Aptamer-Based Point-of-Care Devices: Emerging Technologies and Integration of Computational Methods
Abstract
:1. Introduction
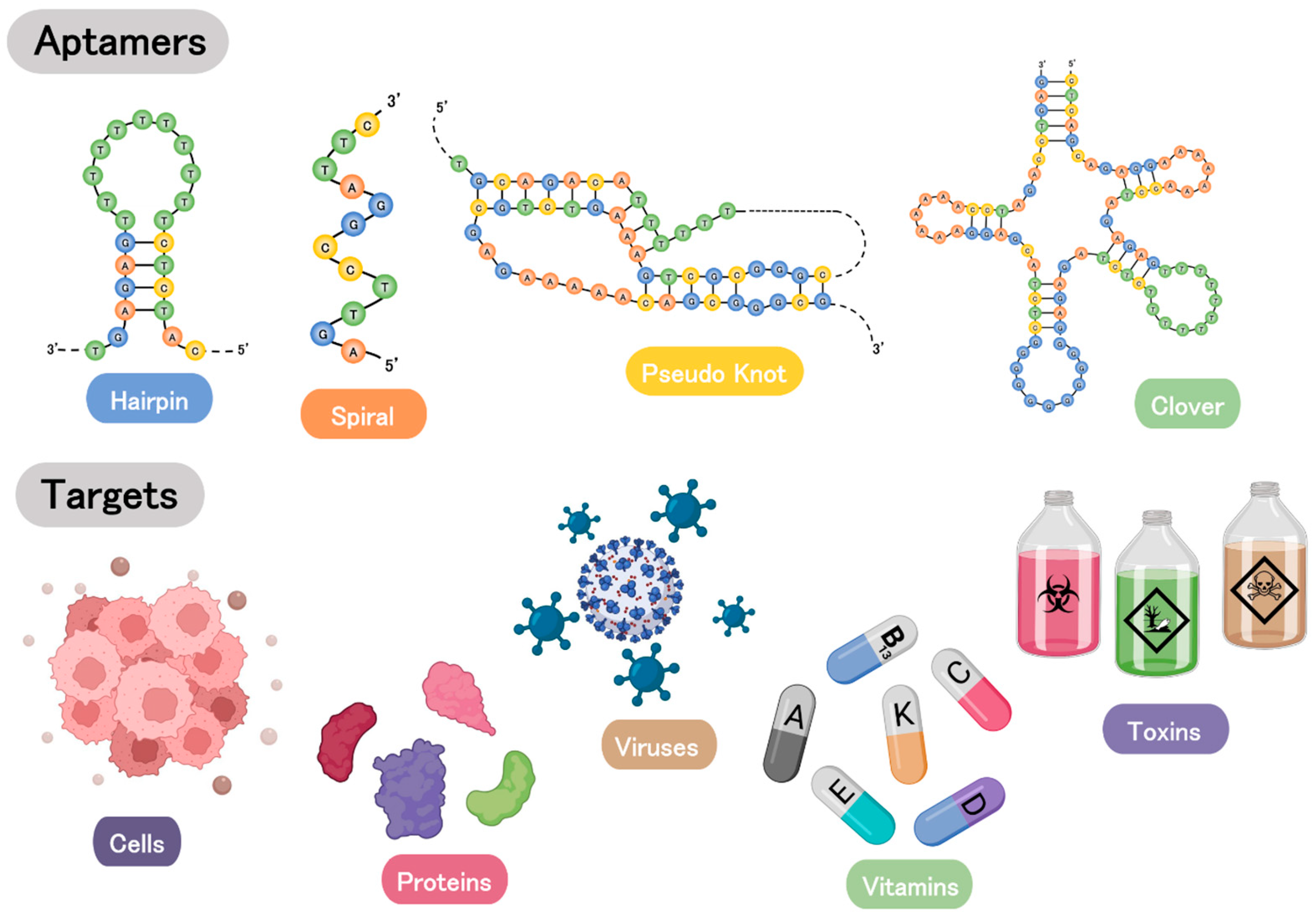
2. Aptamers
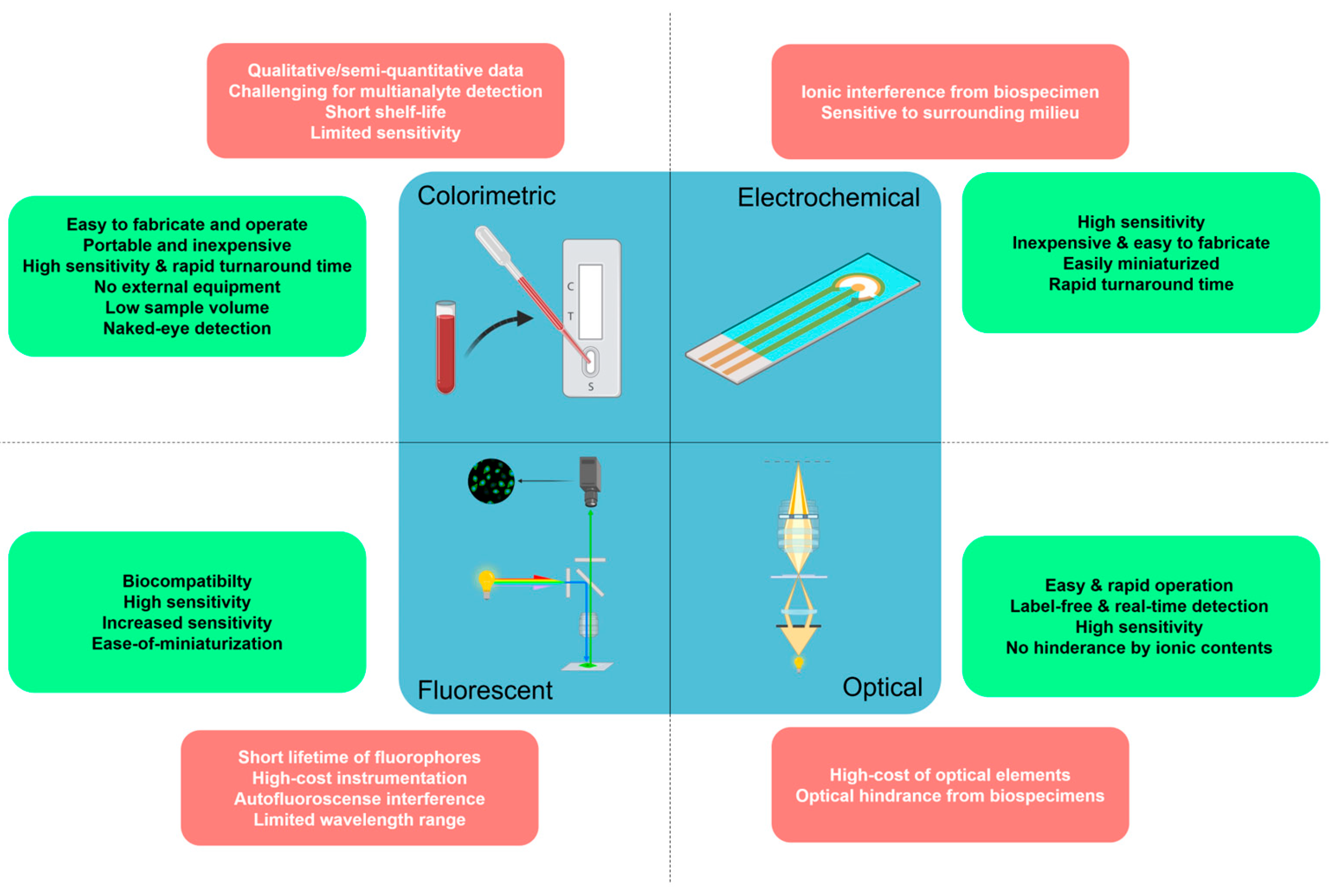
3. Aptasensors in POC-Based Biosensing Platforms
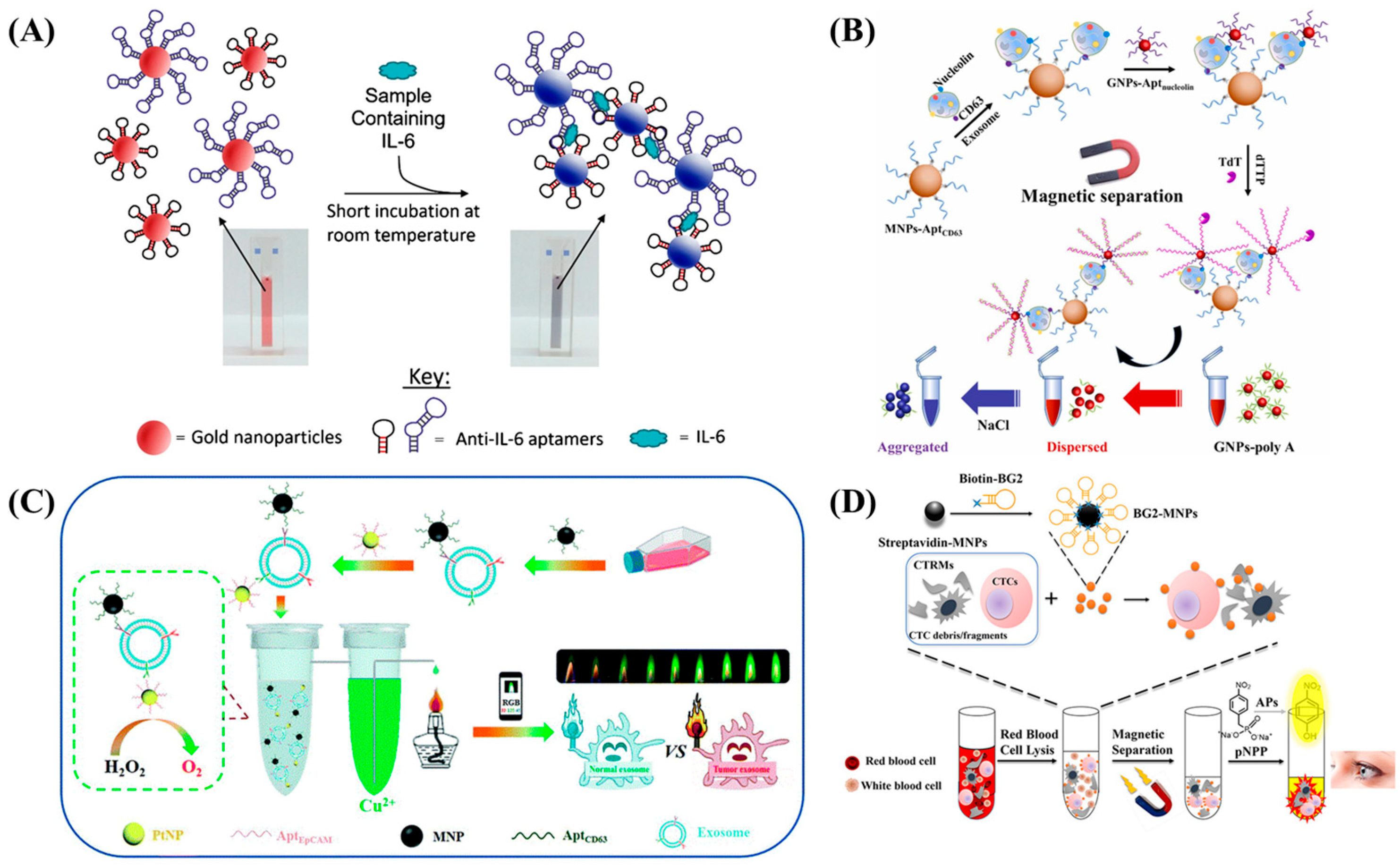
3.1. Colorimetric Aptasensors
3.1.1. Paper-Based Colorimetric Aptasensors
3.1.2. In-Solution Colorimetric Aptasensors
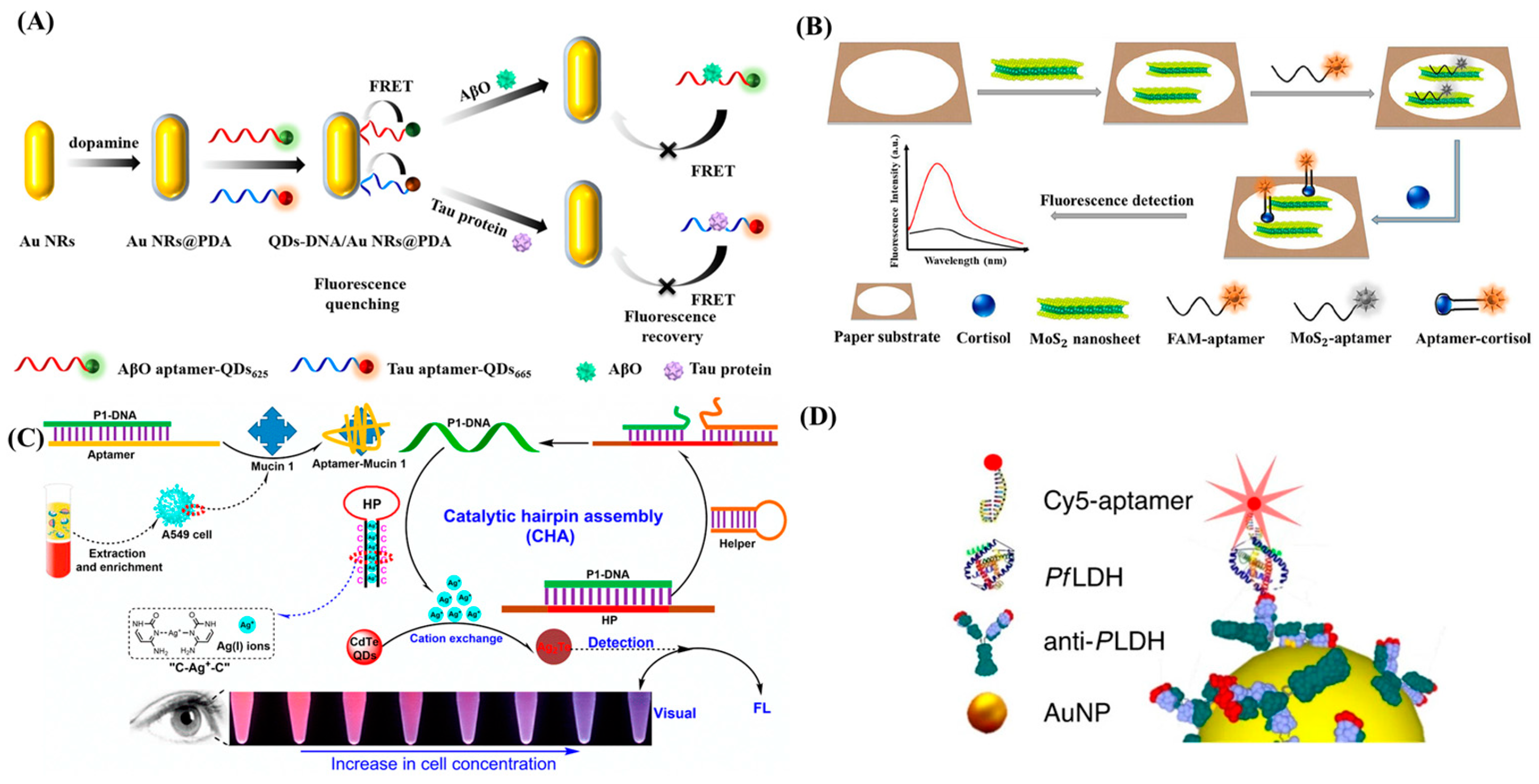
3.2. Fluorescent Aptasensors
3.2.1. Fluorescence Quenching-Based Aptasensors
3.2.2. Metal-Enhanced Fluorescence-Based Aptasensors
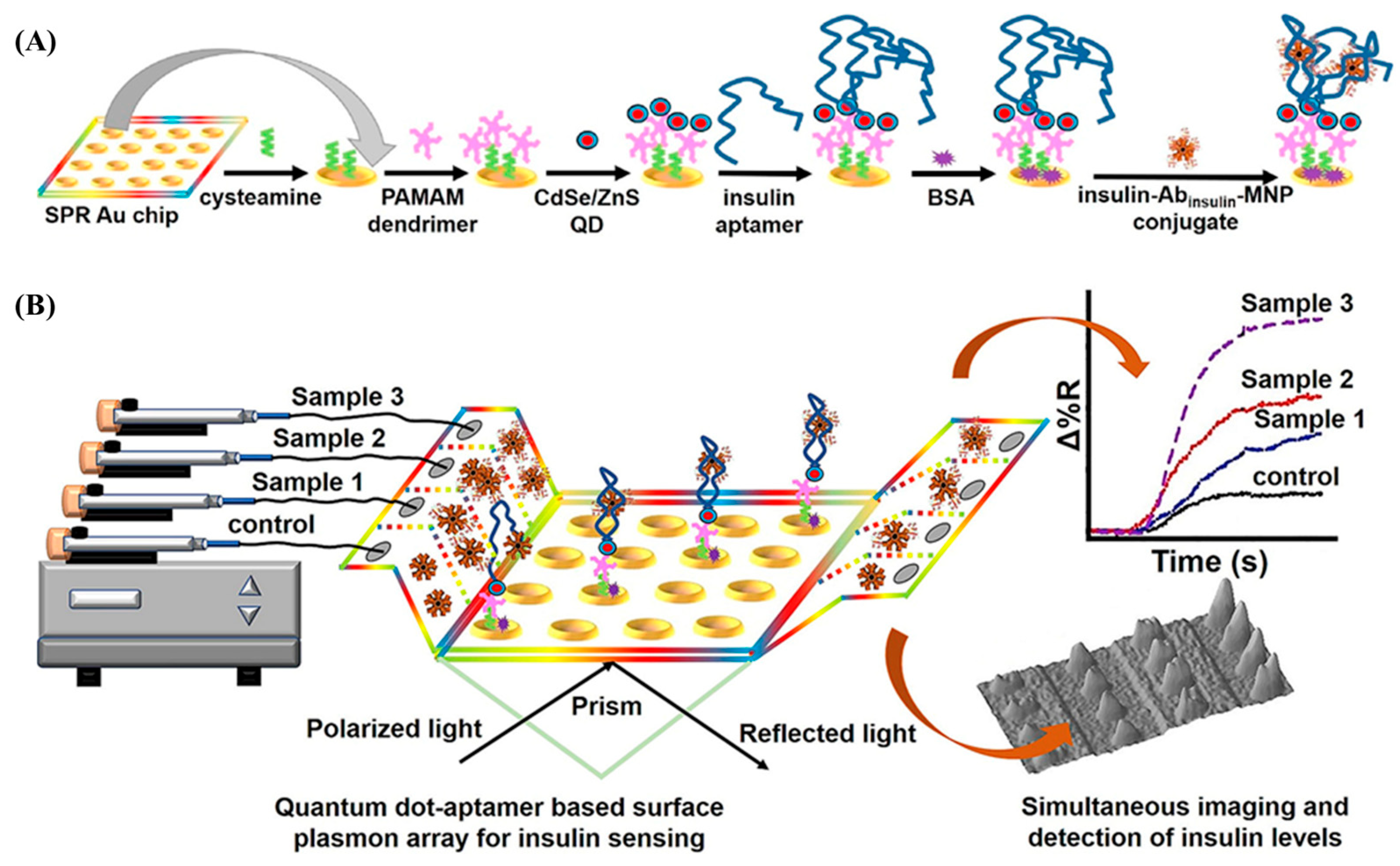
3.3. SPR-Based Aptasensors
3.4. Electrochemical Aptasensors
4. Computational Approaches for Aptamer Modeling and POC Testing Integrations
4.1. Molecular Docking Calculation and Molecular Dynamics Simulation
4.2. Density Functional Theory
4.3. Quantum Mechanics and Molecular Mechanics
4.4. Artificial Intelligence (AI)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, Y.; Liu, C.C. Recent Advances on Electrochemical Biosensing Strategies toward Universal Point-of-Care Systems. Angew. Chemie Int. Ed. 2019, 58, 12355–12368. [Google Scholar] [CrossRef] [PubMed]
- Erdem, Ö.; Eş, I.; Saylan, Y.; Inci, F. Unifying the Efforts of Medicine, Chemistry, and Engineering in Biosensing Technologies to Tackle the Challenges of the COVID-19 Pandemic. Anal. Chem. 2022, 94, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Erdem, Ö.; Derin, E.; Sagdic, K.; Yilmaz, E.G.; Inci, F. Smart Materials-Integrated Sensor Technologies for COVID-19 Diagnosis. Emergent Mater. 2021, 4, 169–185. [Google Scholar] [CrossRef]
- Sagdic, K.; Inci, F. Smart Material-Integrated Systems for Isolation and Profiling of Rare Cancer Cells and Emboli. Adv. Eng. Mater. 2021, 24, 2100857. [Google Scholar] [CrossRef]
- Hu, J.; Cui, X.; Gong, Y.; Xu, X.; Gao, B.; Wen, T.; Lu, T.J.; Xu, F. Portable Microfluidic and Smartphone-Based Devices for Monitoring of Cardiovascular Diseases at the Point of Care. Biotechnol. Adv. 2016, 34, 305–320. [Google Scholar] [CrossRef]
- Wei, T.Y.; Fu, Y.; Chang, K.H.; Lin, K.J.; Lu, Y.J.; Cheng, C.M. Point-of-Care Devices Using Disease Biomarkers to Diagnose Neurodegenerative Disorders. Trends Biotechnol. 2018, 36, 290–303. [Google Scholar] [CrossRef]
- Li, Z.; Leustean, L.; Inci, F.; Zheng, M.; Demirci, U.; Wang, S. Plasmonic-Based Platforms for Diagnosis of Infectious Diseases at the Point-of-Care. Biotechnol. Adv. 2019, 37, 107440. [Google Scholar] [CrossRef]
- Derin, E.; Inci, F. Advances in Biosensor Technologies for Acute Kidney Injury. ACS Sens. 2022, 7, 358–385. [Google Scholar] [CrossRef]
- Crutchfield, C.A.; Thomas, S.N.; Sokoll, L.J.; Chan, D.W. Advances in Mass Spectrometry-Based Clinical Biomarker Discovery. Clin. Proteom. 2016, 13, 1. [Google Scholar] [CrossRef]
- Wang, S.; Lifson, M.A.; Inci, F.; Liang, L.G.; Sheng, Y.F.; Demirci, U. Expert Review of Molecular Diagnostics Advances in Addressing Technical Challenges of Point-of-Care Diagnostics in Resource-Limited Settings. Expert Rev. Mol. Diagn. 2016, 16, 449–459. [Google Scholar] [CrossRef]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-Based Rapid Diagnosis for Point-of-Care Application. Microfluid. Nanofluidics 2023, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the Developing World. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Land, K.J.; Boeras, D.I.; Chen, X.S.; Ramsay, A.R.; Peeling, R.W. Reassured Diagnostics to Inform Disease Control Strategies, Strengthen Health Systems and Improve Patient Outcomes. Nat. Microbiol. 2018, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as Targeted Therapeutics: Current Potential and Challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a Replacement for Antibodies in Enzyme-Linked Immunosorbent Assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of Aptamer Discovery and Technology. Nat. Rev. Chem. 2017, 1, 76. [Google Scholar] [CrossRef]
- Oliveira, R.; Pinho, E.; Sousa, A.L.; Dias, Ó.; Azevedo, N.F.; Almeida, C. Modelling Aptamers with Nucleic Acid Mimics (NAM): From Sequence to Three-Dimensional Docking. PLoS ONE 2022, 17, e0264701. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A Review of Their Chemical Properties and Modifications for Therapeutic Application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Song, K.M.; Lee, S.; Ban, C. Aptamers and Their Biological Applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Hartmann, R.K.; Bindereif, A.; Schn, A. Handbook of RNA Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Meng, H.M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.B. Aptamer-Integrated DNA Nanostructures for Biosensing, Bioimaging and Cancer Therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three Decades of Nucleic Acid Aptamer Technologies: Lessons Learned, Progress and Opportunities on Aptamer Development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Mercier, M.C.; Dontenwill, M.; Choulier, L. Selection of Nucleic Acid Aptamers Targeting Tumor Cell-Surface Protein Biomarkers. Cancers 2017, 9, 69. [Google Scholar] [CrossRef]
- Lyu, C.; Khan, I.M.; Wang, Z. Capture-SELEX for Aptamer Selection: A Short Review. Talanta 2021, 229, 122274. [Google Scholar] [CrossRef]
- Civit, L.; Taghdisi, S.M.; Jonczyk, A.; Haßel, S.K.; Gröber, C.; Blank, M.; Stunden, H.J.; Beyer, M.; Schultze, J.; Latz, E.; et al. Systematic Evaluation of Cell-SELEX Enriched Aptamers Binding to Breast Cancer Cells. Biochimie 2018, 145, 53–62. [Google Scholar] [CrossRef]
- Chen, L.; He, W.; Jiang, H.; Wu, L.; Xiong, W.; Li, B.; Zhou, Z.; Qian, Y. In Vivo SELEX of Bone Targeting Aptamer in Prostate Cancer Bone Metastasis Model. Int. J. Nanomed. 2018, 14, 149–159. [Google Scholar] [CrossRef]
- Shigdar, S.; Agnello, L.; Fedele, M.; Camorani, S.; Cerchia, L. Profiling Cancer Cells by Cell-SELEX: Use of Aptamers for Discovery of Actionable Biomarkers and Therapeutic Applications Thereof. Pharmaceutics 2021, 14, 28. [Google Scholar] [CrossRef]
- Bakhtiari, H.; Palizban, A.A.; Khanahmad, H.; Mofid, M.R. Novel Approach to Overcome Defects of Cell-SELEX in Developing Aptamers against Aspartate β-Hydroxylase. ACS Omega 2021, 6, 11005–11014. [Google Scholar] [CrossRef]
- Bing, T.; Zhang, N.; Shangguan, D. Cell-SELEX, an Effective Way to the Discovery of Biomarkers and Unexpected Molecular Events. Adv. Biosyst. 2019, 3, 1900193. [Google Scholar] [CrossRef]
- Sinha, A.; Gopinathan, P.; Da Chung, Y.; Lin, H.Y.; Li, K.H.; Ma, H.P.; Huang, P.C.; Shiesh, S.C.; Lee, G.B. An Integrated Microfluidic Platform to Perform Uninterrupted SELEX Cycles to Screen Affinity Reagents Specific to Cardiovascular Biomarkers. Biosens. Bioelectron. 2018, 122, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Ghulam, M.; Li, L.; Qu, F. Evolution of Multi-Functional Capillary Electrophoresis for High-Efficiency Selection of Aptamers. Biotechnol. Adv. 2019, 37, 107432. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Okumura, Y.; Amino, T.; Miyachi, Y.; Ogino, C.; Kondo, A. DNA-Duplex Linker for AFM-SELEX of DNA Aptamer against Human Serum Albumin. Bioorg. Med. Chem. Lett. 2017, 27, 954–957. [Google Scholar] [CrossRef]
- Lin, C.H.; Patel, D.J. Structural Basis of DNA Folding and Recognition in an AMP-DNA Aptamer Complex: Distinct Architectures but Common Recognition Motifs for DNA and RNA Aptamers Complexed to AMP. Chem. Biol. 1997, 4, 817–832. [Google Scholar] [CrossRef]
- Yi, J.; Xiao, W.; Li, G.; Wu, P.; He, Y.; Chen, C.; He, Y.; Ding, P.; Kai, T. The Research of Aptamer Biosensor Technologies for Detection of Microorganism. Appl. Microbiol. Biotechnol. 2020, 104, 9877–9890. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, Z.; Liu, D.; Jiang, H.; Zhang, Z.K.; Lu, A.; Zhang, B.T.; Yu, Y.; Zhang, G. Structural Biology for the Molecular Insight between Aptamers and Target Proteins. Int. J. Mol. Sci. 2021, 22, 4093. [Google Scholar] [CrossRef]
- Hu, X.; Tang, L.; Zheng, M.; Liu, J.; Zhang, Z.; Li, Z.; Yang, Q.; Xiang, S.; Fang, L.; Ren, Q.; et al. Structure-Guided Designing Pre-Organization in Bivalent Aptamers. J. Am. Chem. Soc. 2022, 144, 4507–4514. [Google Scholar] [CrossRef]
- Mascini, M. Aptamers and Their Applications. Anal. Bioanal. Chem. 2008, 390, 987–988. [Google Scholar] [CrossRef]
- Ferreira, C.S.M.; Missailidis, S. Aptamer-Based Therapeutics and Their Potential in Radiopharmaceutical Design. Brazilian Arch. Biol. Technol. 2007, 50, 63–76. [Google Scholar] [CrossRef]
- Jayasena, S.D. Aptamers: An Emerging Class of Molecules That Rival Antibodies in Diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef]
- Strehlitz, B.; Nikolaus, N.; Stoltenburg, R. Protein Detection with Aptamer Biosensors. Sensors 2008, 8, 4296–4307. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Sarreshtehdar Emrani, A.; Ramezani, M.; Abnous, K. Aptamer Biosensor for Selective and Rapid Determination of Insulin. Anal. Lett. 2015, 48, 672–681. [Google Scholar] [CrossRef]
- Mackay, S.; Wishart, D.; Xing, J.Z.; Chen, J. Developing Trends in Aptamer-Based Biosensor Devices and Their Applications. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 4–14. [Google Scholar] [CrossRef]
- Daems, E.; Moro, G.; Campos, R.; De Wael, K. Mapping the Gaps in Chemical Analysis for the Characterisation of Aptamer-Target Interactions. TrAC-Trends Anal. Chem. 2021, 142, 116311. [Google Scholar] [CrossRef]
- Zong, C.; Liu, J. The Arsenic-Binding Aptamer Cannot Bind Arsenic: Critical Evaluation of Aptamer Selection and Binding. Anal. Chem. 2019, 91, 10887–10893. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, L.; Zhang, B.T.; Lu, A.; Wang, Y.; Yu, Y.; Zhang, G. Artificial Intelligence in Aptamer–Target Binding Prediction. Int. J. Mol. Sci. 2021, 22, 3605. [Google Scholar] [CrossRef]
- Welch, D.F.; Ginocchio, C.C. Role of Rapid Immunochromatographic Antigen Testing in Diagnosis of Influenza A Virus 2009 H1N1 Infection. J. Clin. Microbiol. 2010, 48, 22–25. [Google Scholar] [CrossRef]
- Chua, A.L.; Yean, C.Y.; Ravichandran, M.; Lim, B.H.; Lalitha, P. A Rapid DNA Biosensor for the Molecular Diagnosis of Infectious Disease. Biosens. Bioelectron. 2011, 26, 3825–3831. [Google Scholar] [CrossRef]
- Wanja, E.; Parker, Z.F.; Odusami, O.; Rowland, T.; Davé, K.; Davé, S.; Turell, M.J. Immuno-Chromatographic Wicking Assay for the Rapid Detection of Dengue Viral Antigens in Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 220–225. [Google Scholar] [CrossRef]
- Chen, A.; Yang, S. Replacing Antibodies with Aptamers in Lateral Flow Immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef]
- Cummins, B.M.; Ligler, F.S.; Walker, G.M. Point-of-Care Diagnostics for Niche Applications. Biotechnol. Adv. 2016, 34, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Dalirirad, S.; Han, D.; Steckl, A.J. Aptamer-Based Lateral Flow Biosensor for Rapid Detection of Salivary Cortisol. ACS Omega 2020, 5, 32890–32898. [Google Scholar] [CrossRef] [PubMed]
- Dalirirad, S.; Steckl, A.J. Lateral Flow Assay Using Aptamer-Based Sensing for on-Site Detection of Dopamine in Urine. Anal. Biochem. 2020, 596, 113637. [Google Scholar] [CrossRef]
- Belsare, S.; Tseng, D.; Ozcan, A.; Coté, G. Monitoring Gestational Diabetes at the Point-of-Care via Dual Glycated Albumin Lateral Flow Assays in Conjunction with a Handheld Reader. Analyst 2022, 147, 5518–5527. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gurung, A.S.; Qiu, W. Lateral Flow Aptasensor for Simultaneous Detection of Platelet-Derived Growth Factor-BB (PDGF-BB) and Thrombin. Molecules 2019, 24, 756. [Google Scholar] [CrossRef]
- Seiler, L.K.; Phung, N.L.; Nikolin, C.; Immenschuh, S.; Erck, C.; Kaufeld, J.; Haller, H.; Falk, C.S.; Jonczyk, R.; Lindner, P.; et al. An Antibody-Aptamer-Hybrid Lateral Flow Assay for Detection of CXCL9 in Antibody-Mediated Rejection after Kidney Transplantation. Diagnostics 2022, 12, 308. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lu, S.Y.; Chen, S.J.; Wang, J.M.; Fu, L.M.; Wu, Y.H. Microfluidic Aptasensor POC Device for Determination of Whole Blood Potassium. Anal. Chim. Acta 2022, 1203, 339722. [Google Scholar] [CrossRef]
- Ranganathan, V.; Srinivasan, S.; Singh, A.; DeRosa, M.C. An Aptamer-Based Colorimetric Lateral Flow Assay for the Detection of Human Epidermal Growth Factor Receptor 2 (HER2). Anal. Biochem. 2020, 588, 113471. [Google Scholar] [CrossRef]
- Moabelo, K.L.; Lerga, T.M.; Jauset-Rubio, M.; Sibuyi, N.R.S.; O’Sullivan, C.K.; Meyer, M.; Madiehe, A.M. A Label-Free Gold Nanoparticles-Based Optical Aptasensor for the Detection of Retinol Binding Protein 4. Biosensors 2022, 12, 1061. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Wei, R.; Ren, J.; Zhou, M.; Yan, C.; Huang, L. An Excellent Colorimetric Aptasensor Integrating Multifunctional SNAs and TdT-Induced Dual Signal Amplification for Rapid Sensitive Detection of Exosomes. Sens. Actuators B Chem. 2023, 380, 133361. [Google Scholar] [CrossRef]
- Giorgi-Coll, S.; Marín, M.J.; Sule, O.; Hutchinson, P.J.; Carpenter, K.L.H. Aptamer-Modified Gold Nanoparticles for Rapid Aggregation-Based Detection of Inflammation: An Optical Assay for Interleukin-6. Microchim. Acta 2020, 187, 13. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, K.; Kwee, S.; Tang, L.; Wang, Z.; Xia, J.; Li, X.J. Gold Nanoparticle Aggregation-Induced Quantitative Photothermal Biosensing Using a Thermometer: A Simple and Universal Biosensing Platform. Anal. Chem. 2020, 92, 2739–2747. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive Aptamer-Based Protein Assays Based on One-Dimensional Core-Shell Nanozymes. Biosens. Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.J. Label-Free Sensitive Detection of Steroid Hormone Cortisol Based on Target-Induced Fluorescence Quenching of Quantum Dots. Langmuir 2020, 36, 7781–7788. [Google Scholar] [CrossRef]
- Lu, X.; Hou, X.; Tang, H.; Yi, X.; Wang, J. A High-Quality CdSe/CdS/ZnS Quantum-Dot-Based FRET Aptasensor for the Simultaneous Detection of Two Different Alzheimer’s Disease Core Biomarkers. Nanomaterials 2022, 12, 4031. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; He, Y.; Huang, K.; Wang, X.; Zhou, R.; Liu, T.; Qu, R.; Zhou, J.; Peng, W.; et al. Homogeneous Visual and Fluorescence Detection of Circulating Tumor Cells in Clinical Samples via Selective Recognition Reaction and Enzyme-Free Amplification. ACS Nano 2021, 15, 11634–11643. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, F.; Zhang, T.; Wang, F.; Li, Y.; Yu, Z.; Jin, X.; Ruan, B. An Fluorescent Aptasensor for Sensitive Detection of Tumor Marker Based on the FRET of a Sandwich Structured QDs-AFP-AuNPs. Talanta 2019, 197, 444–450. [Google Scholar] [CrossRef]
- Choi, J.H.; Lim, J.; Shin, M.; Paek, S.H.; Choi, J.W. CRISPR-Cas12a-Based Nucleic Acid Amplification-Free DNA Biosensor via Au Nanoparticle-Assisted Metal-Enhanced Fluorescence and Colorimetric Analysis. Nano Lett. 2021, 21, 693–699. [Google Scholar] [CrossRef]
- Weng, X.; Fu, Z.; Zhang, C.; Jiang, W.; Jiang, H. A Portable 3D Microfluidic Origami Biosensor for Cortisol Detection in Human Sweat. Anal. Chem. 2022, 94, 3526–3534. [Google Scholar] [CrossRef]
- He, M.; Shang, N.; Zhu, Q.; Xu, J. Paper-Based Upconversion Fluorescence Aptasensor for the Quantitative Detection of Immunoglobulin E in Human Serum. Anal. Chim. Acta 2021, 1143, 93–100. [Google Scholar] [CrossRef]
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Ultrasensitive Antibody-Aptamer Plasmonic Biosensor for Malaria Biomarker Detection in Whole Blood. Nat. Commun. 2020, 11, 6134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ghavaminejad, A.; Ghavaminejad, P.; Samarikhalaj, M.; Giacca, A.; Poudineh, M. Hydrogel Microneedle-Assisted Assay Integrating Aptamer Probes and Fluorescence Detection for Reagentless Biomarker Quantification. ACS Sens. 2022, 7, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Sypabekova, M.; Jolly, P.; Estrela, P.; Kanayeva, D. Electrochemical Aptasensor Using Optimized Surface Chemistry for the Detection of Mycobacterium Tuberculosis Secreted Protein MPT64 in Human Serum. Biosens. Bioelectron. 2019, 123, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sande, M.G.; Ferreira, D.; Rodrigues, J.L.; Melo, L.D.R.; Linke, D.; Silva, C.J.; Moreira, F.T.C.; Sales, M.G.F.; Rodrigues, L.R. Electrochemical Aptasensor for the Detection of the Key Virulence Factor YadA of Yersinia Enterocolitica. Biosensors 2022, 12, 614. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Z.; Yan, Y.; Chen, J.; Yang, R.; Huang, Q.; Jin, M.; Shui, L. Triple Signal-Enhancing Electrochemical Aptasensor Based on Rhomboid Dodecahedra Carbonized-ZIF67 for Ultrasensitive CRP Detection. Biosens. Bioelectron. 2022, 207, 114129. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal-Organic Framework-Functionalized Paper-Based Electrochemical Biosensor for Ultrasensitive Exosome Assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef]
- Yagati, A.K.; Behrent, A.; Beck, S.; Rink, S.; Goepferich, A.M.; Min, J.; Lee, M.H.; Baeumner, A.J. Laser-Induced Graphene Interdigitated Electrodes for Label-Free or Nanolabel-Enhanced Highly Sensitive Capacitive Aptamer-Based Biosensors. Biosens. Bioelectron. 2020, 164, 112272. [Google Scholar] [CrossRef]
- Gosai, A.; Hau Yeah, B.S.; Nilsen-Hamilton, M.; Shrotriya, P. Label Free Thrombin Detection in Presence of High Concentration of Albumin Using an Aptamer-Functionalized Nanoporous Membrane. Biosens. Bioelectron. 2019, 126, 88–95. [Google Scholar] [CrossRef]
- He, C.; Xu, Z.; Sun, T.; Wang, L. Sensitive Electrochemical Aptasensor for Thrombin Detection Based on Graphene Served as Platform and Graphene Oxide as Enhancer. Appl. Biochem. Biotechnol. 2014, 172, 1018–1026. [Google Scholar] [CrossRef]
- Gogola, J.L.; Martins, G.; Gevaerd, A.; Blanes, L.; Cardoso, J.; Marchini, F.K.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Label-Free Aptasensor for P24-HIV Protein Detection Based on Graphene Quantum Dots as an Electrochemical Signal Amplifier. Anal. Chim. Acta 2021, 1166, 338548. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Lee, Y. Point-of-Care Platform for Early Diagnosis of Parkinson’s Disease. ACS Appl. Biomater. 2020, 3, 8997–9001. [Google Scholar] [CrossRef]
- Ou, D.; Sun, D.; Liang, Z.; Chen, B.; Lin, X.; Chen, Z. A Novel Cytosensor for Capture, Detection and Release of Breast Cancer Cells Based on Metal Organic Framework PCN-224 and DNA Tetrahedron Linked Dual-Aptamer. Sens. Actuators B Chem. 2019, 285, 398–404. [Google Scholar] [CrossRef]
- Xue, J.; Li, Y.; Liu, J.; Zhang, Z.; Yu, R.; Huang, Y.; Li, C.; Chen, A.; Qiu, J. Highly Sensitive Electrochemical Aptasensor for SARS-CoV-2 Antigen Detection Based on Aptamer-Binding Induced Multiple Hairpin Assembly Signal Amplification. Talanta 2022, 248, 123605. [Google Scholar] [CrossRef]
- Chanarsa, S.; Jakmunee, J.; Ounnunkad, K. A Sandwich-like Configuration with a Signal Amplification Strategy Using a Methylene Blue/Aptamer Complex on a Heterojunction 2D MoSe2/2D WSe2 Electrode: Toward a Portable and Sensitive Electrochemical Alpha-Fetoprotein Immunoassay. Front. Cell. Infect. Microbiol. 2022, 12, 916357. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-Free Microfluidic Paper-Based Electrochemical Aptasensor for Ultrasensitive and Simultaneous Multiplexed Detection of Cancer Biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Inoue, S.; Seyama, M.; Miura, T.; Horiuchi, T.; Iwasaki, Y.; Takahashi, J.I.; Hayashi, K.; Tamechika, E. A Reliable Aptamer Array Prepared by Repeating Inkjet-Spotting toward on-Site Measurement. Biosens. Bioelectron. 2016, 85, 943–949. [Google Scholar] [CrossRef]
- Hu, W.; Huang, Y.; Chen, C.; Liu, Y.; Guo, T.; Guan, B.O. Highly Sensitive Detection of Dopamine Using a Graphene Functionalized Plasmonic Fiber-Optic Sensor with Aptamer Conformational Amplification. Sens. Actuators B Chem. 2018, 264, 440–447. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Lunelli, L.; Vanzetti, L.; Carafa, V.; Altucci, L.; Zeni, L. SARS-CoV-2 Spike Protein Detection through a Plasmonic D-Shaped Plastic Optical Fiber Aptasensor. Talanta 2021, 233, 122532. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct Quantification of Cancerous Exosomes via Surface Plasmon Resonance with Dual Gold Nanoparticle-Assisted Signal Amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef]
- Jo, S.; Lee, W.; Park, J.; Kim, W.; Kim, W.; Lee, G.; Lee, H.J.; Hong, J.; Park, J. Localized Surface Plasmon Resonance Aptasensor for the Highly Sensitive Direct Detection of Cortisol in Human Saliva. Sens. Actuators B Chem. 2020, 304, 127424. [Google Scholar] [CrossRef]
- Singh, V. Ultrasensitive Quantum Dot-Coupled-Surface Plasmon Microfluidic Aptasensor Array for Serum Insulin Detection. Talanta 2020, 219, 121314. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Lobry, M.; Hassan, E.M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 Breast Cancer Biomarker Detection Using a Sandwich Optical Fiber Assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli Yaraki, M.; Tan, Y.N. Recent Advances in Metallic Nanobiosensors Development: Colorimetric, Dynamic Light Scattering and Fluorescence Detection. Sens. Int. 2020, 1, 100049. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Shaban, S.M.; Kim, D.H. Recent Advances in Aptamer Sensors. Sensors 2021, 21, 979. [Google Scholar] [CrossRef]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent Advances in Aptamer-Based Sensors for Aminoglycoside Antibiotics Detection and Their Applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Application of Various Optical and Electrochemical Aptasensors for Detection of Human Prostate Specific Antigen: A Review. Biosens. Bioelectron. 2019, 142, 111484. [Google Scholar] [CrossRef]
- Rajabnejad, S.H.; Badibostan, H.; Verdian, A.; Karimi, G.R.; Fooladi, E.; Feizy, J. Aptasensors as Promising New Tools in Bisphenol A Detection—An Invisible Pollution in Food and Environment. Microchem. J. 2020, 155, 104722. [Google Scholar] [CrossRef]
- López-Marzo, A.M.; Merkoçi, A. Paper-Based Sensors and Assays: A Success of the Engineering Design and the Convergence of Knowledge Areas. Lab Chip 2016, 16, 3150–3176. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chinnasamy, T.; Lifson, M.A.; Inci, F.; Demirci, U. Flexible Substrate-Based Devices for Point-of-Care Diagnostics. Trends Biotechnol. 2016, 34, 909–921. [Google Scholar] [CrossRef]
- Shafiee, H.; Asghar, W.; Inci, F.; Yuksekkaya, M.; Jahangir, M.; Zhang, M.H.; Durmus, N.G.; Gurkan, U.A.; Kuritzkes, D.R.; Demirci, U. Paper and Flexible Substrates as Materials for Biosensing Platforms to Detect Multiple Biotargets. Sci. Rep. 2015, 5, 8719. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Sheini, A.; Hashemi, P.; Hajian, A.; Bagheri, H. Disposable Paper-Based Biosensors for the Point-of-Care Detection of Hazardous Contaminations—A Review. Biosensors 2021, 11, 316. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.H. Current Advances in Paper-Based Biosensor Technologies for Rapid COVID-19 Diagnosis. BioChip J. 2022, 16, 376–396. [Google Scholar] [CrossRef]
- Mahmoudi, T.; de la Guardia, M.; Baradaran, B. Lateral Flow Assays towards Point-of-Care Cancer Detection: A Review of Current Progress and Future Trends. TrAC Trends Anal. Chem. 2020, 125, 115842. [Google Scholar] [CrossRef]
- Kang, J.; Yeom, G.; Jang, H.; Oh, J.; Park, C.J.; Kim, M.G. Development of Replication Protein A-Conjugated Gold Nanoparticles for Highly Sensitive Detection of Disease Biomarkers. Anal. Chem. 2019, 91, 10001–10007. [Google Scholar] [CrossRef]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; D’Arco, G.; Giraudi, G. Lateral-Flow Immunoassays for Mycotoxins and Phycotoxins: A Review. Anal. Bioanal. Chem. 2013, 405, 467–480. [Google Scholar] [CrossRef]
- Quesada-González, D.; Jairo, G.A.; Blake, R.C.; Blake, D.A.; Merkoçi, A. Uranium (VI) Detection in Groundwater Using a Gold Nanoparticle/Paper-Based Lateral Flow Device. Sci. Rep. 2018, 8, 16157. [Google Scholar] [CrossRef]
- Tripathi, P.; Kumar, A.; Sachan, M.; Gupta, S.; Nara, S. Aptamer-Gold Nanozyme Based Competitive Lateral Flow Assay for Rapid Detection of CA125 in Human Serum. Biosens. Bioelectron. 2020, 165, 112368. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.-C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 333–343. [Google Scholar] [CrossRef]
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Aptamer-Based Lateral Flow Assay on-Site Biosensors. Biosens. Bioelectron. 2021, 186, 113279. [Google Scholar] [CrossRef] [PubMed]
- Alsager, O.A.; Kumar, S.; Hodgkiss, J.M. Lateral Flow Aptasensor for Small Molecule Targets Exploiting Adsorption and Desorption Interactions on Gold Nanoparticles. Anal. Chem. 2017, 89, 7416–7424. [Google Scholar] [CrossRef] [PubMed]
- Derosa’, M.; Liu, H.; Gu, Z.; Velu, S.R.; Derosa, M.C. Lateral Flow Assays for Ochratoxin a Using Metal Nanoparticles: Comparison of “Adsorption–Desorption” Approach to Linkage Inversion Assembled Nano-Aptasensors (LIANA). Analyst 2018, 143, 4566–4574. [Google Scholar] [CrossRef]
- Akceoglu, G.A.; Saylan, Y.; Inci, F. A Snapshot of Microfluidics in Point-of-Care Diagnostics: Multifaceted Integrity with Materials and Sensors. Adv. Mater. Technol. 2021, 6, 2100049. [Google Scholar] [CrossRef]
- Wang, S.Q.; Inci, F.; De Libero, G.; Singhal, A.; Demirci, U. Point-of-Care Assays for Tuberculosis: Role of Nanotechnology/Microfluidics. Biotechnol. Adv. 2013, 31, 438–449. [Google Scholar] [CrossRef]
- Wang, S.Q.; Inci, F.; Chaunzwa, T.L.; Ramanujam, A.; Vasudevan, A.; Subramanian, S.; Fai Ip, A.C.; Sridharan, B.; Gurkan, U.A.; Demirci, U. Portable Microfluidic Chip for Detection of Escherichia Coli in Produce and Blood. Int. J. Nanomed. 2012, 7, 2591. [Google Scholar] [CrossRef]
- Shafiee, H.; Wang, S.Q.; Inci, F.; Toy, M.; Henrich, T.J.; Kuritzkes, D.R.; Demirci, U. Emerging Technologies for Point-of-Care Management of HIV Infection. Annu. Rev. Med. 2015, 66, 387–405. [Google Scholar] [CrossRef]
- Tasoglu, S.; Cumhur Tekin, H.; Inci, F.; Knowlton, S.; Wang, S.Q.; Wang-Johanning, F.; Johanning, G.; Colevas, D.; Demirci, U. Advances in Nanotechnology and Microfluidics for Human Papillomavirus Diagnostics. Proc. IEEE 2015, 103, 161–178. [Google Scholar] [CrossRef]
- Yildiz, U.H.; Inci, F.; Wang, S.Q.; Toy, M.; Tekin, H.C.; Javaid, A.; Lau, D.T.Y.; Demirci, U. Recent Advances in Micro/Nanotechnologies for Global Control of Hepatitis B Infection. Biotechnol. Adv. 2015, 33, 178–190. [Google Scholar] [CrossRef]
- Inan, H.; Wang, S.; Inci, F.; Baday, M.; Zangar, R.; Kesiraju, S.; Anderson, K.S.; Cunningham, B.T.; Demirci, U. Isolation, Detection, and Quantification of Cancer Biomarkers in HPV-Associated Malignancies. Sci. Rep. 2017, 7, 3322. [Google Scholar] [CrossRef]
- Cheng, N.; Du, D.; Wang, X.; Liu, D.; Xu, W.; Luo, Y.; Lin, Y. Recent Advances in Biosensors for Detecting Cancer-Derived Exosomes. Trends Biotechnol. 2019, 37, 1236–1254. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-Based Nanobiosensors. Nanotoday 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Tokel, O.; Inci, F.; Demirci, U. Advances in Plasmonic Technologies for Point of Care Applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef]
- Inci, F.; Tokel, O.; Wang, S.; Gurkan, U.A.; Tasoglu, S.; Kuritzkes, D.R.; Demirci, U. Nanoplasmonic Quantitative Detection of Intact Viruses from Unprocessed Whole Blood. ACS Nano 2013, 7, 4733–4745. [Google Scholar] [CrossRef]
- Ma, X.; He, S.; Qiu, B.; Luo, F.; Guo, L.; Lin, Z. Noble Metal Nanoparticle-Based Multicolor Immunoassays: An Approach toward Visual Quantification of the Analytes with the Naked Eye. ACS Sens. 2019, 4, 782–791. [Google Scholar] [CrossRef]
- Yang, T.; Luo, Z.; Tian, Y.; Qian, C.; Duan, Y. Design Strategies of AuNPs-Based Nucleic Acid Colorimetric Biosensors. TrAC Trends Anal. Chem. 2020, 124, 115795. [Google Scholar] [CrossRef]
- Liang, L.G.; Kong, M.Q.; Zhou, S.; Sheng, Y.F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.J.; Demirci, U.; et al. An Integrated Double-Filtration Microfluidic Device for Isolation, Enrichment and Quantification of Urinary Extracellular Vesicles for Detection of Bladder Cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef]
- Liang, L.G.; Sheng, Y.F.; Zhou, S.; Inci, F.; Li, L.; Demirci, U.; Wang, S.Q. An Integrated Double-Filtration Microfluidic Device for Detection of Extracellular Vesicles from Urine for Bladder Cancer Diagnosis. Methods Mol. Biol. 2017, 1660, 355–364. [Google Scholar] [CrossRef]
- Shirejini, S.Z.; Inci, F. The Yin and Yang of Exosome Isolation Methods: Conventional Practice, Microfluidics, and Commercial Kits. Biotechnol. Adv. 2021, 54, 107814. [Google Scholar] [CrossRef]
- Inci, F. Benchmarking a Microfluidic-Based Filtration for Isolating Biological Particles. Langmuir 2022, 38, 1897–1909. [Google Scholar] [CrossRef]
- Inci, F.; Karaaslan, M.G.; Mataji-Kojouri, A.; Shah, P.A.; Saylan, Y.; Zeng, Y.; Avadhani, A.; Sinclair, R.; Lau, D.T.Y.; Demirci, U. Enhancing the Nanoplasmonic Signal by a Nanoparticle Sandwiching Strategy to Detect Viruses. Appl. Mater. Today 2020, 20, 100709. [Google Scholar] [CrossRef]
- Hu, S.; Fang, X.; Liu, G.; Ma, G.; Ye, F.; Zhao, S. A Gas-Pressure-Assisted Ratiometric Atomic Flame Assay for the Point-of-Care Testing of Tumor-Cell-Derived Exosomes. Analyst 2022, 147, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, T.; Li, X.; Sun, L.; Zhang, Y.; Zhang, Y. Colorimetric Detection of Kanamycin Based on Analyte-Protected Silver Nanoparticles and Aptamer-Selective Sensing Mechanism. Anal. Chim. Acta 2015, 891, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Saraji, M. Optical Aptasensor Based on Silver Nanoparticles for the Colorimetric Detection of Adenosine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jia, K.; Bing, T.; Zhang, Z.; Zhen, X.; Liu, X.; Zhang, N.; Shangguan, D. Detection of Circulating Tumor-Related Materials by Aptamer Capturing and Endogenous Enzyme-Signal Amplification. Anal. Chem. 2020, 92, 5370–5378. [Google Scholar] [CrossRef]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing; Springer: Singapore, 2021; Volume 13, ISBN 4082002100717. [Google Scholar]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Jovin, T.M. FRET Imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef]
- Liu, G.; Feng, D.Q.; Qian, Y.; Wang, W.; Zhu, J.J. Construction of FRET Biosensor for Off-On Detection of Lead Ions Based on Carbon Dots and Gold Nanorods. Talanta 2019, 201, 90–95. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. Förster Resonance Energy Transfer (FRET)-Based Biosensors for Biological Applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef]
- Pehlivan, Z.S.; Torabfam, M.; Kurt, H.; Ow-Yang, C.; Hildebrandt, N.; Yüce, M. Aptamer and Nanomaterial Based FRET Biosensors: A Review on Recent Advances (2014–2019). Microchim. Acta 2019, 186, 563. [Google Scholar] [CrossRef]
- Govorov, A.; Hernández Martínez, P.L.; Demir, H.V. Förster-Type Nonradiative Energy Transfer Models. SpringerBriefs Appl. Sci. Technol. 2016, 1, 19–27. [Google Scholar] [CrossRef]
- Mahata, M.K.; De, R.; Lee, K.T. Near-Infrared-Triggered Upconverting Nanoparticles for Biomedicine Applications. Biomedicines 2021, 9, 756. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H.; Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2022, 213, 2107986. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal Enhanced Fluorescence Biosensing: From Ultra-Violet towards Second Near-Infrared Window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Zheng, G.; Dai, Z.; Ma, Y. Recent Progress in Sensing Application of Metal Nanoarchitecture-Enhanced Fluorescence. Nanoscale Adv. 2021, 3, 2448–2465. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-Enhanced Optical Sensors: A Review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Sundaresan, S.M.; Fothergill, S.M.; Tabish, T.A.; Ryan, M.; Xie, F. Aptamer Biosensing Based on Metal Enhanced Fluorescence Platform: A Promising Diagnostic Tool. Appl. Phys. Rev. 2021, 8, 041311. [Google Scholar] [CrossRef]
- Nicolas, E.; Bertucci, F.; Sabatier, R.; Gonçalves, A. Targeting BRCA Deficiency in Breast Cancer: What Are the Clinical Evidences and the Next Perspectives? Cancers 2018, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Çelik, O.; Saylan, Y.; Göktürk, I.; Yılmaz, F.; Denizli, A. A Surface Plasmon Resonance Sensor with Synthetic Receptors Decorated on Graphene Oxide for Selective Detection of Benzylpenicillin. Talanta 2023, 253, 123939. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, M.; Altintas, Z. Recent Progress in Optical Sensors for Biomedical Diagnostics. Micromachines 2020, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ozen, M.O.; Karaaslan, M.G.; Prator, C.A.; Thanh, C.; Kumar, S.; Torres, L.; Iyer, N.; Munter, S.; Southern, S.; et al. Tunable Fano-Resonant Metasurfaces on a Disposable Plastic-Template for Multimodal and Multiplex Biosensing. Adv. Mater. 2020, 32, 1907160. [Google Scholar] [CrossRef] [PubMed]
- Inci, F.; Filippini, C.; Baday, M.; Ozen, M.O.; Calamak, S.; Durmus, N.G.; Wang, S.; Hanhauser, E.; Hobbs, K.S.; Juillard, F.; et al. Multitarget, Quantitative Nanoplasmonic Electrical Field-Enhanced Resonating Device (NE2RD) for Diagnostics. Proc. Natl. Acad. Sci. USA 2015, 112, E4354–E4363. [Google Scholar] [CrossRef]
- Mataji-Kojouri, A.; Ozen, M.O.; Shahabadi, M.; Inci, F.; Demirci, U. Entangled Nanoplasmonic Cavities for Estimating Thickness of Surface-Adsorbed Layers. ACS Nano 2020, 14, 8518–8527. [Google Scholar] [CrossRef]
- Inci, F.; Celik, U.; Turken, B.; Özer, H.Ö.; Kok, F.N. Construction of P-Glycoprotein Incorporated Tethered Lipid Bilayer Membranes. Biochem. Biophys. Rep. 2015, 2, 115–122. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Preparation of Magnetic Nanoparticles-Assisted Plasmonic Biosensors with Metal Affinity for Interferon-α Detection. Mater. Sci. Eng. B 2022, 280, 115687. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Saylan, Y.; Göktürk, I.; Yılmaz, F.; Denizli, A. Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles. Biosensors 2022, 12, 482. [Google Scholar] [CrossRef]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef]
- Soler, M.; Huertas, C.S.; Lechuga, L.M. Label-Free Plasmonic Biosensors for Point-of-Care Diagnostics: A Review. Expert Rev. Mol. Diagn. 2019, 19, 71–81. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y.; Phang, W.M.; Hashim, U. Aptamer-Based “Point-of-Care Testing”. Biotechnol. Adv. 2016, 34, 198–208. [Google Scholar] [CrossRef]
- Erdem, Ö.; Cihangir, N.; Saylan, Y.; Denizli, A. Comparison of Molecularly Imprinted Plasmonic Nanosensor Performances for Bacteriophage Detection. New J. Chem. 2020, 44, 17654–17663. [Google Scholar] [CrossRef]
- Chang, C.C. Recent Advancements in Aptamer-Based Surface Plasmon Resonance Biosensing Strategies. Biosensors 2021, 11, 233. [Google Scholar] [CrossRef]
- Famulok, M. Oligonucleotide Aptamers That Recognize Small Molecules. Curr. Opin. Struct. Biol. 1999, 9, 324–329. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Wu, Y.; Cook, C.J.; Main, L. Sensitivity Enhancement of Surface Plasmon Resonance Biosensing of Small Molecules. Anal. Biochem. 2005, 343, 125–135. [Google Scholar] [CrossRef]
- Gan, H.; Xu, H. A Novel Aptamer-Based Online Magnetic Solid Phase Extraction Method for Simultaneous Determination of Urinary 8-Hydroxy-2′-Deoxyguanosine and Monohydroxylated Polycyclic Aromatic Hydrocarbons. Talanta 2019, 201, 271–279. [Google Scholar] [CrossRef]
- Guler, E.; Bozokalfa, G.; Demir, B.; Gumus, Z.P.; Guler, B.; Aldemir, E.; Timur, S.; Coskunol, H. An Aptamer Folding-Based Sensory Platform Decorated with Nanoparticles for Simple Cocaine Testing. Drug Test. Anal. 2017, 9, 578–587. [Google Scholar] [CrossRef]
- Ammanath, G.; Delachi, C.G.; Karabacak, S.; Ali, Y.; Boehm, B.O.; Yildiz, U.H.; Alagappan, P.; Liedberg, B. Colorimetric and Fluorometric Profiling of Advanced Glycation End Products. ACS Appl. Mater. Interfaces 2022, 14, 94–103. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and Sensors for Dopamine Detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Chang, K.W.; Li, J.; Yang, C.H.; Shiesh, S.C.; Lee, G. Bin An Integrated Microfluidic System for Measurement of Glycated Hemoglobin Levels by Using an Aptamer-Antibody Assay on Magnetic Beads. Biosens. Bioelectron. 2015, 68, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Duanghathaipornsuk, S.; Shen, B.; Cameron, B.D.; Ijäs, H.; Linko, V.; Kostiainen, M.A.; Kim, D.S. Aptamer-Embedded DNA Origami Cage for Detecting (Glycated) Hemoglobin with a Surface Plasmon Resonance Sensor. Mater. Lett. 2020, 275, 128141. [Google Scholar] [CrossRef]
- Chen, Y.; Nakamoto, K.; Niwa, O.; Corn, R.M. On-Chip Synthesis of RNA Aptamer Microarrays for Multiplexed Protein Biosensing with SPR Imaging Measurements. Langmuir 2012, 28, 8281–8285. [Google Scholar] [CrossRef] [PubMed]
- Dejeu, J.; Bonnet, H.; Coche-Guérente, L.; Defrancq, E.; Spinelli, N.; van der Heyden, A. Negative SPR Signals during Low Molecular Weight Analyte Recognition. Anal. Chem. 2021, 93, 4134–4140. [Google Scholar] [CrossRef]
- Prante, M.; Segal, E.; Scheper, T.; Bahnemann, J.; Walter, J. Aptasensors for Point-of-Care Detection of Small Molecules. Biosensors 2020, 10, 108. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Lunelli, L.; Vanzetti, L.; Pederzolli, C.; Zeni, L.; Pasquardini, L. An Easy Way to Realize SPR Aptasensor: A Multimode Plastic Optical Fiber Platform for Cancer Biomarkers Detection. Talanta 2015, 140, 88–95. [Google Scholar] [CrossRef]
- Daniels, J.; Wadekar, S.; DeCubellis, K.; Jackson, G.W.; Chiu, A.S.; Pagneux, Q.; Saada, H.; Engelmann, I.; Ogiez, J.; Loze-Warot, D.; et al. A Mask-Based Diagnostic Platform for Point-of-Care Screening of COVID-19. Biosens. Bioelectron. 2021, 192, 113486. [Google Scholar] [CrossRef]
- İnci, F. Bioinspired Material-Integrated Sensors for Improving Nanoplasmonic Characteristics. Hacet. J. Biol. Chem. 2022, 50, 193–204. [Google Scholar] [CrossRef]
- Liao, G.; Liu, X.; Yang, X.; Wang, Q.; Geng, X.; Zou, L.; Liu, Y.; Li, S.; Zheng, Y.; Wang, K. Surface Plasmon Resonance Assay for Exosomes Based on Aptamer Recognition and Polydopamine-Functionalized Gold Nanoparticles for Signal Amplification. Microchim. Acta 2020, 187, 251. [Google Scholar] [CrossRef]
- Dillen, A.; Scarpellini, C.; Daenen, W.; Driesen, S.; Zijlstra, P.; Lammertyn, J. Integrated Signal Amplification on a Fiber Optic SPR Sensor Using Duplexed Aptamers. ACS Sens. 2023, 8, 811–821. [Google Scholar] [CrossRef]
- Shama, N.A.; Aşır, S.; Ozsoz, M.; Göktürk, I.; Türkmen, D.; Yılmaz, F.; Denizli, A. Gold-Modified Molecularly Imprinted N-Methacryloyl-(L)-Phenylalanine-Containing Electrodes for Electrochemical Detection of Dopamine. Bioengineering 2022, 9, 87. [Google Scholar] [CrossRef]
- Radi, A.-E. Electrochemical Aptamer-Based Biosensors: Recent Advances and Perspectives. Int. J. Electrochem. 2011, 2011, e863196. [Google Scholar] [CrossRef]
- Jarczewska, M.; Górski, Ł.; Malinowska, E. Electrochemical Aptamer-Based Biosensors as Potential Tools for Clinical Diagnostics. Anal. Methods 2016, 8, 3861–3877. [Google Scholar] [CrossRef]
- Tang, T.; Liu, Y.; Jiang, Y. Recent Progress on Highly Selective and Sensitive Electrochemical Aptamer-Based Sensors. Chem. Res. Chin. Univ. 2022, 38, 866–878. [Google Scholar] [CrossRef]
- Yucel, M.; Akin, O.; Cayoren, M.; Akduman, I.; Palaniappan, A.; Liedberg, B.; Hizal, G.; Inci, F.; Yildiz, U.H. Hand-Held Volatilome Analyzer Based on Elastically Deformable Nanofibers. Anal. Chem. 2018, 90, 5122–5129. [Google Scholar] [CrossRef]
- Aziz, M.; Yelamanchili, V.S. Yersinia Enterocolitica. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Leus, K.; Muylaert, I.; Van Speybroeck, V.; Marin, G.B.; Van Der Voort, P. A Coordinative Saturated Vanadium Containing Metal Organic Framework That Shows a Remarkable Catalytic Activity. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Scientific Bases for the Preparation of Heterogeneous Catalysts; Elsevier: Amsterdam, The Netherlands, 2010; Volume 175, pp. 329–332. [Google Scholar]
- Yang, X.; Tang, Q.; Jiang, Y.; Zhang, M.; Wang, M.; Mao, L. Nanoscale ATP-Responsive Zeolitic Imidazole Framework-90 as a General Platform for Cytosolic Protein Delivery and Genome Editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Y.; Kang, Z.; Liu, X.; Chen, N.; Wang, Q.; Tu, Y.; Wang, L.; Song, S.; Ling, D.; et al. Organelle-Specific Triggered Release of Immunostimulatory Oligonucleotides from Intrinsically Coordinated DNA–Metal–Organic Frameworks with Soluble Exoskeleton. J. Am. Chem. Soc. 2017, 139, 15784–15791. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Li, F. Nucleic Acid-Functionalized Metal-Organic Framework for Ultrasensitive Immobilization-Free Photoelectrochemical Biosensing. Biosens. Bioelectron. 2021, 173, 112832. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Kim, W.; Park, B.; Oh, S.Y.; Kim, T.; Huh, Y.S.; Hwangbo, C.K.; Han, Y.K. Efficient Electron-Mediated Electrochemical Biosensor of Gold Wire for the Rapid Detection of C-Reactive Protein: A Predictive Strategy for Heart Failure. Biosens. Bioelectron. 2019, 142, 111549. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; López, M.Á.; Escarpa, A. Electrochemical Microfluidic Micromotors-Based Immunoassay for C-Reactive Protein Determination in Preterm Neonatal Samples with Sepsis Suspicion. Anal. Chem. 2020, 92, 5048–5054. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous Anodic Aluminum Oxide for Chemical Sensing and Biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Lin, K.C.; Jagannath, B.; Muthukumar, S.; Prasad, S. Sub-Picomolar Label-Free Detection of Thrombin Using Electrochemical Impedance Spectroscopy of Aptamer-Functionalized MoS2. Analyst 2017, 142, 2770–2780. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Chen, R.L.C.; Hsieh, B.C.; Hsiao, H.Y.; Kung, Y.; Hou, Y.T.; Cheng, T.J. Label-Free and Reagentless Capacitive Aptasensor for Thrombin. Biosens. Bioelectron. 2019, 131, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.C. Molecular Design, Synthesis, and Characterization of Conjugated Polymers for Interfacing Electronic Biomedical Devices with Living Tissue. MRS Commun. 2015, 5, 131–152. [Google Scholar] [CrossRef]
- Azadbakht, A.; Roushani, M.; Abbasi, A.R.; Derikvand, Z. Design and Characterization of Electrochemical Dopamine–Aptamer as Convenient and Integrated Sensing Platform. Anal. Biochem. 2016, 507, 47–57. [Google Scholar] [CrossRef]
- Azadbakht, A.; Roushani, M.; Abbasi, A.R.; Derikvand, Z. A Novel Impedimetric Aptasensor, Based on Functionalized Carbon Nanotubes and Prussian Blue as Labels. Anal. Biochem. 2016, 512, 58–69. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Wang, Z.; Liang, Q. A Graphene Oxide-Based Label-Free Electrochemical Aptasensor for the Detection of Alpha-Fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef]
- Upan, J.; Youngvises, N.; Tuantranont, A.; Karuwan, C.; Banet, P.; Aubert, P.H.; Jakmunee, J. A Simple Label-Free Electrochemical Sensor for Sensitive Detection of Alpha-Fetoprotein Based on Specific Aptamer Immobilized Platinum Nanoparticles/Carboxylated-Graphene Oxide. Sci. Rep. 2021, 11, 13969. [Google Scholar] [CrossRef]
- Campos, R.; Kotlyar, A.; Ferapontova, E.E. DNA-Mediated Electron Transfer in DNA Duplexes Tethered to Gold Electrodes via Phosphorothioated DA Tags. Langmuir 2014, 30, 11853–11857. [Google Scholar] [CrossRef]
- Malecka, K.; Ferapontova, E.E. Femtomolar Detection of Thrombin in Serum and Cerebrospinal Fluid via Direct Electrocatalysis of Oxygen Reduction by the Covalent G4-Hemin-Aptamer Complex. ACS Appl. Mater. Interfaces 2021, 13, 37979–37988. [Google Scholar] [CrossRef]
- Joe, C.; Lee, B.H.; Kim, S.H.; Ko, Y.; Gu, M.B. Aptamer Duo-Based Portable Electrochemical Biosensors for Early Diagnosis of Periodontal Disease. Biosens. Bioelectron. 2022, 199, 113884. [Google Scholar] [CrossRef]
- Pothipor, C.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A Gold Nanoparticle-Dye/Poly(3-Aminobenzylamine)/Two Dimensional MoSe2/Graphene Oxide Electrode towards Label-Free Electrochemical Biosensor for Simultaneous Dual-Mode Detection of Cancer Antigen 15-3 and microRNA-21. Colloids Surf. B Biointerfaces 2022, 210, 112260. [Google Scholar] [CrossRef]
- Zhang, J.; Lakshmipriya, T.; Gopinath, S.C.B. Electroanalysis on an Interdigitated Electrode for High-Affinity Cardiac Troponin I Biomarker Detection by Aptamer–Gold Conjugates. ACS Omega 2020, 5, 25899–25905. [Google Scholar] [CrossRef]
- Zargartalebi, H.; Yousefi, H.; Flynn, C.D.; Gomis, S.; Das, J.; Young, T.L.; Chien, E.; Mubareka, S.; McGeer, A.; Wang, H.; et al. Capillary-Assisted Molecular Pendulum Bioanalysis. J. Am. Chem. Soc. 2022, 144, 18338–18349. [Google Scholar] [CrossRef]
- Das, J.; Gomis, S.; Chen, J.B.; Yousefi, H.; Ahmed, S.; Mahmud, A.; Zhou, W.; Sargent, E.H.; Kelley, S.O. Reagentless Biomolecular Analysis Using a Molecular Pendulum. Nat. Chem. 2021, 13, 428–434. [Google Scholar] [CrossRef]
- Khan, N.I.; Maddaus, A.G.; Song, E. A Low-Cost Inkjet-Printed Aptamer-Based Electrochemical Biosensor for the Selective Detection of Lysozyme. Biosensors 2018, 8, 7. [Google Scholar] [CrossRef]
- Miglione, A.; Raucci, A.; Amato, J.; Marzano, S.; Pagano, B.; Raia, T.; Lucarelli, M.; Fuso, A.; Cinti, S. Printed Electrochemical Strip for the Detection of MiRNA-29a: A Possible Biomarker Related to Alzheimer’s Disease. Anal. Chem. 2022, 94, 15558–15563. [Google Scholar] [CrossRef]
- Dutta, S.; Corni, S.; Brancolini, G. Atomistic Simulations of Functionalized Nano-Materials for Biosensors Applications. Int. J. Mol. Sci. 2022, 23, 1484. [Google Scholar] [CrossRef]
- Buglak, A.A.; Samokhvalov, A.V.; Zherdev, A.V.; Dzantiev, B.B. Methods and Applications of in Silico Aptamer Design and Modeling. Int. J. Mol. Sci. 2020, 21, 8420. [Google Scholar] [CrossRef]
- Jain, S.; Nehra, M.; Kumar, R.; Dilbaghi, N.; Hu, T.Y.; Kumar, S.; Kaushik, A.; Li, C. zhong Internet of Medical Things (IoMT)-Integrated Biosensors for Point-of-Care Testing of Infectious Diseases. Biosens. Bioelectron. 2021, 179, 113074. [Google Scholar] [CrossRef]
- Takada, S. Molecular Dynamics Simulations of Biomolecules. J. Soc. Mech. Eng. 2013, 116, 78–80. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Douaki, A.; Garoli, D.; Inam, A.K.M.S.; Angeli, M.A.C.; Cantarella, G.; Rocchia, W.; Wang, J.; Petti, L.; Lugli, P. Smart Approach for the Design of Highly Selective Aptamer-Based Biosensors. Biosensors 2022, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, M.; Roushani, M. Three-Dimensional Modeling of Streptomycin Binding Single-Stranded DNA for Aptamer-Based Biosensors, a Molecular Dynamics Simulation Approach. J. Biomol. Struct. Dyn. 2022, 41, 3430–3439. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Yang, R.; Liu, M.; Feng, H.; Li, N.; Jin, M.; Zhang, M.; Shui, L. A Liquid Crystal-Based Biosensor for Detection of Insulin Driven by Conformational Change of an Aptamer at Aqueous-Liquid Crystal Interface. J. Colloid Interface Sci. 2022, 628, 215–222. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, H.; Chen, H.; Zou, B.; Yang, C.; Zhang, X.; Gao, Y.; Sun, M.; Wang, L. A Rapid and Sensitive Aptamer-Based Biosensor for Amnesic Shellfish Toxin Domoic Acid. Bioengineering 2022, 9, 684. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Mathew, M.; Radhakrishnan, S.; Rout, C.S.; Chakraborty, B. Theoretical Insight on the Biosensing Applications of 2D Materials. J. Phys. Chem. B 2020, 124, 11098–11122. [Google Scholar] [CrossRef]
- Schleder, G.R.; Padilha, A.C.M.; Acosta, C.M.; Costa, M. From DFT to Machine Learning: Recent Approaches to Materials Science—A Review from DFT to Machine Learning: Recent Approaches to Materials Science—A Review. J. Phys. Mater. 2019, 2, 032001. [Google Scholar] [CrossRef]
- Ouyang, X.; Tang, L.; Feng, C.; Peng, B.; Liu, Y.; Ren, X.; Zhu, X.; Tan, J.; Hu, X. Au/CeO2/g-C3N4 Heterostructures: Designing a Self-Powered Aptasensor for Ultrasensitive Detection of Microcystin-LR by Density Functional Theory. Biosens. Bioelectron. 2020, 164, 112328. [Google Scholar] [CrossRef]
- Ouyang, X.; Feng, C.; Zhu, X.; Liao, Y.; Zhou, Z.; Fan, X.; Zhang, Z.; Chen, L.; Tang, L. 3D Printed Bionic Self-Powered Sensing Device Based on Fern-Shaped Nitrogen Doped BiVO4 Photoanode with Enriched Oxygen Vacancies. Biosens. Bioelectron. 2023, 220, 114817. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Umasankar, Y.; Manickam, P.; Nickel, J.C.; Iwasaki, L.R.; Kawamoto, B.K.; Todoki, K.C.; Scott, J.A.M.; Bhansali, S. Disposable Aptamer-Sensor Aided by Magnetic Nanoparticle Enrichment for Detection of Salivary Cortisol Variations in Obstructive Sleep Apnea Patients. Sci. Rep. 2017, 7, 17992. [Google Scholar] [CrossRef]
- Li, Y.; Bu, Y.; Jiang, F.; Dai, X.; Ao, J.P. Fabrication of Ultra-Sensitive Photoelectrochemical Aptamer Biosensor: Based on Semiconductor/DNA Interfacial Multifunctional Reconciliation via 2D-C3N4. Biosens. Bioelectron. 2020, 150, 111903. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, W.; Cai, G.; Huang, Z.; Tao, J.; Tang, D.; Zhu, C. Single-Atom Platinum Nanocatalyst-Improved Catalytic Efficiency with Enzyme-DNA Supermolecular Architectures. Nano Energy 2020, 74, 104931. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Housaindokht, M.R.; Izadyar, M.; Bozorgmehr, M.R.; Verdian, A. Recent Advances in Computational Methods for Biosensor Design. Biotechnol. Bioeng. 2021, 118, 555–578. [Google Scholar] [CrossRef]
- De La Lande, A.; Alvarez-Ibarra, A.; Hasnaoui, K.; Cailliez, F.; Wu, X.; Mineva, T.; Cuny, J.; Calaminici, P.; López-Sosa, L.; Geudtner, G.; et al. Molecular Simulations with In-DeMon2k QM/MM, a Tutorial-Review. Molecules 2019, 24, 1653. [Google Scholar] [CrossRef]
- Karuppaiah, G.; Velayutham, J.; Hansda, S.; Narayana, N.; Bhansali, S.; Manickam, P. Towards the Development of Reagent-Free and Reusable Electrochemical Aptamer-Based Cortisol Sensor. Bioelectrochemistry 2022, 145, 108098. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Palacio, I.; Moreno, M.; Almudena, N.; Mendieta-Moreno, J.I.; Torres, V.B.; García-Hern, M.; Luis, V.; Jelínek, P.; Alpuim, P.; et al. Biosensors and Bioelectronics Attomolar Detection of Hepatitis C Virus Core Protein Powered by Molecular Antenna-like Effect in a Graphene Field-Effect Aptasensor. Biosens. Bioelectron. 2023, 222, 115006. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Ruiz, J.A.R.; Sanjuán, A.M.; Vallejos, S.; García, F.C.; García, J.M. Smart Polymers in Micro and Nano Sensory Devices. Chemosensors 2018, 6, 12. [Google Scholar] [CrossRef]
- Mitchell, T. Machine Learning; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Bhardwaj, T.; Ramana, L.N.; Sharma, T.K. Current Advancements and Future Road Map to Develop ASSURED Microfluidic Biosensors for Infectious and Non-Infectious Diseases. Biosensors 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]


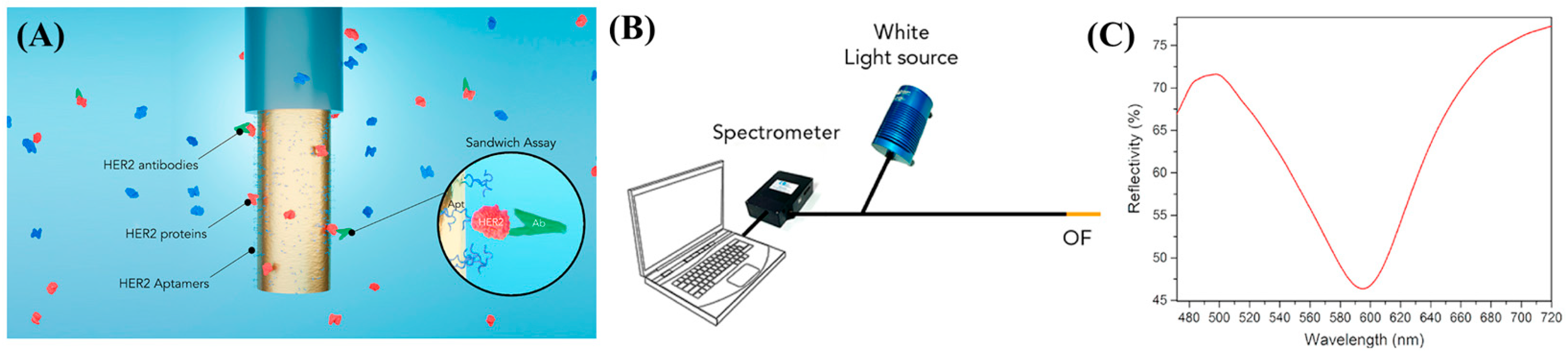

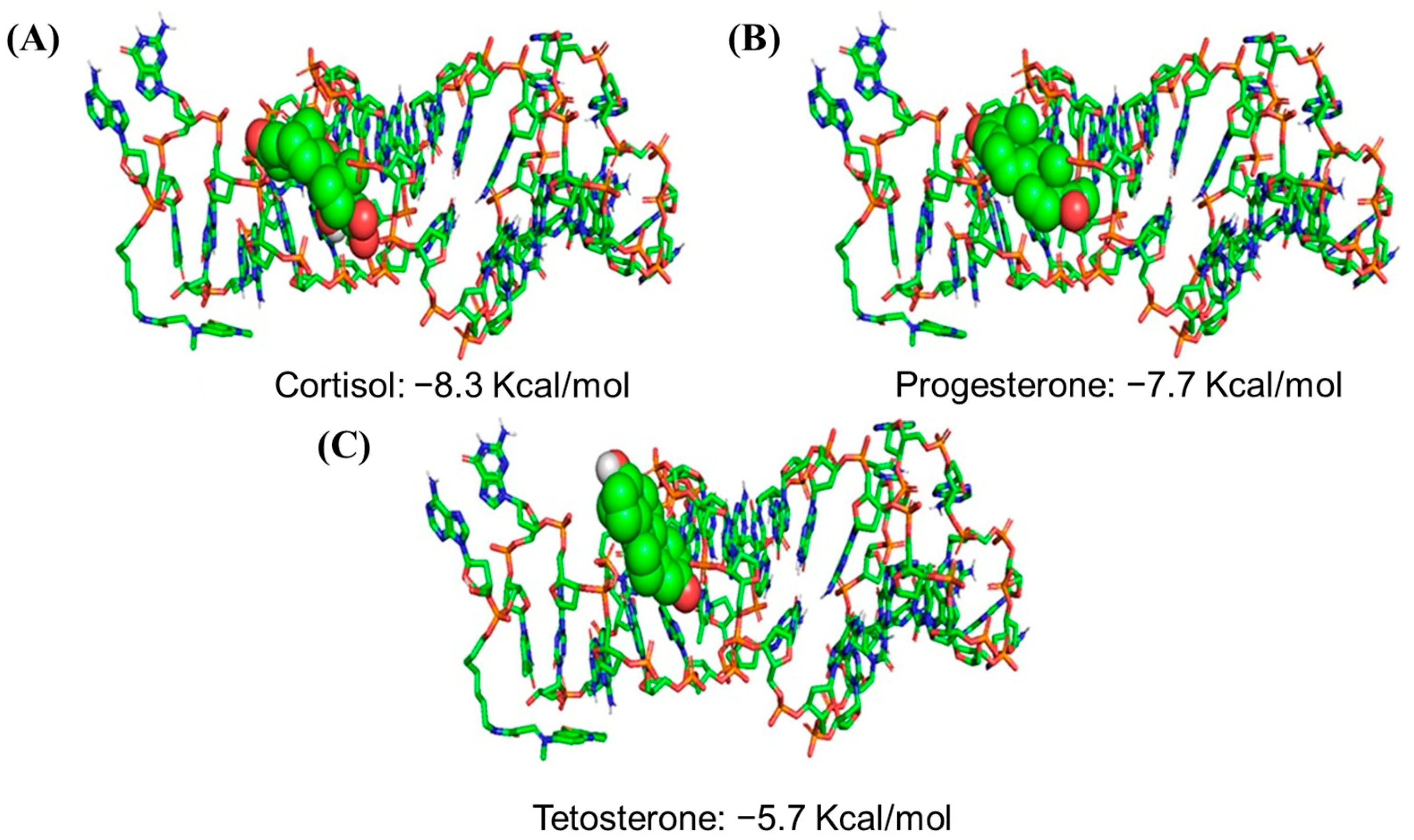
| Target Analyte | Physical Condition | Sensing Principle | Limit of Detection (LOD) | Reference |
|---|---|---|---|---|
| Cortisol | Stress | Colorimetric | 0.37 ng·mL−1 | [53] |
| Dopamine | Alzheimer’s, Parkinson’s, and Huntington’s diseases | Colorimetric | 10 ng·mL−1 | [54] |
| Glycated albumin | GDM | Colorimetric | 0.8 mg·mL−1 and 1.5 mg·mL−1 | [55] |
| PDGF-BB and thrombin | Tumor regions (e.g., liver, gastrointestinal tract) and hemostasis | Colorimetric | 1.0 nM and 1.5 nM | [56] |
| CXCL 9 | Antibody-mediated rejection of kidney transplantation | Colorimetric | 10 pg·mL−1 | [57] |
| K+ | Chronic kidney disease | Colorimetric | 0.01 mM | [58] |
| HER2 | Breast cancer | Colorimetric | 10 nM | [59] |
| RBP4 | Type 2 diabetes mellitus | Colorimetric | 90.76 ± 2.81 nM | [60] |
| Exosomes | Leukemia | Colorimetric | 42 particles·μL−1 | [61] |
| IL-6 | Brain injury or inflammation | Colorimetric | 1.95 μg·mL−1 | [62] |
| Mycobacterium tuberculosis DNA | TB | Colorimetric | 0.28 nM | [63] |
| PDGF-BB | Tumor growth and progression | Colorimetric | 10 fM | [64] |
| Cortisol | Stress | Fluorescent | 1 nM | [65] |
| Aβ and tau protein | Alzheimer | Fluorescent | 50 pM and 10 pM | [66] |
| Mucin 1 | Tumor | Fluorescent | 0.15 fg·mL−1 mucin 1 or 3 CTCs·mL−1 | [67] |
| AFP | Hepatocellular carcinoma | Fluorescent | 400 pg·mL−1 | [68] |
| Mutated BRCA-1 | Breast cancer | Fluorescent | 0.34 fM | [69] |
| Cortisol | Stress | Fluorescent | 6.76 ng·mL−1 | [70] |
| Ig E | Allergic disease | Fluorescent | 0.13 IU·mL−1 | [71] |
| PFLDH | Malaria | Fluorescent | 18 fM (0.6 pg·mL−1) | [72] |
| Glucose, ATP, L-Tyrosinamide, and thrombin | Diabetes, molecular marker for cellular energy, metabolic syndrome and melanoma, and hemostasis | Fluorescent | 1.1 mM, 0.1 mM, 3.5 µM and 25 nM | [73] |
| MPT64 secreted from Mycobacterium tuberculosis | TB | Electrochemical | 81 pM | [74] |
| YadA | Diarrhea, mesenteric lymphadenitis, arthritis, and sepsis | Electrochemical | 7.0 × 104 CFU·mL−1 | [75] |
| CRP | Cerebrovascular diseases, myocardial infectious inflammation, and cancer | Electrochemical | 0.44 pg·mL−1 | [76] |
| Exosomes | Cancer | Electrochemical | 5 × 103 particles·mL−1 | [77] |
| Thrombin | Hemostasis | Electrochemical | 0.12 pM | [78] |
| α-thrombin | Blood clotting cascade | Electrochemical | 10 pM | [79] |
| Thrombin | Anticoagulation and cardiovascular disease | Electrochemical | 1 pM | [80] |
| p24-HIV protein | HIV | Electrochemical | 51.7 pg·mL−1 | [81] |
| α-Syn | Parkinson’s disease | Electrochemical | 1 × 10–6 pM | [82] |
| MCF-7 breast cancer cells | Breast cancer | Electrochemical | 6 cells·mL−1 | [83] |
| SARS-CoV-2 | COVID-19 | Electrochemical | 9.79 fg·mL−1 | [84] |
| AFP | Liver cancer | Electrochemical | 0.65 pg·mL−1 | [85] |
| CEA and NSE | Cancer | Electrochemical | 2 pg·mL−1 and 10 pg·mL−1 | [86] |
| Thrombin | Blood coagulation cascade | SPR | 1 nM | [87] |
| Dopamine | Neurological and psychiatric disorders | SPR | 10−13 M | [88] |
| SARS-CoV-2 spike glycoprotein | COVID-19 | SPR | 36.7 nM | [89] |
| Exosomes | Breast cancer | SPR | 5 × 103 exosomes·mL−1 | [90] |
| Cortisol | Stress | LSPR | 0.1 nM | [91] |
| Insulin | Diabetes | SPR | 5 pM | [92] |
| HER2 proteins | Breast cancer | OF-SPR | 20 g·mL−1 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan, Y.; Atabay, M.; Chowdhury, H.K.; Göktürk, I.; Saylan, Y.; Inci, F. Aptamer-Based Point-of-Care Devices: Emerging Technologies and Integration of Computational Methods. Biosensors 2023, 13, 569. https://doi.org/10.3390/bios13050569
Aslan Y, Atabay M, Chowdhury HK, Göktürk I, Saylan Y, Inci F. Aptamer-Based Point-of-Care Devices: Emerging Technologies and Integration of Computational Methods. Biosensors. 2023; 13(5):569. https://doi.org/10.3390/bios13050569
Chicago/Turabian StyleAslan, Yusuf, Maryam Atabay, Hussain Kawsar Chowdhury, Ilgım Göktürk, Yeşeren Saylan, and Fatih Inci. 2023. "Aptamer-Based Point-of-Care Devices: Emerging Technologies and Integration of Computational Methods" Biosensors 13, no. 5: 569. https://doi.org/10.3390/bios13050569
APA StyleAslan, Y., Atabay, M., Chowdhury, H. K., Göktürk, I., Saylan, Y., & Inci, F. (2023). Aptamer-Based Point-of-Care Devices: Emerging Technologies and Integration of Computational Methods. Biosensors, 13(5), 569. https://doi.org/10.3390/bios13050569








