Wearable Sensors to Evaluate Autonomic Response to Olfactory Stimulation: The Influence of Short, Intensive Sensory Training
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
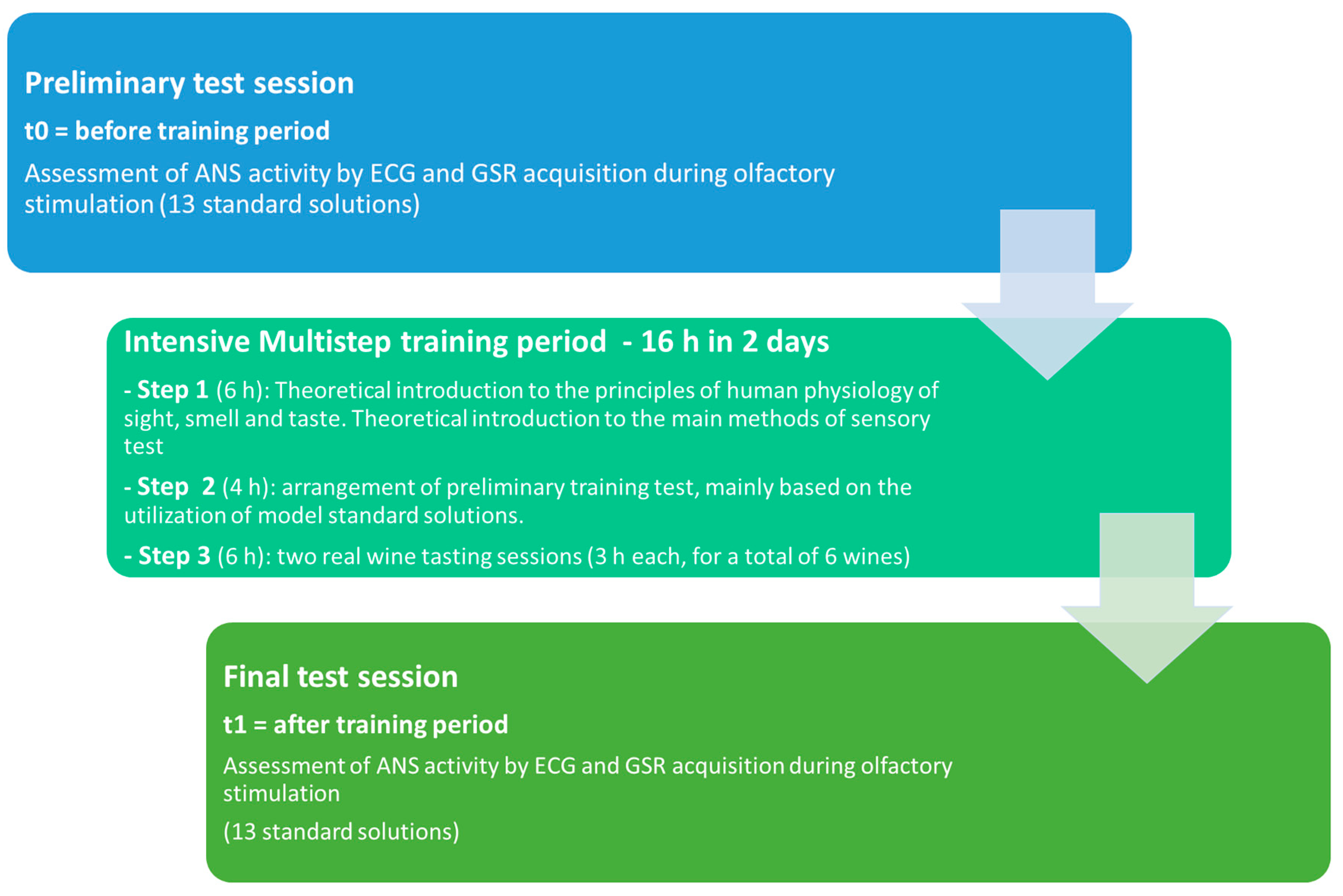
2.2. Training Description
2.3. Solutions Used for the Olfactory Stimulation
2.4. Procedure for ANS Assessment
- (i)
- Baseline (3′ duration): At the beginning of the recording, the subjects sat on a chair in a comfortable way and were asked to stay still and relaxed;
- (ii)
- Task (6′ 40″ duration): During the administration of the compounds, 10 model solutions (previously described) were given to the panelists for the detection of odors. Each of the proposed solutions was administered to the individuals, tested for 10” in both nostrils, at an inter-stimulus interval of 30″. This pause time was intended to clean the nasal cavity from the residuals of the previous compound [29]. At the same time, the panelists were asked to report, on a paper sheet, the identifier for each of the compounds presented;
- (iii)
- Recovery (3′ duration): This phase was analogous to the baseline, after task completion.
2.4.1. ECG Acquisition and Processing
- -
- Features on the time domain:
- Heart rate (HR): number of heart pounds within a time unit. This is measured in beats per minute (bpm), and deals with the sympathetic activity of the ANS [32];
- Root mean square of the successive differences (RMSSD): measured in ms. This is for the root mean square of the differences between the R-R intervals close to each other. Overall, it matches the parasympathetic branch of the ANS [32];
- Number of normal R-R intervals differing for more than 50 ms (NN50): this is the number (or the percentage) of the normal R-R intervals of the ECG signal differing for more than 50 ms from each other. Under resting-state short-term recordings, it deals with the parasympathetic activity of the ANS [32];
- Cardiac sympathetic index (CSI): computed as SD2/SD1. This refers to the sympathetic activity of the ANS. SD1 is the standard deviation of the projection of the Poincaré plot on the perpendicular line to the identity, whereas SD2 is the standard deviation of the projection of the Poincaré plot on the parallel line to the identity [33];
- Cardiac vagal index (CVI): this is obtained by the Poincaré plot; it is calculated as log10 (SD1*SD2), and refers to the parasympathetic activity of the ANS [33].
- -
- Features on the frequency domain:
- Low frequency (LF): when taking into account the frequency spectrum of the ECG signal, the LF represents the power spectral density of the ECG signal at low frequencies (0.04–0.15 Hz). According to the literature, it reflects both the activity of both the sympathetic and parasympathetic nervous systems of the ANS [32];
- High frequency (HF): this represents the power spectral density of the ECG signal at high frequencies (0.15–0.4 Hz), which reflects the parasympathetic activity of the ANS [32];
- Low-to-high frequency components ratio (LF/HF): this mainly reflects the overall sympathovagal balance of the ANS, although the results need to be framed and justified according to the specific measurement condition [32].
2.4.2. GSR Acquisition and Processing
- -
- Global GSR signal: this is the sum of the tonic and phasic components of the GSR signal, measured in microsiemens (µS);
- -
- Tonic GSR: this is mainly related to the slow changes in the electrical skin signal, it is dominant at rest and during most relaxing activities;
- -
- Phasic GSR component: this refers to the quick responses to specific stimuli. In the present study, it can refer to the specific response to sensory (olfactory) stimuli. It is often termed as the skin conductance response (SCR).
2.5. Statistical Analysis
3. Results
3.1. Odor Identification
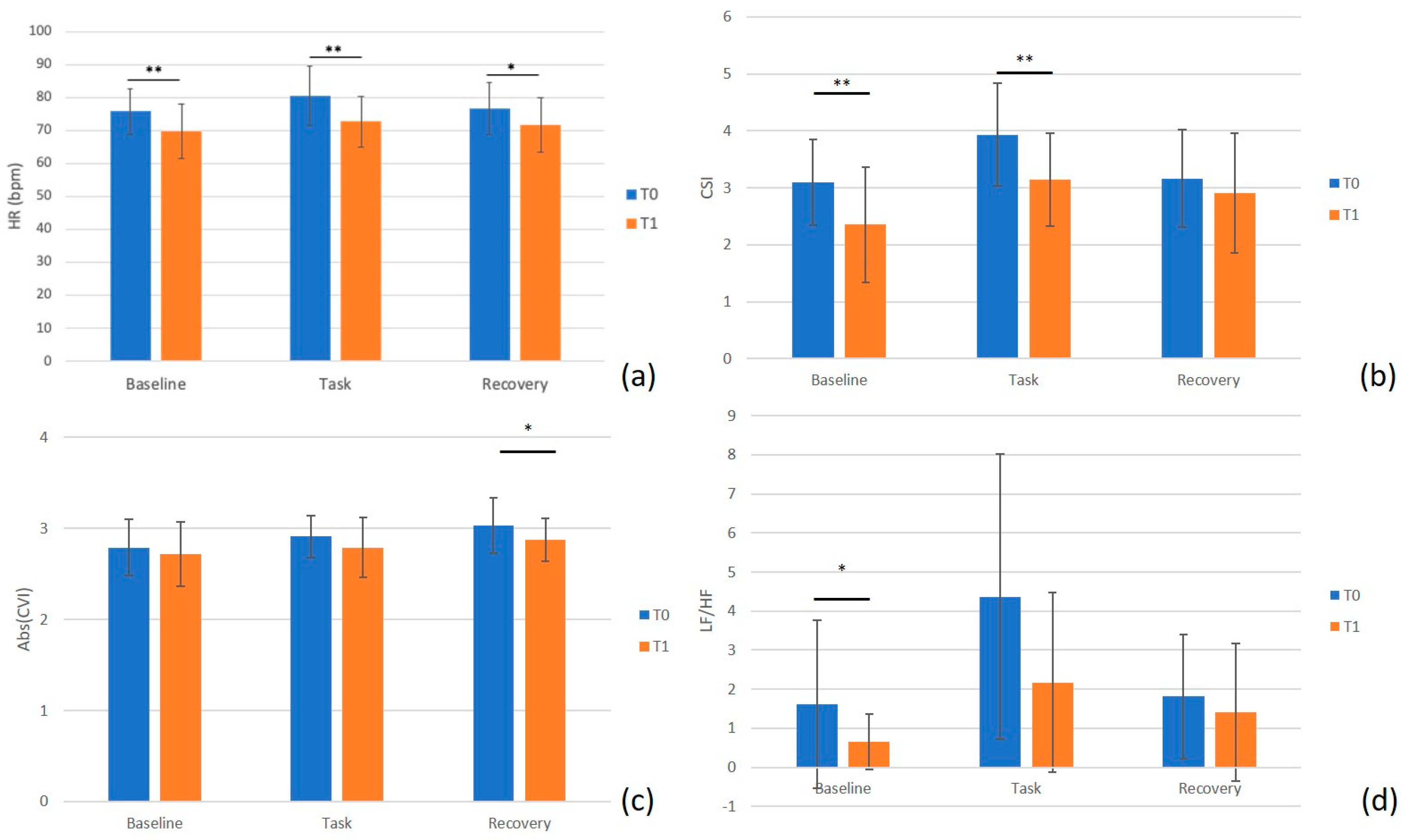
3.2. ECG Signal
3.3. GSR Signal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-Casado, A.; Jiménez-Carvelo, A.M.; Cuadros-Rodríguez, L. Sensory quality control of dry-cured ham: A comprehensive methodology for sensory panel qualification and method validation. Meat Sci. 2019, 149, 149–155. [Google Scholar] [CrossRef]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Munoz, A.M. Sensory evaluation in quality control: An overview, new developments and future opportunities. Food Qual. Prefer. 2002, 13, 329–339. [Google Scholar] [CrossRef]
- Tuorila, H.; Monteleone, E. Sensory food science in the changing society: Opportunities, needs, and challenges. Trends Food Sci. Technol. 2009, 20, 54–62. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Internat. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Modesti, M.; Tonacci, A.; Sansone, F.; Billeci, L.; Bellincontro, A.; Cacopardo, G.; Sanmartin, C.; Taglieri, I.; Venturi, F. E-Senses, Panel Tests and Wearable Sensors: A Teamwork for Food Quality Assessment and Prediction of Consumer’s Choices. Chemosensors 2022, 10, 244. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Fine Wine Flavour Perception and Appreciation: Blending Neuronal Processes, Tasting Methods and Expertise. Trends Food Sci. Technol. 2021, 115, 332–346. [Google Scholar] [CrossRef]
- Ares, G. Methodological challenges in sensory characterization. Curr. Opin. Food Sci. 2015, 3, 1–5. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Introduction to Sensory Evaluation. Sensory Evaluation Practices, 3rd ed.; Academic Press: San Diego, CA, USA, 2004; pp. 1–19. [Google Scholar]
- Dobrushina, O.R.; Arina, G.A.; Dobrynina, L.A.; Novikova, E.S.; Gubanova, M.V.; Belopasova, A.V.; Vorobeva, V.P.; Suslina, A.D.; Pechenkova, E.V.; Perepelkina, O.S.; et al. Sensory Integration in Interoception: Interplay between Top-down and Bottomup Processing. Cortex 2021, 144, 185–197. [Google Scholar] [CrossRef]
- Byrne, D.V.; O’sullivan, M.G.; Dijksterhuis, G.B.; Bredie, W.L.P.; Martens, M. Sensory panel consistency during development of a vocabulary for warmed-over flavour. Food Qual. Prefer. 2001, 12, 171–187. [Google Scholar] [CrossRef]
- Valentin, D.; Chollet, S.; Lelièvre, M.; Abdi, H. Quick and dirty but still pretty good: A review of new descriptive methods in food science. Int. J. Food Sci. Technol. 2012, 47, 1563–1578. [Google Scholar] [CrossRef]
- Alexi, N.; Nanou, E.; Lazo, O.; Guerrero, L.; Grigorakis, K.; Byrne, D.V. Check-All-That-Apply (CATA) with semi-trained assessors: Sensory profiles closer to descriptive analysis or consumer elicited data? Food Qual. Prefer. 2018, 64, 11–20. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Suo, H.; Yuan, X.; Zhou, S.; Ren, H.; Jiang, Y.; Kan, J. Comparison of the performance of the same panel with different training levels: Flash profile versus descriptive analysis. Food Qual. Prefer. 2022, 99, 104582. [Google Scholar] [CrossRef]
- Soudry, Y.; Lemogne, C.; Malinvaud, D.; Consoli, S.-M.; Bonfils, P. Olfactory system and emotion: Common substrates. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 18–23. [Google Scholar] [CrossRef]
- De Luca, R.; Botelho, D. The unconscious perception of smells as a driver of consumer responses: A framework integrating the emotion-cognition approach to scent marketing. AMS Rev. 2021, 11, 145–161. [Google Scholar] [CrossRef]
- Álvarez-Pato, V.M.; Sánchez, C.N.; Domínguez-Soberanes, J.; Méndoza-Pérez, D.E.; Velázquez, R. A Multisensor Data Fusion Approach for Predicting Consumer Acceptance of Food Products. Foods 2020, 9, 774. [Google Scholar] [CrossRef]
- Morquecho-Campos, P.; De Graaf, K.; Boesveldt, S. Smelling our appetite? The influence of food odors on congruent appetite, food preferences and intake. Food Qual. Prefer. 2020, 85, 103959. [Google Scholar] [CrossRef]
- Baccarani, A.; Brand, G.; Dacremont, C.; Valentin, D.; Brochard, R. The influence of stimulus concentration and odor intensity on relaxing and stimulating perceived properties of odors. Food Qual. Prefer. 2021, 87, 104030. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable Sensors for Assessing the Role of Olfactory Training on the Autonomic Response to Olfactory Stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef]
- Huart, C.; Legrain, V.; Hummel, T.; Rombaux, P.; Mouraux, A. Time-Frequency Analysis of Chemosensory Event-Related Potentials to Characterize the Cortical Representation of Odors in Humans. PLoS ONE 2012, 7, e33221. [Google Scholar] [CrossRef]
- Sato, M.; Kodama, N.; Sasaki, T.; Ohta, M. Olfactory evoked potentials: Experimental and clinical studies. J. Neurosurg. 1996, 85, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rupprecht, S.; Sun, X.; Freiberg, D.; Crowell, C.; Cartisano, E.; Vasavada, M.; Yang, Q.X. A Free-breathing fMRI Method to Study Human Olfactory Function. J. Vis. Exp. 2017, 125, e54898. [Google Scholar]
- Tonacci, A.; Billeci, L.; Burrai, E.; Sansone, F.; Conte, R. Comparative Evaluation of the Autonomic Response to Cognitive and Sensory Stimulations through Wearable Sensors. Sensors 2019, 19, 4661. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Di Monte, J.; Meucci, M.B.; Sansone, F.; Pala, A.P.; Billeci, L.; Conte, R. Wearable Sensors to Characterize the Autonomic Nervous System Correlates of Food-Like Odors Perception: A Pilot Study. Electronics 2019, 8, 1481. [Google Scholar] [CrossRef]
- Knaapila, A.; Laaksonen, O.; Virtanen, M.; Yang, B.; Lagström, H.; Sandell, M. Pleasantness, familiarity, and identification of spice odors are interrelated and enhanced by consumption of herbs and food neophilia. Appetite 2017, 109, 190–200. [Google Scholar] [CrossRef]
- Venturi, F.; Andrich, G.; Sanmartin, C.; Taglieri, I.; Scalabrelli, G.; Ferroni, G.; Zinnai, A. Glass and wine: A good example of the deep relationship between drinkware and beverage. J. Wine Res. 2016, 27, 153–171. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Quartacci, M.F.; Sgherri, C.; Andrich, G.; Zinnai, A. A kinetic approach to describe the time evolution of red wine as a function of packaging conditions adopted: Influence of closure and storage position. Food Packag. Shelf Life 2017, 13, 44–48. [Google Scholar] [CrossRef]
- Lötsch, J.; Lange, C.; Hummel, T. A Simple and Reliable Method for Clinical Assessment of Odor Thresholds. Chem. Senses 2004, 29, 311–317. [Google Scholar] [CrossRef]
- Solar, H.; Fernández, E.; Tartarisco, G.; Pioggia, G.; Cvetković, B.; Kozina, S.; Luštrek, M.; Lampe, J. A Non Invasive, Wearable Sensor Platform for Multi-parametric Remote Monitoring in CHF Patients. In Impact Analysis of Solutions for Chronic Disease Prevention and Management; ICOST 2012. Lecture Notes in Computer, Science; Donnelly, M., Paggetti, C., Nugent, C., Mokhtari, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7251. [Google Scholar]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Dodo, N.; Hashimoto, R. Autonomic Nervous System Activity During a Speech Task. Front. Neurosci. 2019, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kendall, M. A New Measure of Rank Correlation. Biometrika 1938, 30, 81–89. [Google Scholar] [CrossRef]
- Pakhomov, S.V.; Thuras, P.D.; Finzel, R.; Eppel, J.; Kotlyar, M. Using consumer-wearable technology for remote assessment of physiological response to stress in the naturalistic environment. PLoS ONE 2020, 15, e0229942. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Sansone, F.; Pala, A.P.; Centrone, A.; Napoli, F.; Domenici, C.; Conte, R. Effect of feeding on neurovegetative response to olfactory stimuli. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), IEEE, Sinaia, Romania, 22–24 June 2017. [Google Scholar]
- Nakanishi, Y.; Sato, Y.; Nakata, H.; Nakahara, H.; Inoue, Y.; Kinoshita, H. The Influence of Emotionally Linked Odors on Autonomic Nerve Activities. Am. J. BioSci. 2021, 9, 147–152. [Google Scholar] [CrossRef]
- Delplanque, S.; Grandjean, D.; Chrea, C.; Aymard, L.; Cayeux, I.; Le Calvé, B.; Velazco, M.I.; Scherer, K.R.; Sander, D. Emotional Processing of Odors: Evidence for a Nonlinear Relation between Pleasantness and Familiarity Evaluations. Chem. Senses 2008, 33, 469–479. [Google Scholar] [CrossRef]
- Luisier, A.-C.; Petitpierre, G.; Bérod, A.C.; Garcia-Burgos, D.; Bensafi, M. Effects of familiarization on odor hedonic responses and food choices in children with autism spectrum disorders. Autism 2018, 23, 1460–1471. [Google Scholar] [CrossRef]
- Joussain, P.; Rouby, C.; Bensafi, M. A pleasant familiar odor influences perceived stress and peripheral nervous system activity during normal aging. Front. Psychol. 2014, 5, 113. [Google Scholar] [CrossRef]
- Brann, J.H.; Firestein, S.J. A lifetime of neurogenesis in the olfactory system. Front. Neurosci. 2014, 8, 182. [Google Scholar] [CrossRef]
- Mouret, A.; Lepousez, G.; Gras, J.; Gabellec, M.M.; Lledo, P.M. Turnover of newborn olfactory bulb neurons optimizes olfaction. J. Neurosci. 2009, 29, 12302–12314. [Google Scholar] [CrossRef]
- Sultan-Styne, K.; Toledo, R.; Walker, C.; Kallkopf, A.; Ribak, C.E.; Guthrie, K.M. Long-term survival of olfactory sensory neurons after target depletion. J. Comp. Neurol. 2009, 515, 696–710. [Google Scholar] [CrossRef]
- Brai, E.; Tonacci, A.; Brugada-Ramentol, V.; D’Andrea, F.; Alberi, L. Intercepting dementia: Awareness and innovation as key tools. Front. Aging Neurosci. 2021, 13, 730727. [Google Scholar] [CrossRef] [PubMed]
- Virmani, A.; Pinto, L.; Binienda, Z.; Ali, S. Food, nutrigenomics, and neurodegeneration—Neuroprotection by what you eat! Mol. Neurobiol. 2013, 48, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Milošević, M.; Arsić, A.; Cvetković, Z.; Vučić, V. Memorable food: Fighting age-related neurodegeneration by precision nutrition. Front. Nutrit. 2021, 8, 688086. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Tonacci, A.; Billeci, L.; Catalucci, T.; Igliozzi, R.; Calderoni, S.; Narzisi, A. Olfactory processing in male children with autism: Atypical odor threshold and identification. J. Autism Dev. Disord. 2017, 47, 3243–3251. [Google Scholar] [CrossRef]
- Tonacci, A.; Calderoni, S.; Billeci, L.; Maestro, S.; Fantozzi, P.; Ciuccoli, F.; Morales, M.A.; Narzisi, A.; Muratori, F. Autistic traits impact on olfactory processing in adolescent girls with Anorexia Nervosa restricting type. Psychiatry Res. 2019, 274, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cosoli, G.; Antognoli, L.; Scalise, L. Methods for the metrological characterization of wearable devices for the measurement of physiological signals: State of the art and future challenges. MethodsX 2023, 10, 102038. [Google Scholar] [CrossRef]
- Pal, D.; Tassanaviboon, A.; Arpnikanondt, C.; Papasratorn, B. Quality of Experience of Smart-Wearables: From Fitness-Bands to Smartwatches. IEEE Consum. Electron. Mag. 2020, 9, 49–53. [Google Scholar] [CrossRef]
- Lara, O.D.; Labrador, M.A. A Survey on Human Activity Recognition using Wearable Sensors. IEEE Commun. Surv. Tutor. 2013, 15, 1192–1209. [Google Scholar] [CrossRef]
- Nahavandi, D.; Alizadehsani, R.; Khosravi, A.; Acharya, U.R. Application of artificial intelligence in wearable devices: Opportunities andchallenges. Comput. Methods Programs Biomed. 2022, 213, 106541. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ding, C.; Hu, X.; Rudin, C. A supervised machine learning semantic segmentation approach for detecting artifacts in plethysmography signals from wearables. Physiol. Meas. 2021, 42, 125003. [Google Scholar] [CrossRef] [PubMed]
- Saadatnejad, S.; Oveisi, M.; Hashemi, M. LSTM-Based ECG Classification for Continuous Monitoring on Personal Wearable Devices. IEEE J. Biomed. Health Inform. 2020, 24, 515–523. [Google Scholar] [CrossRef]
- Amirshahi, A.; Hashemi, M. ECG Classification Algorithm Based on STDP and R-STDP Neural Networks for Real-Time Monitoring on Ultra Low-Power Personal Wearable Devices. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1483–1493. [Google Scholar] [CrossRef] [PubMed]




| Sample Code | Descriptor | Formulation |
|---|---|---|
| 1 | Raspberry | White wine * (100 mL); raspberry juice ** (80 mL) |
| 2 | Grapefruit | White wine * (100 mL); grapefruit juice ** (80 mL) |
| 3 | Orange | White wine * (100 mL); orange juice *** (80 mL) |
| 4 | Pineapple | White wine * (100 mL); pineapple juice *** (80 mL) |
| 5 | Fig | No diluted dried figs (10 g) |
| 6 | Asparagus | White wine * (100 mL); asparagus cooking water (60 mL) ** |
| 7 | Peach | White wine * (100 mL); peach juice *** (80 mL) |
| 8 | Green pepper | Fresh green pepper (20 g) in 100 mL of white wine * |
| 9 | Mango | Dried mango (5 g) in 100 mL of white wine * |
| 10 | Rose | Distilled rose water (4 mL) + 100 mL of white wine * |
| Feature | T0 | T1 | p-Value |
|---|---|---|---|
| Baseline | |||
| HR (bpm) | 75.8 ± 6.9 | 69.8 ± 8.3 | 0.008 ** |
| RMSSD (s) | 0.036 ± 0.013 | 0.052 ± 0.030 | 0.016 * |
| NN50 (n.u.) | 13.833 ± 8.533 | 18.909 ± 14.029 | 0.028 * |
| CSI (n.u.) | 3.093 ± 0.754 | 2.359 ± 1.012 | 0.008 ** |
| CVI (n.u.) | −2.790 ± 0.309 | −2.716 ± 0.352 | 0.286 |
| LF (s2/Hz) | −0.094 ± 0.828 | −0.124 ± 0.782 | 0.091 |
| HF (s2/Hz) | 0.344 ± 0.232 | 0.690 ± 0.488 | 0.026 * |
| LF/HF (n.u.) | 1.617 ± 2.147 | 0.655 ± 0.716 | 0.026 * |
| Task | |||
| HR (bpm) | 80.5 ± 9.0 | 72.6 ± 7.7 | 0.005 ** |
| RMSSD (s) | 0.027 ± 0.008 | 0.037 ± 0.016 | 0.022 * |
| NN50 (n.u.) | 36.150 ± 39.568 | 62.960 ± 61.404 | 0.038 * |
| CSI (n.u.) | 3.928 ± 0.902 | 3.145 ± 0.822 | 0.005 ** |
| CVI (n.u.) | −2.912 ± 0.232 | −2.789 ± 0.329 | 0.139 |
| LF (s2/Hz) | 0.172 ± 0.718 | 0.081 ± 0.834 | 0.169 |
| HF (s2/Hz) | 0.121 ± 0.098 | 0.415 ± 0.484 | 0.005 ** |
| LF/HF (n.u.) | 4.370 ± 3.647 | 2.166 ± 2.302 | 0.093 |
| Recovery | |||
| HR (bpm) | 76.5 ± 7.8 | 71.5 ± 8.2 | 0.010 * |
| RMSSD (s) | 0.027 ± 0.011 | 0.034 ± 0.013 | 0.026 * |
| NN50 (n.u.) | 8.241 ± 5.763 | 11.633 ± 9.131 | 0.058 |
| CSI (n.u.) | 3.162 ± 0.854 | 2.913 ± 1.053 | 0.424 |
| CVI (n.u.) | −3.029 ± 0.304 | −2.874 ± 0.235 | 0.010 * |
| LF (s2/Hz) | −0.091 ± 0.867 | −0.106 ± 0.890 | 0.722 |
| HF (s2/Hz) | 0.307 ± 0.182 | 0.553 ± 0.513 | 0.062 |
| LF/HF (n.u.) | 1.809 ± 1.588 | 1.407 ± 1.752 | 0.062 |
| Feature | T0 | T1 | p-Value |
|---|---|---|---|
| Baseline | |||
| Global (µS) | 2.003 ± 1.683 | 0.994 ± 0.320 | 0.023 * |
| Tonic (µS) | 1.792 ± 1.643 | 0.762 ± 0.420 | 0.019 * |
| Phasic (µS) | 0.210 ± 0.226 | 0.232 ± 0.240 | 0.814 |
| Task | |||
| Global (µS) | 3.099 ± 2.391 | 1.503 ± 0.772 | 0.012 * |
| Tonic (µS) | 2.819 ± 2.278 | 1.164 ± 0.580 | 0.010 * |
| Phasic (µS) | 0.280 ± 0.224 | 0.339 ± 0.339 | 0.530 |
| Recovery | |||
| Global (µS) | 3.145 ± 2.394 | 1.528 ± 0.791 | 0.012 * |
| Tonic (µS) | 2.941 ± 2.366 | 1.279 ± 0.763 | 0.015 * |
| Phasic (µS) | 0.204 ± 0.185 | 0.248 ± 0.234 | 0.695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billeci, L.; Sanmartin, C.; Tonacci, A.; Taglieri, I.; Bachi, L.; Ferroni, G.; Braceschi, G.P.; Odello, L.; Venturi, F. Wearable Sensors to Evaluate Autonomic Response to Olfactory Stimulation: The Influence of Short, Intensive Sensory Training. Biosensors 2023, 13, 478. https://doi.org/10.3390/bios13040478
Billeci L, Sanmartin C, Tonacci A, Taglieri I, Bachi L, Ferroni G, Braceschi GP, Odello L, Venturi F. Wearable Sensors to Evaluate Autonomic Response to Olfactory Stimulation: The Influence of Short, Intensive Sensory Training. Biosensors. 2023; 13(4):478. https://doi.org/10.3390/bios13040478
Chicago/Turabian StyleBilleci, Lucia, Chiara Sanmartin, Alessandro Tonacci, Isabella Taglieri, Lorenzo Bachi, Giuseppe Ferroni, Gian Paolo Braceschi, Luigi Odello, and Francesca Venturi. 2023. "Wearable Sensors to Evaluate Autonomic Response to Olfactory Stimulation: The Influence of Short, Intensive Sensory Training" Biosensors 13, no. 4: 478. https://doi.org/10.3390/bios13040478
APA StyleBilleci, L., Sanmartin, C., Tonacci, A., Taglieri, I., Bachi, L., Ferroni, G., Braceschi, G. P., Odello, L., & Venturi, F. (2023). Wearable Sensors to Evaluate Autonomic Response to Olfactory Stimulation: The Influence of Short, Intensive Sensory Training. Biosensors, 13(4), 478. https://doi.org/10.3390/bios13040478








