Cobalt and Iron Phthalocyanine Derivatives: Effect of Substituents on the Structure of Thin Films and Their Sensor Response to Nitric Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CoPcR4 and FePcR4 and Preparation of Their Films
2.2. Characterization of Metal Phthalocyanines and Their Films
2.3. Study of the Sensor Properties of MPc Films
2.4. Quantum-Chemical Calculations
3. Results and Discussion
3.1. Single Crystal Structure of CoPcCl4 and FePcCl4
3.2. Thin Films of CoPcR4 and FePcR4 (R = H, F, Cl, tBu)
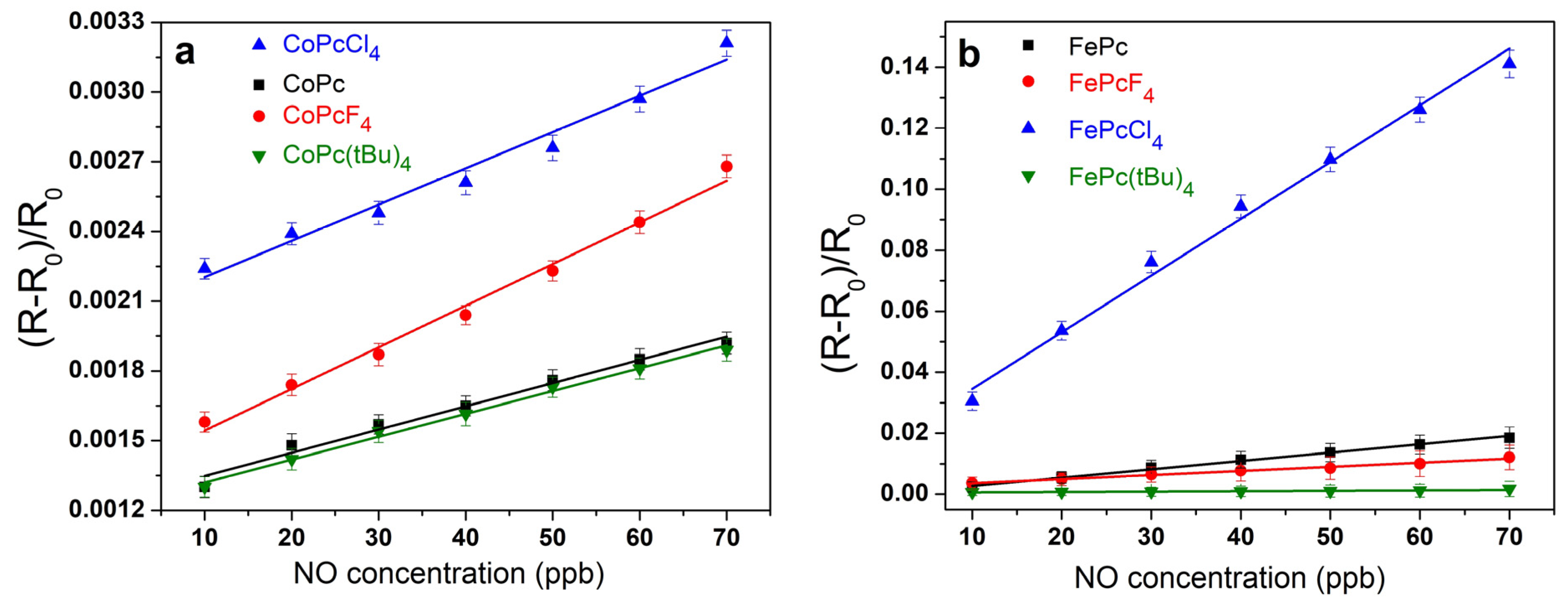
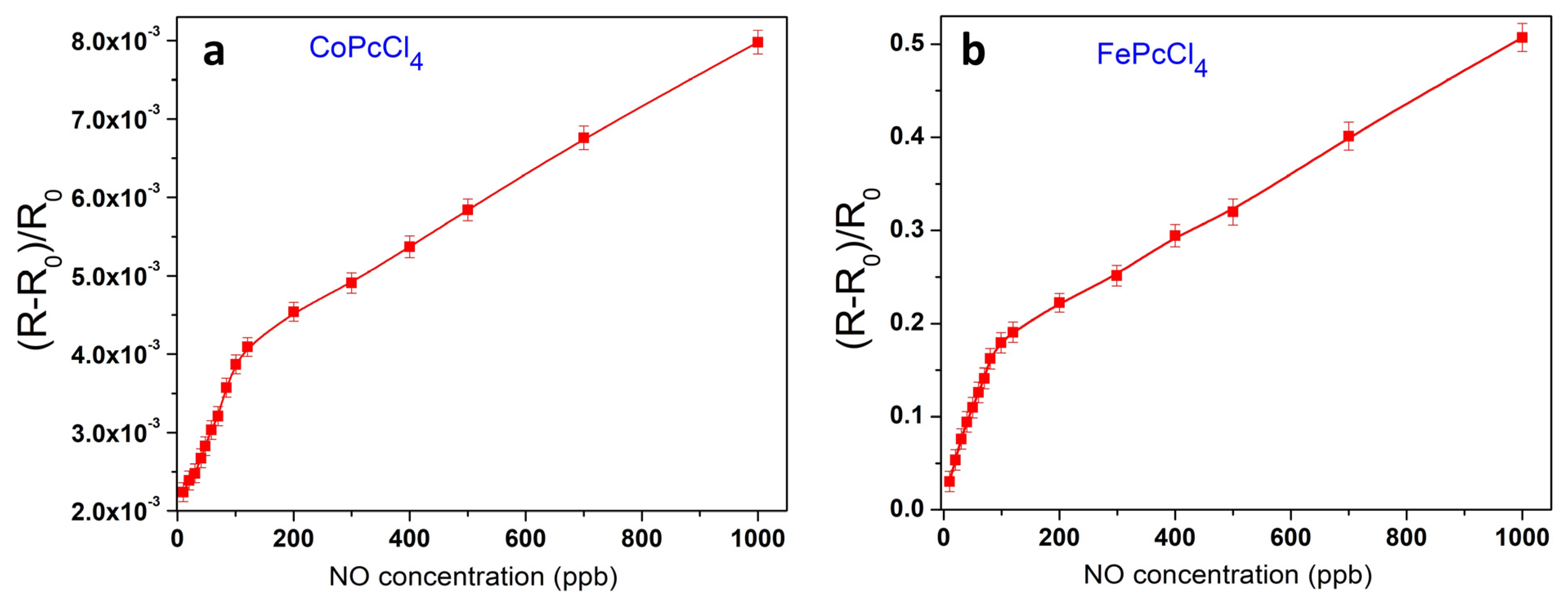
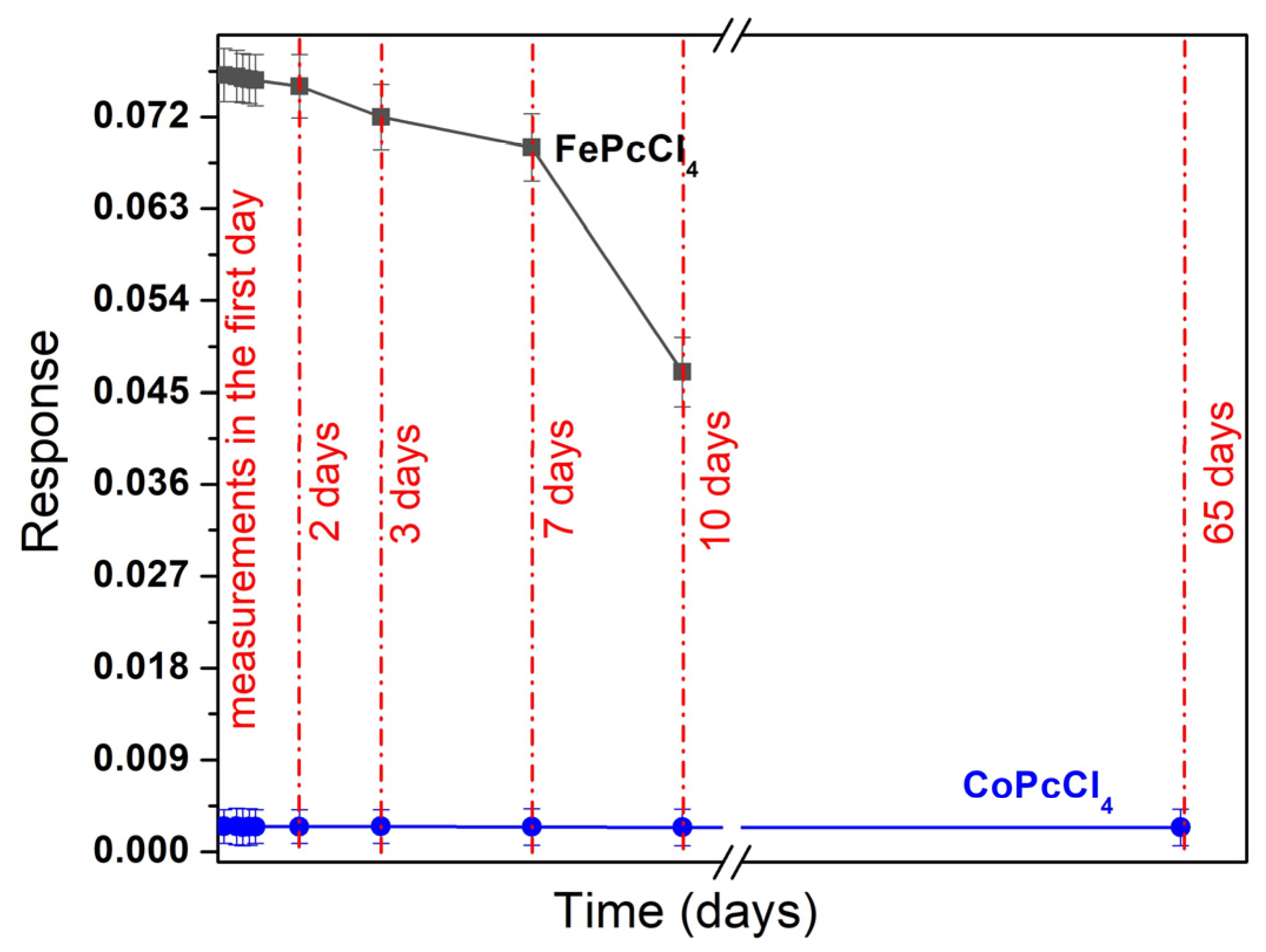
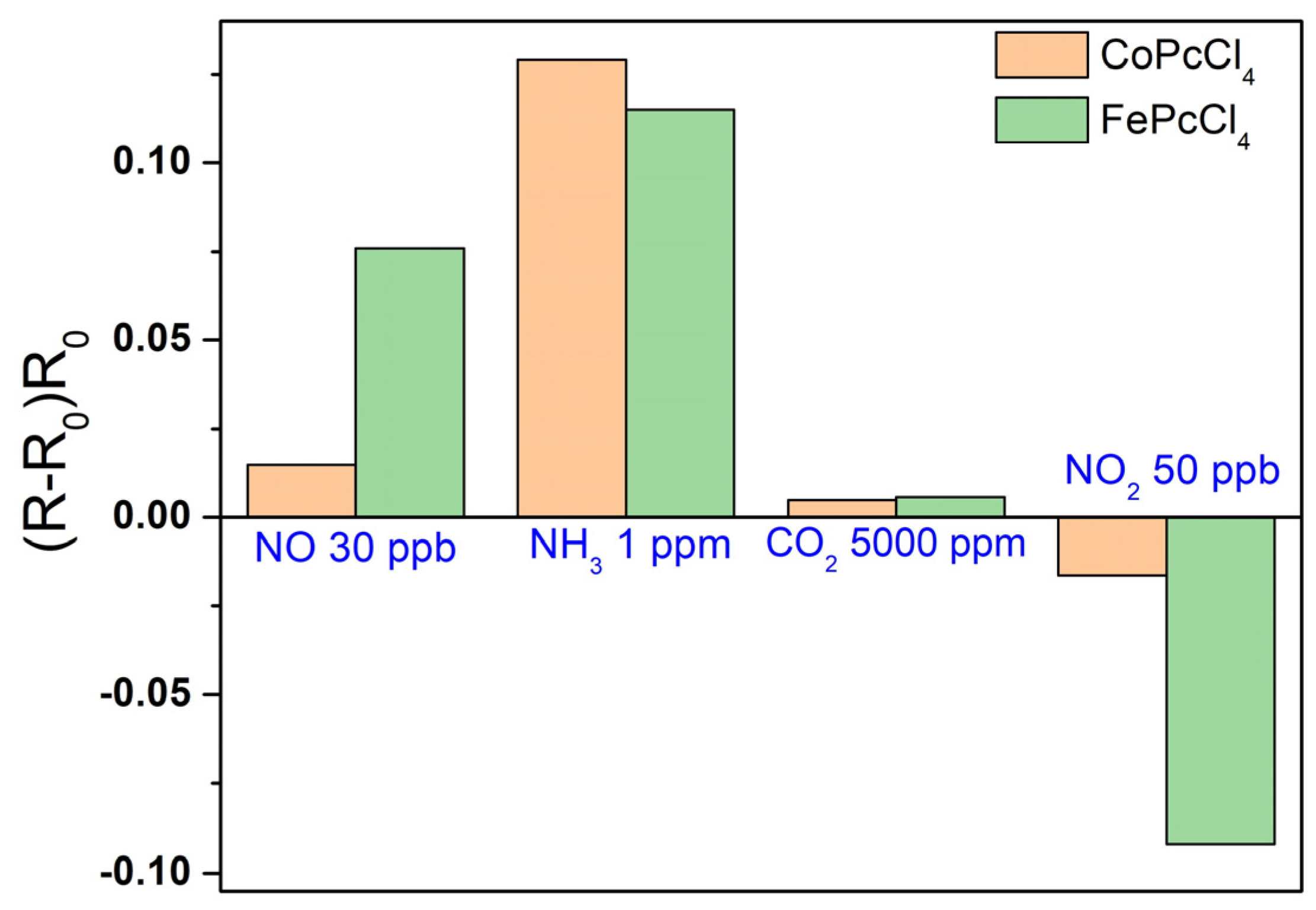
3.3. Sensor Properties of CoPcR4 and FePcR4 (R = H, F, Cl, tBu) Films
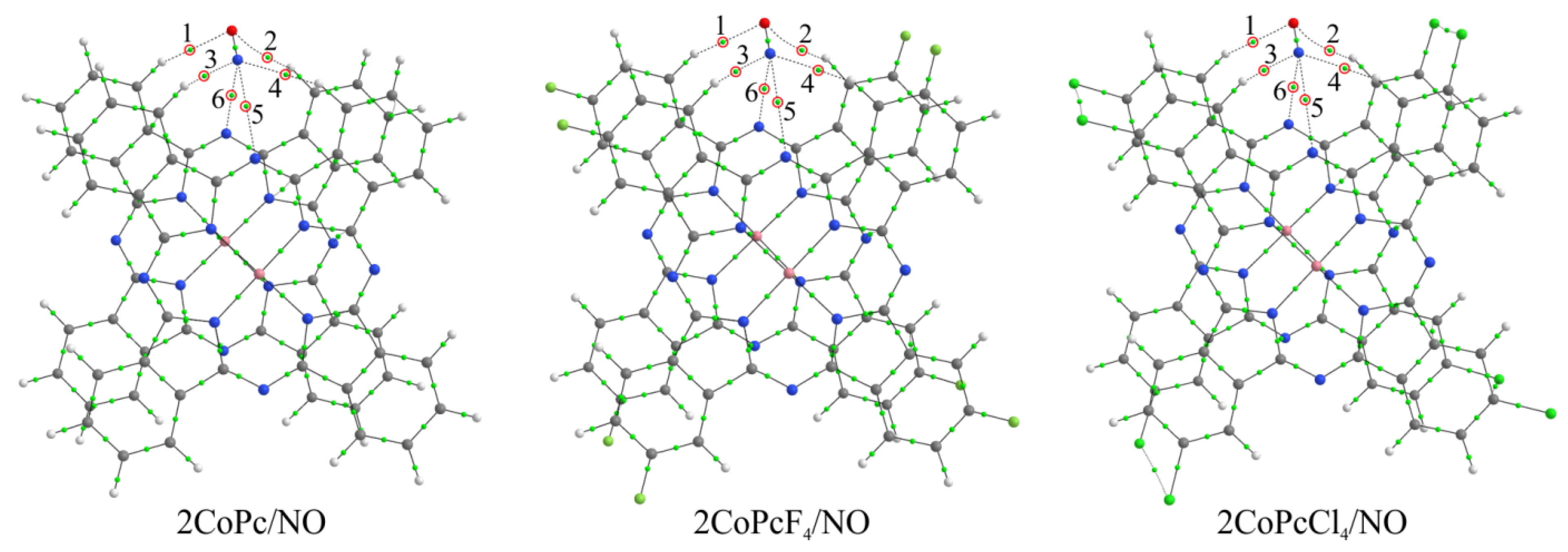
3.4. Quantum-Chemical Modeling of the Interaction between NO and MPcR4 Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasilescu, A.; Hrinczenko, B.; Swain, G.M.; Peteu, S.F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef] [PubMed]
- Pisi, R.; Aiello, M.; Tzani, P.; Marangio, E.; Olivieri, D.; Chetta, A. Measurement of fractional exhaled nitric oxide by a new portable device: Comparison with the standard technique. J. Asthma 2010, 47, 805–809. [Google Scholar] [CrossRef]
- Lim, K.G. Nitric oxide measurement in chronic cough. Lung 2010, 188, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Birajdar, S.N.; Adhyapak, P.V. Palladium-decorated vanadium pentoxide as NOx gas sensor. Ceram. Int. 2020, 46, 27381–27393. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated metal phthalocyanines: Interplay between fluorination degree, films orientation, and ammonia sensing properties. Sensors 2018, 18, 2141. [Google Scholar] [CrossRef]
- Kumawat, L.K.; Mergu, N.; Singh, A.K.; Gupta, V.K. A novel optical sensor for copper ions based on phthalocyanine tetrasulfonic acid. Sens. Actuators B Chem. 2015, 212, 389–394. [Google Scholar] [CrossRef]
- Yang, R.D.; Gredig, T.; Colesniuc, C.N.; Park, J.; Schuller, I.K.; Trogler, W.C.; Kummel, A.C. Ultrathin organic transistors for chemical sensing. Appl. Phys. Lett. 2007, 90, 263506. [Google Scholar] [CrossRef]
- Bouvet, M.; Gaudillat, P.; Suisse, J.M. Phthalocyanine-based hybrid materials for chemosensing. J. Porphyr. Phthalocyanines 2013, 17, 913–919. [Google Scholar] [CrossRef]
- Giancane, G.; Valli, L. State of art in porphyrin Langmuir–Blodgett films as chemical sensors. Adv. Colloid Interface Sci. 2012, 171–172, 17–35. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; van Zyl, W.E. Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 2020, 420, 213359. [Google Scholar] [CrossRef]
- Klyamer, D.; Shutilov, R.; Basova, T. Recent Advances in Phthalocyanine and Porphyrin-Based Materials as Active Layers for Nitric Oxide Chemical Sensors. Sensors 2022, 22, 895. [Google Scholar] [CrossRef] [PubMed]
- Knoben, W.; Crego-Calama, M.; Brongersma, S.H. Comparison of nitric oxide binding to different pure and mixed protoporphyrin IX monolayers. Sens. Actuators B Chem. 2012, 166–167, 349–356. [Google Scholar] [CrossRef]
- Sudarvizhi, A.; Pandian, K.; Oluwafemi, O.S.; Gopinath, S.C.B. Amperometry detection of nitrite in food samples using tetrasulfonated copper phthalocyanine modified glassy carbon electrode. Sens. Actuators B Chem. 2018, 272, 151–159. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, L.; Zhang, S.; Lou, Y.; Zhao, S.; Tan, Q.; He, L.; Du, M. A copper(II) phthalocyanine-based metallo-covalent organic framework decorated with silver nanoparticle for sensitively detecting nitric oxide released from cancer cells. Sens. Actuators B Chem. 2021, 338, 129826. [Google Scholar] [CrossRef]
- Cui, L.; Pu, T.; Liu, Y.; He, X. Layer-by-layer construction of graphene/cobalt phthalocyanine composite film on activated GCE for application as a nitrite sensor. Electrochim. Acta 2013, 88, 559–564. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Devendrachari, M.C.; Khan, F.; Thippeshappa, S.; Kotresh, H.M.N. Highly sensitive and selective detection of nitrite by polyaniline linked tetra amino cobalt (II) phthalocyanine surface functionalized ZnO hybrid electrocatalyst. Surf. Interfaces 2023, 36, 102565. [Google Scholar] [CrossRef]
- Ndebele, N.; Nyokong, T. Electrocatalytic behaviour of chalcone substituted Co, Cu, Mn and Ni phthalocyanines towards the detection of nitrite. J. Electroanal. Chem. 2022, 926, 116951. [Google Scholar] [CrossRef]
- Klyamer, D.; Bonegardt, D.; Basova, T. Fluoro-Substituted Metal Phthalocyanines for Active Layers of Chemical Sensors. Chemosensors 2021, 9, 133. [Google Scholar] [CrossRef]
- Bonegardt, D.; Klyamer, D.; Sukhikh, A.; Krasnov, P.; Popovetskiy, P.; Basova, T. Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. Chemosensors 2021, 9, 137. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Escaño, M.C.S.; Kasai, H. Nitric oxide adsorption effects on metal phthalocyanines. J. Phys. Chem. B 2010, 114, 10017–10021. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Padama, A.A.B.; Escano, M.C.S.; Kasai, H. Theoretical Study on The Adsorption of NO on Metal Macrocycles, Metal=Mn,Fe,Co,Ni,Cu,Zn. ECS Trans. 2013, 45, 91–100. [Google Scholar] [CrossRef]
- Grob, N.M.; Dweik, R.A. Exhaled Nitric Oxide in Asthma: From Diagnosis, to Monitoring, to Screening: Are We There Yet? Chest 2008, 133, 837–839. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. Interpretation of Exhaled Nitric Oxide Levels (FE NO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin films of tetrafluorosubstituted cobalt phthalocyanine: Structure and sensor properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar] [CrossRef]
- Şahin, Z.; Meunier-Prest, R.; Dumoulin, F.; Işci, Ü.; Bouvet, M. Alkylthio-tetrasubstituted μ-Nitrido Diiron Phthalocyanines: Spectroelectrochemistry, Electrical Properties, and Heterojunctions for Ammonia Sensing. Inorg. Chem. 2020, 59, 1057–1067. [Google Scholar] [CrossRef]
- Klyamer, D.; Bonegardt, D.; Krasnov, P.; Sukhikh, A.; Popovetskiy, P.; Basova, T. Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia. Chemosensors 2022, 10, 515. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of fluorosubstitution on the structure of zinc phthalocyanine thin films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ellis, D.E.; Ros, P. Self-consistent molecular Hartree-Fock-Slater calculations I. The computational procedure. Chem. Phys. 1973, 2, 41–51. [Google Scholar] [CrossRef]
- Dunlap, B.I.; Connolly, J.W.D.; Sabin, J.R. On some approximations in applications of Xα theory. J. Chem. Phys. 1979, 71, 3396–3402. [Google Scholar] [CrossRef]
- Van Alsenoy, C. Ab initio calculations on large molecules: The multiplicative integral approximation. J. Comput. Chem. 1988, 9, 620–626. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials (Chem. Phys. Lett. 240 (1995) 283) (PII:0009-2614(95)00621-4). Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atom in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Todd, A. Keith AIMAll, version 19.10.12; TK Gristmill Software: Overland Park, KS, USA, 2019. Available online: aim.tkgristmill.com (accessed on 1 March 2023).
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Basova, T.V.; Krasnov, P.O.; Sukhikh, A.S. Effect of fluorosubstitution and central metals on the molecular structure and vibrational spectra of metal phthalocyanines. J. Mol. Struct. 2019, 1189, 73–80. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Trubin, S.V.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V.; Hassan, A.K. Tetrafluorosubstituted Metal Phthalocyanines: Interplay between Saturated Vapor Pressure and Crystal Structure. Cryst. Growth Des. 2020, 20, 1016–1024. [Google Scholar] [CrossRef]
- Ballirano, P.; Caminiti, R.; Ercolani, C.; Maras, A.; Orrù, M.A. X-ray powder diffraction structure reinvestigation of the α and β forms of cobalt phthalocyanine and kinetics of the α→β phase transition. J. Am. Chem. Soc. 1998, 120, 12798–12807. [Google Scholar] [CrossRef]
- Ercolani, C.; Neri, C.; Porta, P. Synthesis and x-ray data of a stable in air crystalline modification of chromium(II) phthalocyanine (Cr-α-Pc). Inorg. Chim. Acta 1967, 1, 415–418. [Google Scholar] [CrossRef]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Krasnov, P.; Basova, T. Chlorosubstituted copper phthalocyanines: Spectral study and structure of thin films. Molecules 2020, 25, 1620. [Google Scholar] [CrossRef]
- Mason, R.; Williams, G.A.; Fielding, P.E. Structural chemistry of phthalocyaninato-cobalt(II) and -manganese(II). J. Chem. Soc. Dalt. Trans. 1979, 4, 676–683. [Google Scholar] [CrossRef]
- Erk, P. CCDC 112723: Experimental Crystal Structure Determination; CCDC: Cambridge, UK, 2004. [Google Scholar]
- Konarev, D.V.; Ishikawa, M.; Khasanov, S.S.; Otsuka, A.; Yamochi, H.; Saito, G.; Lyubovskaya, R.N. Synthesis, structural and magnetic properties of ternary complexes of (Me4P+)·{[Fe(I)Pc(-2)]-} triptycene and (Me4P+)·{[Fe(I)Pc(-2)]-}·(N,N,N′,N′-tetrabenzyl-p-phenylenediamine)0.5 with iron(I) phthalocyanine anions. Inorg. Chem. 2013, 52, 3851–3859. [Google Scholar] [CrossRef]
- Miki, H.; Matsubara, F.; Nakashima, S.; Ochi, S.; Nakagawa, K.; Matsuguchi, M.; Sadaoka, Y. A fractional exhaled nitric oxide sensor based on optical absorption of cobalt tetraphenylporphyrin derivatives. Sens. Actuators B Chem. 2016, 231, 458–468. [Google Scholar] [CrossRef]
- Jha, R.K.; Nanda, A.; Avasthi, P.; Arya, N.; Yadav, A.; Balakrishnan, V.; Bhat, N. Scalable Approach to Develop High Performance Chemiresistive Nitric Oxide Sensor. IEEE Trans. Nanotechnol. 2022, 21, 177–184. [Google Scholar] [CrossRef]
- Su, P.G.; Li, M.C. Recognition of binary mixture of NO2 and NO gases using a chemiresistive sensors array combined with principal component analysis. Sens. Actuators A Phys. 2021, 331, 112980. [Google Scholar] [CrossRef]
- Ho, K.-C.; Tsou, Y.-H. Chemiresistor-type NO gas sensor based on nickel phthalocyanine thin films. Sens. Actuators B Chem. 2001, 77, 253–259. [Google Scholar] [CrossRef]
- Meng, Z.; Aykanat, A.; Mirica, K.A. Welding Metallophthalocyanines into Bimetallic Molecular Meshes for Ultrasensitive, Low-Power Chemiresistive Detection of Gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Basova, T. Effect of non-peripheral fluorosubstitution on the structure of metal phthalocyanines and their films. Dye. Pigment. 2021, 192, 109442. [Google Scholar] [CrossRef]
- Chia, L.S.; Du, Y.H.; Palale, S.; Lee, P.S. Interaction of Copper Phthalocyanine with Nitrogen Dioxide and Ammonia Investigation Using X-ray Absorption Spectroscopy and Chemiresistive Gas Measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
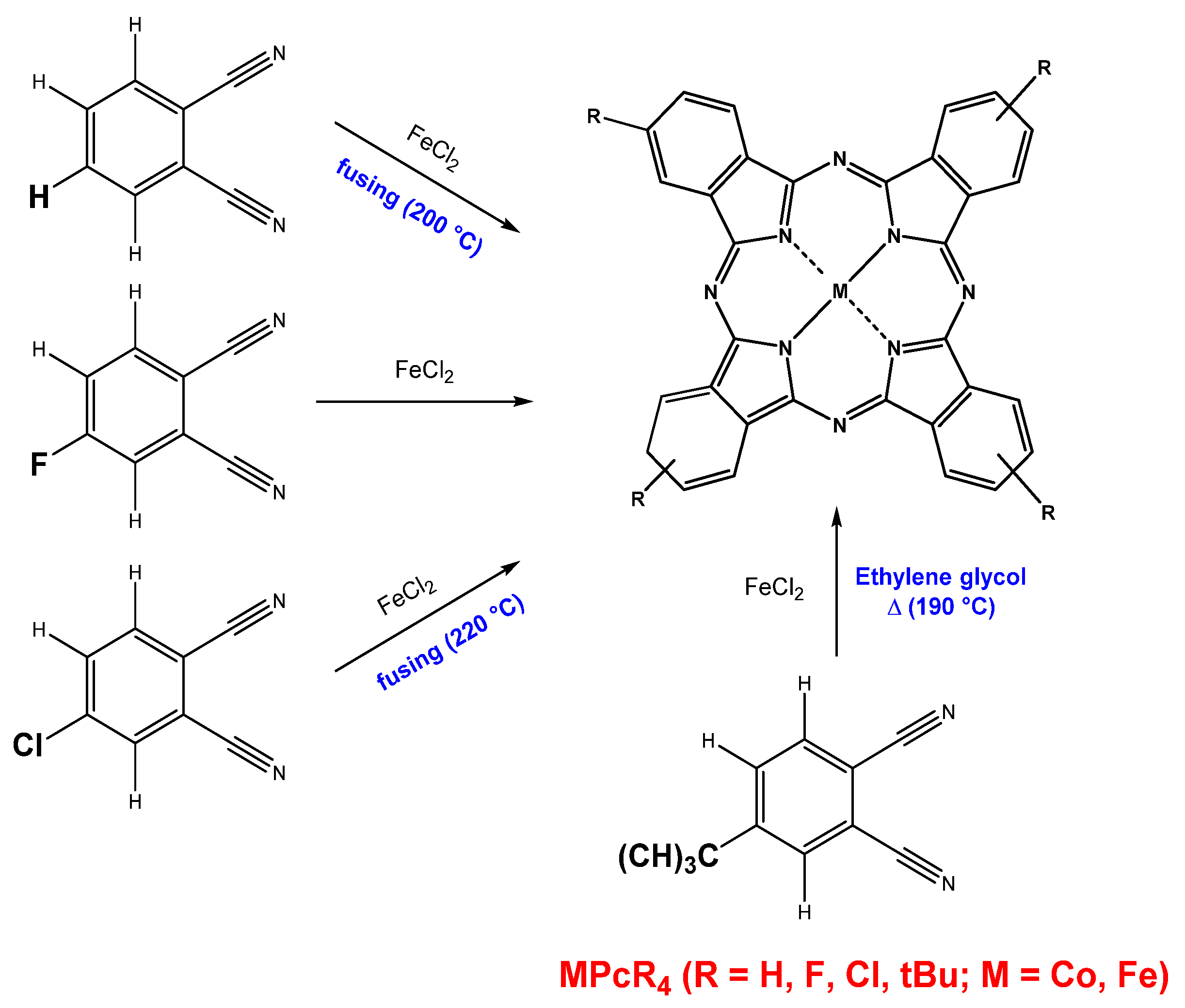

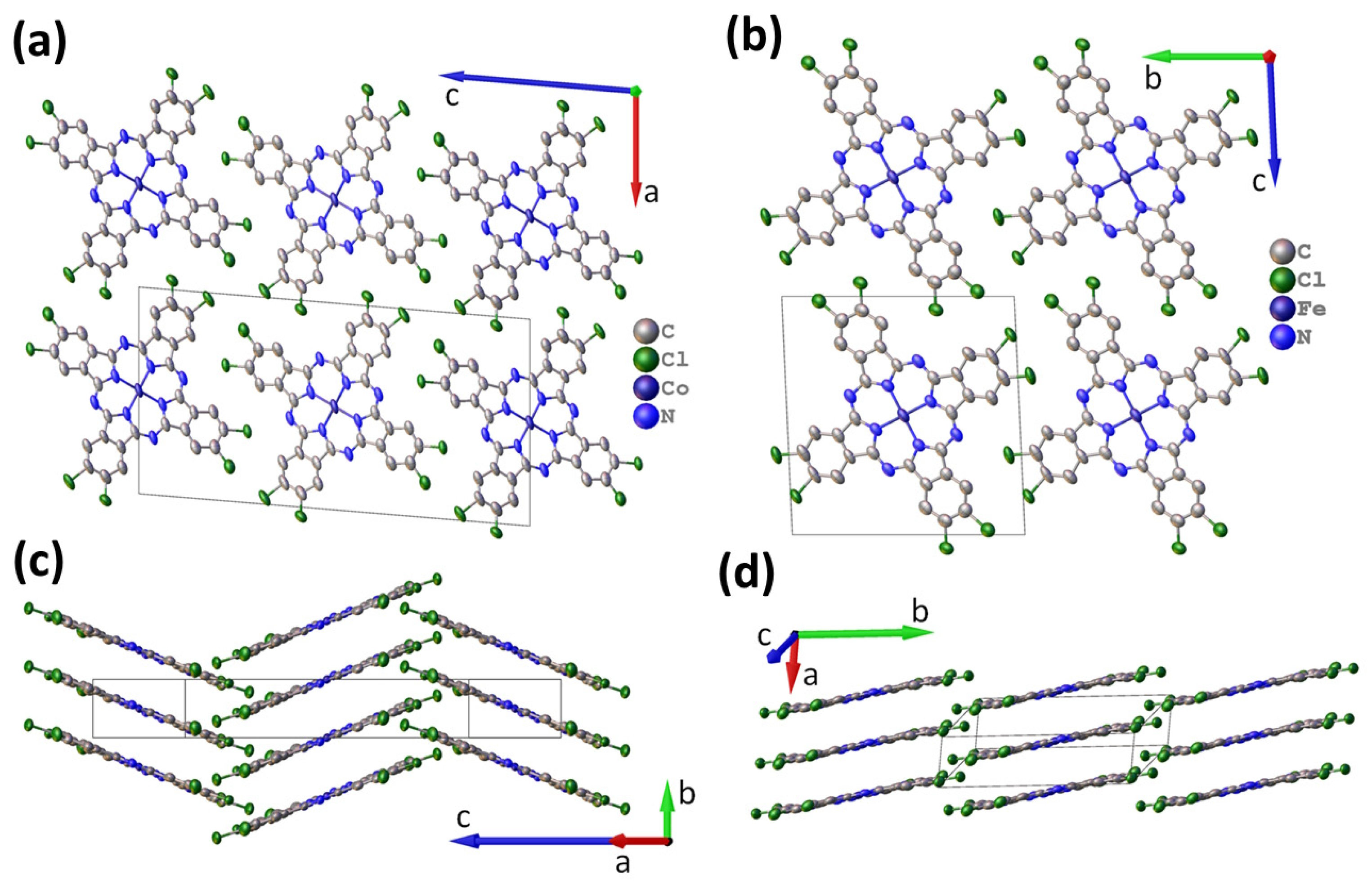
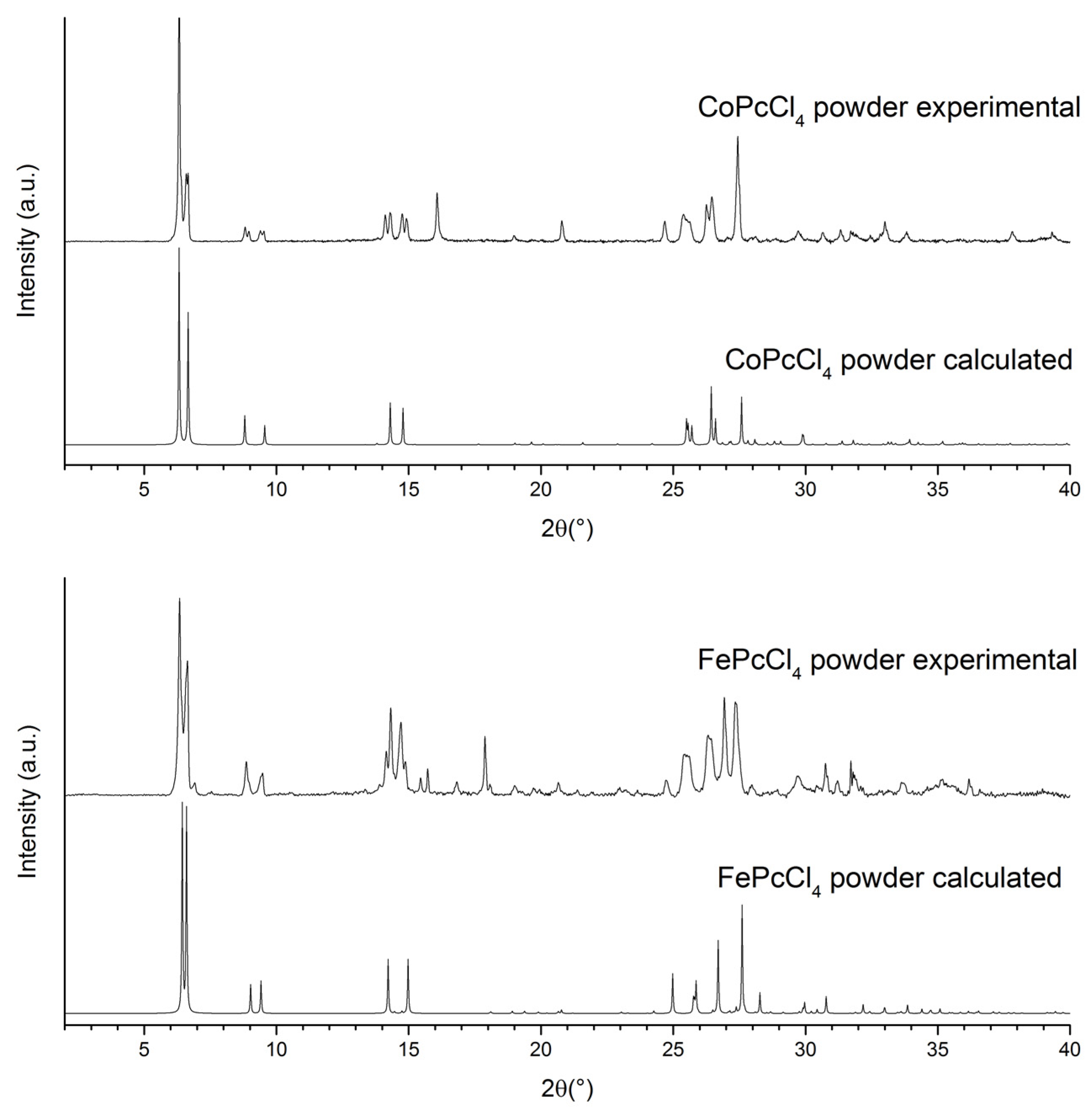






| Compound | CoPcCl4 | FePcCl4 |
|---|---|---|
| Empirical formula | C32H12Cl4CoN8 | C32H12Cl4FeN8 |
| Formula weight | 709.23 | 706.15 |
| Temperature/K | 150 | 150 |
| Crystal system | monoclinic | triclinic |
| Space group | P21/c | P-1 |
| a/Å | 14.0520(16) | 3.5971(3) |
| b/Å | 3.6200(4) | 13.5180(13) |
| c/Å | 26.611(3) | 13.7541(14) |
| α/° | 90 | 92.487(4) |
| β/° | 94.725(5) | 90.116(3) |
| γ/° | 90 | 97.517(3) |
| Volume/Å3 | 1349.0(3) | 662.41(11) |
| Z | 2 | 1 |
| ρcalcg/cm3 | 1.746 | 1.770 |
| μ/mm-1 | 1.075 | 1.017 |
| F(000) | 710.0 | 354.0 |
| Crystal size/mm3 | 0.12 × 0.02 × 0.02 | 0.04 × 0.02 × 0.005 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 4.4 to 51.37 | 4.152 to 51.472 |
| Index ranges | −17 ≤ h ≤ 17, 0 ≤ k ≤ 4, 0 ≤ l ≤ 32 | −4 ≤ h ≤ 4, −16 ≤ k ≤ 16, −16 ≤ l ≤ 16 |
| Reflections collected | 11220 | 7812 |
| Independent reflections | 2932 (Rint = 0.0636, Rsigma = 0.0640) | 2537 (Rint = 0.1003, Rsigma = 0.1331) |
| Data/restraints/parameters | 2932/0/226 | 2537/0/225 |
| Goodness-of-fit on F2 | 1.030 | 0.951 |
| Final R indexes (I >= 2σ (I)) | R1 = 0.0511, wR2 = 0.0983 | R1 = 0.0571, wR2 = 0.0984 |
| Final R indexes (all data) | R1 = 0.1023, wR2 = 0.1168 | R1 = 0.1565, wR2 = 0.1267 |
| Largest diff. peak/hole/e Å−3 | 0.26/−0.31 | 0.26/−0.27 |
| CCDC deposition № | 2231583 | 2231584 |
| Sensing Layer | Sensor Response to 30 ppb of NO | Response/Recovery Time, s | Calculated LOD, ppb |
|---|---|---|---|
| CoPcCl4 | 0.0025 | 15/120 | 7 |
| CoPcF4 | 0.0019 | 20/140 | 8.5 |
| CoPc | 0.0016 | 40/180 | 9 |
| CoPc(tBu)4 | 0.0015 | 20/90 | 9 |
| FePcCl4 | 0.076 | 30/80 | 3 |
| FePcF4 | 0.0065 | 15/290 | 5 |
| FePc | 0.0087 | 45/265 | 4 |
| FePc(tBu)4 | 0.0008 | 15/105 | 10 |
| Aggregate | M = Co | M = Fe | ||||||
|---|---|---|---|---|---|---|---|---|
| m = 1 | m = 2 | m = 3 | m = 4 | m = 1 | m = 2 | m = 3 | m = 4 | |
| MPc/NO-m | −0.098 | −0.025 | −0.022 | – | −0.102 | −0.025 | −0.022 | – |
| MPcF4/NO-m | −0.099 | −0.028 | −0.019 | −0.020 | −0.101 | −0.029 | −0.016 | −0.021 |
| MPcCl4/NO-m | −0.099 | −0.030 | −0.042 | −0.045 | −0.101 | −0.030 | −0.043 | −0.046 |
| 2MPc/NO | −0.093 | −0.095 | ||||||
| 2MPcF4/NO | −0.090 | −0.091 | ||||||
| 2MPcCl4/NO | −0.101 | −0.103 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyamer, D.; Shao, W.; Krasnov, P.; Sukhikh, A.; Dorovskikh, S.; Popovetskiy, P.; Li, X.; Basova, T. Cobalt and Iron Phthalocyanine Derivatives: Effect of Substituents on the Structure of Thin Films and Their Sensor Response to Nitric Oxide. Biosensors 2023, 13, 484. https://doi.org/10.3390/bios13040484
Klyamer D, Shao W, Krasnov P, Sukhikh A, Dorovskikh S, Popovetskiy P, Li X, Basova T. Cobalt and Iron Phthalocyanine Derivatives: Effect of Substituents on the Structure of Thin Films and Their Sensor Response to Nitric Oxide. Biosensors. 2023; 13(4):484. https://doi.org/10.3390/bios13040484
Chicago/Turabian StyleKlyamer, Darya, Wenping Shao, Pavel Krasnov, Aleksandr Sukhikh, Svetlana Dorovskikh, Pavel Popovetskiy, Xianchun Li, and Tamara Basova. 2023. "Cobalt and Iron Phthalocyanine Derivatives: Effect of Substituents on the Structure of Thin Films and Their Sensor Response to Nitric Oxide" Biosensors 13, no. 4: 484. https://doi.org/10.3390/bios13040484
APA StyleKlyamer, D., Shao, W., Krasnov, P., Sukhikh, A., Dorovskikh, S., Popovetskiy, P., Li, X., & Basova, T. (2023). Cobalt and Iron Phthalocyanine Derivatives: Effect of Substituents on the Structure of Thin Films and Their Sensor Response to Nitric Oxide. Biosensors, 13(4), 484. https://doi.org/10.3390/bios13040484








