Thalassemia and Nanotheragnostics: Advanced Approaches for Diagnosis and Treatment
Abstract
:1. Hallmarks of Thalassemia
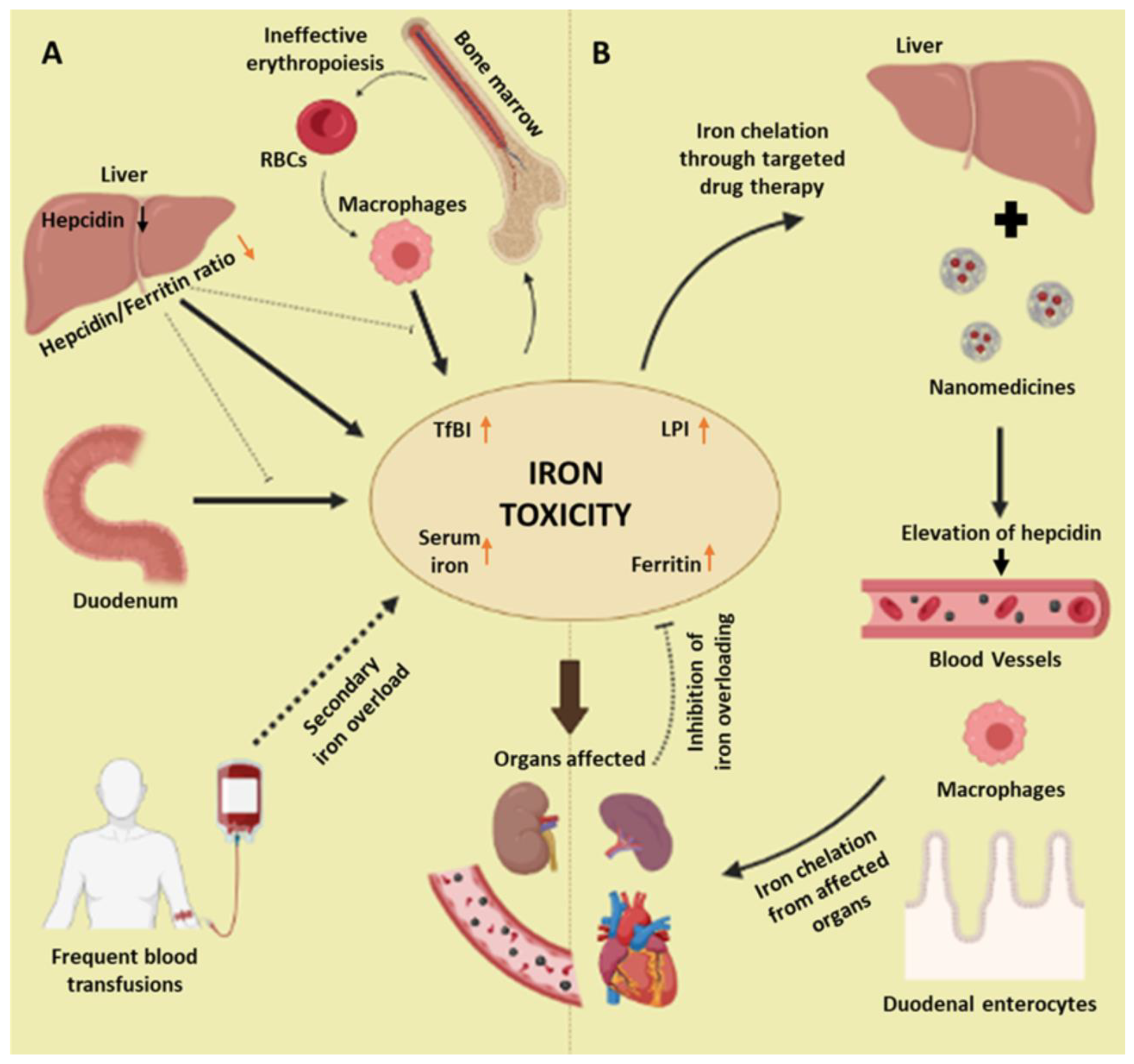
Pathophysiological Pathways
2. Conventional Therapeutic Approaches
2.1. The Complexity of Thalassemia Detection
2.2. Treatments and Complications of Thalassemia
3. Biomedical Landscape of Nanotechnology
4. Nanobiosensor for the Diagnosis of Thalassemia
4.1. Carbon-Based Biosensors
4.2. Quantum Dot-Based Diagnosis
4.3. Metal Nanoparticles-Based Diagnosis
4.3.1. Gold Nanoparticles-Based Diagnosis
4.3.2. Silver Nanoparticles-Based Diagnosis
4.4. Other Nano-Diagnostic Approaches
| Nanoparticles | Modification | Application | Method | Detection Limit | Reference |
|---|---|---|---|---|---|
| Carbon | Co3O4 doped carbon nanofiber (CNF) composite modified on carbon ionic liquid electrode (CILE) | Detection of electrochemical, conformational, and structural alterations of hemoglobin | Immobilization of hemoglobin on the biosensor | 0.1 mmol L−1 | [75] |
| Carbon paste-based electrode modified with 1-ethyl-3-methylimidazolium chloride and CdO-nanoparticle | Determination of deferoxamine and vitamin C in the thalassemic patients | Multivariate curve resolution alternating least (MCR-ALS) algorithm | 0.030 μM | [76] | |
| Graphene | Palladium–graphene (Pd–GR) nanocomposite-modified carbon ionic liquid electrode | Fabrication of third-generation electrochemical biosensor for the detection of hemoglobin | Fixing Hb to Pd–GR nanocomposite | 0.35 mmol L−1 | [78] |
| 3D porous hybrid of graphitic C3N4 nanoparticle decorated in the assembly of graphene and Co2Al layered double hydroxide nanosheets | Production of third-generation biosensor | X-ray diffraction, electron microscopy, X-ray photoelectron spectroscopy | 0.05 mM | [79] | |
| Graphene oxide-tellurium nanowires (TeNWs/GO) | Quantitative determination of hemoglobin β-thalassemia major patients | Detection of electrical response by redox reaction due to electrical stimulus to the biochemical system | 0.29 μM | [70] | |
| Quantum Dot | Coupling of quantum dots with magnetic nanoparticle-based probes | Detection of point mutation in the human beta-globin gene (IVS-II-I G → A point mutation) | Ligase reaction proceeded with the allele-specific probes | - | [81,82] |
| CdS/TiO2 nanocomposite-based molecularly imprinted photo-electrochemical sensor | Detection of hemoglobin under visible light irradiation | Principle of decrease in the photocurrent in the case of attachment of hemoglobin to the sensor | 0.53 pg/mL | [83] | |
| Gold | Calorimetric nanogold probe | Genotyping of subgroups of α-thalassemia 1 and α-thalassemia 2 | Two-step hybridization of target DNA with nanogold mixed probes and nanogold single probes | - | [85] |
| Gold nanoparticle-based systems integrated with nanocrystalline silicon device | Detection of mutation in the b-globin gene | Non-crosslinking hybridization | - | [86] | |
| Nanogold-based universal array | Detection of point mutations from fetal DNA in maternal plasma samples | PCR and ligase detection reaction | - | [87] | |
| Thiol-tagged oligonucleotide probes on the Au nanoparticles- (AuNPs-PAT/rGO/GCE) | Detection of β-thalassemia gene | Hybridization of oligonucleotide with the target sequence | - | [88] | |
| ferrocenoyl cysteine conjugates adsorbed onto gold nanoparticles | Quantitative analysis of hemoglobin | Adsorption | 0.03 μg/mL | [89] | |
| Piezoelectric biosensors based on gold electrodes | Detection of β-thalassemia mutation C→T substitution in the codon 39 of the HBB gene | Immobilization of oligonucleotide probes on the electrodes | - | [90] | |
| Silver | Molecularly imprinted polymers modified by Ag nanoparticles (NPs)/PbTiO3 electrodes | Detection and quantification of hemoglobin | - | 0.23 pM | [91] |
| Silver electrode coupled with Quartz Crystal Microbalance (QCM) | Identification of thalassemia gene mutations | Immobilization of biotinylated probe on the QCM surface using silver electrode | 0.5 μmol/L | [92] | |
| Dendrimer | Dendrimer probe (G3SG) intercalated with electrospun nanofibers | Detection of β-thalassemia gene fragments | Amplified fluorescent sensing | 20 pM | [93] |
| Mesopores | Mesoporous silica nanoparticles (MCM-41) loaded with reporter fluorescein molecules | Detect of thalassemia causing mutation IVS110 (A > G reversion) | Genotyping assay | - | [94] |
| Nickel | NiTe nanorods | Electrochemical analysis of hemoglobin in thalassemic patients | Non-enzymatic sensor-based quantification | 0.012 nM | [95] |
| Cryogel | Molecularly-imprinted cryogel based on lanthanide-chelate | Diagnosis of thalassemia | Cryopolymerization techniques for selective separation of hemoglobin from serum | - | [96] |
5. Nanotechnology for the Treatment of Complications of Thalassemia
| Nanoparticles | Use | Reference |
|---|---|---|
| Enzyme-integrated polymeric membrane | Production of artificial hemoglobin | [110] |
| Deferoxamine-loaded polymeric nanoparticles | Targeted drug delivery | [124] |
| Mithramycin-encoded polymeric micelles (PM-MTH) | Upregulation of γ-globin expression to increase HbF content | [129] |
| Nickel–zinc–iron oxide | Decrease the hemolysis in thalassemia patients | [119] |
| Lipid nanoparticle (LNP)-formulated small interfering RNAs (siRNAs), TLc-A based nanochelator, graphene oxide, MC-AgNPs, cryogel, mesoporous silica nanoparticles, polyrotaxane-based nanochelator | Removal of excess iron from the plasma of β–thalassemia patients | [111,112,113,114,115,116,117,118,120,121,122,123] |
| ZnO nanocrystals–methylene blue nanocomposites, pyro-AgNPs Au nanorods, HRP–AuNP–CaCO3 composites | In vitro sensing of anti-thalassemic iron chelating drug i.e., deferiprone | [125,126,127,128] |
| Supramolecular nano substrate–mediated delivery (SNSMD) | Knockout of the defective HBB gene using CRISPR/Cas9 | [131] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brancaleoni, V.; Di Pierro, E.; Motta, I.; Cappellini, M. Laboratory diagnosis of thalassemia. Int. J. Lab. Hematol. 2016, 38, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Thompson, A.A. Thalassemias. Pediatr. Clin. 2013, 60, 1383–1391. [Google Scholar] [CrossRef]
- Bajwa, H.; Basit, H. Thalassemia; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Huang, H.; Xu, L.; Chen, M.; Lin, N.; Xue, H.; Chen, L.; Wang, Y.; He, D.; Zhang, M.; Lin, Y. Molecular characterization of thalassemia and hemoglobinopathy in Southeastern China. Sci. Rep. 2019, 9, 3493. [Google Scholar] [CrossRef] [Green Version]
- Aydinok, Y. Thalassemia. Hematology 2012, 17 (Suppl. S1), s28–s31. [Google Scholar] [CrossRef]
- El-Beshlawy, A.; El-Ghamrawy, M. Recent trends in treatment of thalassemia. Blood Cells Mol. Dis. 2019, 76, 53–58. [Google Scholar] [CrossRef]
- Tari, K.; Valizadeh Ardalan, P.; Abbaszadehdibavar, M.; Atashi, A.; Jalili, A.; Gheidishahran, M. Thalassemia an update: Molecular basis, clinical features and treatment. Int. J. Biomed. Public Health 2018, 1, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Needs, T.; Gonzalez-Mosquera, L.F.; Lynch, D.T. Beta Thalassemia; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Hardison, R.C. Evolution of hemoglobin and its genes. Cold Spring Harb. Perspect. Med. 2012, 2, a011627. [Google Scholar] [CrossRef] [Green Version]
- Farid, Y.; Bowman, N.S.; Lecat, P. Biochemistry, Hemoglobin Synthesis; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Amjad, F.; Fatima, T.; Fayyaz, T.; Khan, M.A.; Qadeer, M.I. Novel genetic therapeutic approaches for modulating the severity of β-thalassemia. Biomed. Rep. 2020, 13, 1. [Google Scholar] [CrossRef]
- Munkongdee, T.; Chen, P.; Winichagoon, P.; Fucharoen, S.; Paiboonsukwong, K. Update in Laboratory Diagnosis of Thalassemia. Front. Mol. Biosci. 2020, 7, 74. [Google Scholar] [CrossRef]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Tamary, H.; Dgany, O. Alpha-Thalassemia; University of Washington: Seattle, WA, USA, 2020. [Google Scholar]
- Farashi, S.; Harteveld, C.L. Molecular basis of α-thalassemia. Blood Cells Mol. Dis. 2018, 70, 43–53. [Google Scholar] [CrossRef]
- Higgs, D.R. The molecular basis of α-thalassemia. Cold Spring Harb. Perspect. Med. 2013, 3, a011718. [Google Scholar] [CrossRef]
- Mettananda, S.; Higgs, D.R. Molecular basis and genetic modifiers of thalassemia. Hematol. Oncol. Clin. 2018, 32, 177–191. [Google Scholar] [CrossRef]
- Valaei, A.; Karimipoor, M.; Kordafshari, A.; Zeinali, S. Molecular Basis of α-Thalassemia in Iran. Iran. Biomed. J. 2018, 22, 6–14. [Google Scholar] [CrossRef]
- Lee, J.-S.; Cho, S.I.; Park, S.S.; Seong, M.-W. Molecular basis and diagnosis of thalassemia. Blood Res. 2021, 56, 39–43. [Google Scholar] [CrossRef]
- Origa, R. Beta-Thalassemia; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- De Simone, G.; Quattrocchi, A.; Mancini, B.; di Masi, A.; Nervi, C.; Ascenzi, P. Thalassemias: From gene to therapy. Mol. Asp. Med. 2022, 84, 101028. [Google Scholar] [CrossRef]
- Makis, A.; Voskaridou, E.; Papassotiriou, I.; Hatzimichael, E. Novel therapeutic advances in β-thalassemia. Biology 2021, 10, 546. [Google Scholar] [CrossRef]
- Ma, S.-P.; Gao, X.-X.; Zhou, G.-Q.; Zhang, H.-K.; Yang, J.-M.; Wang, W.-J.; Song, S.-M.; Chen, H.-Y.; Lu, D.-R. Reactivation of γ-globin expression using a minicircle DNA system to treat β-thalassemia. Gene 2022, 820, 146289. [Google Scholar] [CrossRef]
- De Dreuzy, E.; Bhukhai, K.; Leboulch, P.; Payen, E. Current and future alternative therapies for beta-thalassemia major. Biomed. J. 2016, 39, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Fibach, E.; Rachmilewitz, E.A. Pathophysiology and treatment of patients with beta-thalassemia—An update. F1000Research 2017, 6, 2156. [Google Scholar] [CrossRef] [Green Version]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzyńska, M.; Rokicki, R.; Koziarska-Rościszewska, M.; Rysz, J. Pathomechanisms of Immunological Disturbances in β-Thalassemia. Int. J. Mol. Sci. 2021, 22, 9677. [Google Scholar] [CrossRef]
- Khandros, E.; Kwiatkowski, J.L. Beta thalassemia: Monitoring and new treatment approaches. Hematol. Oncol. Clin. 2019, 33, 339–353. [Google Scholar] [CrossRef]
- Chaichompoo, P.; Svasti, S.; Smith, D.R. The Roles of Mitophagy and Autophagy in Ineffective Erythropoiesis in β-Thalassemia. Int. J. Mol. Sci. 2022, 23, 10811. [Google Scholar] [CrossRef]
- Soni, S. Gene therapies for transfusion dependent β-thalassemia: Current status and critical criteria for success. Am. J. Hematol. 2020, 95, 1099–1112. [Google Scholar] [CrossRef]
- Costa, E.; Cappellini, M.D.; Rivella, S.; Chilin, A.; Alessi, E.; Riccaboni, M.; Leufkens, H.G.M.; Luzzatto, L. Emergent treatments for β-thalassemia and orphan drug legislations. Drug Discov. Today 2022, 27, 103342. [Google Scholar] [CrossRef]
- Motta, I.; Bou-Fakhredin, R.; Taher, A.T.; Cappellini, M.D. Beta Thalassemia: New Therapeutic Options beyond Transfusion and Iron Chelation. Drugs 2020, 80, 1053–1063. [Google Scholar] [CrossRef]
- Ali, S.; Mumtaz, S.; Shakir, H.A.; Khan, M.; Tahir, H.M.; Mumtaz, S.; Mughal, T.A.; Hassan, A.; Kazmi, S.A.R.; Irfan, M.; et al. Current status of beta-thalassemia and its treatment strategies. Mol. Genet. Genom. Med. 2021, 9, e1788. [Google Scholar] [CrossRef]
- Motta, I.; Ghiaccio, V.; Cosentino, A.; Breda, L. Curing Hemoglobinopathies: Challenges and Advances of Conventional and New Gene Therapy Approaches. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019067. [Google Scholar] [CrossRef]
- Taher, A.T.; Cappellini, M.D. Luspatercept for β-thalassemia: Beyond red blood cell transfusions. Expert Opin. Biol. Ther. 2021, 21, 1363–1371. [Google Scholar] [CrossRef]
- Schriml, L.M.; Mitraka, E.; Munro, J.; Tauber, B.; Schor, M.; Nickle, L.; Felix, V.; Jeng, L.; Bearer, C.; Lichenstein, R.; et al. Human Disease Ontology 2018 update: Classification, content and workflow expansion. Nucleic Acids Res. 2018, 47, D955–D962. [Google Scholar] [CrossRef] [Green Version]
- Claussnitzer, M.; Cho, J.H.; Collins, R.; Cox, N.J.; Dermitzakis, E.T.; Hurles, M.E.; Kathiresan, S.; Kenny, E.E.; Lindgren, C.M.; MacArthur, D.G.; et al. A brief history of human disease genetics. Nature 2020, 577, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.; Marks, L.; May, G.H.W.; Wilson Joanna, B. The genetic basis of disease. Essays Biochem. 2018, 62, 643–723. [Google Scholar] [CrossRef] [Green Version]
- Dou, Y.; Gold, H.D.; Luquette, L.J.; Park, P.J. Detecting Somatic Mutations in Normal Cells. Trends Genet. 2018, 34, 545–557. [Google Scholar] [CrossRef]
- Tambuyzer, E.; Vandendriessche, B.; Austin, C.P.; Brooks, P.J.; Larsson, K.; Miller Needleman, K.I.; Valentine, J.; Davies, K.; Groft, S.C.; Preti, R.; et al. Therapies for rare diseases: Therapeutic modalities, progress and challenges ahead. Nat. Rev. Drug Discov. 2020, 19, 93–111. [Google Scholar] [CrossRef]
- Gurdasani, D.; Barroso, I.; Zeggini, E.; Sandhu, M.S. Genomics of disease risk in globally diverse populations. Nat. Rev. Genet. 2019, 20, 520–535. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; Moufawad El Achkar, C.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.R.; Eng, C.M. Next-generation sequencing to diagnose suspected genetic disorders. N. Engl. J. Med. 2018, 379, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Mole, S.E.; Anderson, G.; Band, H.A.; Berkovic, S.F.; Cooper, J.D.; Kleine Holthaus, S.-M.; McKay, T.R.; Medina, D.L.; Rahim, A.A.; Schulz, A.; et al. Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis. Lancet Neurol. 2019, 18, 107–116. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Amawi, H.A.; Rezigue, M.M.; Hamzat, Y.; Satija, S.; Tambuwala, M.M. Application of Nanomaterials in the Diagnosis and Treatment of Genetic Disorders. In Applications of Nanomaterials in Human Health; Khan, F.A., Ed.; Springer: Singapore, 2020; pp. 125–146. [Google Scholar] [CrossRef]
- Mukhtar, M.; Sargazi, S.; Barani, M.; Madry, H.; Rahdar, A.; Cucchiarini, M. Application of Nanotechnology for Sensitive Detection of Low-Abundance Single-Nucleotide Variations in Genomic DNA: A Review. Nanomaterials 2021, 11, 1384. [Google Scholar] [CrossRef]
- Waris, A.; Ali, A.; Khan, A.U.; Asim, M.; Zamel, D.; Fatima, K.; Raziq, A.; Khan, M.A.; Akbar, N.; Baset, A.; et al. Applications of Various Types of Nanomaterials for the Treatment of Neurological Disorders. Nanomaterials 2022, 12, 2140. [Google Scholar] [CrossRef]
- Mughal, S.S. Diagnosis and treatment of diseases by using metallic nanoparticles—A review. Authorea Prepr. 2022, 3, 27–35. [Google Scholar] [CrossRef]
- Doudna, J.A. The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [Google Scholar] [CrossRef]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The Promises and Challenges of Tumor Mutation Burden as an Immunotherapy Biomarker: A Perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef]
- Anjum, S.; Ishaque, S.; Fatima, H.; Farooq, W.; Hano, C.; Abbasi, B.H.; Anjum, I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals 2021, 14, 707. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosko, S.H.; et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 2015, 9, 6644–6654. [Google Scholar] [CrossRef] [Green Version]
- Talevi, A.; Bellera, C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; Costa, J.S.R.; Coêlho, D.d.F.; Liszbinski, R.B.; Lopes, A.M.; Oliveira-Nascimento, L.; Jesus, M.B.D.; Jozala, A.F.; Ehrhardt, C.; et al. Nanotechnology as a tool to overcome macromolecules delivery issues. Colloids Surf. B Biointerfaces 2023, 222, 113043. [Google Scholar] [CrossRef]
- Chen, G.; Qian, Y.; Zhang, H.; Ullah, A.; He, X.; Zhou, Z.; Fenniri, H.; Shen, J. Advances in cancer theranostics using organic-inorganic hybrid nanotechnology. Appl. Mater. Today 2021, 23, 101003. [Google Scholar] [CrossRef]
- Arnold, A.M.; Bradley, A.M.; Taylor, K.L.; Kennedy, Z.C.; Omberg, K.M. The Promise of Emergent Nanobiotechnologies for In Vivo Applications and Implications for Safety and Security. Health Secur. 2022, 20, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Zaib, S.; Iqbal, J. Nanotechnology: Applications, techniques, approaches, & the advancement in toxicology and environmental impact of engineered nanomaterials. Importance Appl. Nanotechnol. 2019, 8, 1–10. [Google Scholar]
- Er, S.; Laraib, U.; Arshad, R.; Sargazi, S.; Rahdar, A.; Pandey, S.; Thakur, V.K.; Díez-Pascual, A.M. Amino Acids, Peptides, and Proteins: Implications for Nanotechnological Applications in Biosensing and Drug/Gene Delivery. Nanomaterials 2021, 11, 3002. [Google Scholar] [CrossRef]
- Yezdani, U.; Khan, M.G.; Kushwah, N.; Verma, A.; Khan, F. Application of nanotechnology in diagnosis and treatment of various diseases and its future advances in medicine. World J. Pharm. Pharm. Sci. 2018, 7, 1611–1633. [Google Scholar]
- Xu, X.; Liu, C.; Wang, Y.; Koivisto, O.; Zhou, J.; Shu, Y.; Zhang, H. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv. Drug Deliv. Rev. 2021, 176, 113891. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Dung Nguyen, T.T.; Vo, T.K.; Tran, N.-M.-A.; Nguyen, M.K.; Van Vo, T.; Vo, G.V. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Nagraik, R.; Sharma, A.; Kumar, D.; Mukherjee, S.; Sen, F.; Kumar, A.P. Amalgamation of biosensors and nanotechnology in disease diagnosis: Mini-review. Sens. Int. 2021, 2, 100089. [Google Scholar] [CrossRef]
- Meenambiga, S.S.; Sakthiselvan, P.; Hari, S.; Umai, D. Nanotechnology for blood test to predict the blood diseases/blood disorders. In Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood; Chapter 13; Denizli, A., Nguyen, T.A., Rajan, M., Alam, M.F., Rahman, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 285–311. [Google Scholar] [CrossRef]
- Helmi, N.; Bashir, M.; Shireen, A.; Ahmed, I.M. Thalassemia review: Features, dental considerations and management. Electron. Physician 2017, 9, 4003–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, R.E.; Sibmooh, N.; Fucharoen, S.; Friedman, J.M. HbE/β-thalassemia and oxidative stress: The key to pathophysiological mechanisms and novel therapeutics. Antioxid. Redox Signal. 2017, 26, 794–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sana Rafiq, H.; Fatima, B.; Hussain, D.; Mohyuddin, A.; Majeed, S.; Manzoor, S.; Imran, M.; Nawaz, R.; Shabbir, S.; Mukhtar, S.; et al. Selective electrochemical sensing of hemoglobin from blood of β-thalassemia major patients by tellurium nanowires-graphene oxide modified electrode. Chem. Eng. J. 2021, 419, 129706. [Google Scholar] [CrossRef]
- Hussain, K.K.; Moon, J.M.; Park, D.S.; Shim, Y.B. Electrochemical detection of hemoglobin: A review. Electroanalysis 2017, 29, 2190–2199. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef]
- Sun, W.; Gong, S.; Shi, F.; Cao, L.; Ling, L.; Zheng, W.; Wang, W. Direct electrochemistry and electrocatalysis of hemoglobin in graphene oxide and ionic liquid composite film. Mater. Sci. Eng. C 2014, 40, 235–241. [Google Scholar] [CrossRef]
- Alim, S.; Kafi, A.K.M.; Jose, R.; Yusoff, M.M.; Vejayan, J. Enhanced direct electron transfer of redox protein based on multiporous SnO2 nanofiber-carbon nanotube nanocomposite and its application in biosensing. Int. J. Biol. Macromol. 2018, 114, 1071–1076. [Google Scholar] [CrossRef]
- Xie, H.; Luo, G.; Niu, Y.; Weng, W.; Zhao, Y.; Ling, Z.; Ruan, C.; Li, G.; Sun, W. Synthesis and utilization of Co3O4 doped carbon nanofiber for fabrication of hemoglobin-based electrochemical sensor. Mater. Sci. Eng. C 2020, 107, 110209. [Google Scholar] [CrossRef]
- Darabi, R.; Shabani-Nooshabadi, M.; Khoobi, A. A Potential Strategy for Simultaneous Determination of Deferoxamine and Vitamin C Using MCR-ALS with Nanostructured Electrochemical Sensor in Serum and Urine of Thalassemia and Diabetic Patients. J. Electrochem. Soc. 2021, 168, 046514. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Niu, X.; Li, X.; Li, X.; Li, G.; He, B.; Li, Q.; Sun, W. Investigation on direct electrochemical and electrocatalytic behavior of hemoglobin on palladium-graphene modified electrode. Mater. Sci. Eng. C 2017, 80, 135–140. [Google Scholar] [CrossRef]
- Zhan, T.; Tan, Z.; Wang, X.; Hou, W. Hemoglobin immobilized in g-C3N4 nanoparticle decorated 3D graphene-LDH network: Direct electrochemistry and electrocatalysis to trichloroacetic acid. Sens. Actuators B Chem. 2018, 255, 149–158. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef] [PubMed]
- Sharafdarkolaei, S.H.; Motovali-Bashi, M.; Gill, P. Fluorescent detection of poInt. mutation via ligase reaction assisted by quantum dots and magnetic nanoparticle-based probes. RSC Adv. 2017, 7, 25665–25672. [Google Scholar] [CrossRef] [Green Version]
- Heidari Sharafdarkolaee, S.; Motovali-Bashi, M.; Gill, P. The sensitive detection of IVSII-1(G>A) mutation in beta globin gene using a Nano-based ligation genotyping system. Gene 2018, 674, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Liang, Z.; Han, D.; Han, F.; Fu, W.; Wang, W.; Liu, Z.; Niu, L. Molecularly imprinted photo-electrochemical sensor for hemoglobin detection based on titanium dioxide nanotube arrays loaded with CdS quantum dots. Talanta 2021, 224, 121924. [Google Scholar] [CrossRef]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.-S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef] [Green Version]
- Chomean, S.; Wangmaung, N.; Sritongkham, P.; Promptmas, C.; Ittarat, W. Genotyping of α-thalassemias by the colorimetric nanogold probes. Clin. Chim. Acta 2014, 437, 197–202. [Google Scholar] [CrossRef]
- Doria, G.; Franco, R.; Baptista, P. Nanodiagnostics: Fast colorimetric method for single nucleotide polymorphism/mutation detection. IET Nanobiotechnol. 2007, 1, 53–57. Available online: https://digital-library.theiet.org/content/journals/10.1049/iet-nbt_20070001 (accessed on 20 February 2023). [CrossRef] [Green Version]
- Yi, P.; Lu, W.; Guo, J.; Liu, Q.; Chen, Z.; Han, J.; Li, L. Development of a PCR/Ligase Detection Reaction/Nanogold-Based Universal Array Approach for the Detection of Low-Abundant DNA PoInt. Mutations. Cell Biochem. Biophys. 2011, 61, 629–636. [Google Scholar] [CrossRef]
- Gholivand, M.-B.; Akbari, A. A sensitive electrochemical genosensor for highly specific detection of thalassemia gene. Biosens. Bioelectron. 2019, 129, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-C.; Su, X.; Hou, J.; Ferranco, A.; Feng, X.-Z.; Zeng, R.; Chen, Z.; Kraatz, H.-B. Disposable electrochemical sensors for hemoglobin detection based on ferrocenoyl cysteine conjugates modified electrode. Sens. Actuators B Chem. 2019, 282, 130–136. [Google Scholar] [CrossRef]
- Mishra, G.; Saxena, R.; Mishra, A.; Tiwari, A. Recent techniques for the detection of β-thalassemia: A review. J. Biosens. Bioelectron. 2012, 3, 1000123. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Liu, Y.; Xie, W.; Lin, X.; Pan, H. Ag nanoparticles/PbTiO3 with in-situ photocatalytic process and its application in an ultra-sensitive molecularly imprinted hemoglobin detection. Colloids Surf. B Biointerfaces 2022, 217, 112641. [Google Scholar] [CrossRef]
- Wangmaung, N.; Promptmas, C.; Chomean, S.; Sanchomphu, C.; Ittarat, W. Low cost biosensor-based molecular differential diagnosis of α-thalassemia (Southeast Asia deletion). Clin. Chem. Lab. Med. 2013, 51, 1199–1205. [Google Scholar] [CrossRef]
- Wang, H.; Tang, W.; Wei, H.; Zhao, Y.; Hu, S.; Guan, Y.; Pan, W.; Xia, B.; Lia, N.; Liu, F. Integrating dye-intercalated DNA dendrimers with electrospun nanofibers: A new fluorescent sensing platform for nucleic acids, proteins, and cells. J. Mater. Chem. B 2015, 3, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Ercan, M.; Ozalp, V.C.; Tuna, B.G. Genotyping of single nucleotide polymorphism by probe-gated silica nanoparticles. Anal. Biochem. 2017, 537, 78–83. [Google Scholar] [CrossRef]
- Fatima, B.; Saeed, U.; Hussain, D.; Jawad, S.-e.-Z.; Rafiq, H.S.; Majeed, S.; Sumaira Manzoor, S.; Qadir, S.Y.; Ashiq, M.N.; Najam-ul-Haq, M. Facile hydrothermal synthesis of NiTe nanorods for non-enzymatic electrochemical sensing of whole blood hemoglobin in pregnant anemic women. Anal. Chim. Acta 2022, 1189, 339204. [Google Scholar] [CrossRef]
- Dolak, İ.; Canpolat, G.; Onat, R.; Keçili, R.; Baysal, Z.; Ziyadanoğulları, B.; Ersöz, A.; Say, R. A novel lanthanide-chelate based molecularly imprinted cryogel for purification of hemoglobin from blood serum: An alternative method for thalassemia diagnosis. Process Biochem. 2020, 91, 189–196. [Google Scholar] [CrossRef]
- Prabhu, R.; Prabhu, V.; Prabhu, R. Iron overload in beta thalassemia: A review. J. Biosci. Technol. 2009, 1, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Leecharoenkiat, K.; Lithanatudom, P.; Sornjai, W.; Smith, D.R. Iron dysregulation in beta-thalassemia. Asian Pac. J. Trop. Med. 2016, 9, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattamis, A.; Papassotiriou, I.; Palaiologou, D.; Apostolakou, F.; Galani, A.; Ladis, V.; Sakellaropoulos, N.; Papanikolaou, G. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica 2006, 91, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Cappellini, M.D.; Wood, J.C.; Motta, I.; Graziadei, G.; Tamim, H.; Taher, A.T. Elevated liver iron concentration is a marker of increased morbidity in patients with β thalassemia intermedia. Haematologica 2011, 96, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E. Hepcidin in β-thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 31–35. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Marsella, M. Iron Chelation in Thalassemia Major. Clin. Ther. 2015, 37, 2866–2877. [Google Scholar] [CrossRef]
- Berdoukas, V.; Farmaki, K.; Wood, J.C.; Coates, T. Iron chelation in thalassemia: Time to reconsider our comfort zones. Expert Rev. Hematol. 2011, 4, 17–26. [Google Scholar] [CrossRef]
- Peyam, S.; Bansal, D. Dual Oral Iron Chelation in Thalassemia: Need for Robust Evidence. Indian J. Pediatr. 2021, 88, 319–321. [Google Scholar] [CrossRef]
- Chaston, T.B.; Richardson, D.R. Iron chelators for the treatment of iron overload disease: Relationship between structure, redox activity, and toxicity. Am. J. Hematol. 2003, 73, 200–210. [Google Scholar] [CrossRef]
- Chakraborty, N.; Narayanan, V.; Gautam, H.K. Nano-Therapeutics to Treat Acne Vulgaris. Indian J. Microbiol. 2022, 62, 167–174. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Veroniaina, H.; Su, N.; Sha, K.; Jiang, F.; Wu, Z.; Qi, X. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J. Pharm. Sci. 2021, 16, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Yadav, N.; Ahmad, M.; Shadan, M. Blood Substitutes: Possibilities with Nanotechnology. Indian J. Hematol. Blood Transfus. 2014, 30, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikonomidou, P.R.; Casu, C.; Rivella, S. New strategies to target iron metabolism for the treatment of beta thalassemia. Ann. N. Y. Acad. Sci. 2016, 1368, 162–168. [Google Scholar] [CrossRef]
- Nai, A.; Pagani, A.; Mandelli, G.; Lidonnici, M.R.; Silvestri, L.; Ferrari, G.; Camaschella, C. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of β-thalassemia. Blood J. Am. Soc. Hematol. 2012, 119, 5021–5029. [Google Scholar] [CrossRef]
- Bennett, C.F.; Swayze, E.E. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Toudjarska, I.; Sendamarai, A.K.; Racie, T.; Milstein, S.; Bettencourt, B.R.; Hettinger, J.; Bumcrot, D.; Fleming, M.D. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe−/− mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood J. Am. Soc. Hematol. 2013, 121, 1200–1208. [Google Scholar] [CrossRef]
- Casu, C.; Aghajan, M.; Oikonomidou, P.R.; Guo, S.; Monia, B.P.; Rivella, S. Combination of Tmprss6-ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica 2016, 101, e8. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Casu, C.; Gardenghi, S.; Booten, S.; Aghajan, M.; Peralta, R.; Watt, A.; Freier, S.; Monia, B.P.; Rivella, S. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J. Clin. Investig. 2013, 123, 1531–1541. [Google Scholar] [CrossRef] [Green Version]
- Kalanaky, S.; Hafizi, M.; Safari, S.; Mousavizadeh, K.; Kabiri, M.; Farsinejad, A.; Fakharzadeh, S.; Nazaran, M.H. TLc-A, the leading nanochelating-based nanochelator, reduces iron overload in vitro and in vivo. Int. J. Hematol. 2016, 103, 274–282. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Raheb, J.; Akhavan, O.; Arjmand, S.; Mashinchian, O.; Rahman, M.; Abdolahad, M.; Serpooshan, V.; Laurentj, S.; Mahmoudi, M. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale 2015, 7, 8978–8994. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abd-Alkareem, D.; Zainal, I.G.; Ali, S.J. In vitro biochemical evaluation the effect of (Cobalt and Nickel-Zinc) ferrite Nanoparticles on beta-thalassemia major erythrocytes. EurAsian J. Biosci. 2020, 14, 4245–4249. [Google Scholar]
- Tavakoli, S.; Ebrahimzadeh, M.A.; Sameni, F.; Biparva, P.; Mohammadi, H.; Ziar, A.; Mazandarani, A.Z.; Vafaeinejad, S.; Eslami, S. Excess iron ion reduction in a thalassemia model using silver nanoparticles modified by the tannin fraction of Myrtus communis extact. Nanomed. Res. J. 2020, 5, 355–363. [Google Scholar] [CrossRef]
- Ergün, B.; Baydemir, G.; Andaç, M.; Yavuz, H.; Denizli, A. Ion imprinted beads embedded cryogels for in vitro removal of iron from β-thalassemic human plasma. J. Appl. Polym. Sci. 2012, 125, 254–262. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, S.; Heidari, R.; Mohammadi-Samani, S. In vitro and in vivo assessment of EDTA-modified silica nano-spheres with supreme capacity of iron capture as a novel antidote agent. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Simchick, G.A.; Qiao, J.; Ashcraft, M.M.; Cui, S.; Nagy, T.; Zhao, Q.; Xiong, M.P. Reactive Oxygen Species-Triggered Dissociation of a Polyrotaxane-Based Nanochelator for Enhanced Clearance of Systemic and Hepatic Iron. ACS Nano 2021, 15, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Guo, S.; Nie, G.; Anderson, G.J. Hepatospheres formed in quasi-spherical microwells to study the therapeutic efficacy of novel liver-targeted iron chelator-loaded nanoformulations. J. Gastroenterol. Hepatol. 2017, 32, 88. [Google Scholar]
- Singhal, C.; Malhotra, N.; Chauhan, N.; Narang, S.; Pundir, C.S.; Narang, J. Hierarchical electrodeposition of methylene blue on ZnO nanocrystals thin films layered on SnO2/F electrode for in vitro sensing of anti-thalassemic drug. Mater. Sci. Eng. C 2016, 62, 596–604. [Google Scholar] [CrossRef]
- Chavada, V.D.; Bhatt, N.M.; Sanyal, M.; Shrivastav, P.S. Pyrophosphate functionalized silver nanoparticles for colorimetric determination of deferiprone via competitive binding to Fe(III). Microchim. Acta 2017, 184, 4203–4208. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singh, G.; Pundir, C.S. Electrochemical impediometric detection of anti-HIV drug taking gold nanorods as a sensing interface. Biosens. Bioelectron. 2015, 66, 332–337. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singh, G.; Pundir, C.S. Voltammetric detection of anti-HIV replication drug based on novel nanocomposite gold-nanoparticle–CaCO3 hybrid material. Bioprocess Biosyst. Eng. 2015, 38, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Mazzitelli, S.; Brognara, E.; Lampronti, I.; Carugo, D.; Hill, M.; Zhang, X.; Gambari, R.; Nastruzzi, C. Mithramycin encapsulated in polymeric micelles by microfluidic technology as novel therapeutic protocol for beta-thalassemia. Int. J. Nanomed. 2012, 7, 307–324. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, E.F.; Simantirakis, E.; Verras, M.; Barbas, C.; Vassilopoulos, G.; Peterson, K.R.; Athanassiadou, A. Episomal vectors based on S/MAR and the β-globin Replicator, encoding a synthetic transcriptional activator, mediate efficient γ-globin activation in haematopoietic cells. Sci. Rep. 2019, 9, 19765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Chou, S.-J.; Li, J.; Hui, W.; Liu, W.; Sun, N.; Zhang, R.Y.; Zhu, Y.; Tsai, M.L.; Tseng, H.-R.; et al. Supramolecular nanosubstrate—Mediated delivery system enables CRISPR-Cas9 knockin of hemoglobin beta gene for hemoglobinopathies. Sci. Adv. 2020, 6, eabb7107. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Efficacy | Disadvantages | References |
|---|---|---|---|
| Transfusion therapy | Overcomes anemic condition | Iron overload, expensive | [2,5,12,23,25,28,31,32] |
| Iron chelation therapy | Maintains body iron at safe levels. | Disrupted physiological conditions, blurred vision, rashes | [12,21,23,25,28,31,32] |
| HbF induction through hydroxyurea, DNA methylation inhibitors, and short-chain fatty acids | Increases γ-globin production to upregulate total hemoglobin levels, ameliorate anemia, and diminishes phosphatidylserine expression on RBCs. | Ulcers, organ damage, breathing problems, skeletal deformities | [2,12,25,27,28,29,32] |
| Ineffective erythropoiesis signaling modulators | Increases hemoglobin in a dose-dependent fashion by targeting JAK2/STAT5 signaling pathway | Physiological complications, expensive | [27] |
| Bone marrow transplantation | Restore the tissue’s capability of synthesizing functional hemoglobin | Graft-versus-host disease, cataract, organ damage, physiological complications | [31,32] |
| Hematopoietic stem cell transplantation | Reduces intensity or non-myeloablative conditioning and limits iron burden, and comorbidities | Decreased immunity, infections, graft-versus-host disease, death | [2,21,23,25,28,32,33] |
| Splenectomy | Alleviate anemia in non–transfusion-dependent thalassemia, less effective | Infections, sepsis, increased bleeding, injured organs | [2,21,23,31] |
| Gene therapy | Regulates globin genes expression through locus control region and promoter region, may include beta globin replacement or fetal globin reactivation | Genotoxicity, allergic reactions, increased risk of cancer, expensive | [12,22,23,25,28,29,31,32,33] |
| Gene editing | Allow the sustained production and endogenous regulation of the globin proteins by targeting the BCL11A gene, including zinc-finger nucleases (ZFN), transcription activator-like effector nucleases (TALENS), and clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated-nuclease 9 (CRISPR-Cas9) as gene editing tools | Insufficient transduction efficiency, dysregulated transgene expression, increased risk of gene silencing | [12,22,24,29,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, Z.; Qadeer, M.I.; Anjum, I.; Hano, C.; Anjum, S. Thalassemia and Nanotheragnostics: Advanced Approaches for Diagnosis and Treatment. Biosensors 2023, 13, 450. https://doi.org/10.3390/bios13040450
Tariq Z, Qadeer MI, Anjum I, Hano C, Anjum S. Thalassemia and Nanotheragnostics: Advanced Approaches for Diagnosis and Treatment. Biosensors. 2023; 13(4):450. https://doi.org/10.3390/bios13040450
Chicago/Turabian StyleTariq, Zahra, Muhammad Imran Qadeer, Iram Anjum, Christophe Hano, and Sumaira Anjum. 2023. "Thalassemia and Nanotheragnostics: Advanced Approaches for Diagnosis and Treatment" Biosensors 13, no. 4: 450. https://doi.org/10.3390/bios13040450
APA StyleTariq, Z., Qadeer, M. I., Anjum, I., Hano, C., & Anjum, S. (2023). Thalassemia and Nanotheragnostics: Advanced Approaches for Diagnosis and Treatment. Biosensors, 13(4), 450. https://doi.org/10.3390/bios13040450






