Molybdenum Disulfide/Nickel-Metal Organic Framework Hybrid Nanosheets Based Disposable Electrochemical Sensor for Determination of 4-Aminophenol in Presence of Acetaminophen
Abstract
1. Introduction
2. Experimental Section
2.1. Apparatus and Materials
2.2. Fabrication of MoS2
2.3. Fabrication of MoS2/Ni-MOF Hybrid Nanosheets
2.4. SPGE Modification with MoS2/Ni MOF Hybrid Nanosheets
2.5. Preparation of Real Specimens
3. Results and Discussion
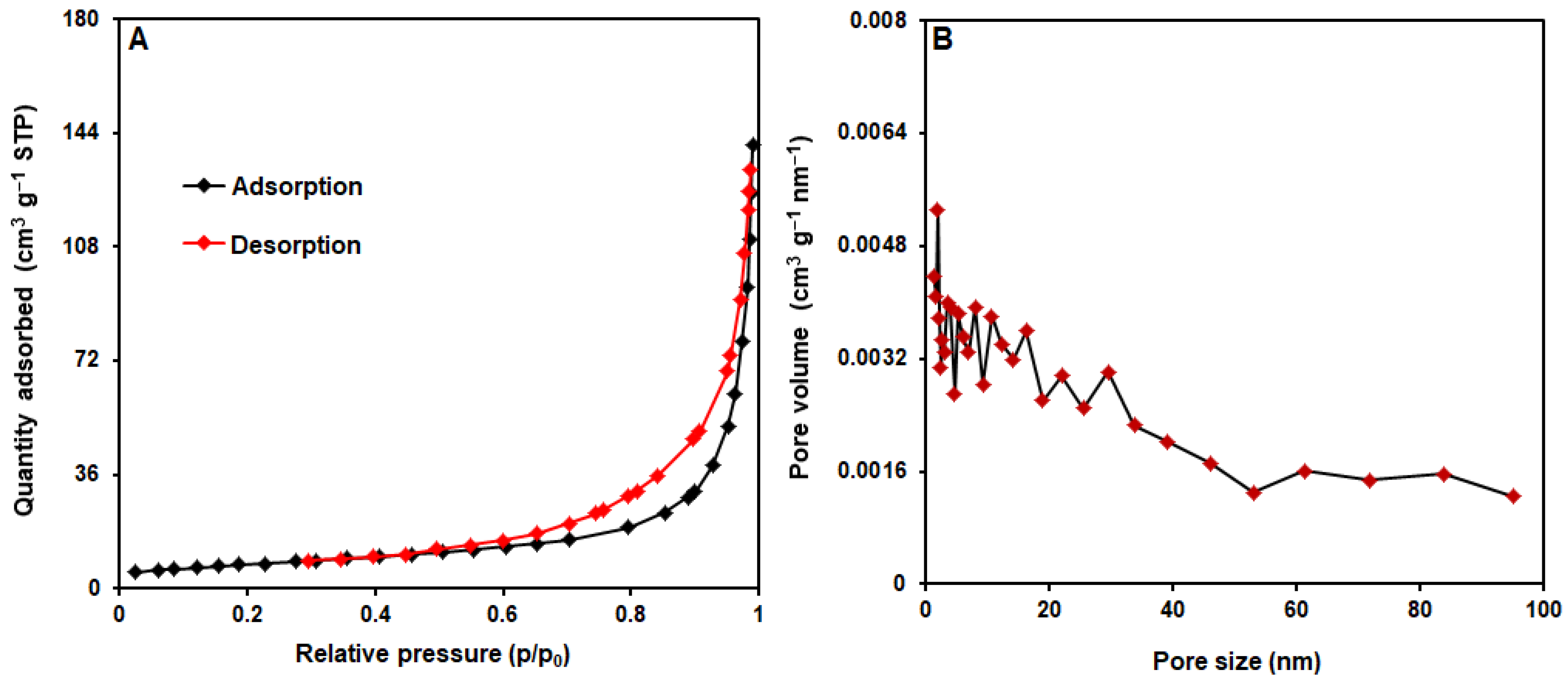
3.1. Characterizations
3.2. Electrochemical Performance of 4-AP on the Surface of MoS2/Ni-MOF/SPGE
3.3. The Effect of the Scanning Rate
3.4. Chronoamperometric Measurements
3.5. Calibration Plot and Detection Limit
| Electrochemical Sensor | Method | Linear Range | LOD | Real Samples | Ref. |
|---|---|---|---|---|---|
| Chitosan-Au nanoparticles-Pd-reduced graphene oxide nanohybrid/glassy carbon electrode | DPV | 1.0–300.0 μM | 0.12 μM | Water | [66] |
| Hemin-molecularly imprinted polymer/glassy carbon electrode | Amperometric | 10.0–90.0 μM | 3.0 μM | Tap and river water | [67] |
| Graphene/hydroxyapatite nanocomposite/glassy carbon electrode | Square wave voltammetry | 0.1–425.0 μM | 0.29 μM | Tap water | [68] |
| Graphene–chitosan composite/glassy carbon electrode | DPV | 0.2–550.0 μM | 0.057 μM | River water, Lake water, Waste water, and Tap water | [69] |
| Graphene–polyaniline nanocomposite/glassy carbon electrode | DPV | 0.2–100.0 μM | 0.065 μM | - | [70] |
| MoS2/Ni-MOF/SPGE | DPV | 0.1–600.0 μM | 0.04 μM | Acetaminophen tablet and tap water | This Work |
3.6. Determination of 4-AP in Presence ACAP
3.7. ACAP and 4-AP Detection in the Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Xing, Z.; Pan, M.; Wang, H.B.; Liu, Y.M. Highly sensitive and selective electrochemical determination of 4-aminophenol based on flower-like Ag-Au nanocomposites modified glassy carbon electrode. J. Electrochem. Soc. 2020, 167, 126504. [Google Scholar] [CrossRef]
- Sun, L.; Yang, M.; Guo, H.; Zhang, T.; Wu, N.; Wang, M.; Yang, W. COOH-MWCNT connected COF and chemical activated CTF as a novel electrochemical sensing platform for simultaneous detection of acetaminophen and p-aminophenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129092. [Google Scholar] [CrossRef]
- Rahman, M.M. Selective and sensitive 4-Aminophenol chemical sensor development based on low-dimensional Ge-doped ZnO nanocomposites by electrochemical method. Microchem. J. 2020, 157, 104945. [Google Scholar] [CrossRef]
- Chen, S.; Huang, R.; Zou, J.; Liao, D.; Yu, J.; Jiang, X. A sensitive sensor based on MOFs derived nanoporous carbons for electrochemical detection of 4-aminophenol. Ecotoxicol. Environ. Saf. 2020, 191, 110194. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Reddy, T.M.; Palakollu, V.N.; Karpoormath, R.; Rao, Y.S.; Venkataprasad, G.; Gopal, P. Multi walled carbon nanotubes supported CuO-Au hybrid nanocomposite for the effective application towards the electrochemical determination of acetaminophen and 4-aminophenol. Synt. Metals 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, H.; Wu, N.; Cao, Y.; Wang, M.; Zhang, L.; Yang, W. Highly sensitive determination of acetaminophen and 4-aminophenol based on COF/3D NCNF-T/Au NPs composite electrochemical sensing platform. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127624. [Google Scholar] [CrossRef]
- Ariavand, S.; Ebrahimi, M.; Fooladi, E. The Simultaneous Spectrophotometric Determination of Acetaminophen, Celecoxib, Diazepam, and Famotidine in Environmental Samples by Partial Least Squares. Chem. Methodol. 2021, 5, 82–89. [Google Scholar]
- Dou, N.; Zhang, S.; Qu, J. Simultaneous detection of acetaminophen and 4-aminophenol with an electrochemical sensor based on silver–palladium bimetal nanoparticles and reduced graphene oxide. RSC Adv. 2019, 9, 31440–31446. [Google Scholar] [CrossRef]
- Calam, T.T. A modified pencil graphite electrode with 2-thiobarbituric acid for the efficient and cheap voltammetric sensing of 4-aminophenol in water samples and child syrup sample. J. Food Compos. Anal. 2021, 98, 103809. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Yu, S.; Yang, Q.; Tong, Y.; Ye, B.C. Electrochemical sensor based on N-doped carbon dots decorated with manganese oxide nanospheres for simultaneous detection of p-aminophenol and paracetamol. Analyst 2021, 146, 5135–5142. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, M.; Zhang, L. 3D multiporous Co, N co-doped MoO2/MoC nanorods hybrids as improved electrode materials for highly sensitive simultaneous determination of acetaminophen and 4-aminophenol. Electrochim. Acta 2019, 302, 56–64. [Google Scholar] [CrossRef]
- Bloomfield, M.S. A sensitive and rapid assay for 4-aminophenol in paracetamol drug and tablet formulation, by flow injection analysis with spectrophotometric detection. Talanta 2002, 58, 1301–1310. [Google Scholar] [CrossRef]
- Easwaramoorthy, D.; Yu, Y.C.; Huang, H.J. Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal. Chim. Acta 2001, 439, 95–100. [Google Scholar] [CrossRef]
- Merrikhi Khosroshahi, A.; Aflaki, F.; Saemiyan, N.; Abdollahpour, A.; Asgharian, R. Simultaneous determination of paracetamol, 4-Aminophenol, 4-Chloroacetanilid, Benzyl alcohol, Benzaldehyde and EDTA by HPLC methodin paracetamol injection ampoule. J. Pharma. Health Sci. 2016, 4, 61–69. [Google Scholar]
- Chu, Q.; Jiang, L.; Tian, X.; Ye, J. Rapid determination of acetaminophen and p-aminophenol in pharmaceutical formulations using miniaturized capillary electrophoresis with amperometric detection. Anal. Chim. Acta 2008, 606, 246–251. [Google Scholar] [CrossRef]
- Palanna, M.; Mohammed, I.; Aralekallu, S.; Nemakal, M.; Sannegowda, L.K. Simultaneous detection of paracetamol and 4-aminophenol at nanomolar levels using biocompatible cysteine-substituted phthalocyanine. New J. Chem. 2020, 44, 1294–1306. [Google Scholar] [CrossRef]
- Peyman, H. Design and Fabrication of Modified DNA-Gp Nano-Biocomposite Electrode for Industrial Dye Measurement and Optical Confirmation. Prog. Chem. Biochem. Res. 2022, 5, 391–405. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Aflatoonian, M.R.; Makarem, A. Glutathione detection at carbon paste electrode modified with ethyl 2-(4-ferrocenyl-[1,2,3] triazol-1-yl) acetate, ZnFe2O4nano-particles and ionic liquid. J. Electrochem. Sci. Eng. 2022, 12, 209–217. [Google Scholar] [CrossRef]
- Almandil, N.B.; Ibrahim, M.; Ibrahim, H.; Kawde, A.N.; Shehatta, I.; Akhtar, S. A hybrid nanocomposite of CeO 2–ZnO–chitosan as an enhanced sensing platform for highly sensitive voltammetric determination of paracetamol and its degradation product p-aminophenol. RSC adv. 2019, 9, 15986–15996. [Google Scholar] [CrossRef]
- Beitollahi, H.; Raoof, J.B.; Hosseinzadeh, R. Electroanalysis and simultaneous determination of 6-thioguanine in the presence of uric acid and folic acid using a modified carbon nanotube paste electrode. Anal. Sci. 2011, 27, 991. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Maleki, N.; Moradlou, O. A selective and sensitive method for simultaneous determination of traces of paracetamol and p-aminophenol in pharmaceuticals using carbon ionic liquid electrode. Electroanalysis 2008, 20, 2158–2162. [Google Scholar] [CrossRef]
- Taleat, Z.; Ardakani, M.M.; Naeimi, H.; Beitollahi, H.; Nejati, M.; Zare, H.R. Electrochemical behavior of ascorbic acid at a 2, 2’-[3, 6-dioxa-1, 8-octanediylbis (nitriloethylidyne)]-bis-hydroquinone carbon paste electrode. Anal. Sci. 2008, 24, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Harismah, K.; Mirzaei, M.; Da’i, M.; Roshandel, Z.; Salarrezaei, E. In silico investigation of nanocarbon biosensors for diagnosis of COVID-19. Eurasian Chem. Commun. 2021, 3, 95–102. [Google Scholar]
- Scandurra, G.; Antonella, A.; Ciofi, C.; Saitta, G.; Lanza, M. Electrochemical detection of p-aminophenol by flexible devices based on multi-wall carbon nanotubes dispersed in electrochemically modified nafion. Sensors 2014, 14, 8926–8939. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. Simultaneous determination of droxidopa and carbidopa using a carbon nanotubes paste electrode. Sens. Actuators B Chem. 2013, 188, 923–930. [Google Scholar] [CrossRef]
- Peyman, H.; Roshanfekr, H.; Babakhanian, A.; Jafari, H. PVC Membrane Electrode Modified by Lawson as Synthetic Derivative Ionophore for Determination of Cadmium in Alloy and Wastewater. Chem. Methodol. 2021, 5, 446–453. [Google Scholar]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar] [CrossRef]
- Mohabis, R.M.; Fazeli, F.; Amini, I.; Azizkhani, V. An overview of recent advances in the detection of ascorbic acid by electrochemical techniques. J. Electrochem. Sci. Eng. 2022, 12, 1081–1098. [Google Scholar]
- Beitollahi, H.; Sheikhshoaie, I. Novel nanostructure-based electrochemical sensor for simultaneous determination of dopamine and acetaminophen. Mater. Sci. Eng. C 2012, 32, 375–380. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, Y.; Liu, S. A novel enhanced electrochemical sensor based on the peroxidase-like activity of Fe3O4@ Au/MOF for the detection of p-aminophenol. J. Appl. Electrochem. 2022, 52, 989–1002. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Zhao, J.; Zhang, R.; Zhao, N.; Ren, H.; Li, Y. Simultaneous determination of paracetamol and p-aminophenol using glassy carbon electrode modified with nitrogen-and sulfur-co-doped carbon dots. Microchim. Acta 2019, 186, 733. [Google Scholar] [CrossRef] [PubMed]
- Roshanfekr, H. A Simple Specific Dopamine Aptasensor Based on Partially Reduced Graphene Oxide–AuNPs composite. Prog. Chem. Biochem. Res. 2023, 6, 79–88. [Google Scholar]
- Shi, P.; Xue, R.; Wei, Y.; Lei, X.; Ai, J.; Wang, T.; Yang, W. Gold nanoparticles/tetraaminophenyl porphyrin functionalized multiwalled carbon nanotubes nanocomposites modified glassy carbon electrode for the simultaneous determination of p-acetaminophen and p-aminophenol. Arab. J. Chem. 2020, 13, 1040–1051. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Aflatoonian, M.R.; Makarem, A. A sensitive Cu (salophen) modified screen-printed electrode for simultaneous determination of dopamine and uric acid. J. Electrochem. Sci. Eng. 2022, 12, 199–208. [Google Scholar] [CrossRef]
- Miraki, M.; Karimi-Maleh, H.; Taher, M.A.; Cheraghi, S.; Karimi, F.; Agarwal, S.; Gupta, V.K. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 2019, 278, 672–676. [Google Scholar] [CrossRef]
- Mustafa, Y.F.; Chehardoli, G.; Habibzadeh, S.; Arzehgar, Z. Electrochemical detection of sulfite in food samples. J. Electrochem. Sci. Eng. 2022, 12, 1061–1079. [Google Scholar] [CrossRef]
- Calam, T.T.; Uzun, D. Rapid and Selective Determination of Vanillin in the Presence of Caffeine, its Electrochemical Behavior on an Au Electrode Electropolymerized with 3-amino-1, 2, 4-triazole-5-thiol. Electroanalysis 2019, 31, 2347–2358. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with Multi-Wall Carbon Nano-Tubes (MWCNTs/N, N-Bis (salicyliden)-1, 3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Cerda, V.; Rennan, G.O.A.; Ferreira, S.L. Revising Flow-through Cells for Amperometric and Voltammetric Detections Using Stationary Mercury and Bismuth Screen Printed Electrodes. Prog. Chem. Biochem. Res. 2022, 5, 351–366. [Google Scholar]
- Zaid Almarbd, Z.; Mutter Abbass, N. Synthesis and characterization of TiO2, Ag2O, and graphene oxide nanoparticles with polystyrene as a nonocomposites and some of their applications. Eurasian Chem. Commun. 2022, 4, 1033–1043. [Google Scholar]
- Zheng, S.; Li, B.; Tang, Y.; Li, Q.; Xue, H.; Pang, H. Ultrathin nanosheet-assembled [Ni3(OH)2(PTA)2(H2O)4]·2H2O hierarchical flowers for high-performance electrocatalysis of glucose oxidation reactions. Nanoscale 2018, 10, 13270–13276. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Li, J.; Liu, Y.M. Electrochemical sensing based on layered MoS2–graphene composites. Sens. Actuators B Chem. 2013, 178, 671–677. [Google Scholar] [CrossRef]
- Su, S.; Sun, H.; Xu, F.; Yuwen, L.; Wang, L. Highly sensitive and selective determination of dopamine in the presence of ascorbic acid using gold nanoparticles-decorated MoS2 nanosheets modified electrode. Electroanalysis 2013, 25, 2523–2529. [Google Scholar] [CrossRef]
- Kukkar, M.; Sharma, A.; Kumar, P.; Kim, K.H.; Deep, A. Application of MoS2 modified screen-printed electrodes for highly sensitive detection of bovine serum albumin. Anal. Chim. Acta 2016, 939, 101–107. [Google Scholar] [CrossRef]
- Wu, Q.; Ji, C.; Zhang, L.; Shi, Q.; Wu, Y.; Tao, H. A simple sensing platform based on a 1T@ 2H-MoS 2/cMWCNTs composite modified electrode for ultrasensitive detection of illegal Sudan I dye in food samples. Anal. Methods 2022, 14, 549–559. [Google Scholar] [CrossRef]
- Shayegan, H.; Safarifard, V.; Taherkhani, H.; Rezvani, M.A. Efficient removal of cobalt(II) ion from aqueous solution using amide-functionalized metal-organic framework. J. Appl. Organomet. Chem. 2022, 2, 109–118. [Google Scholar]
- Taghavi, R.; Rostamnia, S. Four-Component Synthesis of Polyhydroquinolines via Unsymmetrical Hantzsch Reaction Employing Cu-IRMOF-3 as a Robust Heterogeneous Catalyst. Chem. Methodol. 2022, 6, 639–648. [Google Scholar]
- Akeremale, O.K. Metal-Organic Frameworks (MOFs) as Adsorbents for Purification of Dye-Contaminated Wastewater: A Review. J. Chem. Rev. 2022, 4, 1–14. [Google Scholar]
- Ko, M.; Mendecki, L.; Mirica, K.A. Conductive two-dimensional metal–organic frameworks as multifunctional materials. Chem. Commun. 2018, 54, 7873–7891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.T.; Yin, H.; An, P.; Tang, Z. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar]
- Zhu, D.; Liu, J.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. Engineering 2D metal–organic framework/MoS2 interface for enhanced alkaline hydrogen evolution. Small 2019, 15, 1805511. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. 2D layered metal organic framework nanosheets as an emerging platform for electrochemical sensing. J. Electrochem. Soc. 2020, 167, 136502. [Google Scholar]
- Zhao, H.; Du, X.; Dong, H.; Jin, D.; Tang, F.; Liu, Q.; Li, Y. Electrochemical immunosensor based on Au/Co-BDC/MoS2 and DPCN/MoS2 for the detection of cardiac troponin I. Biosens. Bioelectron. 2021, 175, 112883. [Google Scholar] [CrossRef]
- Ge, Y.; Chu, H.; Chen, J.; Zhuang, P.; Feng, Q.; Smith, W.R.; Shen, J. Ultrathin MoS2 nanosheets decorated hollow CoP heterostructures for enhanced hydrogen evolution reaction. ACS Sust. Chem. Eng. 2019, 7, 10105–10111. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Xiong, Q.; Zhang, S.; Zhao, C.; Gong, W.; Zhao, H. Dramatically enhanced ambient ammonia electrosynthesis performance by in-operando created Li–S interactions on MoS2 electrocatalyst. Adv. Energy Mater. 2019, 9, 1803935. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Q.; Guo, Z.; Zhang, M.; Zhou, M.; Zhai, Z.; Xu, Y. Self-assembly of MoS2 nanosheet on functionalized pomelo peel derived carbon and its electrochemical sensor behavior toward taxifolin. Inorg. Chem. Commun. 2021, 129, 108631. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Yang, W.; Guo, H.; Fan, T.; Zhao, X.; Zhang, L.; Guan, Q.; Yang, W. MoS2/Ni(OH)2 composites derived from in situ grown Ni-MOF coating MoS2 as electrode materials for supercapacitor and electrochemical sensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126178. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Shanmugapriya, K.; Sriram, S. Photocatalytic activity of MoS2 nanoparticles: An experimental and DFT analysis. Appl. Phys. A 2019, 125, 817. [Google Scholar] [CrossRef]
- Feng, W.; Chen, L.; Qin, M.; Zhou, X.; Zhang, Q.; Miao, Y.; He, C. Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy. Sci. Rep. 2015, 5, 17422. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, S.; Qu, J. Electrochemical sensor based on palladium-reduced graphene oxide modified with gold nanoparticles for simultaneous determination of acetaminophen and 4-aminophenol. Talanta 2018, 178, 188–194. [Google Scholar] [CrossRef]
- Neto, J.D.R.M.; Santos, W.D.J.R.; Lima, P.R.; Tanaka, S.M.C.N.; Tanaka, A.A.; Kubota, L.T. A hemin-based molecularly imprinted polymer (MIP) grafted onto a glassy carbon electrode as a selective sensor for 4-aminophenol amperometric. Sens. Actuators B Chem. 2011, 152, 220–225. [Google Scholar] [CrossRef]
- Lavanya, N.; Sudhan, N.; Kanchana, P.; Radhakrishnan, S.; Sekar, C. A new strategy for simultaneous determination of 4-aminophenol, uric acid and nitrite based on a graphene/hydroxyapatite composite modified glassy carbon electrode. RSC Adv. 2015, 5, 52703–52709. [Google Scholar] [CrossRef]
- Yin, H.; Ma, Q.; Zhou, Y.; Ai, S.; Zhu, L. Electrochemical behavior and voltammetric determination of 4-aminophenol based on graphene–chitosan composite film modified glassy carbon electrode. Electrochim. Acta 2010, 55, 7102–7108. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.H.; Yang, C.P.; Yu, M.; Liu, P. Graphene–polyaniline composite film modified electrode for voltammetric determination of 4-aminophenol. Sens. Actuators B Chem. 2011, 157, 669–674. [Google Scholar] [CrossRef]









| Sample | Spiked | Found | Recovery (%) | R.S.D. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 4-AP | ACAP | 4-AP | ACAP | 4-AP | ACAP | 4-AP | ACAP | |
| Acetaminophen tablets | 0 | 0 | - | 4.0 | - | - | - | 3.5 |
| 5.0 | 2.0 | 4.9 | 6.2 | 98.0 | 103.3 | 1.9 | 2.9 | |
| 6.0 | 3.0 | 6.1 | 6.9 | 101.7 | 98.6 | 2.7 | 2.1 | |
| 7.0 | 4.0 | 7.3 | 7.9 | 104.3 | 98.7 | 3.2 | 1.8 | |
| 8.0 | 5.0 | 7.7 | 9.2 | 96.2 | 102.2 | 2.8 | 2.2 | |
| Tap water | 0 | 0 | - | - | - | - | - | - |
| 5.5 | 6.0 | 5.6 | 5.9 | 101.8 | 98.3 | 2.1 | 3.6 | |
| 6.5 | 8.0 | 6.3 | 8.2 | 97.0 | 102.5 | 3.0 | 2.3 | |
| 7.5 | 10.0 | 7.6 | 9.7 | 101.3 | 97.0 | 1.9 | 2.8 | |
| 8.5 | 12.0 | 8.4 | 12.2 | 98.8 | 101.7 | 2.4 | 1.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourandish, Z.; Sheikhshoaie, I.; Maghsoudi, S. Molybdenum Disulfide/Nickel-Metal Organic Framework Hybrid Nanosheets Based Disposable Electrochemical Sensor for Determination of 4-Aminophenol in Presence of Acetaminophen. Biosensors 2023, 13, 524. https://doi.org/10.3390/bios13050524
Dourandish Z, Sheikhshoaie I, Maghsoudi S. Molybdenum Disulfide/Nickel-Metal Organic Framework Hybrid Nanosheets Based Disposable Electrochemical Sensor for Determination of 4-Aminophenol in Presence of Acetaminophen. Biosensors. 2023; 13(5):524. https://doi.org/10.3390/bios13050524
Chicago/Turabian StyleDourandish, Zahra, Iran Sheikhshoaie, and Shahab Maghsoudi. 2023. "Molybdenum Disulfide/Nickel-Metal Organic Framework Hybrid Nanosheets Based Disposable Electrochemical Sensor for Determination of 4-Aminophenol in Presence of Acetaminophen" Biosensors 13, no. 5: 524. https://doi.org/10.3390/bios13050524
APA StyleDourandish, Z., Sheikhshoaie, I., & Maghsoudi, S. (2023). Molybdenum Disulfide/Nickel-Metal Organic Framework Hybrid Nanosheets Based Disposable Electrochemical Sensor for Determination of 4-Aminophenol in Presence of Acetaminophen. Biosensors, 13(5), 524. https://doi.org/10.3390/bios13050524




