Fluorescent Sensing Platforms for Detecting and Imaging the Biomarkers of Alzheimer’s Disease
Abstract
1. Introduction
2. Biomarkers for AD Diagnosis
3. Enhanced Fluorescence-Sensing Platforms for the Detection of AD Blood Biomarkers
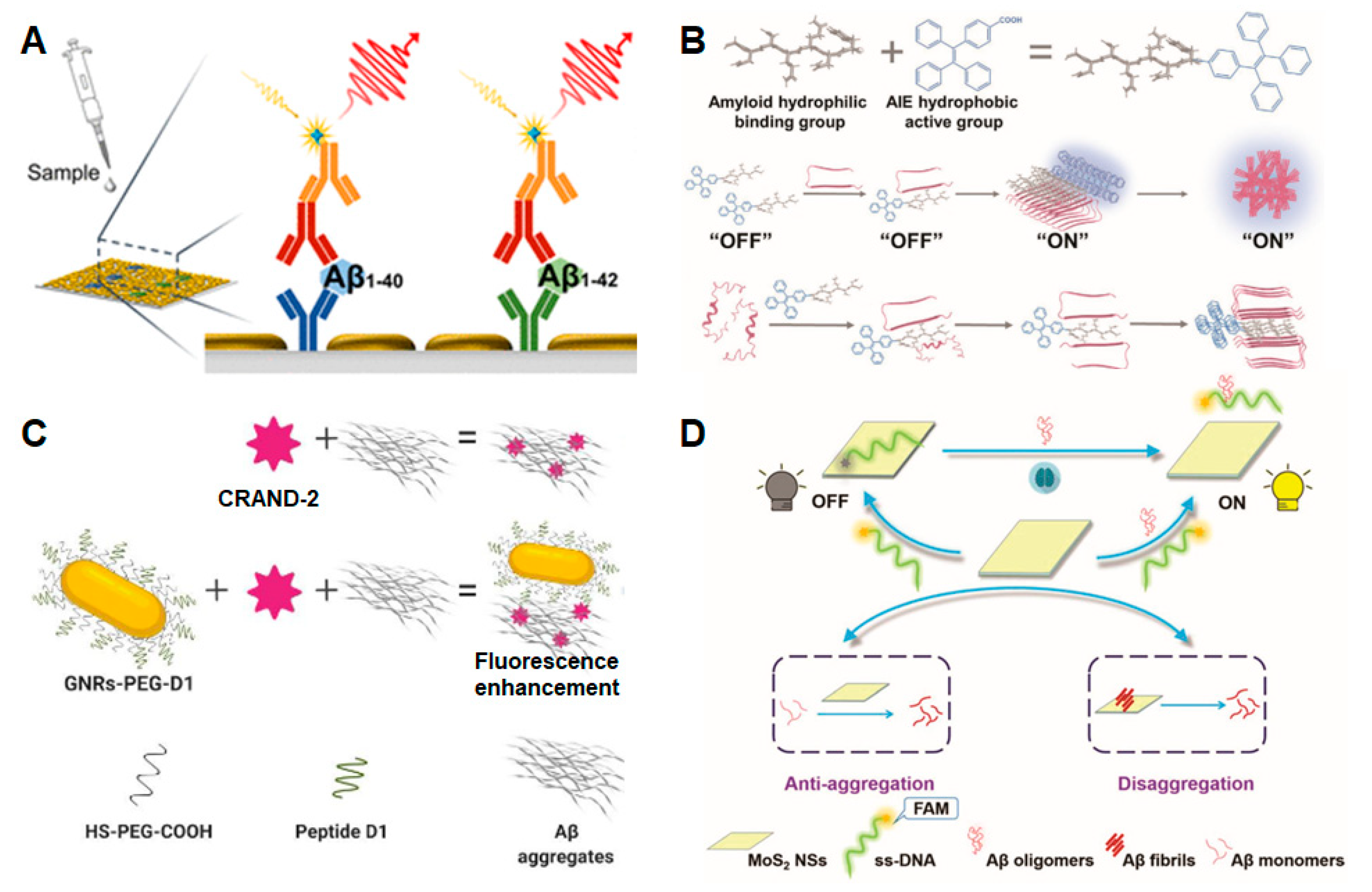
3.1. Amyloid-β Peptide
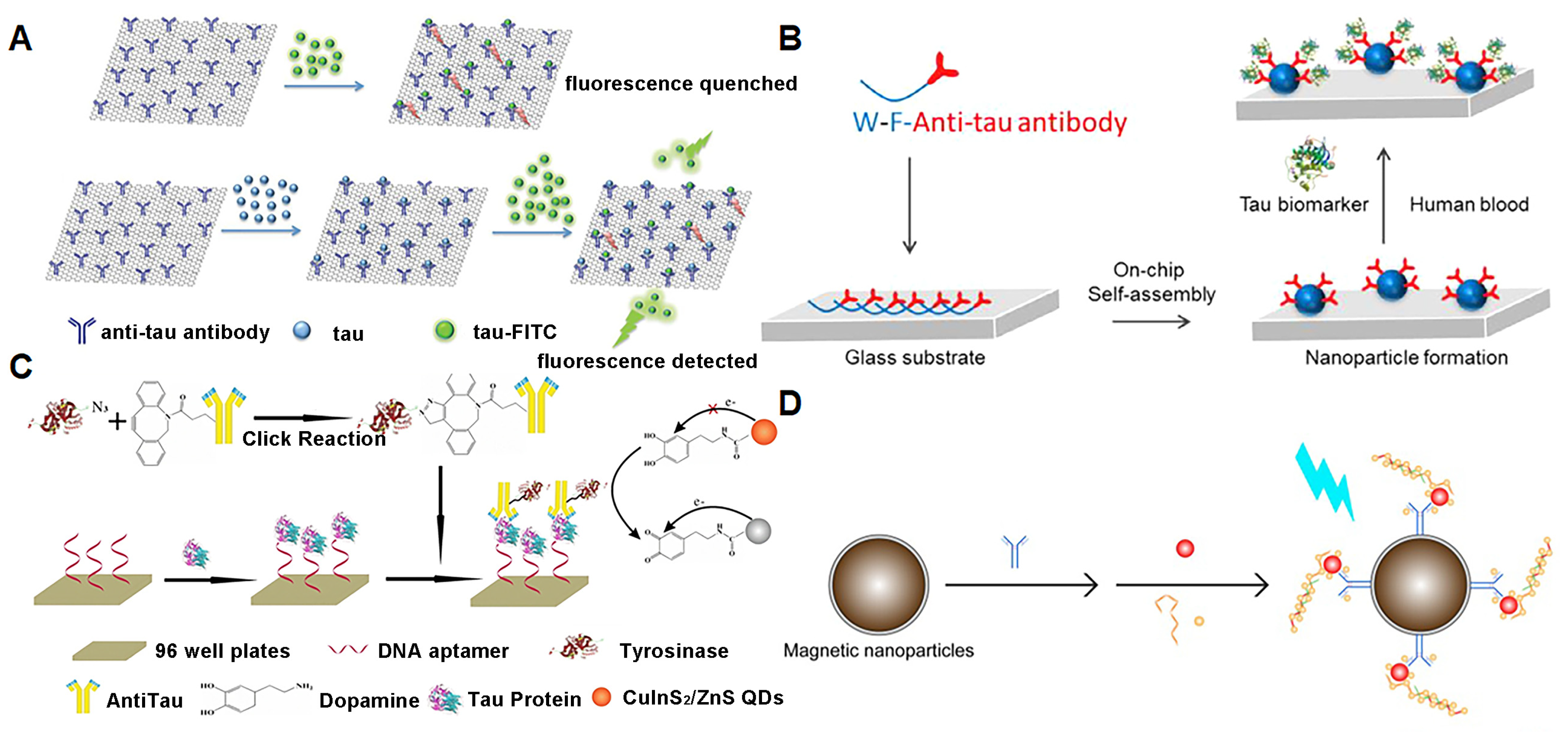
3.2. Tau
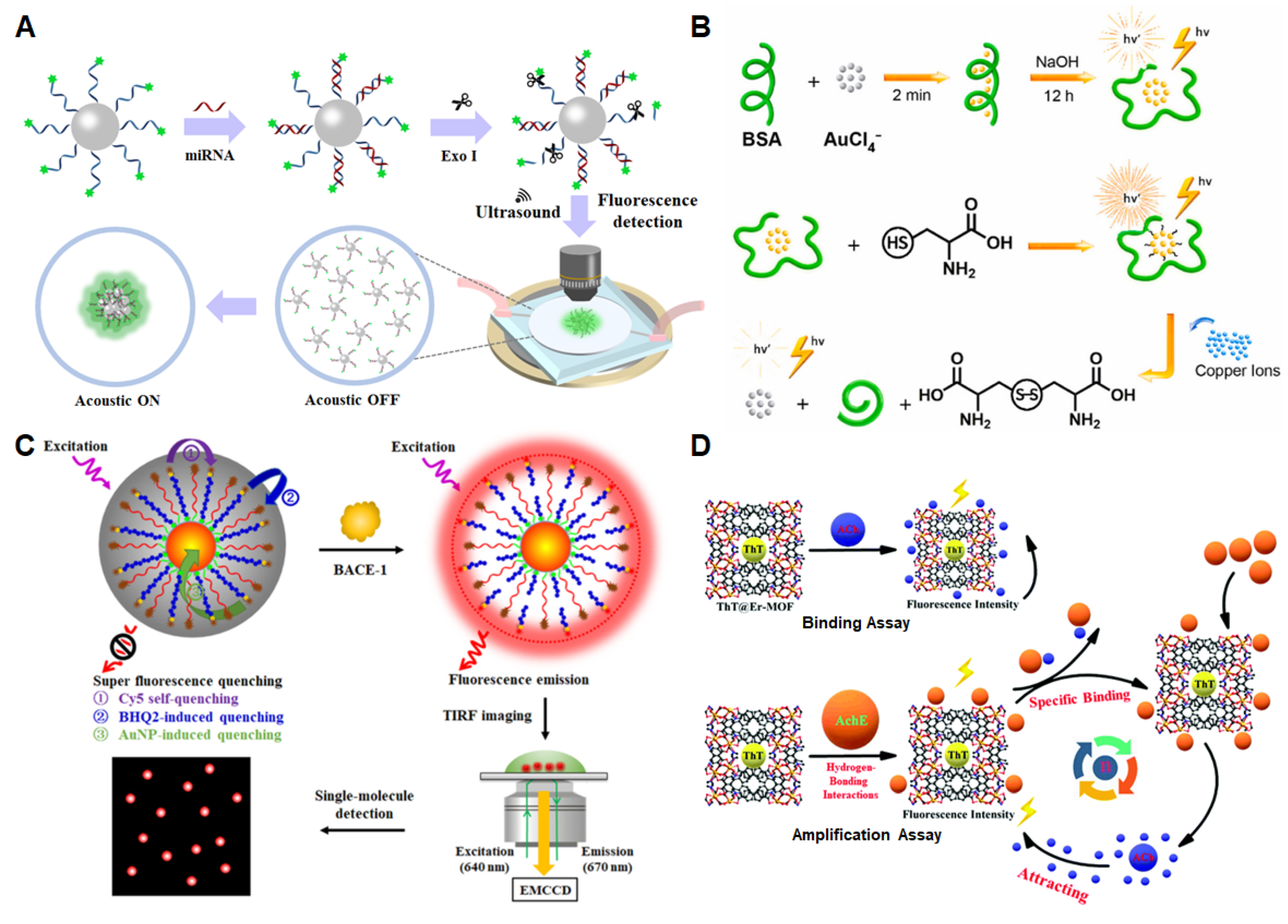
3.3. Other Biomarkers in the Blood
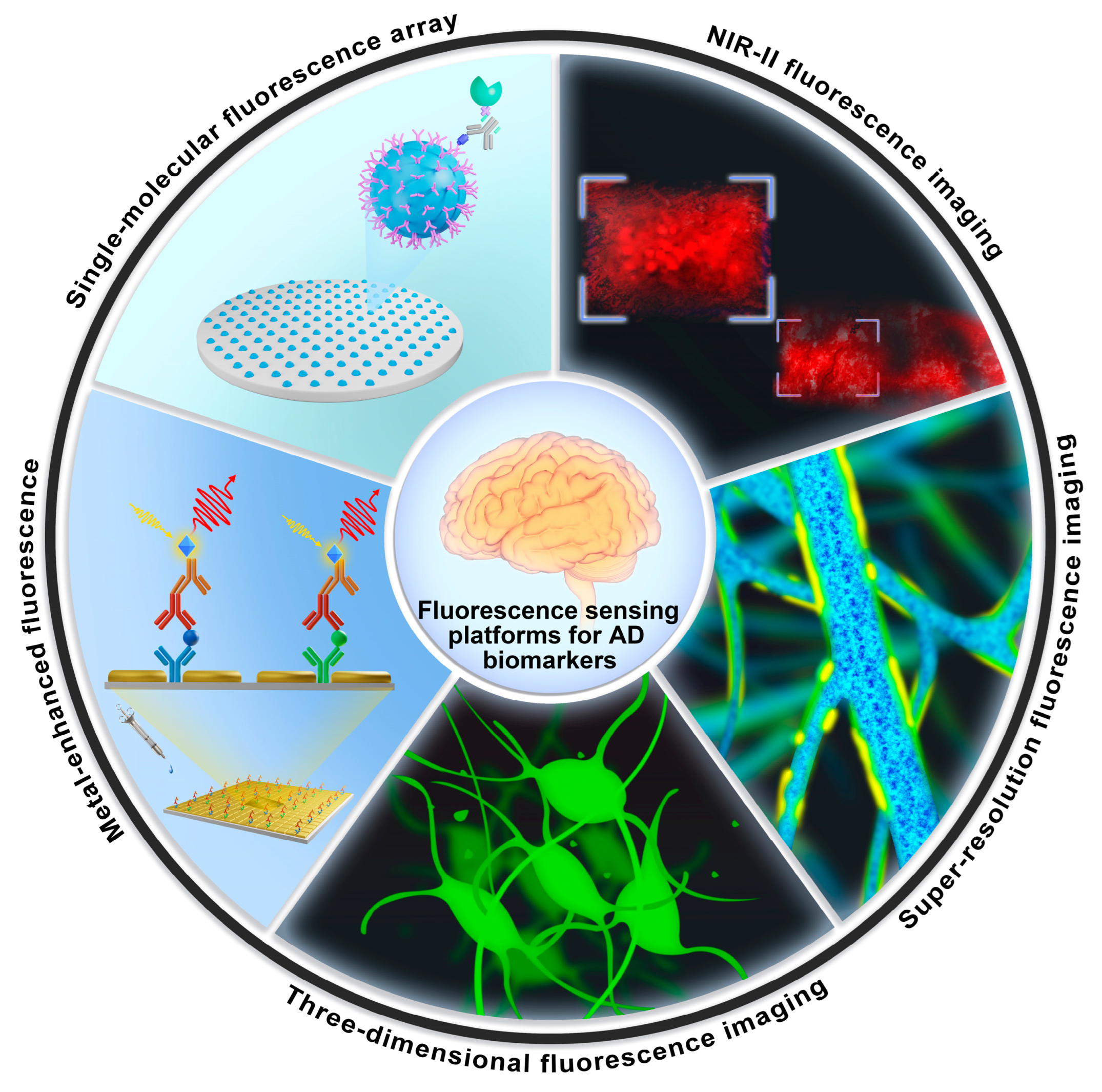
4. Fluorescence-Sensing Platforms for Imaging AD Biomarkers in the Brain
5. Conclusions and Prospects
- (1)
- Application of novel composite nanomaterials. The accuracy and sensitivity of the analytical method can be significantly improved via the development of special structures and biocompatible nanomaterials combined with fluorescence enhancement technology.
- (2)
- In-depth application of near-infrared fluorescence probes. The biggest defect of fluorescence detection is the decrease in resolution with the increase in fluorescence emission depth, and thus, only the outer surface of the brain can be identified. Furthermore, the fluorescence wavelength is below 550 nm, and some biological substances themselves also fluoresce in this range, which interferes with the detection of organisms in vivo [12,13,15]. With the advent of near-infrared fluorescence imaging, the above problems are gradually solved. The wavelength range of NIR fluorescence imaging is between 700 nm and 2500 nm, which greatly enhances the penetration depth and reduces the interference of biomaterials themselves [88].
- (3)
- Simultaneous detection of different forms of aggregates. For example, Aβ has many aggregation forms, and the current method still utilizes the single detection of only one form. Simultaneous detection of different aggregation forms can not only reduce the cost but also save time and samples, leading to the method being simple, fast and sensitive for the trace analysis of AD biomarkers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Pichet Binette, A.; Groot, C.; Smith, R.; Strandberg, O.; Palmqvist, S.; Stomrud, E.; Tideman, P.; Ohlsson, T.; Jögi, J.; et al. Amyloid-beta and tau PET scans predict clinical progression in cognitively unimpaired people. Nat. Med. 2022, 28, 2267–2268. [Google Scholar]
- Sebastian, P.; Shorena, J.; Yakeel, T.Q.; Henrik, Z.; Francisco, L.; Erik, S.; Yi, S.; Yinghua, C.; Geidy, E.S.; Antoine, L.; et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Frank, B.; Ally, M.; Brekke, B.; Zetterberg, H.; Blennow, K.; Sugarman, M.A.; Ashton, N.J.; Karikari, T.K.; Tripodis, Y.; Martin, B.; et al. Plasma p-tau181 shows stronger network association to Alzheimer’s disease dementia than neurofilament light and total tau. Alzheimers Dement. 2021, 18, 1523–1536. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Strom, A.; Fonseca, C.; Iaccarino, L.; Wolf, A.; Spina, S.; Allen, I.E.; Cobigo, Y.; Heuer, H.; et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: A retrospective diagnostic performance study. Lancet Neurol. 2021, 20, 739–752. [Google Scholar] [CrossRef]
- Moscoso, A.; Karikari, T.K.; Grothe, M.J.; Ashton, N.J.; Lantero-Rodriguez, J.; Snellman, A.; Zetterberg, H.; Blennow, K.; Scholl, M. CSF biomarkers and plasma p-tau181 as predictors of longitudinal tau accumulation: Implications for clinical trial design. Alzheimers Dement. 2022, 18, 2614–2626. [Google Scholar] [CrossRef]
- Yeo, S.K.; Shepelytskyi, Y.; Grynko, V.; Albert, M.S. Molecular Imaging of Fluorinated Probes for Tau Protein and Amyloid-β Detection. Molecules 2020, 25, 3413. [Google Scholar] [CrossRef]
- Xu, M.Y.; Li, R.H.; Li, X.; Lv, G.L.; Li, S.P.; Sun, A.Y.; Zhou, Y.F.; Yi, T. NIR fluorescent probes with good water-solubility for detection of amyloid beta aggregates in Alzheimer’s disease. J. Mater. Chem. B 2019, 7, 5535–5540. [Google Scholar] [CrossRef]
- Si, G.F.; Zhou, S.J.; Xu, G.Y.; Wang, J.F.; Wu, B.X.; Zhou, S.S. A curcumin-based NIR fluorescence probe for detection of amyloid-beta (A beta) plaques in Alzheimer’s disease. Dyes Pigments 2019, 163, 509–515. [Google Scholar] [CrossRef]
- Sato, T.; Hotsumi, M.; Makabe, K.; Konno, H. Design, synthesis and evaluation of curcumin-based fluorescent probes to detect A beta fibrils. Bioorg. Med. Chem. Lett. 2018, 28, 3520–3525. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Narayanaswamy, N.; Murugan, N.A.; Viccaro, K.; Lee, H.G.; Shah, K.; Govindaraju, T. A beta plaque-selective NIR fluorescence probe to differentiate Alzheimer’s disease from tauopathies. Biosens. Bioelectron. 2017, 98, 54–61. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Li, Y.; Li, H.W.; Wong, M.S. Versatile fluorescent probes for near-infrared imaging of amyloid-beta species in Alzheimer’s disease mouse model. J. Mater. Chem. B 2019, 7, 1986–1995. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.-Y.; Wang, X.-B. Advances in Detection Methods of β-Amyloid Protein. Chin. J. Anal. Chem. 2018, 46, 1339–1349. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2022, e2107986. [Google Scholar] [CrossRef]
- Hu, S.; Yang, C.; Luo, H. Current trends in blood biomarker detection and imaging for Alzheimer’s disease. Biosens. Bioelectron. 2022, 210, 114278. [Google Scholar] [CrossRef]
- Aliyan, A.; Cook, N.P.; Marti, A.A. Interrogating Amyloid Aggregates using Fluorescent Probes. Chem. Rev. 2019, 119, 11819–11856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhuang, D.; Wang, J.; Huang, H.; Li, R.; Wu, C.; Deng, Y.; Hu, G.; Guo, B. Recent advances in small molecular near-infrared fluorescence probes for a targeted diagnosis of the Alzheimer disease. Analyst 2022, 147, 4701–4723. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Clark, I.A.; Vissel, B. Questions concerning the role of amyloid-beta in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 2018, 136, 663–689. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Zamanian, J.; Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Hosseinzadeh, H.; Danesh, N.M. Current progress in aptamer-based sensing tools for ultra-low level monitoring of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2022, 197, 113789. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s Disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef]
- Ge, L.H.; Liu, Z.C.; Tian, Y. A novel two-photon ratiometric fluorescent probe for imaging and sensing of BACE1 in different regions of AD mouse brain. Chem. Sci. 2020, 11, 2215–2224. [Google Scholar] [CrossRef]
- Singh, N.; Benoit, M.R.; Zhou, J.; Das, B.; Davila-Velderrain, J.; Kellis, M.; Tsai, L.-H.; Hu, X.; Yan, R. BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1. Sci. Adv. 2022, 8, eabo1286. [Google Scholar] [CrossRef]
- Roberts, J.A.; Varma, V.R.; An, Y.; Varma, S.; Candia, J.; Fantoni, G.; Tiwari, V.; Anerillas, C.; Williamson, A.; Saito, A.; et al. A brain proteomic signature of incipient Alzheimer’s disease in young APOE ε4 carriers identifies novel drug targets. Sci. Adv. 2021, 7, eabi8178. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.-P.; Wang, S.; Yang, W.; Wen, Y.; Zhang, X. An ultrasensitive electrochemical immunosensor for apolipoprotein E4 based on fractal nanostructures and enzyme amplification. Biosens. Bioelectron. 2015, 71, 396–400. [Google Scholar] [CrossRef]
- Singh, N.A.; Tosakulwong, N.; Graff-Radford, J.; Machulda, M.M.; Pham, N.T.T.; Sintini, I.; Weigand, S.D.; Schwarz, C.G.; Senjem, M.L.; Carrasquillo, M.M.; et al. APOE epsilon4 influences medial temporal atrophy and tau deposition in atypical Alzheimer’s disease. Alzheimers Dement. 2022, 19, 784–796. [Google Scholar] [CrossRef]
- Mahan, T.E.; Wang, C.; Bao, X.; Choudhury, A.; Ulrich, J.D.; Holtzman, D.M. Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Aβ accumulation and plaque-related pathology in a mouse model of amyloidosis. Mol. Neurodegener. 2022, 17, 13. [Google Scholar] [CrossRef]
- Niu, Y.X.; Ding, T.; Liu, J.M.; Zhang, G.L.; Tong, L.L.; Cheng, X.F.; Yang, Y.M.; Chen, Z.Z.; Tang, B. Fluorescence switch of gold nanoclusters stabilized with bovine serum albumin for efficient and sensitive detection of cysteine and copper ion in mice with Alzheimer ‘s disease. Talanta 2021, 223, 121745. [Google Scholar] [CrossRef]
- Yao, Y.S.; Li, W.H.; Han, Q.Q.; Lv, G.L.; Li, C.X.; Sun, A.Y. A Pyridyl Zn (II) Chelate for the Fluorescent Detection of A beta Fibrils. Z. Anorg. Allg. Chem. 2022, 648, e202200070. [Google Scholar] [CrossRef]
- Guo, Y.X.; Hu, Z.Y.; Wang, Z.H. Recent Advances in the Application Peptide and Peptoid in Diagnosis Biomarkers of Alzheimer’s Disease in Blood. Front. Mol. Neurosci. 2021, 14, 778955. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Yang, H.L.; Fang, S.Q.; Tang, Y.W.; Wang, C.; Luo, H.; Qu, L.L.; Zhao, J.H.; Shi, C.J.; Yin, F.C.; Wang, X.B.; et al. A hemicyanine derivative for near-infrared imaging of beta-amyloid plaques in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 179, 736–743. [Google Scholar] [CrossRef]
- Lim, J.; Kim, S.; Oh, S.J.; Han, S.M.; Moon, S.Y.; Kang, B.; Seo, S.B.; Jang, S.; Son, S.U.; Jung, J.; et al. miRNA sensing hydrogels capable of self-signal amplification for early diagnosis of Alzheimer’s disease. Biosens. Bioelectron. 2022, 209, 114279. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.H.; Park, C.B. Chemical sensing platforms for detecting trace-level Alzheimer’s core biomarkers. Chem. Soc. Rev. 2020, 49, 5446–5472. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 5562–5566. [Google Scholar] [CrossRef] [PubMed]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef]
- Woo, N.; Kim, S.K.; Sun, Y.; Kang, S.H. Enhanced capillary electrophoretic screening of Alzheimer based on direct apolipoprotein E genotyping and one-step multiplex PCR. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 290–299. [Google Scholar] [CrossRef]
- Theendakara, V.; Peters-Libeu, C.A.; Bredesen, D.E.; Rao, R.V. Transcriptional Effects of ApoE4: Relevance to Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 5243–5254. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.W.; Dai, H.X.; Zhang, H.G.; Liu, J.J.; Ma, S.D.; Chen, X.G. A peptide-WS2 nanosheet based biosensing platform for determination of beta-secretase and screening of its inhibitors. Analyst 2018, 143, 4585–4591. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. Role of BACE1 in Alzheimer’s synaptic function. Transl. Neurodegener. 2017, 6, 23. [Google Scholar] [CrossRef]
- Kou, X.; Chen, D.; Chen, N. The Regulation of microRNAs in Alzheimer’s Disease. Front. Neurol. 2020, 11, 288. [Google Scholar] [CrossRef]
- Sheinerman, K.S.; Toledo, J.B.; Tsivinsky, V.G.; Irwin, D.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Chen-Plotkin, A.; Wolk, D.A.; McCluskey, L.F.; et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimers Res. Ther. 2017, 9, 89. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Halvorsen, K. DNA-Based Smart Reagent for Detecting Alzheimer’s Associated MicroRNAs. ACS Sens. 2021, 6, 3176–3181. [Google Scholar] [CrossRef]
- Jiao, C.; Liu, Y.; Lu, W.; Zhang, P.; Wang, Y. Molecular Fluorescence Probe for Detecting Reactive Nitrogen/Reactive Oxygen. Chin. J. Org. Chem. 2019, 39, 591–616. [Google Scholar] [CrossRef]
- Wang, P.; Yu, L.; Gong, J.; Xiong, J.; Zi, S.; Xie, H.; Zhang, F.; Mao, Z.; Liu, Z.; Kim, J.S. An Activity-Based Fluorescent Probe for Imaging Fluctuations of Peroxynitrite (ONOO−) in the Alzheimer’s Disease Brain. Angew. Chem. Int. Ed. Engl. 2022, 61, e202206894. [Google Scholar]
- Oyarzun, M.P.; Tapia-Arellano, A.; Cabrera, P.; Jara-Guajardo, P.; Kogan, M.J. Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease. Sensors 2021, 21, 2067. [Google Scholar] [CrossRef]
- Han, H.F.; Yen, H.C.; Wu, H.C.; Tan, H.Y.; Xu, W.; Jiang, H.S.; Tsai, P.J.; Qian, K.; Wu, Y.C.; Chen, C.C. Ultrasensitive Detection of Alzheimer’s Amyloids on a Plasmonic-Gold Platform. ACS Appl. Mater. Interfaces 2021, 13, 57036–57042. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, D.; Gong, X.; Zheng, J. Dual-Functional, Multi-Targeting GNNQQNY-AIE Conjugates as Amyloid Probes and Amyloid Modulators via Amyloid Cross-Seeding Principle. Adv. Funct. Mater. 2022, 32, 2208022. [Google Scholar] [CrossRef]
- Jara-Guajardo, P.; Cabrera, P.; Celis, F.; Soler, M.; Berlanga, I.; Parra-Munoz, N.; Acosta, G.; Albericio, F.; Guzman, F.; Campos, M.; et al. Gold Nanoparticles Mediate Improved Detection of beta-amyloid Aggregates by Fluorescence. Nanomaterials 2020, 10, 690. [Google Scholar] [CrossRef]
- Kong, L.N.; Zhou, X.G.; Shi, G.Y.; Yu, Y.Y. Molybdenum disulfide nanosheets-based fluorescent "off-to-on" probe for targeted monitoring and inhibition of beta-amyloid oligomers. Analyst 2020, 145, 6369–6377. [Google Scholar] [CrossRef]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, Synthesis, and Testing of Difluoroboron-Derivatized Curcumins as Near-Infrared Probes for in Vivo Detection of Amyloid-β Deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, L.N.; Li, W.W.; Ma, Z.Y.; Shuo, S.; Yao, T.M. Controlled fluorescence quenching by antibody-conjugated graphene oxide to measure tau protein. R. Soc. Open Sci. 2018, 5, 171808. [Google Scholar] [CrossRef]
- Leming, S.; Yang, L.; Yuerong, W.; Dingchang, L. Blood-based Alzheimer’s disease diagnosis using fluorescent peptide nanoparticle arrays. Chin. Chem. Lett. 2021, 33, 1946–1950. [Google Scholar]
- Chen, L.; Lin, J.W.; Yi, J.Q.; Weng, Q.H.; Zhou, Y.; Han, Z.Z.; Li, C.Y.; Chen, J.H.; Zhang, Q. A tyrosinase-induced fluorescence immunoassay for detection of tau protein using dopamine-functionalized CuInS2/ZnS quantum dots. Anal. Bioanal. Chem. 2019, 411, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.N.; Xu, D.; Ho, S.L.; He, D.; Wong, M.S.; Li, H.W. Highly sensitive quantification of Alzheimer’s disease biomarkers by aptamer-assisted amplification. Theranostics 2019, 9, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, Y.; Xu, T.L.; Cheng, G.Z.; Cai, H.; Zhang, X.J. Acoustic aggregation-induced separation for enhanced fluorescence detection of Alzheimer’s biomarker. Talanta 2021, 233, 122517. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Ulmann, P.A.; Han, M.S.; Mirkin, C.A. A DNA-gold nanoparticle-based colorimetric competition assay for the detection of cysteine. Nano Lett. 2008, 8, 529–533. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Q.; Xu, Q.F.; Zhang, C.Y. Self-Assembly of Superquenched Gold Nanoparticle Nanosensors for Lighting up BACE-1 in Live Cells. Anal. Chem. 2021, 93, 15124–15132. [Google Scholar] [CrossRef]
- Wang, X.Z.; Du, J.; Xiao, N.N.; Zhang, Y.; Fei, L.; LaCoste, J.D.; Huang, Z.; Wang, Q.; Wang, X.R.; Ding, B. Driving force to detect Alzheimer’s disease biomarkers: Application of a thioflavine T@Er-MOF ratiometric fluorescent sensor for smart detection of presenilin 1, amyloid beta-protein and acetylcholine. Analyst 2020, 145, 4646–4663. [Google Scholar] [CrossRef]
- Frederiksen, H.R.; Holst, B.; Mau-Holzmann, U.A.; Freude, K.; Schmid, B. Generation of two isogenic iPSC lines with either a heterozygous or a homozygous E280A mutation in the PSEN1 gene. Stem Cell Res. 2019, 35, 101403. [Google Scholar] [CrossRef]
- Albishri, H.M.; Abd El-Hady, D. Hyphenation of enzyme/graphene oxide-ionic liquid/glassy carbon biosensors with anodic differential pulse stripping voltammetry for reliable determination of choline and acetylcholine in human serum. Talanta 2019, 200, 107–114. [Google Scholar] [CrossRef]
- Liu, X.W.; Li, X.; Xu, S.L.; Guo, S.J.; Xue, Q.W.; Wang, H.S. Efficient ratiometric fluorescence probe based on dual-emission luminescent lanthanide coordination polymer for amyloid beta-peptide detection. Sens. Actuator B Chem. 2022, 352, 131052. [Google Scholar] [CrossRef]
- Hamd-Ghadareh, S.; Salimi, A.; Parsa, S.; Mowla, S.J. Development of three-dimensional semi-solid hydrogel matrices for ratiometric fluorescence sensing of Amyloid beta peptide and imaging in SH-SY5 cells: Improvement of point of care diagnosis of Alzheimer’s disease biomarker. Biosens. Bioelectron. 2022, 199, 113895. [Google Scholar] [CrossRef]
- Fang, W.K.; Liu, L.; Zhang, L.L.; Liu, D.; Liu, Y.; Tang, H.W. Detection of Amyloid beta Oligomers by a Fluorescence Ratio Strategy Based on Optically Trapped Highly Doped Upconversion Nanoparticles-SiO2@Metal-Organic Framework Microspheres. Anal. Chem. 2021, 93, 12447–12455. [Google Scholar] [CrossRef]
- Yin, Y.M.; Chen, G.F.; Gong, L.; Ge, K.Z.; Pan, W.Z.; Li, N.; Machuki, J.O.; Yu, Y.Y.; Geng, D.Q.; Dong, H.F.; et al. DNAzyme-Powered Three-Dimensional DNA Walker Nanoprobe for Detection Amyloid beta-Peptide Oligomer in Living Cells and in Vivo. Anal. Chem. 2020, 92, 9247–9256. [Google Scholar] [CrossRef]
- Ren, H.X.; Zhong, Q.L.; Miao, Y.B.; Wen, X.W.; Wu, G.Y.; Wang, H.L.; Zhang, Y. A label-free reusable aptasensor for Alzheimer’s disease. Microchim. Acta 2020, 187, 515. [Google Scholar] [CrossRef]
- Ren, H.X.; Miao, Y.B.; Zhang, Y.D. An aptamer based fluorometric assay for amyloid-beta oligomers using a metal-organic framework of type Ru@MIL-101(Al) and enzyme-assisted recycling. Microchim. Acta 2020, 187, 514. [Google Scholar] [CrossRef]
- Liu, C.; Lu, D.; You, X.; Shi, G.; Deng, J.; Zhou, T. Carbon dots sensitized lanthanide infinite coordination polymer nanoparticles: Towards ratiometric fluorescent sensing of cerebrospinal Aβ monomer as a biomarker for Alzheimer’s disease. Anal. Chim. Acta 2020, 1105, 147–154. [Google Scholar] [CrossRef]
- Chen, W.; Gao, G.; Jin, Y.; Deng, C. A facile biosensor for Aβ40O based on fluorescence quenching of prussian blue nanoparticles. Talanta 2020, 216, 120930. [Google Scholar] [CrossRef]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef]
- Rahaie, M.; Noroozi, S.K. A nanobiosensor based on graphene oxide and DNA binding dye for multi-microRNAs detection. Biosci. Rep. 2019, 39, BSR20181404. [Google Scholar] [CrossRef]
- Kim, H.I.; Yim, D.; Jeon, S.J.; Kang, T.W.; Hwang, I.J.; Lee, S.; Yang, J.K.; Ju, J.M.; So, Y.; Kim, J.H. Modulation of oligonucleotide-binding dynamics on WS2 nanosheet interfaces for detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2020, 165, 112401. [Google Scholar] [CrossRef]
- Huang, D.; Cao, Y.; Yang, X.; Liu, Y.; Zhang, Y.; Li, C.; Chen, G.; Wang, Q. A Nanoformulation-Mediated Multifunctional Stem Cell Therapy with Improved Beta-Amyloid Clearance and Neural Regeneration for Alzheimer’s Disease. Adv. Mater. 2021, 33, 2006357. [Google Scholar] [CrossRef] [PubMed]
- Vagenknecht, P.; Luzgin, A.; Ono, M.; Ji, B.; Higuchi, M.; Noain, D.; Maschio, C.A.; Sobek, J.; Chen, Z.; Konietzko, U.; et al. Non-invasive imaging of tau-targeted probe uptake by whole brain multi-spectral optoacoustic tomography. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2137–2152. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.S.; Yang, J.; Lee, J.H.; Kwon, Y.; Calvo-Rodriguez, M.; Bao, K.; Ahn, S.; Kashiwagi, S.; Kumar, A.T.N.; Bacskai, B.J.; et al. Near-infrared fluorescence lifetime imaging of amyloid-β aggregates and tau fibrils through the intact skull of mice. Nat. Biomed. Eng. 2023, 7, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Gilvesy, A.; Husen, E.; Magloczky, Z.; Mihaly, O.; Hortobágyi, T.; Kanatani, S.; Heinsen, H.; Renier, N.; Hökfelt, T.; Mulder, J.; et al. Spatiotemporal characterization of cellular tau pathology in the human locus coeruleus–pericoerulear complex by three-dimensional imaging. Acta Neuropathol. 2022, 144, 651–676. [Google Scholar] [CrossRef]
- Wang, Y.X.; Qiu, Y.T.; Sun, A.Y.; Xiong, Y.H.; Tan, H.Y.; Shi, Y.Q.; Yu, P.; Roy, G.; Zhang, L.; Yan, J.W. Dual-functional AIE fluorescent probes for imaging beta-amyloid plaques and lipid droplets. Anal. Chim. Acta 2020, 1133, 109–118. [Google Scholar] [CrossRef]
- Yujun, H.; Yong, W.; Sofie, L.; Beimeng, Y.; Yue, W.; Stephanie, C.; Mark, P.M.; Deborah, L.C.; Vilhelm, A.B. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING [Neuroscience]. Proc. Natl. Acad. Sci. USA 2021, 24, 3. [Google Scholar]
- Hokari, R.; Tomioka, A. The role of lymphatics in intestinal inflammation. Inflamm. Regen. 2021, 41, 25. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, F.; Ge, Y.; Peng, K.; Li, X.; Li, Y.; Xu, Y. Development of Near-Infrared Fluorescent Probes for Use in Alzheimer’s Disease Diagnosis. Bioconjug. Chem. 2020, 31, 2–15. [Google Scholar] [CrossRef]

| Nanomaterials | Biomarkers | LOD | Clinical Samples | References |
|---|---|---|---|---|
| Cu-BTC/Tb | Aβ40 | 0.3 nM | Human blood | [70] |
| 3D hydrogel | Aβ | 0.5 pM | Human serum | [71] |
| pGOLD | Aβ | 0.1 pg/mL | Human blood | [54] |
| H-USM/BHQ-1 | Aβ oligomers | 28.4 pg/mL | / | [72] |
| AuNP-RAMRA | Aβ oligomers | 22.3 pM | AD mice | [73] |
| ThT@Er-MOF | AchE | 0.03226 nM | / | [67] |
| L-MOF | Aβ oligomers | 0.4 pg/mL | Human serum | [74] |
| LMOF/Apt-Au | Aβ oligomers | 0.3 pM | Human serum | [75] |
| CDs@Eu/GMP | Aβ | 0.17 nM | Brain tissue of rat | [76] |
| MoS2 NSs | Aβ oligomers | 3.1 nM | Brain tissue of AD mice | [57] |
| FAM-AptAβ@PBNPs | Aβ oligomers | 1.0 nM | Human CSF | [77] |
| CuInS2/ZnS quantum dots | Tau protein | 9.3 pM | Human serum | [62] |
| GO | Tau protein | 0.14 nM | Human samples | [60] |
| WS2 nanosheet | BACE1 | 66 pM | Rat CSF | [46] |
| GQD-CM | APOE4 | 18.6 pg/mL | Human plasma | [78] |
| AuNPs | BACE-1 | 26.48 pM | / | [66] |
| PS nanoparticle | miRNA-101 | 5 fM | Human serum | [64] |
| GOX-SYBR | miRNA-137, miRNA-142 | 82 nM | Human serum | [79] |
| WS2 nanosheets | miR-29a | 745 pM | Human serum | [80] |
| BSA-AuNCs | Cu2+ | 0.1465 μM | Mice sample | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, Y.; Liu, Q. Fluorescent Sensing Platforms for Detecting and Imaging the Biomarkers of Alzheimer’s Disease. Biosensors 2023, 13, 515. https://doi.org/10.3390/bios13050515
Liu X, Liu Y, Liu Q. Fluorescent Sensing Platforms for Detecting and Imaging the Biomarkers of Alzheimer’s Disease. Biosensors. 2023; 13(5):515. https://doi.org/10.3390/bios13050515
Chicago/Turabian StyleLiu, Xingyun, Yibiao Liu, and Qiong Liu. 2023. "Fluorescent Sensing Platforms for Detecting and Imaging the Biomarkers of Alzheimer’s Disease" Biosensors 13, no. 5: 515. https://doi.org/10.3390/bios13050515
APA StyleLiu, X., Liu, Y., & Liu, Q. (2023). Fluorescent Sensing Platforms for Detecting and Imaging the Biomarkers of Alzheimer’s Disease. Biosensors, 13(5), 515. https://doi.org/10.3390/bios13050515






