
- Liu, D.; Wu, F. Biosynthesis of Pd nanoparticle using onion extract for electrochemical determination of carbendazim. Int. J. Electrochem. Sci. 2017, 12, 2125–2134. [Google Scholar] [CrossRef]
- Bolat, G.; Abaci, S. Non-enzymatic electrochemical sensing of malathion pesticide in tomato and apple samples based on gold nanoparticles-chitosan-ionic liquid hybrid nanocomposite. Sensors 2018, 18, 773. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, J.; Wu, Y. A simple and rapid chemosensor for colorimetric detection of dimethoate pesticide based on the peroxidase-mimicking catalytic activity of gold nanoparticles. Anal. Methods 2019, 11, 5337–5347. [Google Scholar] [CrossRef]
- Kushwaha, A.; Singh, G.; Sharma, M. Colorimetric sensing of chlorpyrifos through negative feedback inhibition of the catalytic activity of silver phosphate oxygenase nanozymes. RSC Adv. 2020, 10, 13050–13065. [Google Scholar] [CrossRef] [Green Version]
- Wannasri, N.; Uppachai, P.; Butwong, N.; Jantrasee, S.; Isa, I.M.; Loiha, S.; Srijaranai, S.; Mukdasai, S. A facile nonenzymatic electrochemical sensor based on copper oxide nanoparticles deposited on activated carbon for the highly sensitive detection of methyl parathion. J. Appl. Electrochem. 2022, 52, 595–606. [Google Scholar] [CrossRef]
- Sun, M.; Huang, S.; Su, G.; Wang, X.; Lu, Z.; Wang, Y.; Liu, T.; Jiang, Y.; Song, C.; Rao, H. Synthesis of pH-switchable Pt/Co3O4 nanoflowers: Catalytic mechanism, four-enzyme activity and smartphone biosensing applications. Chem. Eng. J. 2022, 437, 134414. [Google Scholar] [CrossRef]
- Qi, Y.; Xiu, F.; Zheng, M.; Li, B. A simple and rapid chemiluminescence aptasensor for acetamiprid in contaminated samples: Sensitivity, selectivity and mechanism. Biosens. Bioelectron. 2016, 83, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, H.; Hu, M.; Bai, M.; Guo, Y.; Sun, X. Effective electrochemiluminescence aptasensor for detection of atrazine residue. Sensors 2022, 22, 3430. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Wang, S.; Guo, Y.; Zhu, G. Development of a one-step strip for the detection of triazophos residues in environmental samples. Anal. Biochem. 2008, 377, 202–208. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An amperometric acetylcholinesterase sensor based on Fe3O4 nanoparticle/multi-walled carbon nanotube-modified ITO-coated glass plate for the detection of pesticides. Electrochim. Acta 2012, 67, 79–86. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Li, H.; Li, F. pH-response quantum dots with orange-red emission for monitoring the residue, distribution, and variation of an organophosphorus pesticide in an agricultural crop. J. Agric. Food Chem. 2021, 69, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Li, X.; Yan, Y.; Li, C.; Pan, J. A core-shell surface magnetic molecularly imprinted polymers with fluorescence for λ-cyhalothrin selective recognition. Anal. Bioanal. Chem. 2014, 406, 7213–7220. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Malik, A. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Hirsch, G.; Karna, S.P.; Srivastava, A.K. Potentiometric stripping analysis of methyl and ethyl parathion employing carbon nanoparticles and halloysite nanoclay modified carbon paste electrode. Anal. Chim. Acta 2012, 735, 37–45. [Google Scholar] [CrossRef]
- Chu, C.; Lin, P.; Li, J.; Kirankumar, R.; Tsai, C.; Chen, N.; Wen, Z.; Hsieh, S. A novel SERS substrate based on discarded oyster shells for rapid detection of organophosphorus pesticide. Coatings 2022, 12, 506. [Google Scholar] [CrossRef]
- Qian, S.; Lin, H. Colorimetric sensor array for detection and identification of organophosphorus and carbamate pesticides. Anal. Chem. 2015, 87, 5395–5400. [Google Scholar] [CrossRef] [PubMed]
- Pabbi, M.; Kaur, A.; Mittal, S.K.; Jindal, R. A surface expressed alkaline phosphatase biosensor modified with flower shaped ZnO for the detection of chlorpyrifos. Sens. Actuators B Chem. 2018, 258, 215–227. [Google Scholar] [CrossRef]
- Ehzari, H.; Safari, M.; Samimi, M.; Shamsipur, M.; Bagher Gholivand, M. A highly sensitive electrochemical biosensor for chlorpyrifos pesticide detection using the adsorbent nanomatrix contain the human serum albumin and the Pd:CdTe quantum dots. Microchem. J. 2022, 179, 107424. [Google Scholar] [CrossRef]
- Mane, P.C.; Shinde, M.D.; Varma, S.; Chaudhari, B.P.; Fatehmulla, A.; Shahabuddin, M.; Amalnerkar, D.P.; Aldhafiri, A.M.; Chaudhari, R.D. Highly sensitive label-free bio-interfacial colorimetric sensor based on silk fibroin-gold nanocomposite for facile detection of chlorpyrifos pesticide. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, N.; Thakur, H.; Bharti, A.; Kaur, N. Chitosan-iron oxide nanocomposite based electrochemical aptasensor for determination of malathion. Anal. Chim. Acta 2016, 939, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Hou, J.; Jiang, J.; Ai, S. Innovative approach for the electrochemical detection of non-electroactive organophosphorus pesticides using oxime as electroactive probe. Anal. Chim. Acta 2015, 885, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Tefera, M.; Admassie, S.; Tessema, M.; Mehretie, S. Electrochemical sensor for determination of fenitrothion at multi-wall carbon nanotubes modified glassy carbon electrode. Anal. Bioanal. Chem. Res. 2015, 2, 139–150. [Google Scholar]
- Pajooheshpour, N.; Rezaei, M.; Hajian, A.; Afkhami, A.; Sillanpää, M.; Arduini, F.; Bagheri, H. Protein templated Au-Pt nanoclusters-graphene nanoribbons as a high performance sensing layer for the electrochemical determination of diazinon. Sens. Actuators B Chem. 2018, 275, 180–189. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Sadeghzadeh, S.; Rezayi, M.; Sany, S. Diazinon electrochemical biosensor mediated by aptamer and nanoscale porous carbon derived from ZIF-8. Sens. Actuators B Chem. 2023, 381, 133424. [Google Scholar] [CrossRef]
- Cioffi, A.; Mancini, M.; Gioia, V.; Cinti, S. Office paper-based electrochemical strips for organophosphorus pesticide monitoring in agricultural soil. Environ. Sci. Technol. 2021, 55, 8859–8865. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Hermsen, A.; Lamers, D.; Schoettl, J.; Mayer, C.; Jaeger, M. In-field detection method for imidacloprid by surface enhanced Raman spectroscopy. Toxicol. Environ. Chem. 2022, 104, 36–54. [Google Scholar] [CrossRef]
- Tao, Z.; Deng, J.; Wang, Y.; Chen, H.; Ding, Y.; Hua, X.; Wang, M. Competitive immunoassay for simultaneous detection of imidacloprid and thiacloprid by upconversion nanoparticles and magnetic nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 23471–23479. [Google Scholar] [CrossRef]
- Kumar, S.; Karfa, P.; Madhuri, R.; Sharma, P.K. Designing of fluorescent and magnetic imprinted polymer for rapid, selective and sensitive detection of imidacloprid via activators regenerated by the electron transfer-atom transfer radical polymerization (ARGET-ATRP) technique. J. Phys. Chem. Solids 2018, 116, 222–233. [Google Scholar] [CrossRef]
- Xiu, F.; Lu, Y.; Qi, Y.; Wang, Y.; He, J. Ultrasensitive and practical chemiluminescence sensing pesticide residue acetamiprid in agricultural products and environment: Combination of synergistically coupled co-amplifying signal and smart interface engineering. Talanta 2021, 235, 122811. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Ding, Y.; Tao, Z.; You, H.; Hua, X.; Wang, M. Development of an upconversion fluorescence dna probe for the detection of acetamiprid by magnetic nanoparticles separation. Food Chem. 2018, 257, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hua, X.; Ma, M.; Liu, J.; Zhou, L.; Wang, M. Detecting clothianidin residues in environmental and agricultural samples using rapid, sensitive enzyme-linked immunosorbent assay and gold immunochromatographic assay. Sci. Total Environ. 2014, 499, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cao, Y.; Tian, Y.; Qi, Y.; Fang, G.; Wang, S. A molecularly imprinted fluorescence nanosensor based on upconversion metal-organic frameworks for alpha-cypermethrin specific recognition. Microchim. Acta 2020, 187, 632. [Google Scholar] [CrossRef]
- Karabiberoğlu, U.K.; Koçak, Ç.C.; Dursun, Z. Electrochemical determination of dicofol at nickel nanowire modified poly(p-aminophenol) film electrode. Electroanalysis 2019, 31, 1304–1310. [Google Scholar] [CrossRef]
- Rodrigues, G.; Miyazaki, C.M.; Rubira, R.J.G.; Constantino, C.J.L.; Ferreira, M. Layer-by-layer films of graphene nanoplatelets and gold nanoparticles for methyl parathion sensing. ACS Appl. Nano Mater. 2019, 2, 1082–1091. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, D.; Yu, Y.; Zhou, T.; Shi, G. Differential pulse voltammetric determination of methyl parathion based on multiwalled carbon nanotubes-poly(acrylamide) nanocomposite film modified electrode. J. Hazard. Mater. 2012, 217–218, 315–322. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, D.; Zhao, W.; Yang, Q.; Kajiura, H.; Li, Y.; Zhou, T.; Shi, G. A molecularly imprinted polymer based on functionalized multiwalled carbon nanotubes for the electrochemical detection of parathion-methyl. Analyst 2012, 137, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, W.; Yin, Z. Covalent fabrication of methyl parathion hydrolase on gold nanoparticles modified carbon substrates for designing a methyl parathion biosensor. Biosens. Bioelectron. 2014, 53, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, H.; Liu, Y.; Ren, C.; Chen, H. A new fluorescence probing strategy for the detection of parathion-methyl based on n-doped carbon dots and methyl parathion hydrolase. Chin. Chem. Lett. 2017, 28, 1675–1680. [Google Scholar] [CrossRef]
- Tümay, S.O.; Şenocak, A.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. A new perspective for electrochemical determination of parathion and chlorantraniliprole pesticides via carbon nanotube-based thiophene-ferrocene appended hybrid nanosensor. Sens. Actuators B Chem. 2021, 345, 130344. [Google Scholar] [CrossRef]
- Sreedhar, N.Y.; Sunil Kumar, M.; Krishnaveni, K. Sensitive determination of chlorpyrifos using ag/cu alloy nanoparticles and graphene composite paste electrode. Sens. Actuators B Chem. 2015, 210, 475–482. [Google Scholar] [CrossRef]
- Srinivasan, S.; Nesakumar, N.; Rayappan, J.B.B.; Kulandaiswamy, A.J. Electrochemical detection of imidacloprid using cu-rgo composite nanofibers modified glassy carbon electrode. Bull. Environ. Contam. Tox. 2020, 104, 449–454. [Google Scholar] [CrossRef]
- Bruzaca, E.E.S.; de Oliveira, R.C.; Duarte, M.S.S.; Sousa, C.P.; Morais, S.; Correia, A.N.; de Lima-Neto, P. Electrochemical sensor based on multi-walled carbon nanotubes for imidacloprid determination. Anal. Methods 2021, 13, 2124–2136. [Google Scholar] [CrossRef]
- Zhang, D.; Lang, X.; Hui, N.; Wang, J. Dual-mode electrochemical biosensors based on chondroitin sulfate functionalized polypyrrole nanowires for ultrafast and ultratrace detection of acetamiprid pesticide. Microchem. J. 2022, 179, 107530. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Adriano, A.M.; Rubira, R.J.G.; Constantino, C.J.L.; Ferreira, M. Combining electrochemically reduced graphene oxide and layer-by-layer films of magnetite nanoparticles for carbofuran detection. J. Environ. Chem. Eng. 2020, 8, 104294. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Nanomaterial-sensors for herbicides detection using electrochemical techniques and prospect applications. TrAC Trends Anal. Chem. 2021, 135, 116178. [Google Scholar] [CrossRef]
- Butmee, P.; Tumcharern, G.; Songsiriritthigul, C.; Durand, M.J.; Thouand, G.; Kerr, M.; Kalcher, K.; Samphao, A. Enzymatic electrochemical biosensor for glyphosate detection based on acid phosphatase inhibition. Anal. Bioanal. Chem. 2021, 413, 5859–5869. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. Doubly imprinted polymer nanofilm-modified electrochemical sensor for ultra-trace simultaneous analysis of glyphosate and glufosinate. Biosens. Bioelectron. 2014, 59, 81–88. [Google Scholar] [CrossRef]
- Wang, D.; Lin, B.; Cao, Y.; Guo, M.; Yu, Y. A highly selective and sensitive fluorescence detection method of glyphosate based on an immune reaction strategy of carbon dot labeled antibody and antigen magnetic beads. J. Agric. Food Chem. 2016, 64, 6042–6050. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Li, Y.; Yang, H.; Hu, P.; Lu, S.; Ren, H.; Liu, Z.; Soo Park, K.; Zhou, Y. Dual-functionalized gold nanoparticles probe based bio-barcode immuno-PCR for the detection of glyphosate. Food Chem. 2021, 338, 128133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shu, Y.; Li, J.; Liang, A.; Jiang, Z. Silver nanosol RRS aptamer assay of trace glyphosate based on gold-doped polystyrene nanocatalytic amplification. Microchem. J. 2022, 176, 107252. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, R.; Yu, B.; Liang, A.; Jiang, Z. A highly sensitive gold nanosol SERS aptamer assay for glyphosate with a new cof nanocatalytic reaction of glycol-Au(iii). Sens. Actuators B Chem. 2021, 344, 130288. [Google Scholar] [CrossRef]
- Muñoz, R.; Guevara-Lara, A.; Santos, J.L.M.; Miranda, J.M.; Rodriguez, J.A. Determination of glyphosate in soil samples using CdTe/Cds quantum dots in capillary electrophoresis. Microchem. J. 2019, 146, 582–587. [Google Scholar] [CrossRef]
- Rajaram, R.; Gurusamy, T.; Ramanujam, K.; Neelakantan, L. Electrochemical determination of paraquat using gold nanoparticle incorporated multiwalled carbon nanotubes. J. Electrochem. Soc. 2022, 169, 47522. [Google Scholar] [CrossRef]
- Pourakbari, Z.; Sheykhan, M.; Aliakbar, A. A new poly carboxylic catex polymer-gold nanoparticles modified electrode for determination of paraquat by voltammetry method. J. Environ. Chem. Eng. 2020, 8, 104284. [Google Scholar] [CrossRef]
- Niu, L.M.; Liu, F.; Wang, W.; Lian, K.Q.; Ma, L.; Shi, H.M.; Kang, W.J. Electrochemical behavior of paraquat on a highly ordered biosensor based on an unmodified dna-3d gold nanoparticle composite and its application. Electrochim. Acta 2015, 153, 190–199. [Google Scholar] [CrossRef]
- Botta, R.; Eiamchai, P.; Horprathum, M.; Limwichean, S.; Chananonnawathorn, C.; Patthanasettakul, V.; Maezono, R.; Jomphoak, A.; Nuntawong, N. 3d structured laser engraves decorated with gold nanoparticle sers chips for paraquat herbicide detection in environments. Sens. Actuators B Chem. 2020, 304, 127327. [Google Scholar] [CrossRef]
- Ali, S.; Shah, M.R.; Hussain, S.; Khan, S.; Latif, A.; Ahmad, M.; Ali, M. A facile approach based on functionalized silver nanoparticles as a chemosensor for the detection of paraquat. J. Clust. Sci. 2022, 33, 413–420. [Google Scholar] [CrossRef]
- Siangproh, W.; Somboonsuk, T.; Chailapakul, O.; Songsrirote, K. Novel colorimetric assay for paraquat detection on-silica bead using negatively charged silver nanoparticles. Talanta 2017, 174, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Q.; Patterson, E.; Sayer, C.; Powles, S. Dinitroaniline herbicide resistance and mechanisms in weeds. Front. Plant Sci. 2021, 12, 634018. [Google Scholar] [CrossRef] [PubMed]
- Shokri, R.; Amjadi, M.; Manzoori, J.L. A chemiluminescent probe for highly sensitive detection of trifluralin based on cobalt ion-complexed boron nitride quantum dots as efficient nanocatalysts. Microchem. J. 2022, 181, 107759. [Google Scholar] [CrossRef]
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green synthesis of fluorescent carbon dots from cherry tomatoes for highly effective detection of trifluralin herbicide in soil samples. ChemistrySelect 2020, 5, 1956–1960. [Google Scholar] [CrossRef]
- Gogoi, J.; Chowdhury, D. Calcium-modified carbon dots derived from polyethylene glycol: Fluorescence-based detection of trifluralin herbicide. J. Mater. Sci. 2020, 55, 11597–11608. [Google Scholar] [CrossRef]
- Mirabi-Semnakolaii, A.; Daneshgar, P.; Moosavi-Movahedi, A.A.; Rezayat, M.; Norouzi, P.; Nemati, A.; Farhadi, M. Sensitive determination of herbicide trifluralin on the surface of copper nanowire electrochemical sensor. J. Solid State Electr. 2011, 15, 1953–1961. [Google Scholar] [CrossRef]
- Haghighi, M.; Irandoust, M.; Shariati-Rad, M. Simultaneous determination of antinonin and trifluralin by electrochemical method and net analyte signal interferent modeling. Microchem. J. 2019, 146, 34–40. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Moharramnezhad, M. A promising electrochemiluminescence herbicide sensor based on ternary nanocomposite and boron nitride quantum dots for trace analysis of tribenuron-methyl in environmental samples. Microchem. J. 2021, 168, 106518. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Niu, Y.; Li, S.; Luo, R. Electrochemical sensor based on molecularly imprinted composite membrane of poly(o-aminothiophenol) with gold nanoparticles for sensitive determination of herbicide simazine in environmental samples. Sens. Actuators B Chem. 2017, 249, 747–755. [Google Scholar] [CrossRef]
- Serge, M.; Karangayssouf, B.R.; Issa, T.; Fadilatou, S.; Koulibalybazoumana, S.I.; Ignas, T.; Emmanuel, N. Electrochemical determination of diuron in soil using a nanocrystalline cellulose modified carbon paste electrode. Int. J. Electrochem. Sci. 2021, 16, 210552. [Google Scholar] [CrossRef]
- Figueiredo-Filho, L.C.S.; Sartori, E.R.; Fatibello-Filho, O. Electroanalytical determination of the linuron herbicide using a cathodically pretreated boron-doped diamond electrode: Comparison with a boron-doped diamond electrode modified with platinum nanoparticles. Anal. Methods 2015, 7, 643–649. [Google Scholar] [CrossRef]
- Prabhu, K.; Malode, S.J.; Shetti, N.P.; Kulkarni, R.M. Analysis of herbicide and its applications through a sensitive electrochemical technique based on MWCNTs/ZnO/CPE fabricated sensor. Chemosphere 2022, 287, 132086. [Google Scholar] [CrossRef] [PubMed]
- Gopi, P.K.; Mutharani, B.; Chen, S.; Chen, T.; Eldesoky, G.E.; Ali, M.A.; Wabaidur, S.M.; Shaik, F.; Tzu, C.Y. Electrochemical sensing base for hazardous herbicide aclonifen using gadolinium niobate (GdNbO4) nanoparticles-actual river water and soil sample analysis. Ecotox. Environ. Saf. 2021, 207, 111285. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Vernekar, P.R.; Nayak, D.S.; Shetty, N.S.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Electro-sensing base for herbicide aclonifen at graphitic carbon nitride modified carbon electrode—Water and soil sample analysis. Microchem. J. 2019, 149, 103976. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Luo, Y.; Shen, F.; Sun, C. Efficient fluorescence resonance energy transfer between oppositely charged CdTe quantum dots and gold nanoparticles for turn-on fluorescence detection of glyphosate. Talanta 2014, 125, 385–392. [Google Scholar] [CrossRef]
- Butmee, P.; Samphao, A.; Tumcharern, G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. [Google Scholar] [CrossRef]
- Habekost, A. Rapid and sensitive spectroelectrochemical and electrochemical detection of glyphosate and AMPA with screen-printed electrodes. Talanta 2017, 162, 583–588. [Google Scholar] [CrossRef]
- Wen, X.; Fei, J.; Chen, X.; Yi, L.; Ge, F.; Huang, M. Electrochemical analysis of trifluralin using a nanostructuring electrode with multi-walled carbon nanotubes. Environ. Pollut. 2008, 156, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Bruhl, C.A.; Imfeld, G.; Knabel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmuller, A. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lin, S.; Liu, Y.; Zhao, H.; Liu, B.; Wang, L. Lab-on-paper surface-enhanced Raman spectroscopy platform based on self-assembled Au@Ag nanocube monolayer for on-site detection of thiram in soil. J. Raman Spectrosc. 2019, 50, 916–925. [Google Scholar] [CrossRef]
- Hashemi, F.; Rastegarzadeh, S.; Pourreza, N. A combination of dispersive liquid-liquid microextraction and surface plasmon resonance sensing of gold nanoparticles for the determination of ziram pesticide. J. Sep. Sci. 2017, 41, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, P.; Fu, C.; Liu, S.; Juang, R. Facile synthesis of chitosan-carbon nanofiber composite supported copper nanoparticles for electrochemical sensing of carbendazim. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126934. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Biosynthesis of gold nanoparticles using green alga pithophora oedogonia with their electrochemical performance for determining carbendazim in soil. Int. J. Electrochem. Sci. 2016, 11, 4550–4559. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Foucaud, Y.; Badawi, M.; Aminabhavi, T.M. Graphene/g-carbon nitride (Go/G-C3N4) nanohybrids as a sensor material for the detection of methyl parathion and carbendazim. Chemosphere 2022, 292, 133450. [Google Scholar] [CrossRef]
- Khare, N.G.; Dar, R.A.; Srivastava, A.K. Determination of carbendazim by adsorptive stripping differential pulse voltammetry employing glassy carbon paste electrode modified with graphene and amberlite XAD 2 resin. Electroanalysis 2015, 27, 1915–1924. [Google Scholar] [CrossRef]
- Truong, P.L.; Duyen, V.T.C.; Toi, V.V.; Hien, M.D. Rapid detection of tebuconazole based on aptasensor and aggregation of silver nanoparticles. J. Nanomater. 2021, 2021, 5532477. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. Molecularly imprinted mesoporous silica embedded with carbon dots and semiconductor quantum dots as a ratiometric fluorescent sensor for diniconazole. Biosens. Bioelectron. 2017, 96, 121–126. [Google Scholar] [CrossRef]
- Duan, D.; Ye, J.; Cai, X.; Li, K. Cobalt(II)-ion-exchanged Zn-bio-MOF-1 derived CoS/ZnS composites modified electrochemical sensor for chloroneb detection by differential pulse voltammetry. Microchim. Acta 2021, 188, 111. [Google Scholar] [CrossRef] [PubMed]
- Sheng, E.; Lu, Y.; Tan, Y.; Xiao, Y.; Li, Z.; Dai, Z. Ratiometric fluorescent quantum dot-based biosensor for chlorothalonil detection via an inner-filter effect. Anal. Chem. 2020, 92, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Dong, T.; Xiao, S.; Lin, L.; He, Y.; Qu, F. Quantitative determination of thiabendazole in soil extracts by surface-enhanced Raman spectroscopy. Molecules 2018, 23, 1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somasundaram, L.; Coats, J.R.; Racke, K.D.; Stahr, H.M. Application of the microtox system to assess the toxicity of pesticides and their hydrolysis metabolites. Bull. Environ. Contam. Tox. 1990, 44, 254–259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Qiu, M.; Wang, J.; Liu, Y. Recent Advances in Nanoparticle-Based Optical Sensors for Detection of Pesticide Residues in Soil. Biosensors 2023, 13, 415. https://doi.org/10.3390/bios13040415
Zhang C, Qiu M, Wang J, Liu Y. Recent Advances in Nanoparticle-Based Optical Sensors for Detection of Pesticide Residues in Soil. Biosensors. 2023; 13(4):415. https://doi.org/10.3390/bios13040415
Chicago/Turabian StyleZhang, Chunhong, Mingle Qiu, Jinglin Wang, and Yongchun Liu. 2023. "Recent Advances in Nanoparticle-Based Optical Sensors for Detection of Pesticide Residues in Soil" Biosensors 13, no. 4: 415. https://doi.org/10.3390/bios13040415
APA StyleZhang, C., Qiu, M., Wang, J., & Liu, Y. (2023). Recent Advances in Nanoparticle-Based Optical Sensors for Detection of Pesticide Residues in Soil. Biosensors, 13(4), 415. https://doi.org/10.3390/bios13040415





