An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Instrumentation
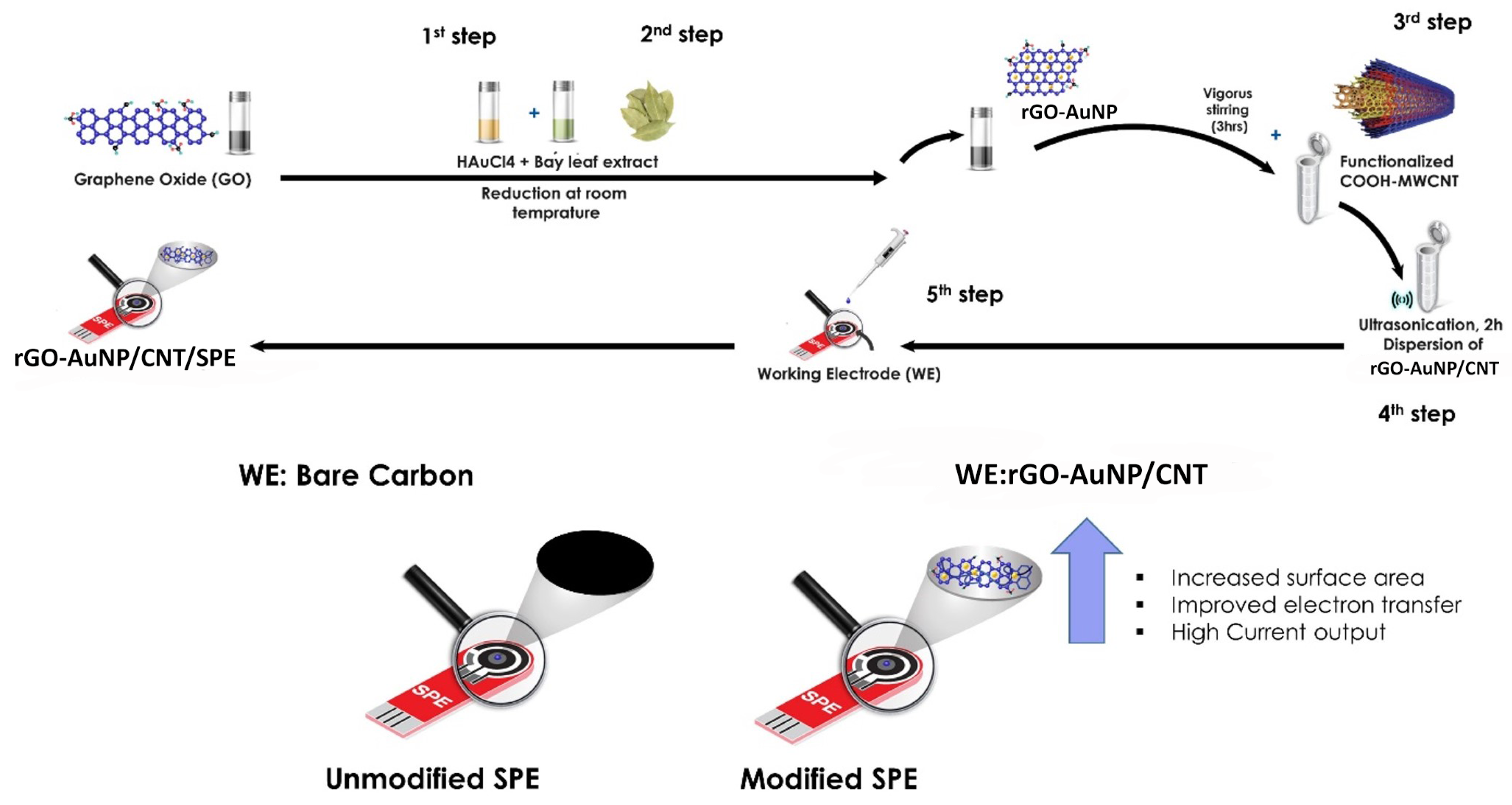
Synthesis of rGO-AuNPs/CNT and Electrode Fabrication
3. Results
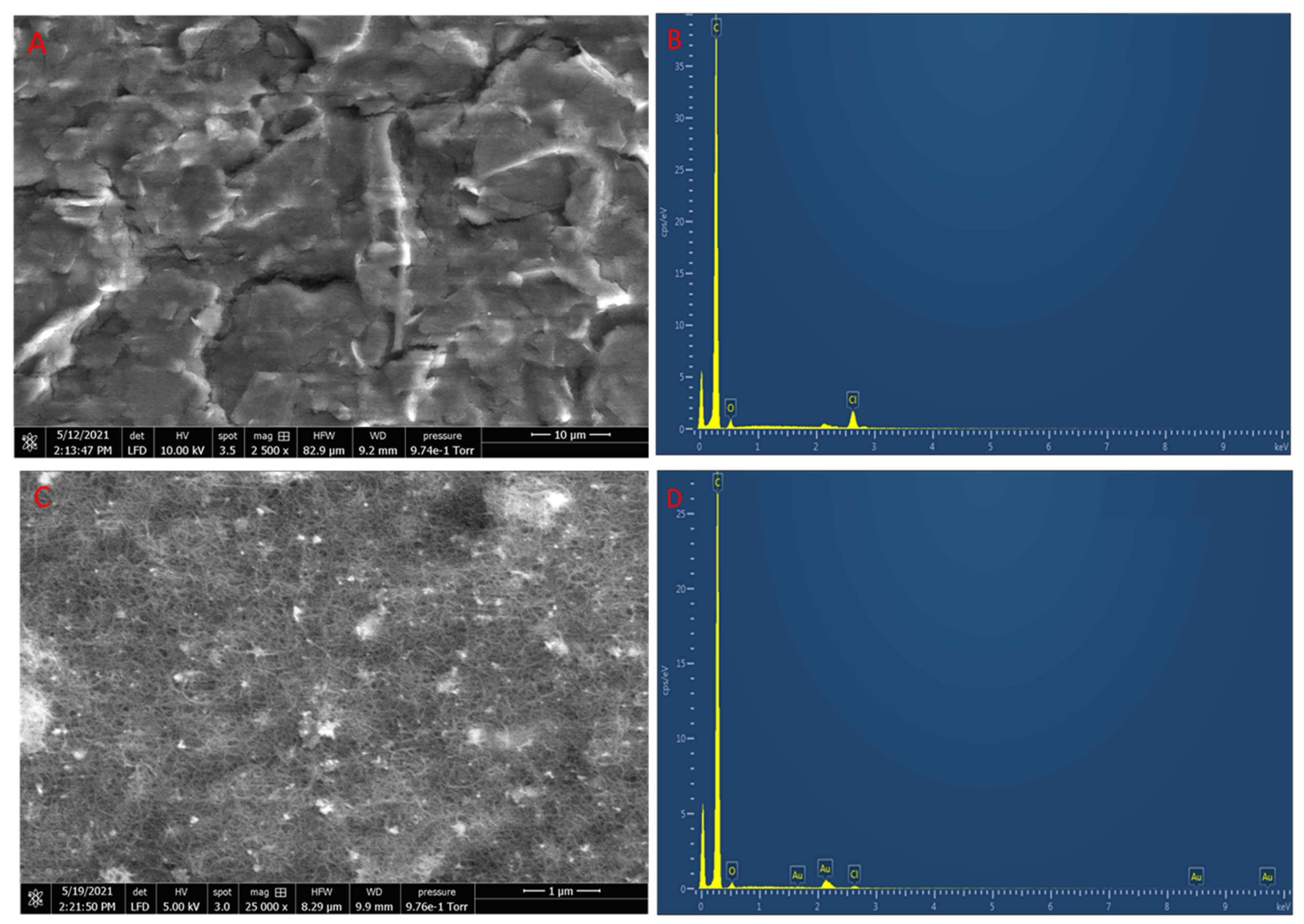
3.1. Characterisation of rGO-AuNPs/CNT
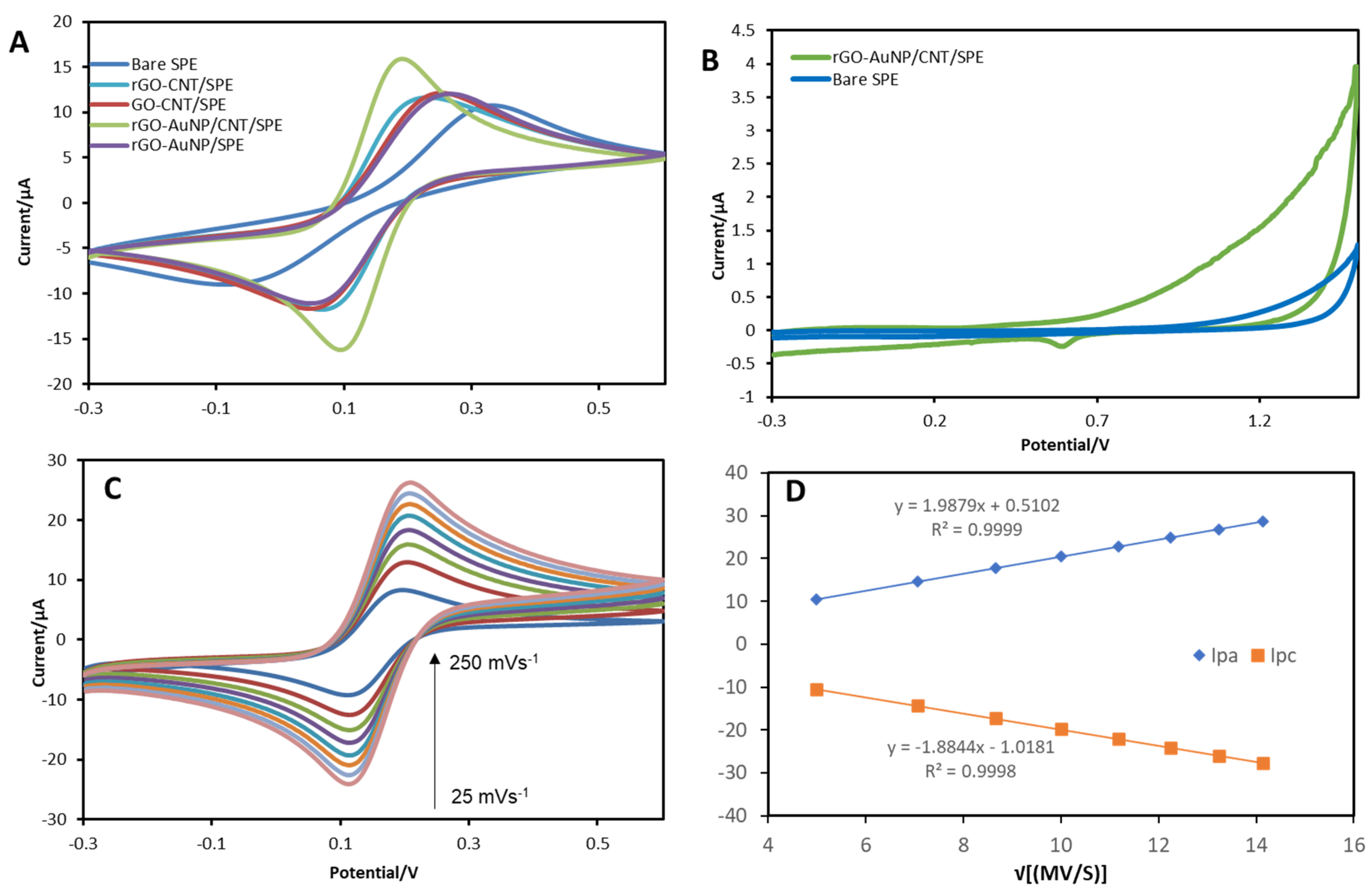
3.2. Electrochemical Characterisation
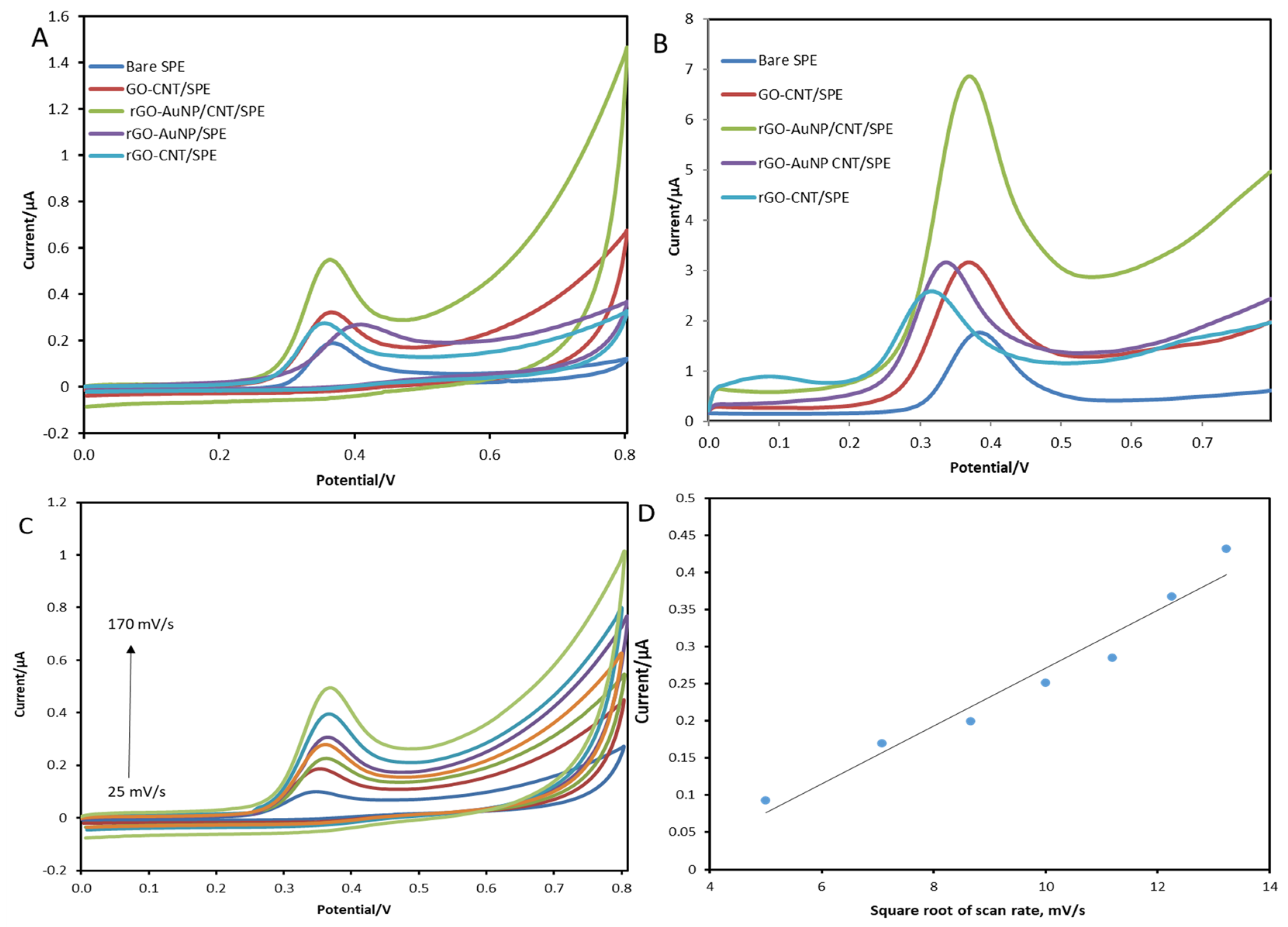
Voltammetric Behaviour of 17β-Estradiol
3.3. Effect of pH
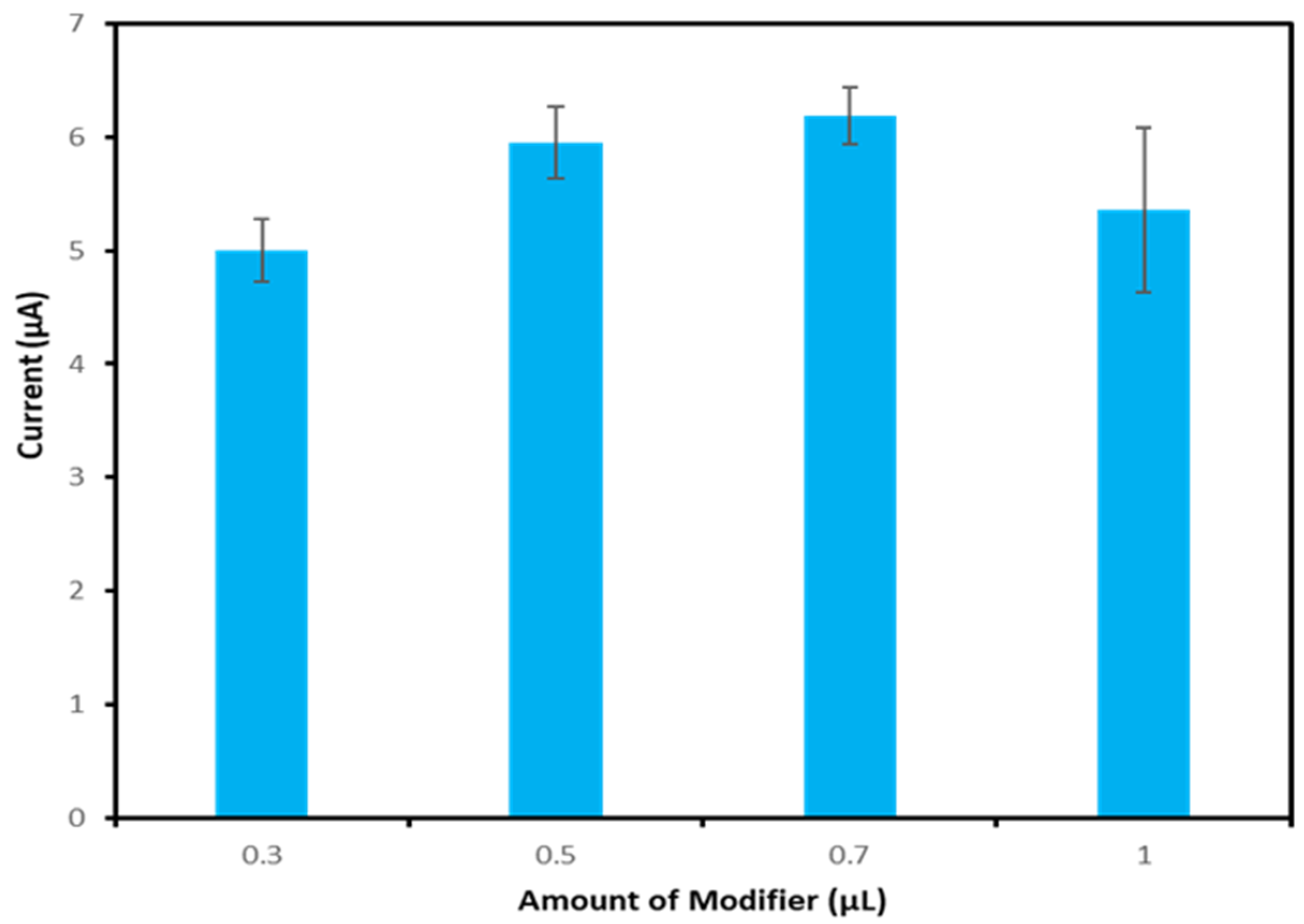
3.4. Optimisation of Modifier
3.5. Chemometric Fourier Analysis
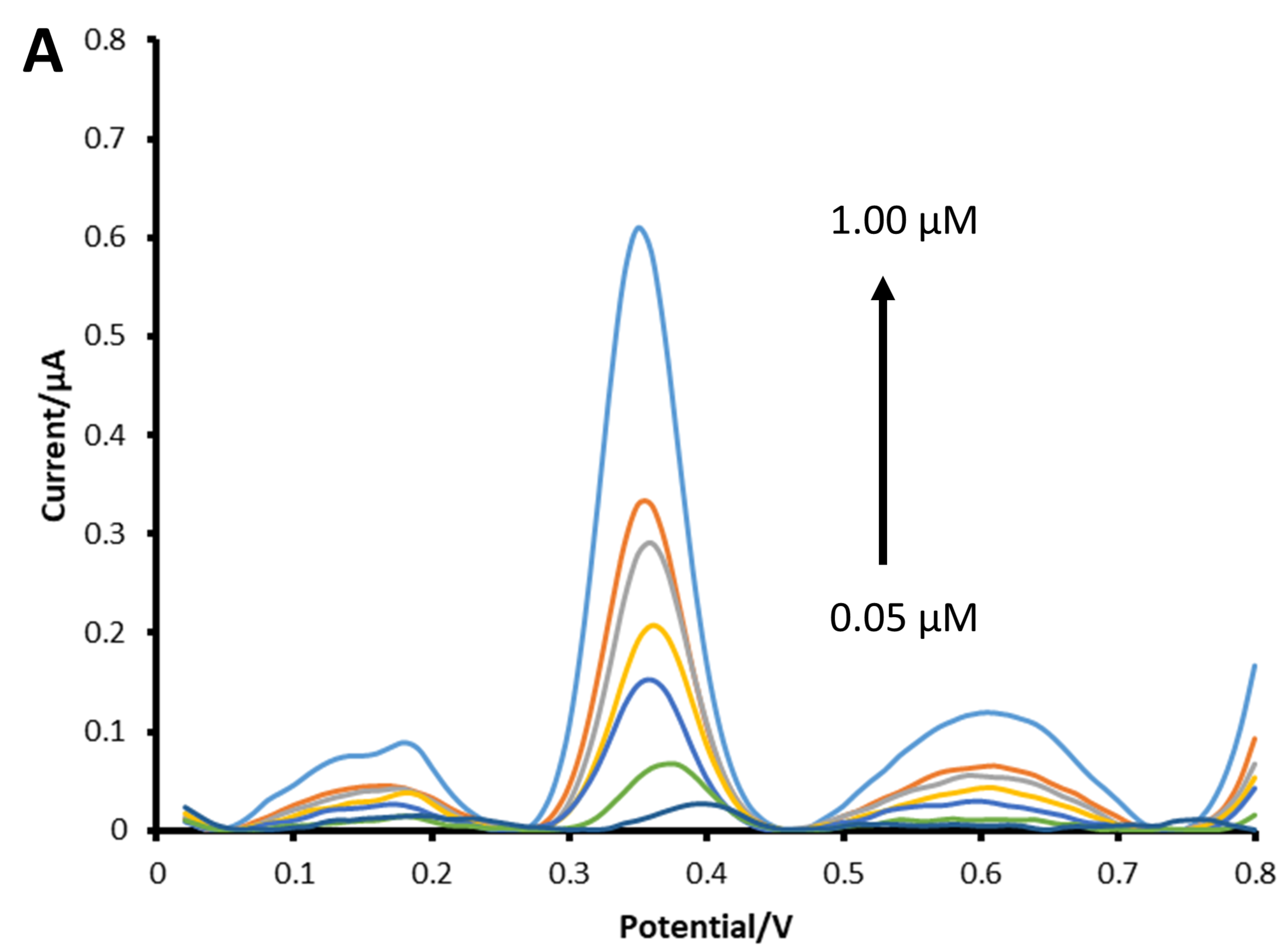
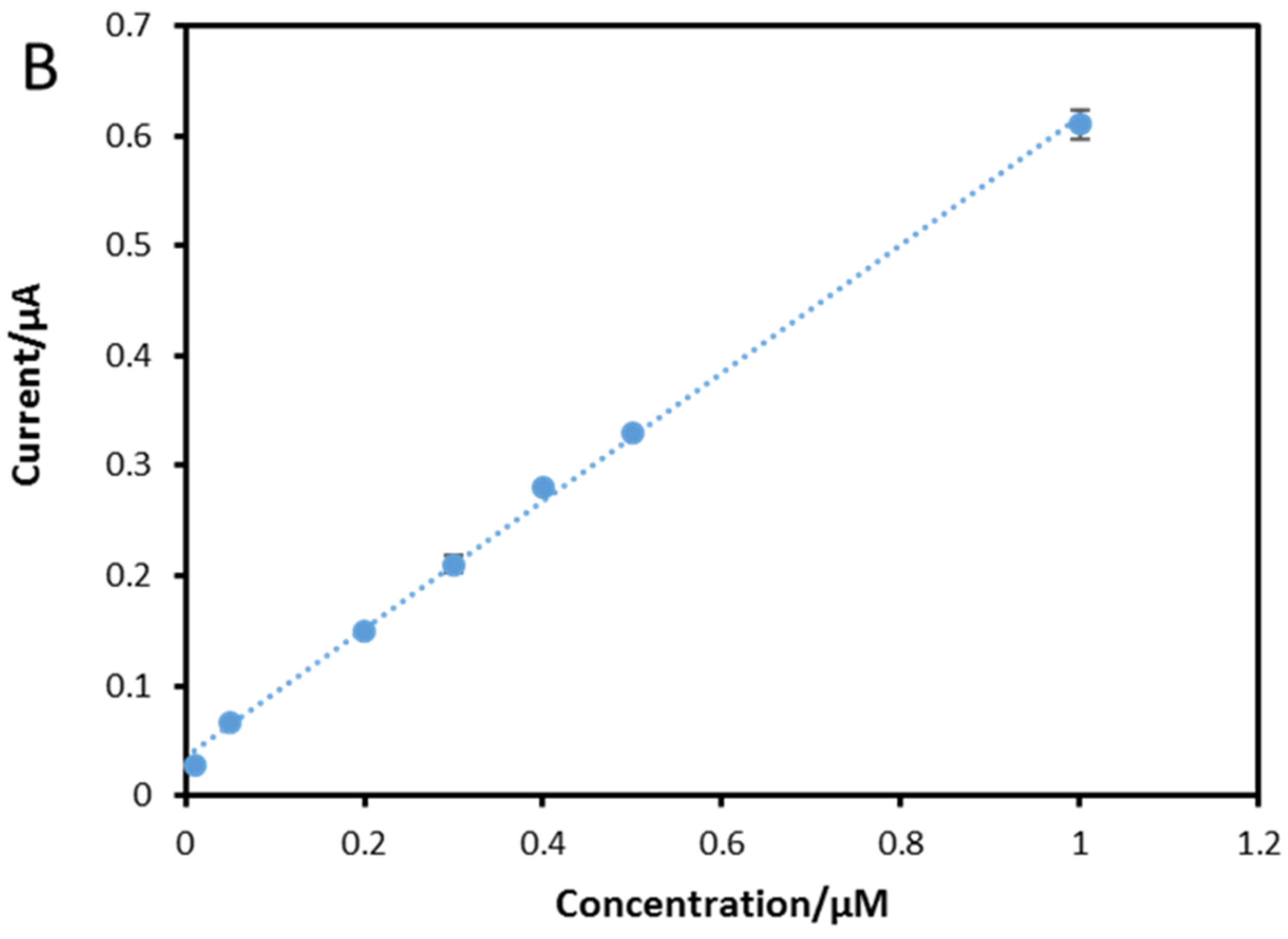
3.6. Calibration Curve and Limit of Detection
3.7. Reproducibility and Interference Studies
3.8. Analysis of E2 in Drinking Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Dang, Z.; Yin, H.; Liu, Y. Making waves: Improving removal performance of conventional wastewater treatment plants on endocrine disrupting compounds (EDCs): Their conjugates matter. Water Res. 2021, 188, 116469. [Google Scholar] [CrossRef] [PubMed]
- Pillon, A.; Boussioux, A.-M.; Escande, A.; Ait-Aissa, S.; Gomez, E.; Fenet, H.; Ruff, M.; Moras, D.; Vignon, F.; Duchesne, M.-J.; et al. Binding of Estrogenic Compounds to Recombinant Estrogen Receptor-α: Application to Environmental Analysis. Environ. Health Perspect. 2005, 113, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Thomas, J.L.; Liu, W.-C.; Zhang, Z.-X.; Liu, B.-D.; Yang, C.-H.; Lin, H.-Y. A multichannel system integrating molecularly imprinted conductive polymers for ultrasensitive voltammetric determination of four steroid hormones in urine. Microchim. Acta 2019, 186, 695. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Piano, M. Electrochemical (Bio) Sensors for Environmental and Food Analyses. Biosensors 2018, 8, 57. [Google Scholar] [CrossRef]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Hu, S.; Wu, K.; Yi, H.; Cui, D. Voltammetric behavior and determination of estrogens at Nafion-modified glassy carbon electrode in the presence of cetyltrimethylammonium bromide. Anal. Chim. Acta 2002, 464, 209–216. [Google Scholar] [CrossRef]
- Moreira, F.; Santana, E.R.; Spinelli, A. Ionic liquid-supported magnetite nanoparticles as electrode modifier materials for estrogens sensing. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Leandro, C.S.; Mattoso, L.H.; Correa, D.S. Layer-by-Layer assembled films of chitosan and multi-walled carbon nanotubes for the electrochemical detection of 17α-ethinylestradiol. J. Electroanal. Chem. 2015, 755, 215–220. [Google Scholar] [CrossRef]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Daverio, D.; Dellavedova, P.; Balzamo, S.; Calabretta, E.; Ubaldi, V. Determination of estrogenic endocrine disruptors in water at sub-ng L−1 levels in compliance with Decision 2015/495/EU using offline-online solid phase extraction concentration coupled with high performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 147, 1186–1191. [Google Scholar] [CrossRef]
- Barreiros, L.; Queiroz, J.F.; Magalhães, L.M.; Silva, A.M.; Segundo, M.A. Analysis of 17-β-estradiol and 17-α-ethinylestradiol in biological and environmental matrices—A review. Microchem. J. 2016, 126, 243–262. [Google Scholar] [CrossRef]
- Pereira, A.C.; Braga, G.B.; Oliveira, A.E.F.; Silva, R.C.; Borges, K.B. Synthesis and characterization of molecularly imprinted polymer for ethinylestradiol. Chem. Pap. 2019, 73, 141–149. [Google Scholar] [CrossRef]
- Zhang, G.; Li, T.; Zhang, J.; Chen, A. A simple FRET-based turn-on fluorescent aptasensor for 17β-estradiol determination in environmental water, urine and milk samples. Sens. Actuators B Chem. 2018, 273, 1648–1653. [Google Scholar] [CrossRef]
- Liu, X.; Deng, K.; Wang, H.; Li, C.; Zhang, S.; Huang, H. Aptamer based ratiometric electrochemical sensing of 17β-estradiol using an electrode modified with gold nanoparticles, thionine, and multiwalled carbon nanotubes. Microchim. Acta 2019, 186, 347. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Santos, A.M.; Cincotto, F.H.; Moraes, F.C.; Fatibello-Filho, O.; Sotomayor, M.D. A new electrochemical platform based on low cost nanomaterials for sensitive detection of the amoxicillin antibiotic in different matrices. Talanta 2019, 206, 120252. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.; Moraes, F.C. Voltammetric determination of ethinylestradiol using screen-printed electrode modified with functionalized graphene, graphene quantum dots and magnetic nanoparticles coated with molecularly imprinted polymers. Talanta 2021, 224, 121804. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Deng, Q.; Long, J. Highly sensitive electrochemical sensor for estradiol based on the signal amplification strategy of Cu-BDC frameworks. J. Solid State Electrochem. 2018, 22, 487–493. [Google Scholar] [CrossRef]
- Ji, L.; Wang, Y.; Wu, K.; Zhang, W. Simultaneous determination of environmental estrogens: Diethylstilbestrol and estradiol using Cu-BTC frameworks-sensitized electrode. Talanta 2016, 159, 215–221. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Fernandes, I.P.; Oliveira, S.C.B.; Shahrokhian, S.; Oliveira-Brett, A.M. Anodic behavior of clioquinol at a glassy carbon electrode. Bioelectrochemistry 2011, 80, 175–181. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Mathiyarasu, J. Graphene-Carbon Nanotubes Modified Electrochemical Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.; Banks, C.E. Graphene Oxide Bulk-Modified Screen-Printed Electrodes Provide Beneficial Electroanalytical Sensing Capabilities. Biosensors 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Arduini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-Printed Electrodes Modified with Carbon Nanomaterials: A Comparison among Carbon Black, Carbon Nanotubes and Graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Velický, M.; Bradley, D.F.; Cooper, A.J.; Hill, E.W.; Kinloch, I.A.; Mishchenko, A.; Novoselov, K.S.; Patten, H.V.; Toth, P.S.; Valota, A.T.; et al. Electron Transfer Kinetics on Mono- and Multilayer Graphene. ACS Nano 2014, 8, 10089–10100. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Moraes, F.C.; Rossi, B.; Donatoni, M.C.; de Oliveira, K.T.; Pereira, E.C. Sensitive determination of 17β-estradiol in river water using a graphene based electrochemical sensor. Anal. Chim. Acta 2015, 881, 37–43. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Grosse, W.; Champavert, J.; Gambhir, S.; Wallace, G.G.; Moulton, S.E. Aqueous dispersions of reduced graphene oxide and multi wall carbon nanotubes for enhanced glucose oxidase bioelectrode performance. Carbon 2013, 61, 467–475. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Ren, L.; Wang, X.; Liu, T. Graphene Oxide-Assisted Dispersion of Pristine Multiwalled Carbon Nanotubes in Aqueous Media. J. Phys. Chem. C 2010, 114, 11435–11440. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Balandin, A. Thermal properties of graphene and nanostructured carbon materials. Nature Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Keeley, G.P.; O’Neill, A.; Holzinger, M.; Cosnier, S.; Coleman, J.N.; Duesberg, G.S. DMF-exfoliated graphene for electrochemical NADH detection. Phys. Chem. Chem. Phys. 2011, 13, 7747–7750. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Ranjan, K.R.; Mukherjee, M.D.; Solanki, P.R. Bioinspired synthesis of hematite nanoparticles-reduced graphene oxide composite for application in bisphenol a detection: A new in-sight. Biosens. Bioelectron. X 2022, 11, 100217. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Electrochemically Exfoliated Graphene and Graphene Oxide for Energy Storage and Electrochemistry Applications. Chem. A Eur. J. 2016, 22, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.N.L.; Van Toan, P.; Hao, N.Q. Using AuNPs-modified screen-printed electrode in the development of molecularly imprinted polymer for artificial bioreceptor fabrication to improve biosensor sensitivity for 17 β -estradiol detection. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015015. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Ye, J.; Zhan, S.; Xia, B.; Lv, J.; Xu, H.; Du, G.; Wang, L. A Label-Free Colorimetric Biosensor for 17β-Estradiol Detection Using Nanoparticles Assembled by Aptamer and Cationic Polymer. Aust. J. Chem. 2016, 69, 12–19. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Yildirim, N.; Long, F.; Gao, C.; He, M.; Shi, H.-C.; Gu, A.Z. Aptamer-Based Optical Biosensor for Rapid and Sensitive Detection of 17β-Estradiol In Water Samples. Environ. Sci. Technol. 2012, 46, 3288–3294. [Google Scholar] [CrossRef]
- Hasyim, M.F.; Wahab, W.A.; Pratiwi, M.W. Synthesis and Characterization of Gold Nanoparticles Using the Bioreductor Bay Leaf (Syzygium polyanthum). J. Akta Kim. Indones. (Indones. Chim. Acta) 2020, 13, 79–84. [Google Scholar] [CrossRef]
- Ismail, Z. Green reduction of graphene oxide by plant extracts: A short review. Ceram. Int. 2019, 45, 23857–23868. [Google Scholar] [CrossRef]
- Altintas, Z.; Guerreiro, A.; Piletsky, S.A.; Tothill, I.E. NanoMIP based optical sensor for pharmaceuticals monitoring. Sens. Actuators B Chem. 2015, 213, 305–313. [Google Scholar] [CrossRef]
- Singh, C.; Sharma, V.; Naik, P.K.; Khandelwal, V.; Singh, H. A green biogenic approach for synthesis of gold and silver nanoparticles using zingiber officinale. Dig. J. Nanomater. Biostructures 2011, 6, 535–542. [Google Scholar]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Molecularly imprinted electrochemical aptasensor for the attomolar detection of bisphenol A. Microchim. Acta 2018, 185, 265. [Google Scholar] [CrossRef]
- Zhao, W.-R.; Kang, T.-F.; Lu, L.-P.; Shen, F.-X.; Cheng, S.-Y. A novel electrochemical sensor based on gold nanoparticles and molecularly imprinted polymer with binary functional monomers for sensitive detection of bisphenol A. J. Electroanal. Chem. 2017, 786, 102–111. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, N.; Ma, Z. Prussian blue–gold nanoparticles-ionic liquid functionalized reduced graphene oxide nanocomposite as label for ultrasensitive electrochemical immunoassay of alpha-fetoprotein. Anal. Chim. Acta 2014, 829, 15–21. [Google Scholar] [CrossRef]
- Petrucci, R.; Bortolami, M.; Di Matteo, P.; Curulli, A. Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials 2022, 12, 959. [Google Scholar] [CrossRef]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N., Jr. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J. Electroanal. Chem. 2019, 848, 113319. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Kiely, J.; Luxton, R. Metal oxide decorated carbon nanocomposite electrodes for propofol monitoring. Biosens. Bioelectron. X 2022, 12, 100286. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Chen, J.; Fang, G. Green synthesis and characterization of gold nanoparticles using lignin nanoparticles. Nanomaterials 2020, 10, 1869. [Google Scholar] [CrossRef] [PubMed]
- Aunkor, M.T.H.; Mahbubul, I.M.; Saidur, R.; Metselaar, H.S.C. The green reduction of graphene oxide. RSC Adv. 2016, 6, 27807–27828. [Google Scholar] [CrossRef]
- Shanmugam, M.; Kim, K. Electrodeposited gold dendrites at reduced graphene oxide as an electrocatalyst for nitrite and glucose oxidation. J. Electroanal. Chem. 2016, 776, 82–92. [Google Scholar] [CrossRef]
- Mhamane, D.; Ramadan, W.; Fawzy, M.; Rana, A.; Dubey, M.; Rode, C.; Lefez, B.; Hannoyer, B.; Ogale, S. From graphite oxide to highly water dispersible functionalized graphene by single step plant extract-induced deoxygenation. Green Chem. 2011, 13, 1990–1996. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Q.; Cheng, H.; Zhang, H.; Wang, S. Green reduction of graphene oxide via Lycium barbarum extract. J. Solid State Chem. 2017, 246, 351–356. [Google Scholar] [CrossRef]
- Grennan, K.; Killard, A.J.; Smyth, M.R. Physical Characterizations of a Screen-Printed Electrode for Use in an Amperometric Biosensor System. Electroanalysis 2001, 13, 745–750. [Google Scholar] [CrossRef]
- Morrin, A.; Killard, A.; Smyth, M.R. Electrochemical Characterization of Commercial and Home-Made Screen-Printed Carbon Electrodes. Anal. Lett. 2003, 36, 2021–2039. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Cokeliler, D.; Gunasekaran, S. Reduced Graphene Oxide/Carbon Nanotube/Gold Nanoparticles Nanocomposite Functionalized Screen-Printed Electrode for Sensitive Electrochemical Detection of Endocrine Disruptor Bisphenol A. Electroanalysis 2015, 27, 2527–2536. [Google Scholar] [CrossRef]
- Gonçalves, G.; Marques, P.; Granadeiro, C.; Nogueira, H.S.; Singh, M.K.; Grácio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Lo, T.W.; Aldous, L.; Compton, R.G. The use of nano-carbon as an alternative to multi-walled carbon nanotubes in modified electrodes for adsorptive stripping voltammetry. Sens. Actuators B Chem. 2012, 162, 361–368. [Google Scholar] [CrossRef]
- Yang, J.; Strickler, J.R.; Gunasekaran, S. Indium tin oxide-coated glass modified with reduced graphene oxide sheets and gold nanoparticles as disposable working electrodes for dopamine sensing in meat samples. Nanoscale 2012, 4, 4594–4602. [Google Scholar] [CrossRef]
- Jian, J.-M.; Fu, L.; Ji, J.; Lin, L.; Guo, X.; Ren, T.-L. Electrochemically reduced graphene oxide/gold nanoparticles composite modified screen-printed carbon electrode for effective electrocatalytic analysis of nitrite in foods. Sens. Actuators B Chem. 2018, 262, 125–136. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.; Metters, J.P.; Kadara, R.O.; Banks, C.E. The fabrication, characterisation and electrochemical investigation of screen-printed graphene electrodes. Phys. Chem. Chem. Phys. 2014, 16, 4598–4611. [Google Scholar] [CrossRef]
- Zhang, W.; Bas, A.D.; Ghali, E.; Yeonuk, C.H.O.I. Passive behavior of gold in sulfuric acid medium. Trans. Nonferrous Met. Soc. China 2015, 25, 2037–2046. [Google Scholar] [CrossRef]
- Panchompoo, J.; Aldous, L.; Downing, C.; Crossley, A.; Compton, R.G. Facile synthesis of Pd nanoparticle modified carbon black for electroanalysis: Application to the detection of hydrazine. Electroanalysis 2011, 23, 1568–1578. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Wing Fen, Y. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef]
- Garry, L.M. The Electrochemical Characterisation of Carbon-based Nanomaterials and their Application in the Detection of Heavy Metal Ions. Ph.D. Thesis, National University of Ireland Maynooth, Maynooth, Ireland, 2013. [Google Scholar]
- He, Y.Q.; Liu, S.P.; Kong, L.; Liu, Z.F. A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2861–2866. [Google Scholar] [CrossRef]
- Presa, M.J.R.; Gassa, L.M.; Azzaroni, O.; Gervasi, C.A. Estimating Diffusion Coefficients of Probe Molecules into Polyelectrolyte Brushes by Electrochemical Impedance Spectroscopy. Anal. Chem. 2009, 81, 7936–7943. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Dawson, K.; Strutwolf, J.; Herzog, G.; Arrigan, D.; Quinn, A.; O’Riordan, A.J. Nanofabrication of robust nanoelectrodes for electrochemical Applications. ECS Trans. 2010, 28, 29. [Google Scholar] [CrossRef]
- Hassan, N.; Holze, R. A comparative electrochemical study of electrosorbed 2- and 4-mercaptopyridines and their application as corrosion inhibitors at C60 steel. J. Chem. Sci. 2009, 121, 693–701. [Google Scholar] [CrossRef]
- Smajdor, J.; Piech, R.; Ławrywianiec, M.; Paczosa-Bator, B. Glassy carbon electrode modified with carbon black for sensitive estradiol determination by means of voltammetry and flow injection analysis with amperometric detection. Anal. Biochem. 2018, 544, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Topçuoğulları, D. Voltammetric determination of 17-β-estradiol by cysteamine self-assembled gold nanoparticle modified fumed silica decorated graphene nanoribbon nanocomposite. Sens. Actuators B Chem. 2017, 250, 85–90. [Google Scholar] [CrossRef]
- Bös, M. Kalman filtering for the evaluation of the current-time function in d.c. polarography. Talanta 1986, 33, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, S.-X.; Bond, A.M.; Marken, F. Large-Amplitude Fourier Transformed High-Harmonic Alternating Current Cyclic Voltammetry: Kinetic Discrimination of Interfering Faradaic Processes at Glassy Carbon and at Boron-Doped Diamond Electrodes. Anal. Chem. 2004, 76, 3619–3629. [Google Scholar] [CrossRef] [PubMed]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Azzaroni, O.; Gervasi, C. Characterization of responsive polymer brushes at solid/liquid interfaces by electrochemical impedance spectroscopy. Funct. Polym. Film. 2011, 2, 809–830. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- De Carvalho, R.M.; Mello, C.; Kubota, L.T. Simultaneous determination of phenol isomers in binary mixtures by differential pulse voltammetry using carbon fibre electrode and neural network with pruning as a multivariate calibration tool. Anal. Chim. Acta 2000, 420, 109–121. [Google Scholar] [CrossRef]
- Górski, Ł.; Ciepiela, F.; Jakubowska, M. Automatic baseline correction in voltammetry. Electrochim. Acta 2014, 136, 195–203. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Yu, J.; Lian, W.; Cui, M.; Xu, W.; Huang, J. Electrochemical immunosensor based on graphene–polyaniline composites and carboxylated graphene oxide for estradiol detection. Sens. Actuators B Chem. 2013, 188, 99–105. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Analytical methodology for the electro-catalytic determination of estradiol and progesterone based on graphene quantum dots and poly(sulfosalicylic acid) co-modified electrode. Talanta 2017, 174, 243–255. [Google Scholar] [CrossRef]



| Electrode-Design-Modifier | Applied Technique | Sensor Type | Linear Range | Detection Limit | Reference | |
|---|---|---|---|---|---|---|
| Glassy carbon electrode (GCE) | 2 GR–PANI | 1 DPV | Immunosensor | 0.04–7.00 ng/mL | 0.02 ng/mL | [85] |
| Glassy carbon electrode (GCE) | 3 RGO/CuTthP | 1 DPV | Electrochemical sensor | 0.1–1.0 μM | 5.3 nM | [27] |
| Carbon paste electrode | 4 GNR-FS-Au-CA | 1 DPV | Electrochemical sensor | 0.1–5.0 μM | 7.4 nM | [77] |
| Glassy carbon electrode (GCE) | 5 GQDs-PSSA/GO | 1 DPV | Electrochemical sensor | 0.001–6.0 μM | 0.23 nM | [86] |
| Screen-printed carbon electrode | 6 rGO/AuNP/CNT) | 1 DPV | Electrochemical sensor | 0.05–1.0 μM | 3 nM | This work |
| Added (μM) | Found (μM) | RSD | Recoveries (%) |
|---|---|---|---|
| 0.5 | 0.46 | 2.7 | 92 |
| 0.5 | 0.45 | 2.5 | 90 |
| 0.9 | 0.96 | 3.2 | 106 |
| 0.9 | 0.92 | 2.8 | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors 2023, 13, 491. https://doi.org/10.3390/bios13040491
Musa AM, Kiely J, Luxton R, Honeychurch KC. An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors. 2023; 13(4):491. https://doi.org/10.3390/bios13040491
Chicago/Turabian StyleMusa, Auwal M., Janice Kiely, Richard Luxton, and Kevin C. Honeychurch. 2023. "An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol" Biosensors 13, no. 4: 491. https://doi.org/10.3390/bios13040491
APA StyleMusa, A. M., Kiely, J., Luxton, R., & Honeychurch, K. C. (2023). An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors, 13(4), 491. https://doi.org/10.3390/bios13040491






