Highly Sensitive Detection of Urea Using Si Electrolyte-Gated Transistor with Low Power Consumption
Abstract
:1. Introduction
2. Materials and Methods
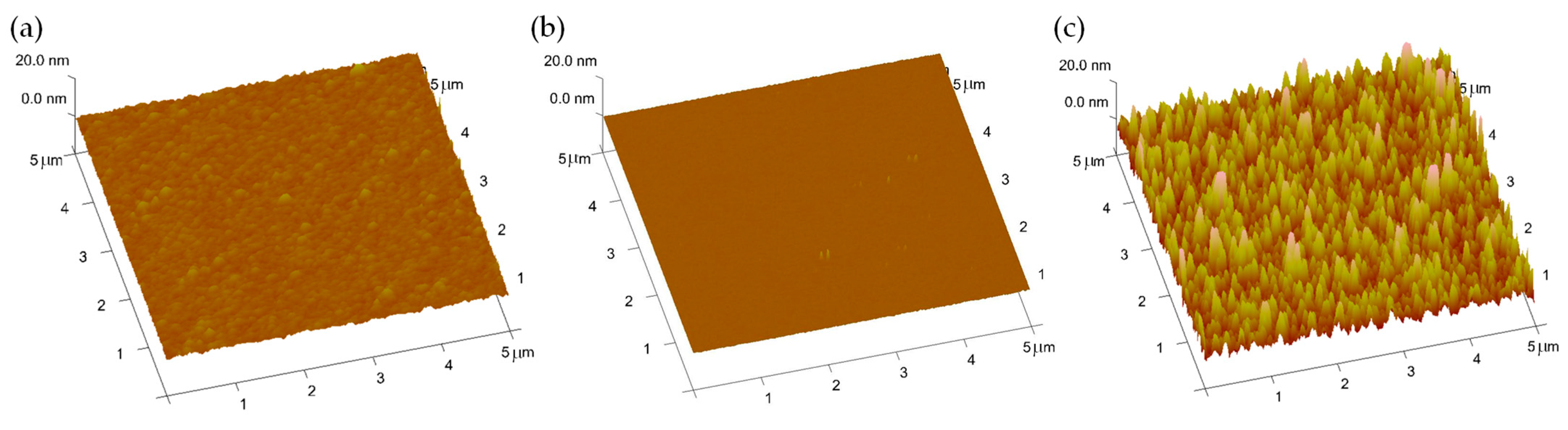
2.1. Material Preparation and Electrical Characterization
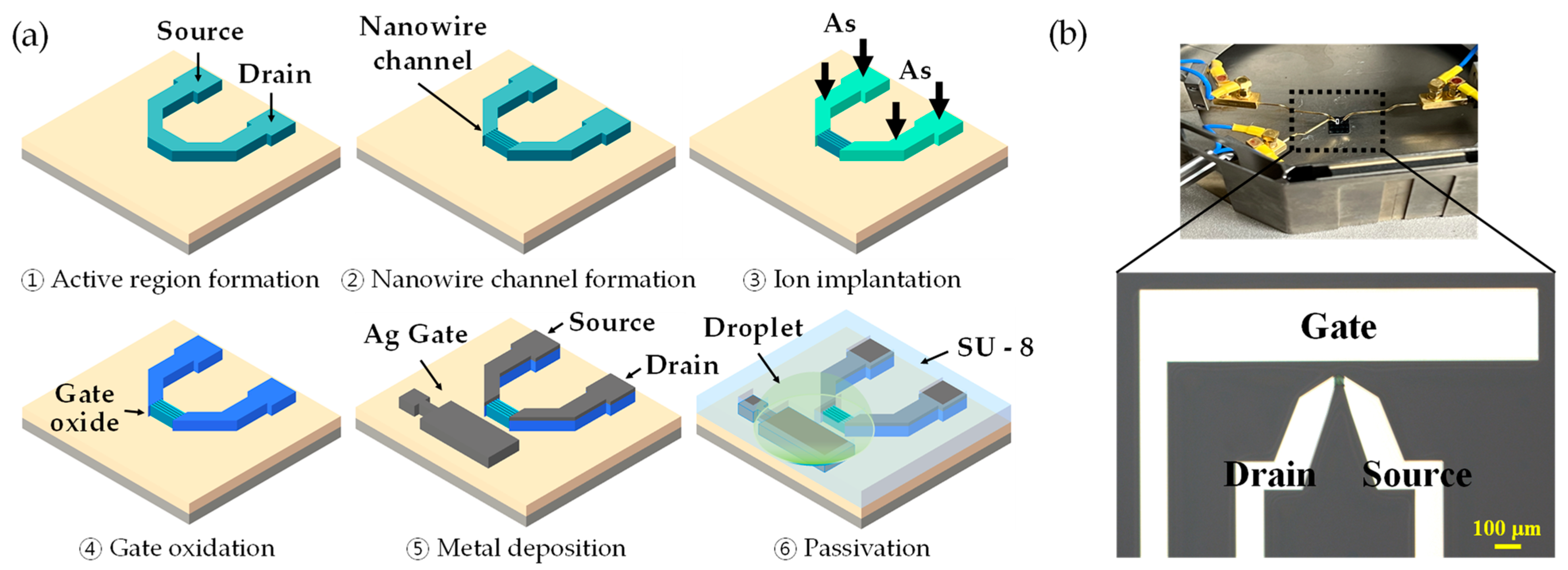
2.2. Fabrication of EGTs
2.3. Functionalization of EGTs
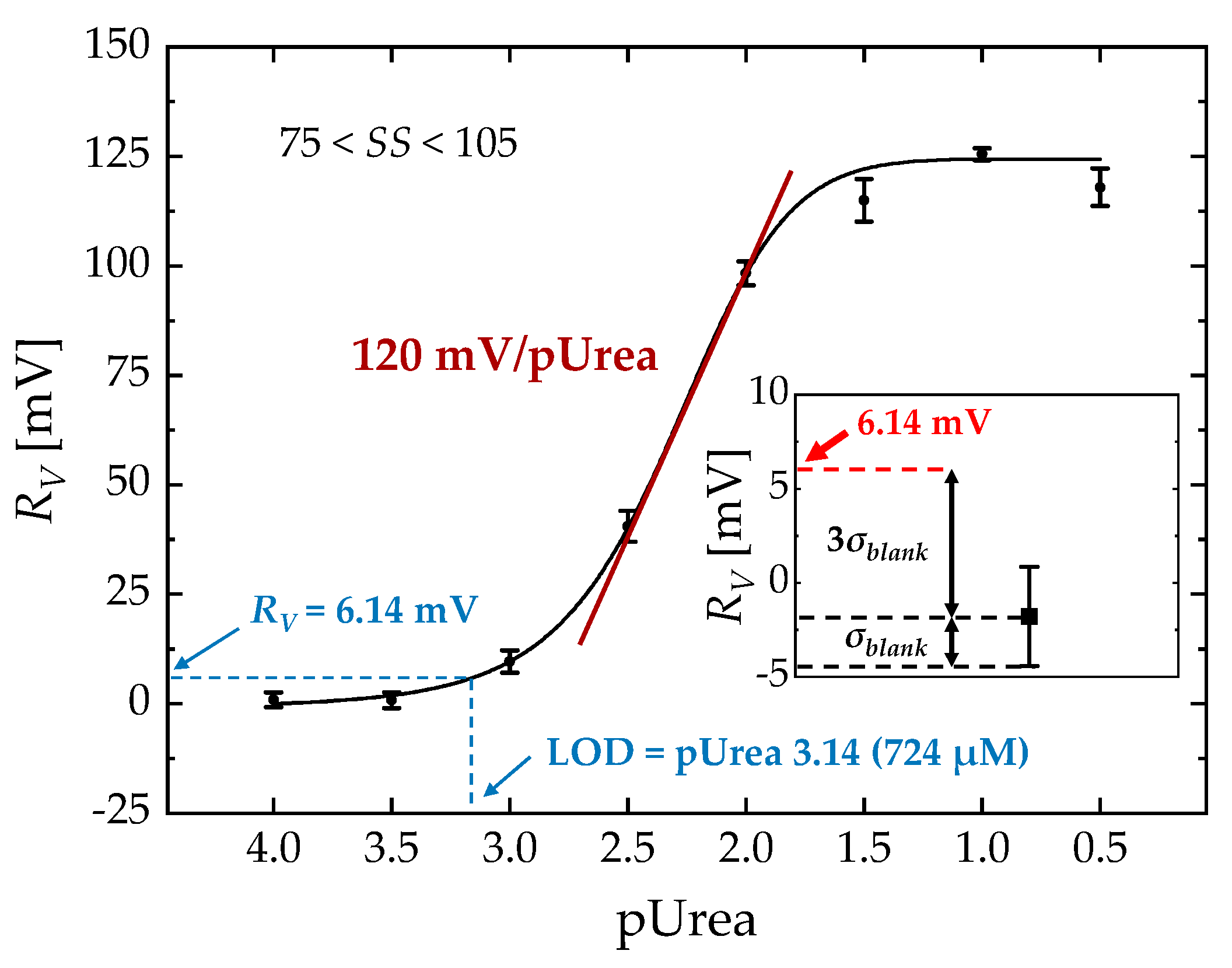
3. Results and Discussion
3.1. Intrinsic Electrical Characteristics
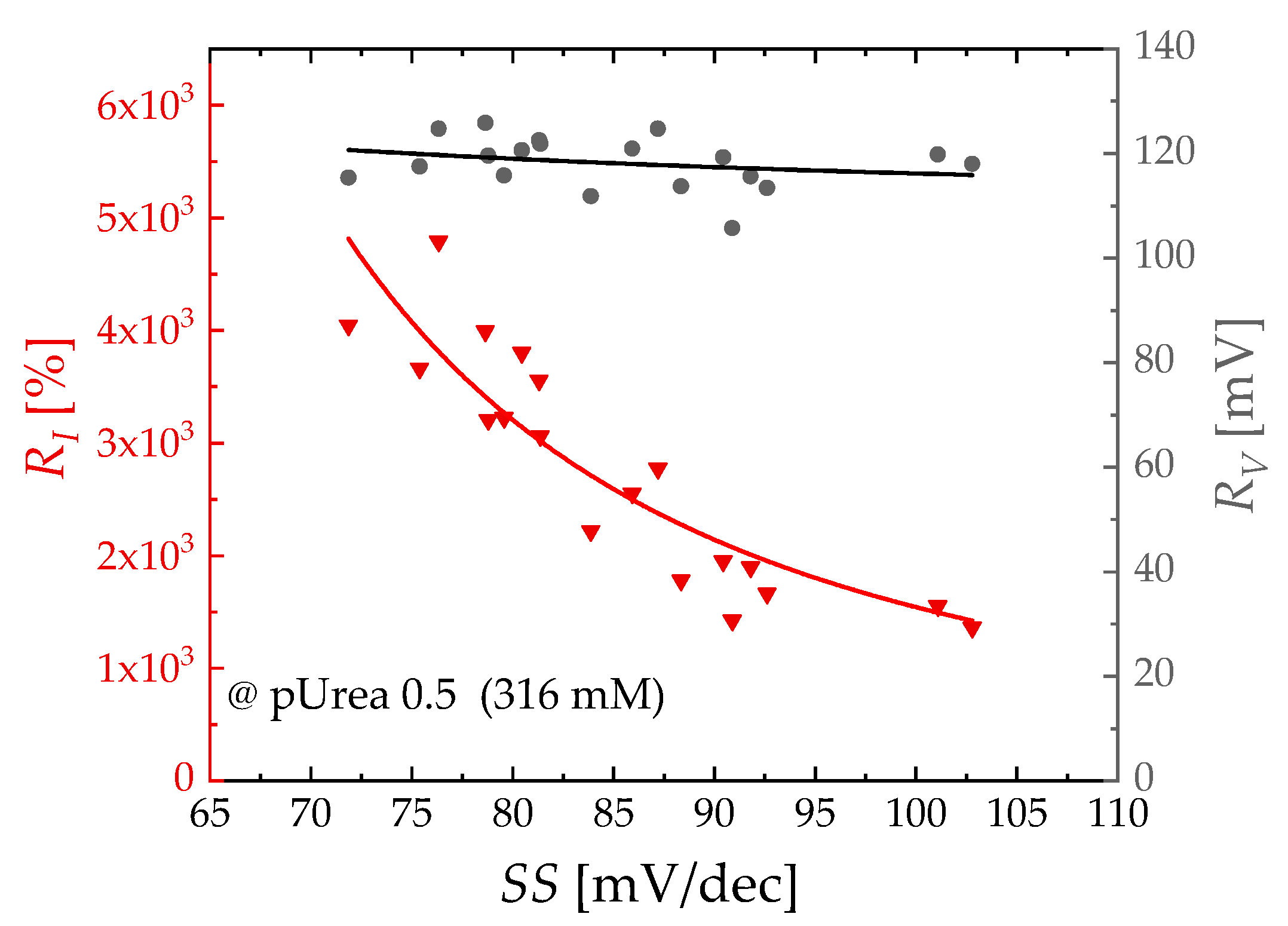
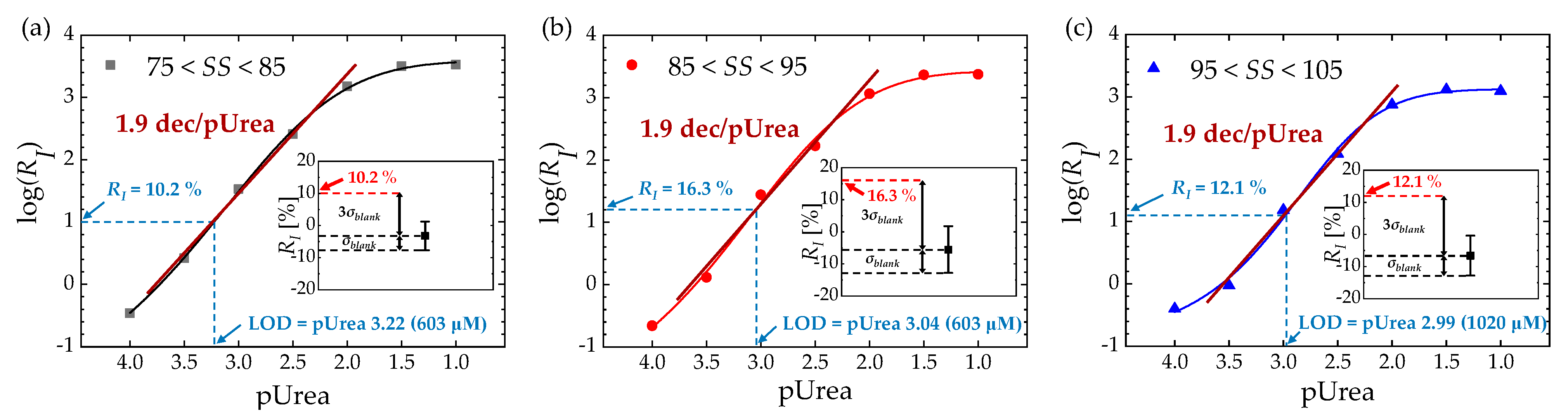
3.2. Sensing Characteristics
3.3. Selectivity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botewad, S.N.; Gaikwad, D.K.; Girhe, N.B.; Thorat, H.N.; Pawar, P.P. Urea biosensors: A comprehensive review. Biotechnol. Appl. Biochem. 2021, 70, 485–501. [Google Scholar] [CrossRef]
- Rosental, H.L. Determination of urea in blood and urine with diacetyl monoxime. Anal. Chem. 1955, 27, 1980–1982. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Zhang, L.; Zhang, S.; Zhang, L. A reversible, colorimetric, pH-responsive indole-based hydrogel and its application in urea detection. RSC Adv. 2019, 9, 24299–24304. [Google Scholar] [CrossRef]
- Choi, C.K.; Shaban, S.M.; Moon, B.S.; Pyun, D.G.; Kim, D.H. Smartphone-assisted point-of-care colorimetric biosensor for the detection of urea via pH-mediated AgNPs growth. Anal. Chim. Acta 2021, 1170, 338630. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Zhang, Q.Y.; Huang, H.Y.; Ren, C.J.; Ouyang, S.; Zhao, Q. L-noradrenaline functionalized near-infrared fluorescence CdSeTe probe for the determination of urea and bioimaging of HepG2 Cells. Talanta 2017, 171, 16–24. [Google Scholar] [CrossRef]
- Deng, H.H.; Li, K.L.; Zhuang, Q.Q.; Peng, H.P.; Zhuang, Q.Q.; Liu, A.L.; Xia, X.H.; Chen, W. An ammonia-based etchant for attaining copper nanoclusters with green fluorescence emission. Nanoscale 2018, 10, 6467–6473. [Google Scholar] [CrossRef]
- Pang, S. A pH sensitive fluorescent carbon dots for urea and urease detection. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 752–760. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, P.Y.; Ju, S.P. Preparation of Ni nanotube-modified electrodes via galvanic displacement on sacrificial Zn templates: Solvent effects and attempts for non-enzymatic electrochemical detection of urea. Microchem. J. 2020, 158, 105172. [Google Scholar] [CrossRef]
- Rogers, K.R. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiu, Y.-S. Gate-Recessed AlGaN/GaN ISFET urea biosensor fabricated by photoelectrochemical method. IEEE Sens. J. 2016, 16, 1518–1523. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Park, J.H.; Hahn, Y.B. A comprehensive biosensor integrated with a ZnO nanorod FET array for selective detection of glucose, cholesterol and urea. Chem. Commun. (Camb.) 2015, 51, 11968–11971. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.O.; Mulato, M. Urea detection using commercial field effect transistors. ECS J. Solid State Sci. Technol. 2018, 7, Q3014–Q3019. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Y.; Zhu, R.; Chen, Y.; Liu, X.; Xu, J.; Li, M.; Wang, D. Urea detection of electrochemical transistor sensors based on polyanline (PANI)/MWCNT/cotton yarns. Electroanalysis 2021, 33, 2406–2416. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.Q.; Park, H.K.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.R.; Wang, X.F.; Yu, J.Y.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Van der Spiegel, J.; Lauks, I.; Chan, P.; Babic, D. The extended gate chemically sensitive field effect transistor as multi-species microprobe. Sens. Actuators 1983, 4, 291–298. [Google Scholar] [CrossRef]
- Chi, L.-L.; Chou, J.-C.; Chung, W.-Y.; Sun, T.-P.; Hsiung, S.-K. Study on extended gate field effect transistor with tin oxide sensing membrane. Mater. Chem. Phys. 2000, 63, 19–23. [Google Scholar] [CrossRef]
- Macchia, E.; Sarcina, L.; Picca, R.A.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. Ultra-low HIV-1 p24 detection limits with a bioelectronic sensor. Anal. Bioanal. Chem. 2020, 412, 811–818. [Google Scholar] [CrossRef]
- Kim, D.; Jin, B.; Kim, S.A.; Choi, W.; Shin, S.; Park, J.; Shim, W.B.; Kim, K.; Lee, J.S. An ultrasensitive silicon-based electrolyte-gated transistor for the detection of peanut allergens. Biosensors 2022, 12, 24. [Google Scholar] [CrossRef]
- Guo, K.Y.; Wustoni, S.; Koklu, A.; Diaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [Google Scholar] [CrossRef]
- Macchia, E.; Manoli, K.; Holzer, B.; Di Franco, C.; Ghittorelli, M.; Torricelli, F.; Alberga, D.; Mangiatordi, G.F.; Palazzo, G.; Scamarcio, G.; et al. Single-molecule detection with a millimetre-sized transistor. Nat. Commun. 2018, 9, 3223. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-modulated transistor detects odorant binding protein chiral interactions. Nat. Commun. 2015, 6, 6010. [Google Scholar] [CrossRef] [PubMed]
- Corso, C.D.; Dickherber, A.; Hunt, W.D. An investigation of antibody immobilization methods employing organosilanes on planar ZnO surfaces for biosensor applications. Biosens. Bioelectron. 2008, 24, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Shoorideh, K.; Chui, C.O. Optimization of the sensitivity of FET-based biosensors via biasing and surface charge engineering. IEEE Trans. Electron Devices 2012, 59, 3104–3110. [Google Scholar] [CrossRef]
- Vico, L.D.; Sørensen, M.H.; Iversen, L.; Rogers, D.M.; Sørensen, B.S.; Brandbyge, M.; Nygård, J.; Martinez, K.L.; Jensen, J.H. Quantifying signal changes in nano-wire based biosensors. Nanoscale 2011, 3, 706–717. [Google Scholar] [CrossRef]
- Hideshima, S.; Hinou, H.; Ebihara, D.; Sato, R.; Kuroiwa, S.; Nakanishi, T.; Nishimura, S.-I.; Osaka, T. Attomolar detection of influenza A virus hemagglutinin human H1 and avian H5 using glycan-blotted field effect transistor biosensor. Anal. Chem. 2013, 85, 5641–5644. [Google Scholar] [CrossRef]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett 2009, 10, 547–552. [Google Scholar] [CrossRef]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef]
- Brews, J.R. A charge-sheet model of the MOSFET. Solid State Electron. 1978, 21, 345–355. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Gao, A.R.; Lu, N.; Wang, Y.L.; Li, T. Robust ultrasensitive tunneling-FET biosensor for point-of-care diagnostics. Sci. Rep. 2016, 6, 22554. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Wang, I.S.; Lin, Y.-T.; Huang, C.-H.; Lu, T.-F.; Lue, C.-E.; Pijanowska, D.G.; Hua, M.-Y.; Lai, C.-S. Low cost and flexible electrodes with NH3 plasma treatments in extended gate field effect transistors for urea detection. Sens. Actuators B: Chem. 2013, 187, 274–279. [Google Scholar] [CrossRef]



| FET Type | Dynamic Range (pUrea) | Urea Sensitivity (RI) (dec/pUrea) | Urea Sensitivity (RV) (mV/pUrea) | Power Consumption | Ref |
|---|---|---|---|---|---|
| AlGaN/GaN ion-sensitive FET | 1.6–3.4 (RI) | 0.24 (Linear regime) | – | 6 W | [10] |
| ZnO nanorod FET | – | 0.27 (Linear regime) | – | – | [11] |
| EGFET Membrane: ITO layer | – | – | 62.4 (Linear regime) | 500 nW | [32] |
| EGFET Membrane: SnO2:F layer | 1–3.1 (RV) | 0.42 (Linear regime) | 109 (Linear regime) | 25 mW | [12] |
| Si-based EGT | 2.0–3.4 (RI) 1.8–2.9 (RV) | 1.9 (SubVTH regime) | 120 (SubVTH regime) | 0.3 nW | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Jin, B.; Shin, S.; Do, J.; Son, J.; Kim, K.; Lee, J.-S. Highly Sensitive Detection of Urea Using Si Electrolyte-Gated Transistor with Low Power Consumption. Biosensors 2023, 13, 565. https://doi.org/10.3390/bios13050565
Choi W, Jin B, Shin S, Do J, Son J, Kim K, Lee J-S. Highly Sensitive Detection of Urea Using Si Electrolyte-Gated Transistor with Low Power Consumption. Biosensors. 2023; 13(5):565. https://doi.org/10.3390/bios13050565
Chicago/Turabian StyleChoi, Wonyeong, Bo Jin, Seonghwan Shin, Jeonghyeon Do, Jongmin Son, Kihyun Kim, and Jeong-Soo Lee. 2023. "Highly Sensitive Detection of Urea Using Si Electrolyte-Gated Transistor with Low Power Consumption" Biosensors 13, no. 5: 565. https://doi.org/10.3390/bios13050565
APA StyleChoi, W., Jin, B., Shin, S., Do, J., Son, J., Kim, K., & Lee, J.-S. (2023). Highly Sensitive Detection of Urea Using Si Electrolyte-Gated Transistor with Low Power Consumption. Biosensors, 13(5), 565. https://doi.org/10.3390/bios13050565







