Flavocytochrome b2-Mediated Electroactive Nanoparticles for Developing Amperometric L-Lactate Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Enzyme
2.2. Synthesis of NPs
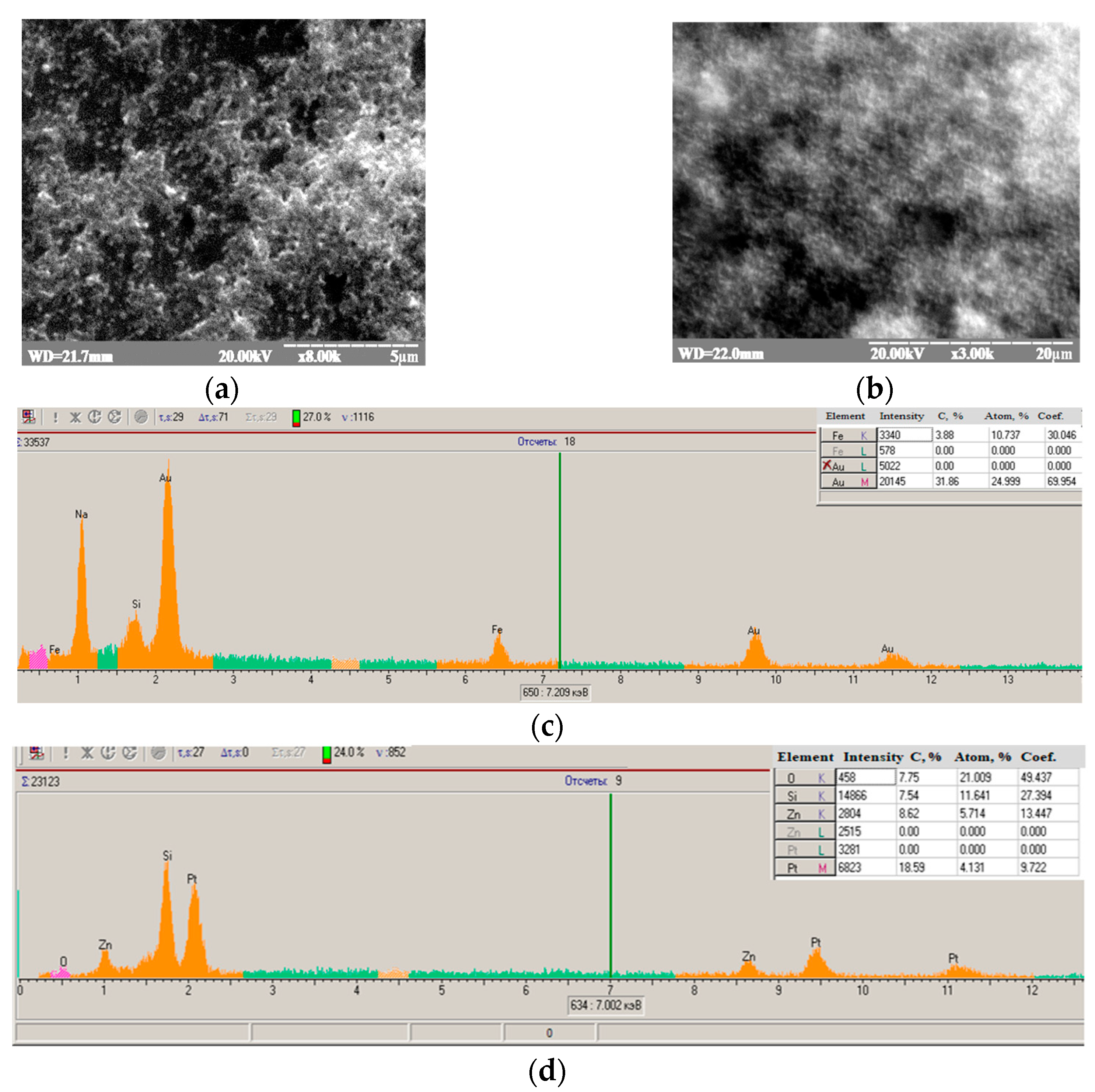
2.3. Modification of Graphite Electrodes with Nanoparticles and Their Characterization
2.4. Development, Characterization, and Application of Bioelectrodes
2.5. Reference Method for Lact Determination in Real Sample
3. Results
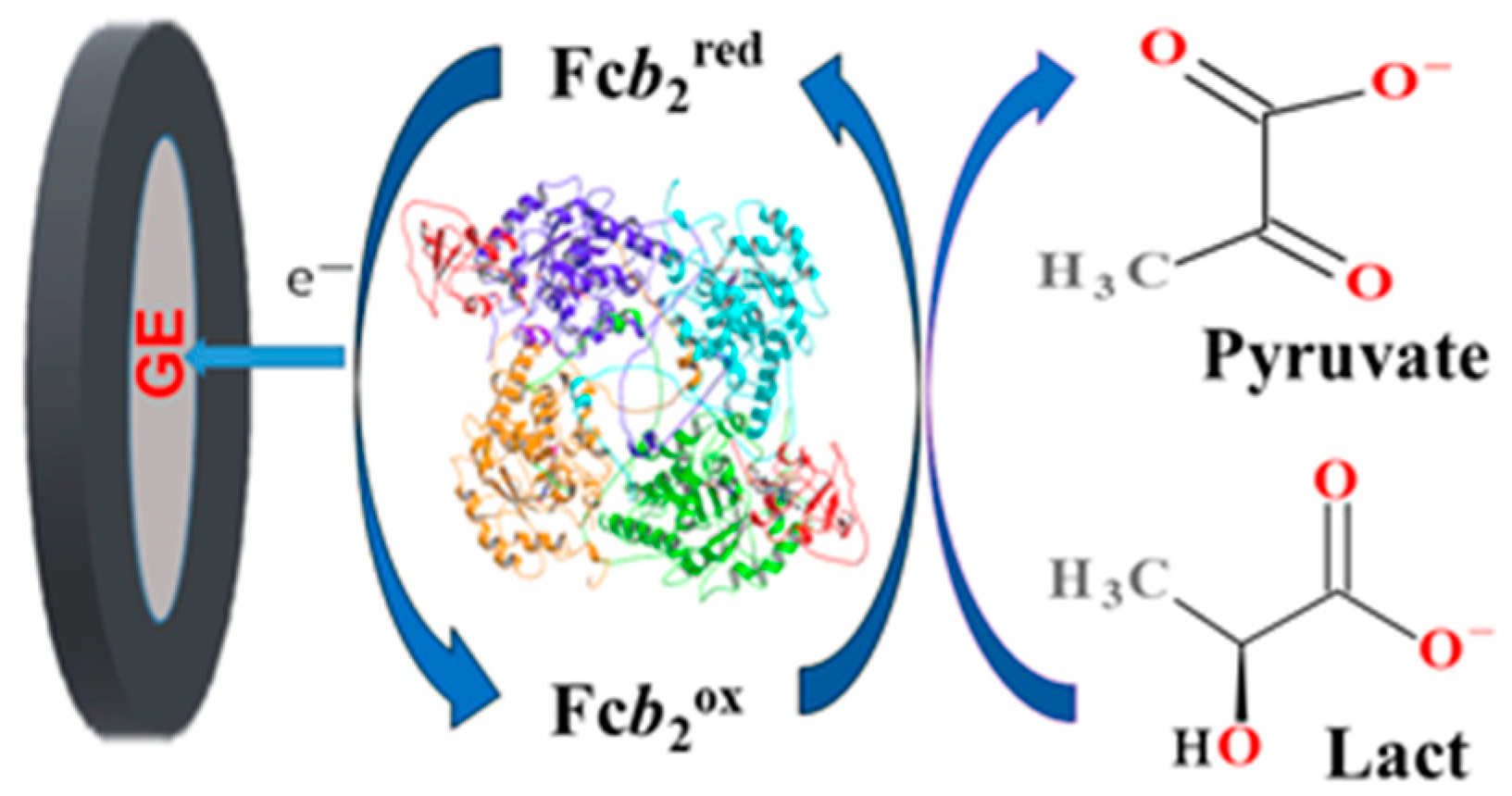
3.1. Development of Fcb2-Based Amperometric Biosensors
3.2. Selection of the Optimal Redox Nanomediators and Their Properties
3.3. Development of Fcb2/NPs-Based Amperometric Biosensors
3.4. Optimization of Lact Sensing for the Fcb2/AuHCF/GE
3.5. Application of the Fcb2/AuHCF/GE for Lact Determination in the Real Samples
3.6. Ways to Enhance the Sensitivity of the Fcb2-Based ABS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



| ABS | Sensitivity, A·M−1·m−2 | Linear Range, up to, mM | Imax, µA | KMapp, mM | Total Fcb2, m-Units |
|---|---|---|---|---|---|
| Fcb2/GE | 60 | 0.16 | 0.23 | 0.44 | 250 |
| Fcb2/GE + PMS | 565 | 0.08 | 1.70 | 0.30 | 250 |
| Fcb2/GE + PMS | 195 | 0.20 | 0.90 | 0.39 | 500 |
| Fcb2/AuHCF/GE | 124 | 0.35 | 0.48 | 0.10 | 250 |
| Fcb2/AuHCF/GE + PMS | 769 | 0.07 | 1.65 | 0.33 | 250 |
| Fcb2/PtZn/GE | 135 | 0.44 | 0.75 | 0.43 | 250 |
| Fcb2/PtZn/GE + PMS | 1436 | 0.12 | 2.74 | 0.18 | 250 |
| Enzyme | Working Potential, V | Sensitivity, A M−1m−2 | Linear Range, up to, mM | LOD, mM | Stability (days) | Reference |
|---|---|---|---|---|---|---|
| LOX | −0.1 | 5233 | 0.14 | 0.001 | 5 | [22] |
| 0.15 | 7974 | 0.1 | 0.003 | 4.5 | [21] | |
| 0.3 | 450 | 3 | 0.05 | * NR | [20] | |
| 0.52 | 280 | 0.6 | 0.001 | NR | [61] | |
| 0.8 | 156 | 1.2 | 0.012 | 14 | [62] | |
| 0.6 | 400 | 0.35 | 0.032 | NR | [63] | |
| 0.15 | NR | 1 | 0.75 × 10−3 | NR | [64] | |
| 0.4 | 4300 | 0.7 | 0.022 | 30 | [65] | |
| ** rFcb | 0.25 | 48.6 | 2.7 | 0.3 | NR | [28] |
| rFcb2 | 0.25 | 106 | 2 | 6 | NR | [28] |
| rLDH | 0 | 287.5 | 8 | 10 | 0.3 | [37] |
| 0.1 | 638.3 | 6 | 14 | 0.9 | ||
| LDH | 0.6 | 83 | 0.12 | NR | 7 | [66] |
| 0.1 | 34.5 | 55 | 0.001 | 210 | [67] | |
| 0 | 34.6 | 10 | 0.1 | NR | [68] | |
| Fcb2 | −0.75 | 565 | 0.08 | 0.015 | 5 | This paper |
| 769 | 0.07 | 0.010 | 7 | |||
| 1436 | 0.12 | 0.010 | 7 |
References
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M.Ł. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional foods and the immune system: A review. Eur. J. Clin. Nutr. 2002, 56, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Sushanto Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Albiach, R.; Pozuelo de Felipe, M.J.; Angulo, S.; Morosini, M.-I.; Bravo, D.; Baquero, F.; del Campo, R. Molecular analysis of yogurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. Am. J. Clin. Nutr. 2008, 87, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Natella, F.; Zinno, P.; Guantario, B.; Canali, R.; Schifano, E.; De Angelis, M.; Nikoloudaki, O.; Gobbetti, M.; Perozzi, G.; et al. Colonization Ability and Impact on Human Gut Microbiota of Foodborne Microbes From Traditional or Probiotic-Added Fermented Foods: A Systematic Review. Front. Nutr. 2021, 8, 689084. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Bakker, J.; Postelnicu, R.; Mukherjee, V. Lactate: Where Are We Now? Crit. Care. Clin. 2020, 36, 115–124. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; Sierra-Padilla, A.; Palacios-Santander, J.M.; Fernández-Alba, J.J.; Macías, C.G.; Cubillana-Aguilera, L. What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications. Biosensors 2022, 12, 919. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Rattu, G.; Murali Krishna, P. Enzyme-Free Colorimetric Nanosensor for the Rapid Detection of Lactic Acid in Food Quality Analysis. J. Agric. Food Res. 2022, 7, 100268. [Google Scholar] [CrossRef]
- Pranveer, S. Chapter 1—Electrochemical Biosensing: Progress and Perspectives. In Electrochemical Biosensors; Singh, P., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–31. ISBN 9780323906326. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Neethirajan, S. Chapter 7–Nanoparticles as Biosensors for Food Quality and Safety Assessment. In Nanomaterials for Food Applications; Rubio, A.L., Rovira, M.J.F., Sanz, M.M., Gomez-Mascaraque, L.G., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–202. ISBN 9780128141304. [Google Scholar] [CrossRef]
- Villalonga, A.; Sánchez, A.; Mayol, B.; Reviejo, J.; Villalonga, R. Electrochemical biosensors for food bioprocess monitoring. Curr. Opin. Food Sci. 2022, 43, 18–26. [Google Scholar] [CrossRef]
- Escalona-Villalpando, R.A.; Viveros-Palma, K.; Espinosa-Lagunes, F.I.; Rodríguez-Morales, J.A.; Arriaga, L.G.; Macazo, F.C.; Minteer, S.D.; Ledesma-García, J. Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection. Biosensors 2022, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, I.; Pack, S.P.; Lee, G.; Hong, Y. Colorimetric Sensing of Lactate in Human Sweat Using Polyaniline Nanoparticles-Based Sensor Platform and Colorimeter. Biosensors 2022, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors Based on Electrochemical Lactate Detection: A Comprehensive Review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Hiraka, K.; Kojima, K.; Tsugawa, W.; Asano, R.; Ikebukuro, K.; Sode, K. Rational engineering of Aerococcus viridans L-lactate oxidase for the mediator modification to achieve quasi-direct electron transfer type lactate sensor. Biosens. Bioelectron. 2020, 151, 111974. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Liu, Y.; Shi, L.; Yang, D. A cascade-triggered ratiometric fluorescent sensor based on nanocomposite for lactate determination. Sens. Actuators B Chem. 2022, 355, 131295. [Google Scholar] [CrossRef]
- Hickey, D.P.; Reid, R.C.; Milton, R.D.; Minteer, S.D. A self-powered amperometric lactate biosensor based on lactate oxidase immobilized in dimethylferrocene-modified LPEI. Biosens. Bioelectron. 2016, 77, 26–31. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive microneedle-based biosensor array for simultaneous lactate and glucose monitoring in artificial interstitial fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Smutok, O.; Kavetskyy, T.; Prokopiv, T.; Serkiz, R.; Šauša, O.; Novák, I.; Švajdlenková, H.; Maťko, I.; Gonchar, M.; Katz, E. Biosensor Based on Peroxidase-Mimetic Nanozyme and Lactate Oxidase for Accurate L-Lactate Analysis in Beverages. Biosensors 2022, 12, 1042. [Google Scholar] [CrossRef]
- Haas, E.; Horecker, B.L.; Hogness, T.R. Cytochrome b2. Science 1942, 95, 406. [Google Scholar] [CrossRef]
- Bach, S.J.; Dixon, M.; Zerfas, L.G. Yeast lactic dehydrogenase and cytochrome b2. Biochem J. 1946, 40, 229–239. [Google Scholar] [CrossRef]
- Lederer, F. Flavocytochrome b2. In Chemistry and Biochemistry of Flavoenzymes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; p. 90. ISBN 9781351070584. [Google Scholar] [CrossRef]
- Lederer, F. On the First Steps of Lactate Oxidation by Bakers’ Yeast L-(+)-Lactate Dehydrogenase (Cytochrome b2). Eur. J. Biochem. 1974, 46, 393. [Google Scholar] [CrossRef] [PubMed]
- Smutok, O.; Kavetskyy, T.; Gonchar, M.; Katz, E. Microbial L- and D-Lactate Selective Oxidoreductases as a Very Prospective but Still Uncommon Tool in Commercial Biosensors. ChemElectroChem 2021, 8, 4725–4731. [Google Scholar] [CrossRef]
- Smutok, O.; Dmytruk, K.; Kavetskyy, T.; Sibirny, A.; Gonchar, M. Flavocytochrome b2 of the Methylotrophic Yeast Ogataea polymorpha: Construction of Overproducers, Purification, and Bioanalytical Application. In Flavin and Flavoproteins; Methods Molecular Biology; Springer: New York, NY, USA, 2021; pp. 249–260. ISBN 978-1-0716-1285-9. [Google Scholar]
- Schachinger, F.; Chang, H.; Scheiblbrandner, S.; Ludwig, R. Amperometric Biosensors Based on Direct Electron Transfer Enzymes. Molecules 2021, 26, 4525. [Google Scholar] [CrossRef]
- Garjonyte, R.; Malinauskas, A. Investigation of Saccharomyces cerevisiae- and mediator-based carbon paste electrodes as amperometric biosensors for lactic acid. Sens. Actuat. B Chem. 2003, 96, 509–515. [Google Scholar] [CrossRef]
- Staškevičiene, S.L.; Čenas, N.K.; Kulys, J.J. Reagentless lactate electrodes based on electrocatalytic oxidation of flavocytochrome b2. Anal. Chim. Acta 1991, 243, 167–171. [Google Scholar] [CrossRef]
- Brunt, C.E.; Cox, M.C.; Thurgood, A.G.P.; Moore, G.R.; Reid, G.A.; Chapman, S.K. Isolation and characterization of the cytochrome domain of flavocytochrome b2 expressed independently in Escherichia coli. Biochem. J. 1992, 283, 87–90. [Google Scholar] [CrossRef]
- Silvestrini, M.C.; Tegoni, M.; Célerier, J.; Desbois, A.; Gervais, M. Expression in Escherichia coli of the flavin and the haem domains of Hansenula anomala flavocytochrome b2 (flavodehydrogenase and b2 core) and characterization of the recombinant proteins. Biochem. J. 1993, 295 Pt 2, 501–508. [Google Scholar] [CrossRef]
- Hiraka, K.; Tsugawa, W.; Asano, R.; Yokus, M.A.; Ikebukuro, K.; Daniele, M.A.; Sode, K. Rational design of direct electron transfer type l-lactate dehydrogenase for the development of multiplexed biosensor. Biosens. Bioelectron. 2021, 176, 112933. [Google Scholar] [CrossRef]
- Tsvik, L.; Steiner, B.; Herzog, P.; Haltrich, D.; Sützl, L. Flavin Mononucleotide-Dependent L-Lactate Dehydrogenases: Expanding the Toolbox of Enzymes for L-Lactate Biosensors. ACS Omega 2022, 7, 41480–41492. [Google Scholar] [CrossRef] [PubMed]
- Boyarski, A.; Shlush, N.; Paz, S.; Eichler, J.; Alfonta, L. Electrochemical characterization of a dual cytochrome-containing lactate dehydrogenase. Bioelectrochemistry 2023, 152, 108406. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Herzallh, N.S.; Meirovich, M.M.; Bachar, O.; Frech, L.; Cohen, Y.; Yehezkeli, O. An Oxygen-Insensitive Biosensor and a Biofuel Cell Device Based on FMN L-Lactate Dehydrogenase. Bioelectrochemistry 2023, 149, 108316. [Google Scholar] [CrossRef]
- Gaida, G.; Stel’mashchuk, S.; Smutok, O.; Gonchar, M. A new method of visualization of the enzymatic activity of flavocytochrome b2 in electrophoretograms. Appl. Biochem. Microbiol. 2003, 39, 221–223. [Google Scholar] [CrossRef]
- Gayda, G.; Demkiv, O.; Klepach, H.; Gonchar, M.; Nisnevitch, M. Effective Technologies for Isolating Yeast Oxido-Reductases of Analytical Importance. In Non-Conventional Yeasts: From Basic Research to Application; Andriy, S., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 5, pp. 119–151. ISBN 978-3-030-21110-3. [Google Scholar] [CrossRef]
- Smutok, O.; Gayda, G.; Gonchar, M.; Schuhmann, W. A novel L-lactate-selective biosensor based on the use of flavocytochrome b2 from methylotrophic yeast Hansenula polymorpha. Biosens. Bioelectron. 2005, 20, 1285–1290. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Demkiv, O.; Darmohray, L.; Gonchar, M.; Nisnevitch, M. Amperometric Biosensors for L-Arginine Determination Based on L-Arginine Oxidase and Peroxidase-Like Nanozymes. Appl. Sci. 2021, 11, 7024. [Google Scholar] [CrossRef]
- Mugle, D.; Jadhav, G. Short review on chemical bath deposition of thin film and characterization. AIP Conf. Proc. 2016, 1728, 020597. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Kavetskyy, T.; Gonchar, M. Nanozymes with reductase-like activities: Electrochemical and antioxidant properties. RSC Adv. 2022, 12, 2026–2035. [Google Scholar] [CrossRef]
- Demkiv, O.; Gayda, G.; Stasyuk, N.; Brahinetz, O.; Gonchar, M.; Nisnevitch, M. Nanomaterials as Redox Mediators in Laccase-Based Amperometric Biosensors for Catechol Assay. Biosensors 2022, 12, 741. [Google Scholar] [CrossRef]
- Demkiv, O.; Stasyuk, N.; Gayda, G.; Gonchar, M. Highly sensitive amperometric sensor based on laccase-mimicking metal-based hybrid nanozymes for adrenaline analysis in pharmaceuticals. Catalysts 2021, 11, 1510. [Google Scholar] [CrossRef]
- European Union. Commission Recommendation of 10 June 2022 on the definition of nanomaterial (Text with EEA relevance). Off. J. 2022, 229, 1–5. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022H0614(01) (accessed on 16 May 2023).
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Londero, A.; Hamet, M.F.; De Antoni, G.L.; Garrote, G.L.; Abraham, A.G. Kefir grains as a starter for whey fermentation at different temperatures: Chemical and microbiological characterisation. J. Dairy Res. 2012, 79, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Deeth, H.C.; Tamime, A.Y. Yogurt: Nutritive and Therapeutic Aspects. J. Food Prot. 1981, 44, 78–86. [Google Scholar] [CrossRef]
- Warren, J.J.; Gray, H.B. 8.02—Electron Transfer Proteins. In Comprehensive Coordination Chemistry III; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–18. ISBN 9780081026892. [Google Scholar] [CrossRef]
- Stasyuk, N.; Demkiv, O.; Gayda, G.; Zakalskiy, A.; Klepach, H.; Bisko, N.; Gonchar, M.; Nisnevitch, M. Highly Porous 3D Gold Enhances Sensitivity of Amperometric Biosensors Based on Oxidases and CuCe Nanoparticles. Biosensors 2022, 12, 472. [Google Scholar] [CrossRef]
- Tegoni, M.; Janot, J.M.; Labeyrie, F. Inhibition of L-lactate: Cytochrome-c reductase (flavocytochrome b2) by product binding to the semiquinone transient. Loss of reactivity towards monoelectronic acceptors. Eur. J. Biochem. 1990, 190, 329–342. [Google Scholar] [CrossRef]
- Blumberger, J. Electron transfer and transport through multi-heme proteins: Recent progress and future directions. Curr. Opin. Chem. Biol. 2018, 47, 24–31. [Google Scholar] [CrossRef]
- Bertrand, P.; Janot, J.M.; Benosman, H.; Gayda, J.P.; Labeyrie, F. An EPR study of the interactions between heme and flavin in yeast flavocytochrome b2. Eur. Biophys. J. 1987, 14, 273–278. [Google Scholar] [CrossRef]
- White, P.; Manson, F.D.; Brunt, C.E.; Chapman, S.K.; Reid, G.A. The importance of the interdomain hinge in intramolecular electron transfer in flavocytochrome b2. Biochem. J. 1993, 291 Pt 1, 89–94. [Google Scholar] [CrossRef]
- Black, M.T.; Gunn, F.J.; Chapman, S.K.; Reid, G.A. Structural basis for the kinetic differences between flavocytochromes b2 from the yeasts Hansenula anomala and Saccharomyces cerevisiae. Biochem. J. 1989, 263, 973–976. [Google Scholar] [CrossRef]
- Capeillère-Blandin, C. Electron-transfer steps involved in the reactivity of Hansenula anomala flavocytochrome b2 as deduced from deuterium isotope effects and simulation studies. Biochem. J. 1991, 274 Pt 1, 207–217. [Google Scholar] [CrossRef]
- Lederer, F. Another look at the interaction between mitochondrial cytochrome c and flavocytochrome b2. Eur. Biophys. J. 2011, 40, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Boubacar, O.A.K.; Pethe, S.; Mahy, J.-P.; Lederer, F. Flavocytochrome b2: Reactivity of Its Flavin with Molecular Oxygen. Biochemistry 2007, 46, 13080–13088. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Lederer, F.; Alliel, P.M. On the Transhydrogenase Activity of Baker’s Yeast Flavocytochrome b2. Eur. J. Biochem. 1983, 134, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Luo, N.; Yan, X.; Zhao, Y.; Zhang, G.; Zhang, Y. A highly sensitive electrochemical biosensor based on zinc oxide nanotetrapods for L-lactic acid detection. Nanoscale 2012, 4, 3438–3443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, X.; Fang Kang, Z.; Fang, X.; Zheng, X.; Zhao, L.; Du, H.; Zhang, Y. Zinc oxide nanowires-based electrochemical biosensor for L-lactic acid amperometric detection. J. Nanoparticle Res. 2014, 16, 2398. [Google Scholar] [CrossRef]
- Ozoglu, O.; Uzunoglu, A.; Unal, M.A.; Gumustas, M.; Ozkan, S.A.; l Korukluoglu, M.; Altuntas, E.G. Electrochemical detection of lactate produced by foodborne presumptive lactic acid bacteria. JBB 2023, 135, 313–320. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual range lactate oxidase-based screen printed amperometric biosensor for analysis of lactate in diversified samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef]
- Zanini, V.P.; de Mishima, B.L.; Solís, V. An amperometric biosensor based on lactate oxidase immobilized in laponite–chitosan hydrogel on a glassy carbon electrode. Application to the analysis of L-lactate in food samples. Sens. Actuat. 2011, 155, 75–80. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Chen, S.-Y.; Liaw, H.-W. Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitosan nanocomposite for application to lactate biosensors. Sens. Actuat. B Chem. 2007, 125, 474–481. [Google Scholar] [CrossRef]
- Narwal, V.; Sharma, M.; Rani, S.; Pundir, C.S. An ultrasensitive amperometric determination of lactate by lactate dehydrogenase nanoparticles immobilized onto Au electrode. Int. J. Biol. Macromol. 2018, 115, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Aguiar, M.R.; Kisner, A.; Macedo, D.V.; Kubota, L.T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue co-immobilized on multi-wall carbon-nanotube, Sens. Actuat. B Chem. 2007, 124, 269–276. [Google Scholar] [CrossRef]


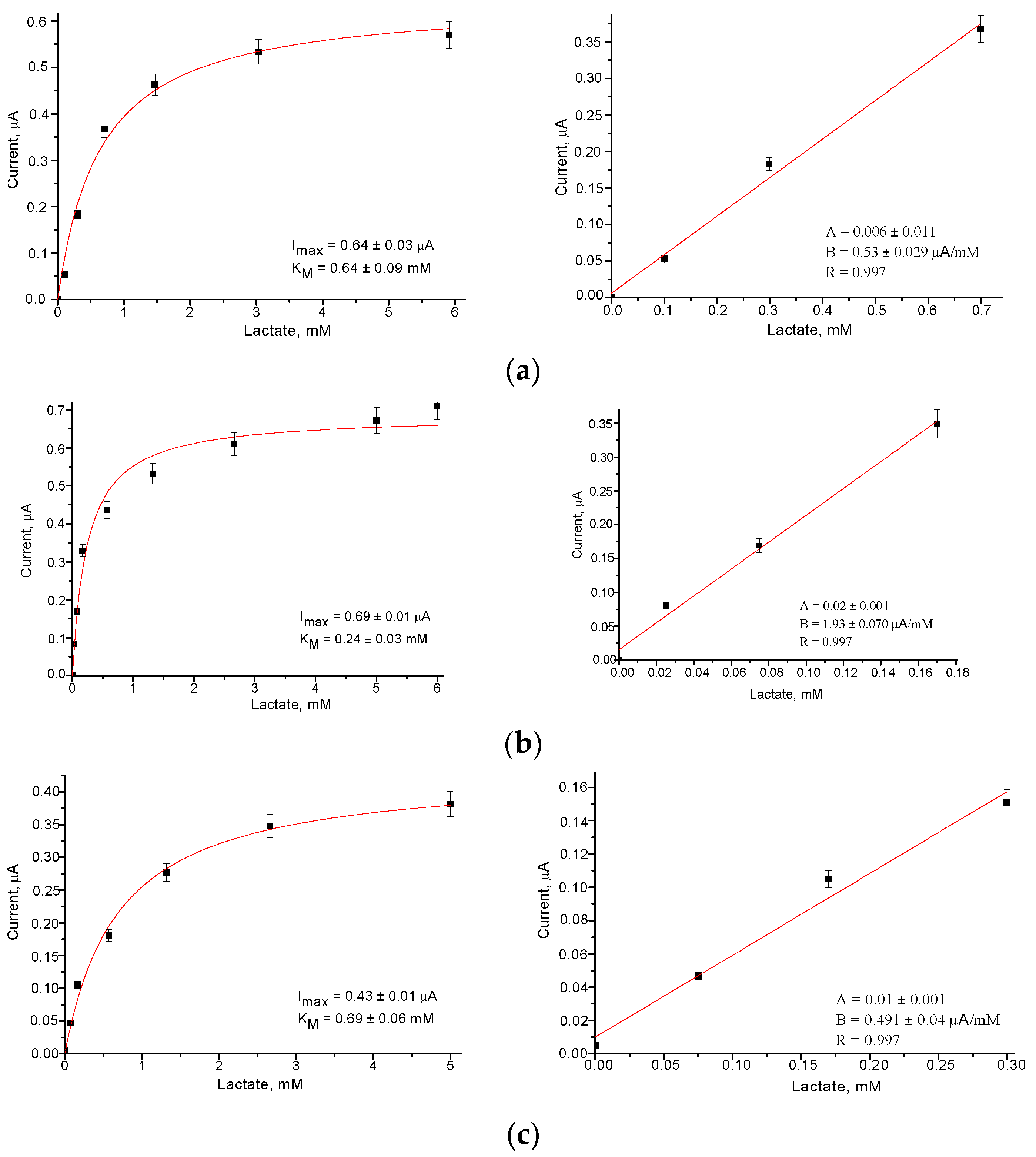
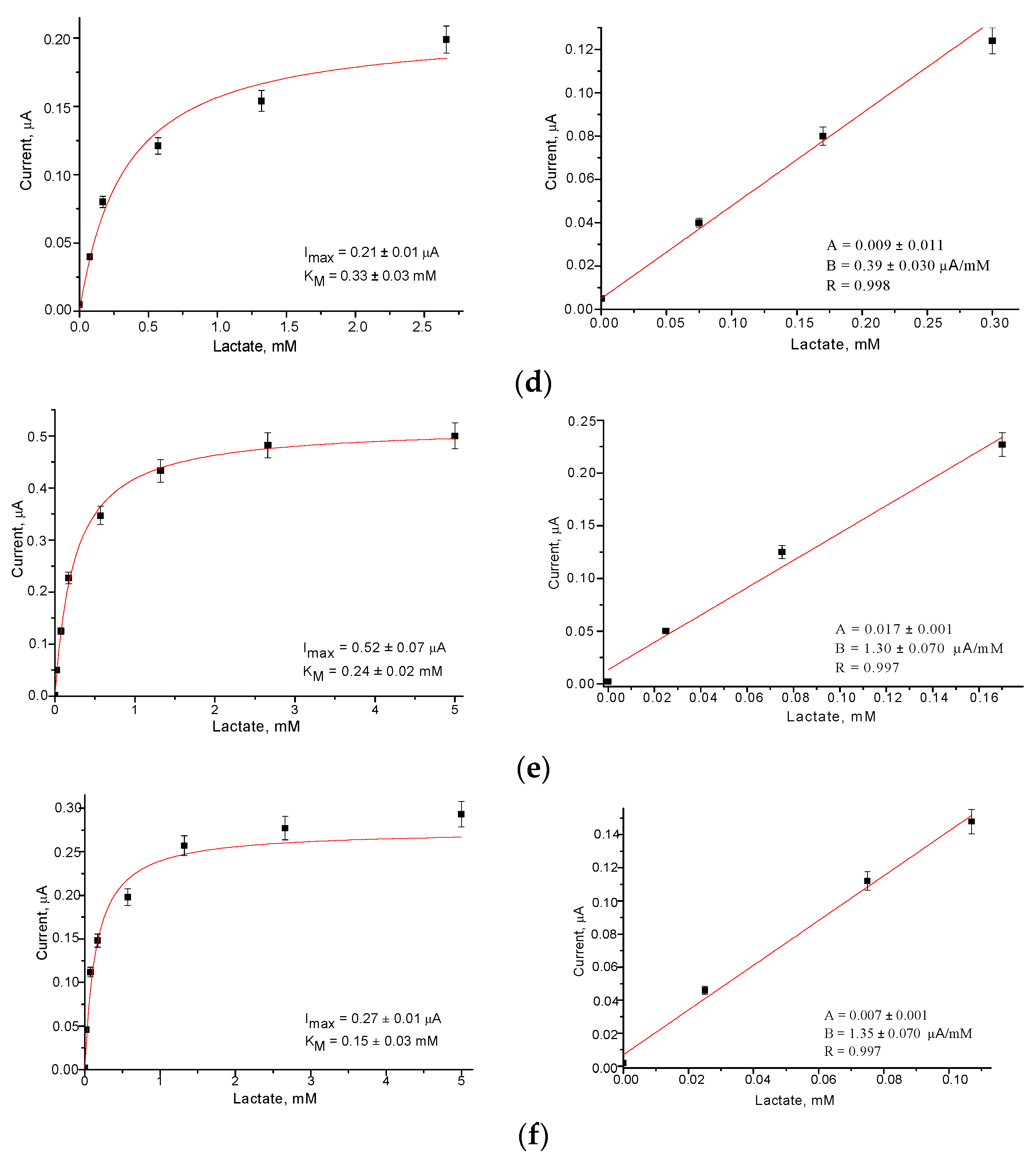

| No | Nanomediator | Sensitivity, A·M−1·m−2 | Linear Range, up to mM | LOD, mM | Imax, µA | KMapp, mM |
|---|---|---|---|---|---|---|
| 1 | – | 72 | 0.70 | 0.05 | 0.64 | 0.64 |
| 2 | PtHCF | 53 | 0.30 | 0.05 | 0.21 | 0.33 |
| 3 | PdHCF | 70 | 0.30 | 0.02 | 0.43 | 0.70 |
| 4 | gCuHCF | 80 | 0.30 | 0.01 | 0.84 | 1.90 |
| 5 | PtZn | 178 | 0.18 | 0.02 | 0.52 | 0.24 |
| 6 | NiPtPd | 185 | 0.11 | 0.01 | 0.27 | 0.15 |
| 7 | AuHCF | 253 | 0.18 | 0.01 | 0.69 | 0.24 |
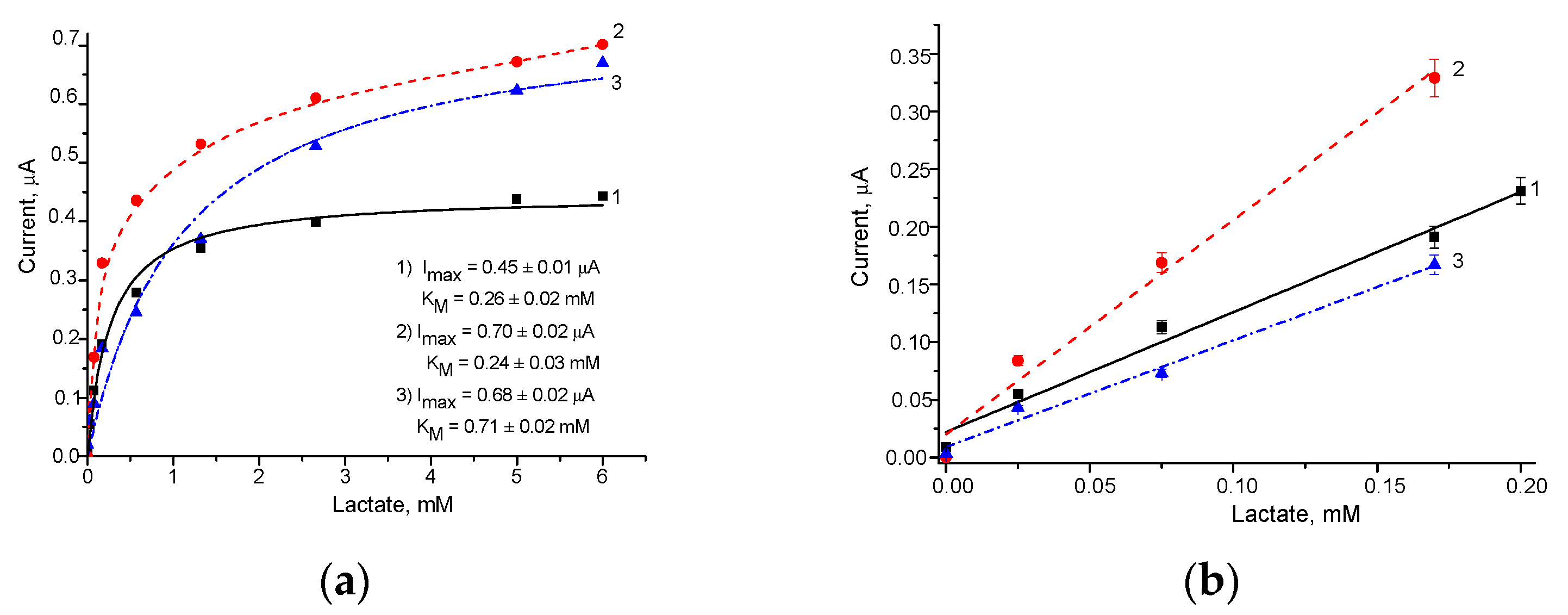
| No | ABS | Total Fcb2, mU | Sensitivity, A·M−1·m−2 | Linear Range, up to mM | Imax, µA | KMapp, mM |
|---|---|---|---|---|---|---|
| 1 | Fcb2/AuHCF/GE | 13 | 142 | 0.20 | 0.45 | 0.26 |
| 2 | Fcb2/AuHCF/GE | 25 | 253 | 0.17 | 0.70 | 0.24 |
| 3 | Fcb2/AuHCF/GE | 50 | 126 | 0.17 | 0.68 | 0.71 |
| 4 | Fcb2/AuHCF/GE | 250 | 124 | 0.35 | 0.48 | 0.10 |
| 5 | Fcb2/GE | 25 | 72 | 0.70 | 0.64 | 0.64 |
| 6 | Fcb2/GE | 250 | 60 | 0.16 | 0.23 | 0.44 |
| No | Yogurt | ABS Method | CV, % | Reference Method | CV, % |
|---|---|---|---|---|---|
| 1 | Lactel | 61 ± 4.5 | 7.4 | 63 ± 4.0 | 6.3 |
| 2 | Activia | 72 ± 1.0 | 1.4 | 71 ± 5.4 | 7.6 |
| 3 | Choodo | 94.5 ± 2.5 | 2.6 | 95 ± 7.0 | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demkiv, O.; Gayda, G.; Stasyuk, N.; Moroz, A.; Serkiz, R.; Kausaite-Minkstimiene, A.; Gonchar, M.; Nisnevitch, M. Flavocytochrome b2-Mediated Electroactive Nanoparticles for Developing Amperometric L-Lactate Biosensors. Biosensors 2023, 13, 587. https://doi.org/10.3390/bios13060587
Demkiv O, Gayda G, Stasyuk N, Moroz A, Serkiz R, Kausaite-Minkstimiene A, Gonchar M, Nisnevitch M. Flavocytochrome b2-Mediated Electroactive Nanoparticles for Developing Amperometric L-Lactate Biosensors. Biosensors. 2023; 13(6):587. https://doi.org/10.3390/bios13060587
Chicago/Turabian StyleDemkiv, Olha, Galina Gayda, Nataliya Stasyuk, Anna Moroz, Roman Serkiz, Asta Kausaite-Minkstimiene, Mykhailo Gonchar, and Marina Nisnevitch. 2023. "Flavocytochrome b2-Mediated Electroactive Nanoparticles for Developing Amperometric L-Lactate Biosensors" Biosensors 13, no. 6: 587. https://doi.org/10.3390/bios13060587
APA StyleDemkiv, O., Gayda, G., Stasyuk, N., Moroz, A., Serkiz, R., Kausaite-Minkstimiene, A., Gonchar, M., & Nisnevitch, M. (2023). Flavocytochrome b2-Mediated Electroactive Nanoparticles for Developing Amperometric L-Lactate Biosensors. Biosensors, 13(6), 587. https://doi.org/10.3390/bios13060587






