Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Apparatus
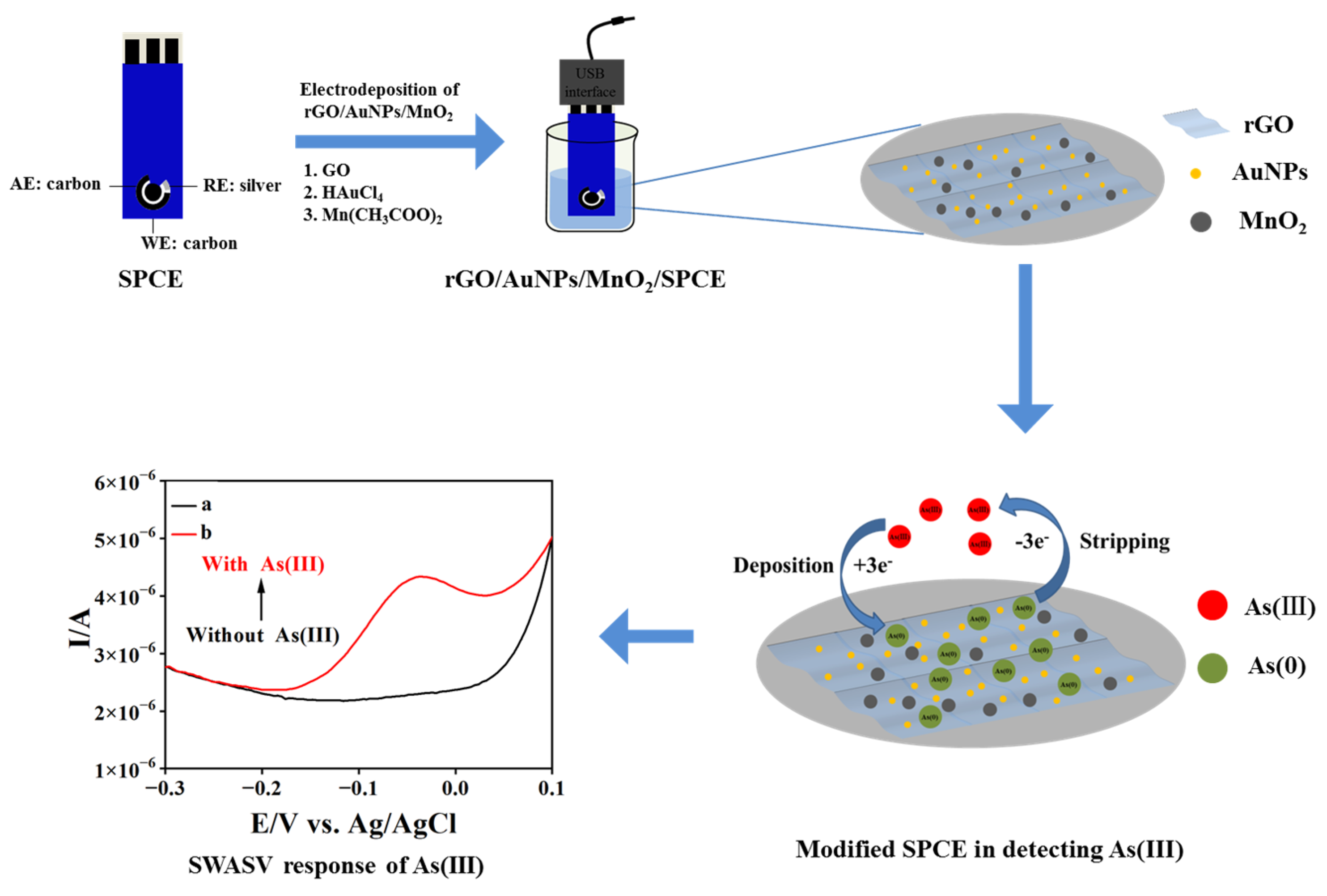
2.3. Fabrication of the Nanocomposite-Modified Electrode
2.4. Electrochemical Measurements
2.5. Detection of Water Samples
3. Results and Discussion
3.1. Preparation and Characterization of Nanocomposite-Modified Electrodes
3.2. Electrochemical Responses of rGO/AuNPs/MnO2/SPCE toward As(III)
3.3. Optimization of Sensing Conditions toward As(III)
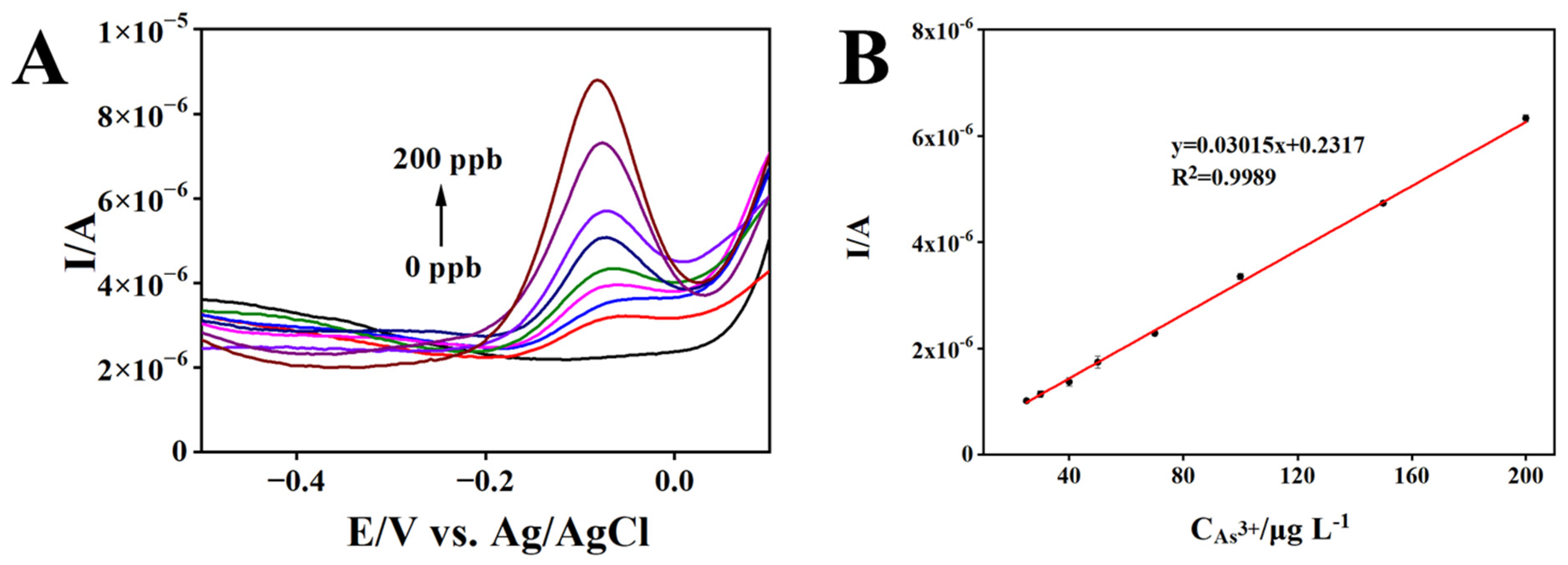
3.4. Analytical Performance of rGO/AuNPs/MnO2/SPCE for As(III) Detection
3.5. Selectivity, Reproducibility, and Stability Measurement
3.6. Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Rosen, B.P. Biosensors for inorganic and organic arsenicals. Biosensors 2014, 4, 494–512. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Wang, Q.Q.; Naranmandura, H. Molecular mechanisms of arsenic toxicity. Adv. Mol. Toxicol. 2015, 9, 77–107. [Google Scholar] [CrossRef]
- Guo, X.H.; Peng, X.X.; Li, Q.; Mo, J.M.; Du, Y.P.; Wang, Z. Ultra-sensitive determination of inorganic arsenic valence by solution cathode glow discharge-atomic emission spectrometry coupled with hydride generation. J. Anal. At. Spectrom. 2017, 32, 2416–2422. [Google Scholar] [CrossRef]
- Yang, L.L.; Gao, L.R.; Zhang, D.Q. Speciation analysis of arsenic in traditional Chinese medicines by hydride generation-atomic fluorescence spectrometry. Anal. Sci. 2003, 19, 897–902. [Google Scholar] [CrossRef]
- Donnell, A.M.; Nahan, K.; Holloway, D.; Vonderheide, A.P. Determination of arsenic in sinus wash and tap water by inductively coupled plasma–mass spectrometry. J. Chem. Educ. 2016, 93, 738–741. [Google Scholar] [CrossRef]
- Tašev, K.; Karadjova, I.; Stafilov, T. Determination of inorganic and total arsenic in wines by hydride generation atomic absorption spectrometry. Microchim. Acta 2005, 149, 55–60. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.; Gupta, V.; Sundramoorthy, A.K.; Arya, S. Involvement of metal organic frameworks in wearable electrochemical sensor for efficient performance. Trends Environ. Anal. Chem. 2023, 38, e00200. [Google Scholar] [CrossRef]
- Forsberg, G.; O’Laughlin, J.W.; Megargle, R.G. Determination of arsenic by anodic stripping voltammetry and differential pulse anodic stripping voltammetry. Anal. Chem. 1975, 47, 1586–1592. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Tavares, E.D.F.L.; Saczk, A.A.; Okumura, L.L.; Cardoso, M.D.G.; Magriotis, Z.M.; Oliveira, M.F.D. Cathodic stripping voltammetric determination of arsenic in sugarcane brandy at a modified carbon nanotube paste electrode. Food Chem. 2014, 154, 38–43. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Sampath, S. In-situ formation of graphene–lead oxide composite and its use in trace arsenic detection. Sens. Actuators B Chem. 2011, 160, 306–311. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.Y.; Xiong, S.Q.; Liu, J.H.; Huang, X.J. Electrochemical detection of arsenic(III) completely free from noble metal: Fe3O4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal. Chem. 2013, 85, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, P.H.; Xu, W.H.; Wei, Y.; Li, L.N.; Huang, Y.Y.; Sun, Y.F.; Chen, X.; Liu, J.H.; Huang, X.J. Reliable electrochemical sensing arsenic(III) in nearly groundwater pH based on efficient adsorption and excellent electrocatalytic ability of AuNPs/CeO2-ZrO2 nanocomposite. Sens. Actuators B Chem. 2018, 255, 226–234. [Google Scholar] [CrossRef]
- Dai, X.; Compton, R.G. Detection of As(III) via oxidation to As(V) using platinum nanoparticle modified glassy carbon electrodes: Arsenic detection without interference from copper. Analyst 2006, 131, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.O.; Mafa, J.P.; Mabuba, N.; Arotiba, O.A. Nanogold modified glassy carbon electrode for the electrochemical detection of arsenic in water. Russ. J. Electrochem. 2017, 53, 170–177. [Google Scholar] [CrossRef]
- Touilloux, R.; Tercier-Waeber, M.L.; Bakker, E. Direct arsenic(III) sensing by a renewable gold plated Ir-based microelectrode. Analyst 2015, 140, 3526–3534. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Xie, Q.J.; Sun, L.G.; Gu, T.A.; Li, Z.; Bu, L.J.; Yao, S.Z.; Tu, X.M.; Luo, X.B.; et al. Electrodeposition of electroreduced graphene oxide-Au nanoparticles composite film at glassy carbon electrode for anodic stripping voltammetric analysis of trace arsenic(III). Sens. Actuators B Chem. 2013, 188, 894–901. [Google Scholar] [CrossRef]
- Li, S.S.; Zhou, W.Y.; Li, Y.X.; Jiang, M.; Guo, Z.; Liu, J.H.; Huang, X.J. Noble-metal-free Co0.6Fe2.4O4 nanocubes self-assembly monolayer for highly sensitive electrochemical detection of As(III) based on surface defects. Anal. Chem. 2018, 90, 1263–1272. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive electrochemical detection of arsenic (III) using gold nanoparticle modified carbon nanotubes via anodic stripping voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef]
- Mafakheri, E.; Salimi, A.; Hallaj, R.; Ramazani, A.; Kashi, M.A. Synthesis of iridium oxide nanotubes by electrodeposition into polycarbonate template: Fabrication of chromium(III) and arsenic(III) electrochemical sensor. Electroanalysis 2011, 23, 2429–2437. [Google Scholar] [CrossRef]
- Li, S.S.; Zhou, W.Y.; Jiang, M.; Guo, Z.; Liu, J.H.; Zhang, L.Z.; Huang, X.J. Surface Fe(II)/Fe(III) cycle promoted ultra-highly sensitive electrochemical sensing of arsenic(III) with dumbbell-like Au/Fe3O4 nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef]
- Jiang, T.J.; Guo, Z.; Liu, J.H.; Huang, X.J. Gold electrode modified with ultrathin SnO2 nanosheets with high reactive exposed surface for electrochemical sensing of As(III). Electrochim. Acta 2016, 191, 142–148. [Google Scholar] [CrossRef]
- Salimi, A.; Mamkhezri, H.; Hallaj, R.; Soltanian, S. Electrochemical detection of trace amount of arsenic(III) at glassy carbon electrode modified with cobalt oxide nanoparticles. Sens. Actuators B Chem. 2008, 129, 246–254. [Google Scholar] [CrossRef]
- Chowdhury, A.N.; Ferdousi, S.; Islam, M.M.; Okajima, T.; Ohsaka, T. Arsenic detection by nanogold/conducting-polymer-modified glassy carbon electrodes. J. Appl. Polym. Sci. 2007, 104, 1306–1311. [Google Scholar] [CrossRef]
- Salunke, R.S.; Kasar, C.K.; Bangar, M.A.; Chavan, P.G.; Shirale, D.J. Electrodeposition of gold nanoparticles decorated single polypyrrole nanowire for arsenic detection in potable water: A chemiresistive sensor device. J. Mater. Sci. Mater. Electron. 2017, 28, 14672–14677. [Google Scholar] [CrossRef]
- Li, W.W.; Kong, F.Y.; Wang, J.Y.; Chen, Z.D.; Fang, H.L.; Wang, W. Facile one-pot and rapid synthesis of surfactant-free Au-reduced graphene oxide nanocomposite for trace arsenic (III) detection. Electrochim. Acta 2015, 157, 183–190. [Google Scholar] [CrossRef]
- Ahmed, A.; Singh, A.; Young, S.J.; Gupta, V.; Singh, M.; Arya, S. Synthesis techniques and advances in sensing applications of reduced graphene oxide (rGO) composites: A review. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107373. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and its derivatives: Synthesis and application in the electrochemical detection of analytes in sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, P.K.; Satpati, A.K. Gold nano particle and reduced graphene oxide composite modified carbon paste electrode for the ultra trace detection of arsenic (III). Electroanalysis 2017, 29, 1400–1409. [Google Scholar] [CrossRef]
- Wu, S.G.; Zhao, Q.P.; Zhou, L.; Zhang, Z.X. Stripping analysis of trace arsenic based on the MnOx/AuNPs composite film modified electrode in alkaline media. Electroanalysis 2014, 26, 1840–1849. [Google Scholar] [CrossRef]
- Devi, P.; Bansod, B.; Kaur, M.; Bagchi, S.; Nayak, M.K. Co-electrodeposited rGO/MnO2 nanohybrid for arsenite detection in water by stripping voltammetry. Sens. Actuators B Chem. 2016, 237, 652–659. [Google Scholar] [CrossRef]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Guo, S.J.; Fang, Y.X.; Han, L.; Wang, E.K.; Dong, S.J. One-step electrochemical approach to the synthesis of Graphene/MnO2 nanowall hybrids. Nano Res. 2011, 4, 648–657. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.F.; Xiao, P.; Garcia, B.B.; Guo, Q.; Champion, R.; Cao, G.Z. Hydrous manganese dioxide nanowall arrays growth and their Li+ ions intercalation electrochemical properties. Chem. Mater. 2008, 20, 1376–1380. [Google Scholar] [CrossRef]
- Liu, D.W.; Garcia, B.B.; Zhang, Q.F.; Guo, Q.; Zhang, Y.H.; Sepehri, S.; Cao, G.Z. Mesoporous hydrous manganese dioxide nanowall arrays with large lithium ion energy storage capacities. Adv. Funct. Mater. 2009, 19, 1015–1023. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Shamspur, T. Designing an Au/reduced graphene oxide modified carbon paste electrode for the electrochemical quantification of agnuside. Sens. Actuators B Chem. 2019, 290, 188–194. [Google Scholar] [CrossRef]
- El-badawy, F.M.; Mohamed, M.A.; El-Desoky, H.S. Fabrication of an electrochemical sensor based on manganese oxide nanoparticles supported on reduced graphene oxide for determination of subnanomolar level of anti-hepatitis C daclatasvir in the formulation and biological models. Microchem. J. 2020, 157, 104914. [Google Scholar] [CrossRef]
- Dai, X.; Nekrassova, O.; Hyde, M.E.; Compton, R.G. Anodic stripping voltammetry of arsenic (III) using gold nanoparticle-modified electrodes. Anal. Chem. 2004, 76, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Sharma, C.; Kumar, P.; Kumar, M.; Bansod, B.K.S.; Nayak, M.K.; Singla, M.L. Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J. Hazard. Mater. 2017, 322, 85–94. [Google Scholar] [CrossRef]
- Song, Y.; Swain, G.M. Total inorganic arsenic detection in real water samples using anodic stripping voltammetry and a gold-coated diamond thin-film electrode. Anal. Chim. Acta 2007, 593, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Yu, J.G.; Xiao, W. Hierarchically porous MnO2 microspheres with enhanced adsorption performance. J. Mater. Chem. A 2013, 1, 11682–11690. [Google Scholar] [CrossRef]
- Xue, W.L.; Yi, H.; Lu, Y.L.; Xia, L.; Meng, D.L.; Song, S.X.; Li, Y.T.; Wu, L.; Farías, M.E. Combined electrosorption and chemisorption of As(III) in aqueous solutions with manganese dioxide as the electrode. Environ. Technol. Innov. 2021, 24, 101832. [Google Scholar] [CrossRef]
- Aguirre, M.d.C.; Rivas, B.L.; Basáez, L.; Peña-Farfal, C. Electrochemical detection of arsenite with silver electrodes in inorganic electrolyte and natural system mixtures. J. Braz. Chem. Soc. 2011, 22, 2362–2370. [Google Scholar] [CrossRef]
- Huang, J.F.; Chen, H.H. Gold-nanoparticle-embedded nafion composite modified on glassy carbon electrode for highly selective detection of arsenic(III). Talanta 2013, 116, 852–859. [Google Scholar] [CrossRef]
- Boonpeng, P.; Sooksamiti, P.; Lapanantnoppakhun, S.; Jakmunee, J. Determination of inorganic arsenic by anodic stripping voltammetry with gold nanoparticles modified screen-printed carbon electrode and anion exchange column preconcentration. Chiang Mai J. Sci 2019, 46, 106–117. [Google Scholar]
- Sedki, M.; Zhao, G.; Ma, S.C.; Jassby, D.; Mulchandani, A. Linker-free magnetite-decorated gold nanoparticles (Fe3O4-Au): Synthesis, characterization, and application for electrochemical detection of arsenic (III). Sensors 2021, 21, 883. [Google Scholar] [CrossRef]
- Thotiyl, M.M.O.; Basit, H.; Sánchez, J.A.; Goyer, C.; Coche-Guerente, L.; Dumy, P.; Sampath, S.; Labbé, P.; Moutet, J.-C. Multilayer assemblies of polyelectrolyte-gold nanoparticles for the electrocatalytic oxidation and detection of arsenic(III). J. Colloid Interface Sci. 2012, 383, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Han, X.J.; Fan, H.L.; Liu, Y.Q. Electrochemical sensing toward trace As(III) based on mesoporous MnFe2O4/Au hybrid nanospheres modified glass carbon electrode. Sensors 2016, 16, 935. [Google Scholar] [CrossRef]
- Hu, H.B.; Lu, W.J.; Liu, X.N.; Meng, F.C.; Zhu, J.X. A high-response electrochemical As(III) sensor using Fe3O4–rGO nanocomposite materials. Chemosensors 2021, 9, 150. [Google Scholar] [CrossRef]
- Méndez Cortés, S.P.; Galán Vidal, C.A.; Rodríguez Ávila, J.A.; Álvarez Romero, G.A.; Páez Hernández, M.E. Square wave anodic stripping voltammetry determination of arsenic (III) onto carbon electrodes by means of co-deposition with silver. J. Mex. Chem. Soc. 2018, 62, 314–322. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Z.; Li, L.N.; Huang, Y.Y.; Liu, J.H.; Zhou, Q.; Chen, X.; Huang, X.J. Electrochemical determination of arsenic(III) with ultra-high anti-interference performance using Au–Cu bimetallic nanoparticles. Sens. Actuators B Chem. 2016, 231, 70–78. [Google Scholar] [CrossRef]
- Huang, H.Q.; Li, Y.Y.; Chen, S.H.; Liu, Z.G.; Cui, Y.M.; Li, H.Q.; Guo, Z.; Huang, X.J. Noble-metal-free Fe3O4/Co3S4 nanosheets with oxygen vacancies as an efficient electrocatalyst for highly sensitive electrochemical detection of As(III). Anal. Chim. Acta 2022, 1189, 339208. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wei, Y.Y.; Shen, W.; Dong, X.; Yang, M.; Wei, J. Ultrahigh sensitivity electroanalysis of trace As(III) in water and human serum via gold nanoparticles uniformly anchored to Co3O4 porous microsheets. Electrochim. Acta 2021, 368, 137605. [Google Scholar] [CrossRef]





| Electrode | Technique | Modification Method | LDR (μg L−1) | LOD (μg L−1) | Service Life (days) | Reference |
|---|---|---|---|---|---|---|
| AuNPs/SPCE | ASV | Electrodeposition | 30–150 | 8.9 | - | [44] |
| Fe3O4/AuNPs/GCE | SWASV | Drop casting | 1–100 | 0.22 | - | [45] |
| PDDA/AuNPs/GCE | DPV | Layer-by-layer assembly | 0–7492 | 4.36 | - | [46] |
| MnFe2O4/AuNPs/GCE | SWASV | Drop casting | 10–110 | 3.37 | 10 | [47] |
| Fe3O4/rGO/GCE | SWV | Drop casting | 1–20 | 1.19 | 15 | [48] |
| Ag/SPCE | SWASV | Electrodeposition | 10–80 | 8.4 | - | [49] |
| AuNPs/CeO2-ZrO2/GCE | SWASV | Drop casting | 0.5–15 | 0.137 | - | [12] |
| Au89Cu11/GCE | SWASV | Drop casting | 10–100 | 2.09 | - | [50] |
| AuNPs/rGO/CPE | ASV | Electrodeposition | 1–20 | 0.13 | 30 | [28] |
| Fe3O4/Co3S4/SPCE | SWASV | Drop casting | 1–10 | 0.691 | - | [51] |
| AuNPs/CNTs/GCE | SWV | Drop casting | 0.75–7.5 | 0.1 | - | [18] |
| AuNPs/Co3O4/SPCE | SWASV | Drop casting | 0.1–1/1–20 | 0.09/0.79 | - | [52] |
| rGO/AuNPs/MnO2/SPCE | SWASV | Electrodeposition | 25–200 | 2.4 | 42 | This work |
| Sample | Added (μg L−1) | Found (μg L−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 50.0 | 53.38 | 106.8 | 2.7 |
| 100.0 | 110.3 | 110.3 | 7.6 | |
| 150.0 | 139.1 | 92.8 | 8.5 | |
| Mountain spring water | 50.0 | 57.28 | 114.6 | 9.4 |
| 100.0 | 108.3 | 108.3 | 9.2 | |
| 150.0 | 158.2 | 105.5 | 3.0 | |
| mineral water | 50.0 | 48.72 | 97.4 | 7.9 |
| 100.0 | 105.6 | 105.6 | 8.8 | |
| 150.0 | 163.8 | 109.2 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, T.; Su, L.; Wu, X. Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water. Biosensors 2023, 13, 563. https://doi.org/10.3390/bios13050563
Wu Y, Zhang T, Su L, Wu X. Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water. Biosensors. 2023; 13(5):563. https://doi.org/10.3390/bios13050563
Chicago/Turabian StyleWu, Yanqing, Tao Zhang, Lishen Su, and Xiaoping Wu. 2023. "Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water" Biosensors 13, no. 5: 563. https://doi.org/10.3390/bios13050563
APA StyleWu, Y., Zhang, T., Su, L., & Wu, X. (2023). Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water. Biosensors, 13(5), 563. https://doi.org/10.3390/bios13050563





