Recent Aptamer-Based Biosensors for Cd2+ Detection
Abstract
:1. Introduction
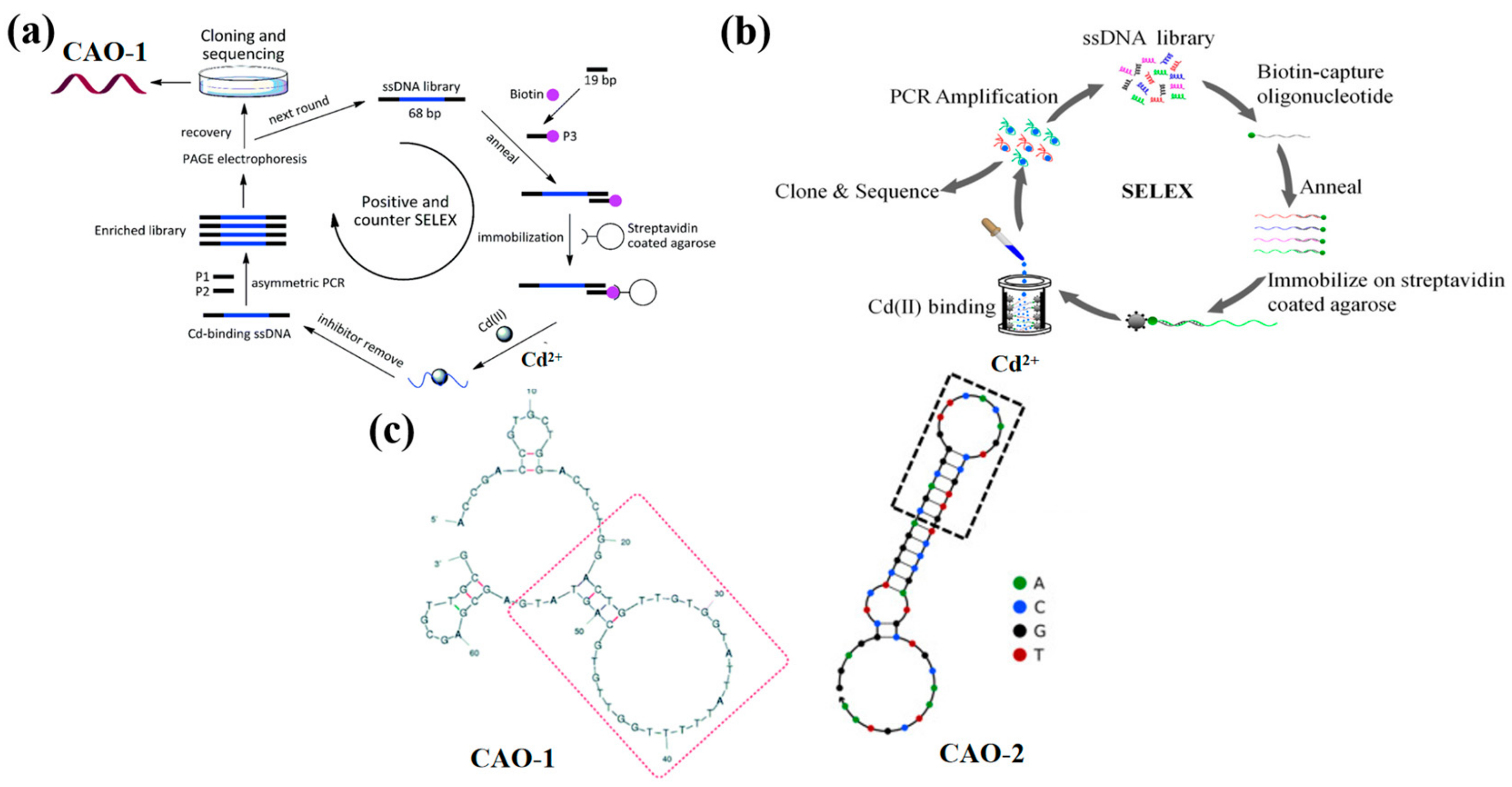
2. DNA Aptamers for Cd2+
3. Electrochemical Biosensors
3.1. Gold Nanoparticles (AuNPs)
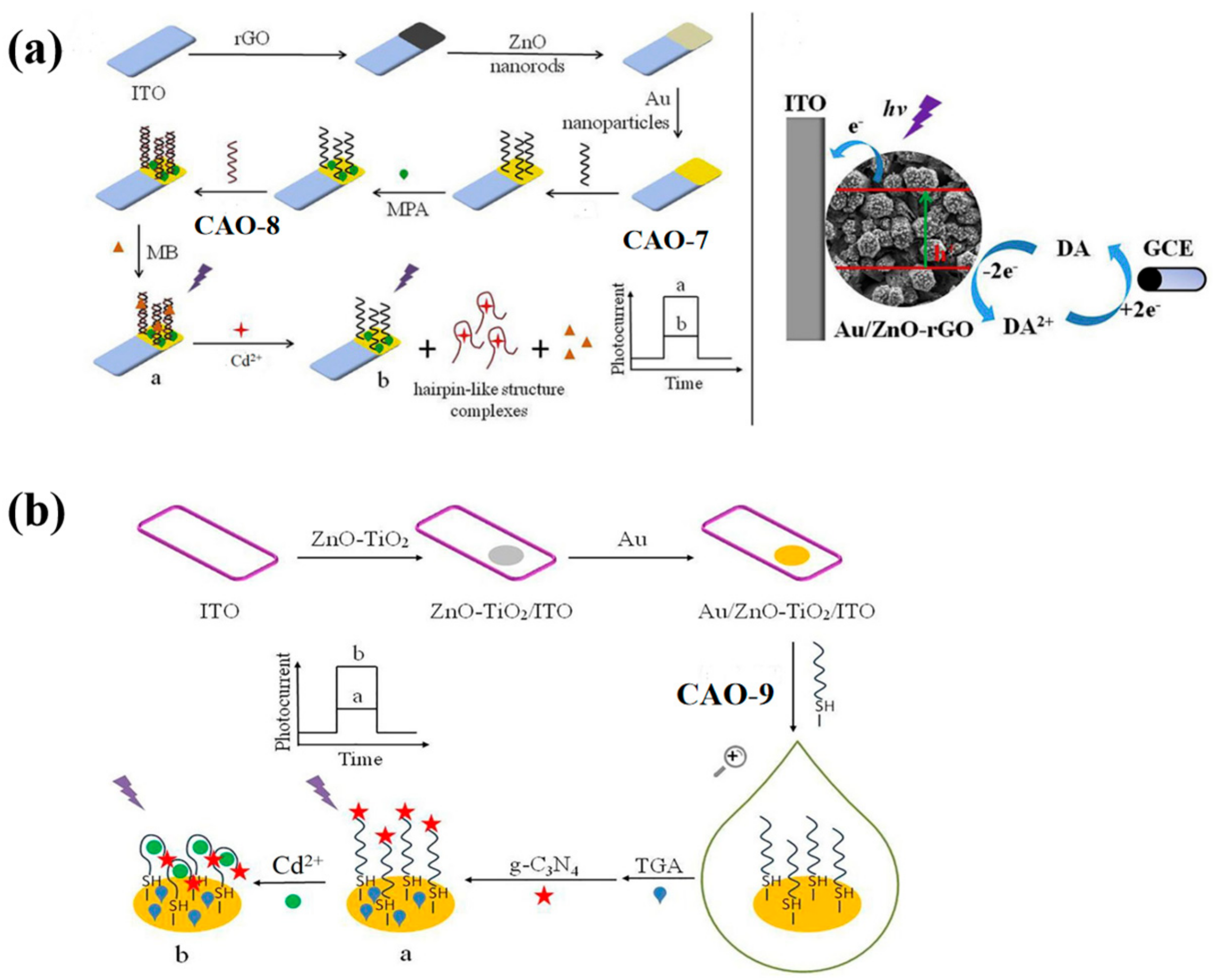
3.2. ZnO Nanocomposites
3.3. Other Materials
4. Fluorescent Biosensors
4.1. Label-Free
4.2. Labeled Method
4.3. Signal Amplification
5. Colorimetric Biosensors
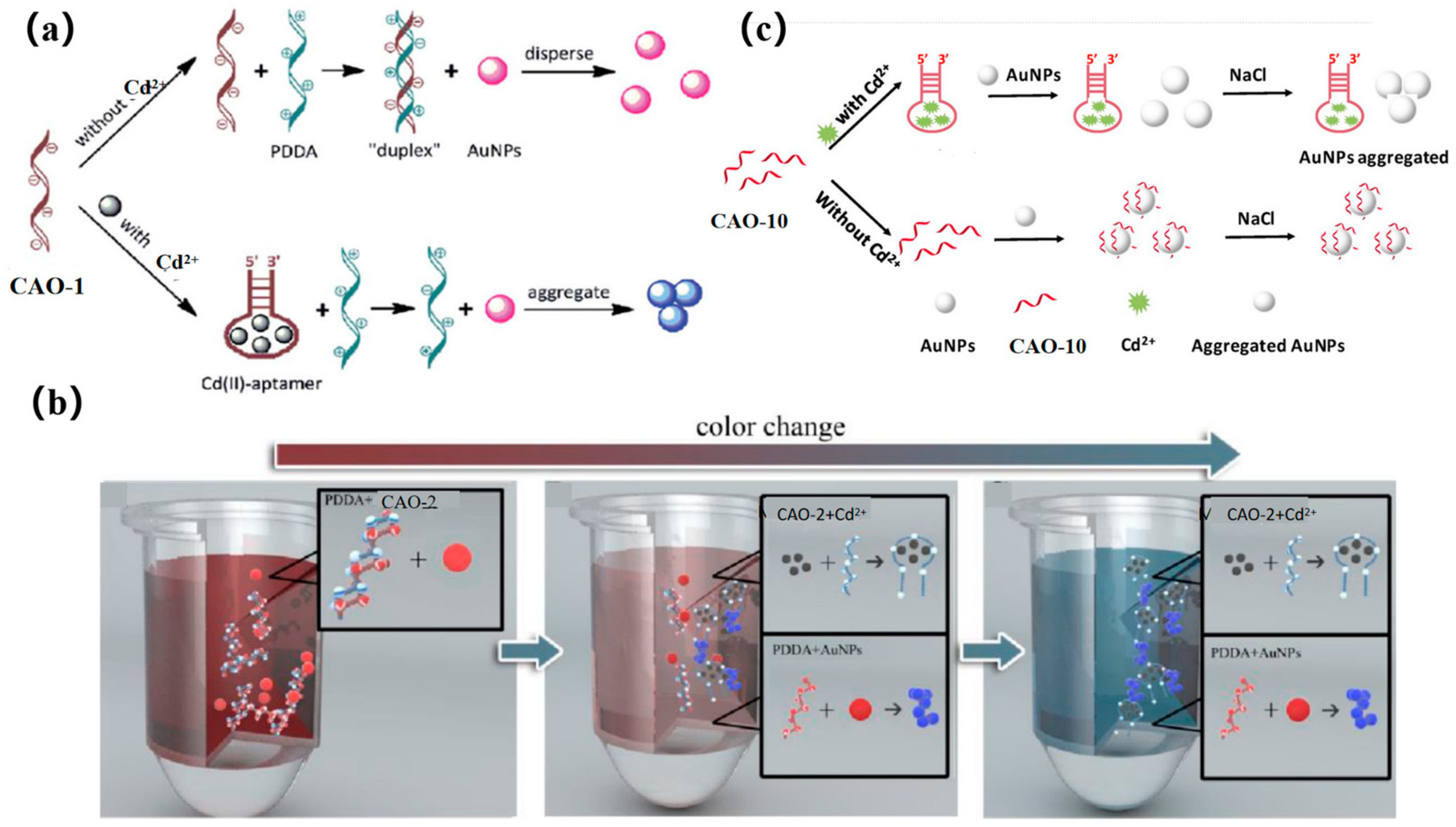
5.1. Gold Nanoparticles
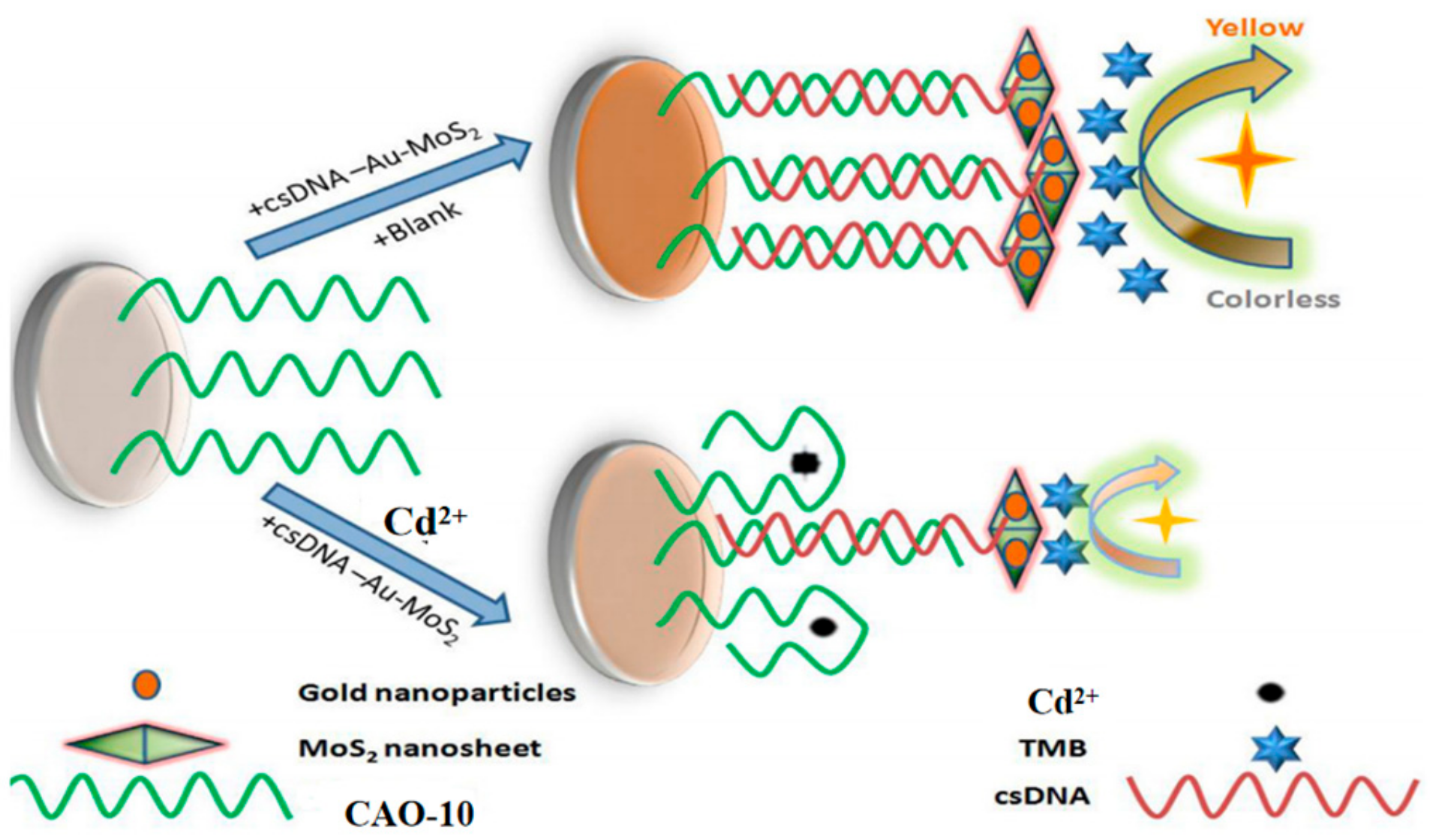
5.2. Other Nanoparticles
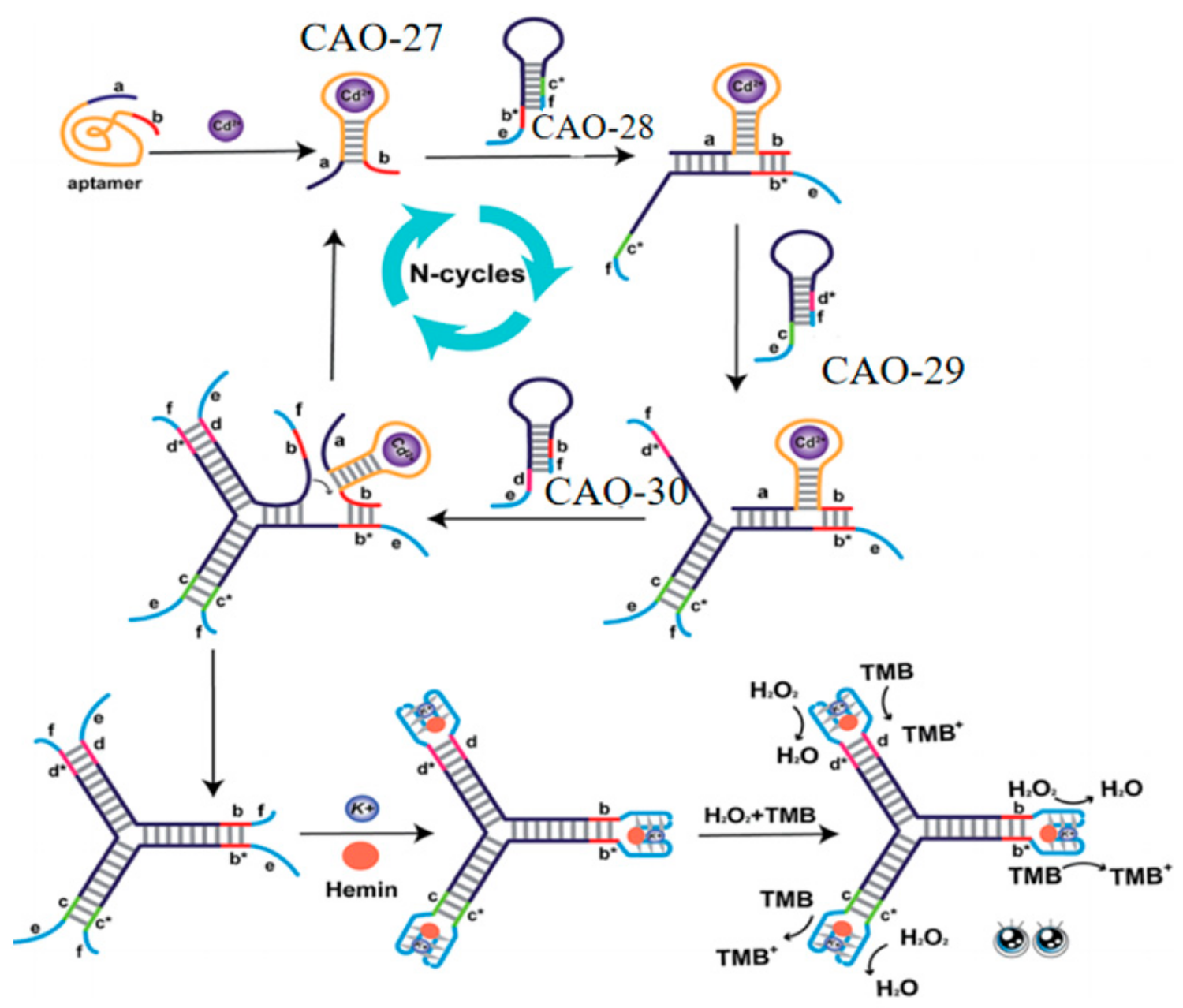
5.3. G-4/Hemin-Assisted Method
| Method | No. | LOD | Detection Range | Application | Ref. |
|---|---|---|---|---|---|
| Electrochemical detection | CAO-4 | 0.04995 pM | 0.001~100 nM | Tap water | [37] |
| CAO-5 | 0.14 ppb | 1~50 ppb | Drinking water | [38] | |
| CAO-6 | 7 × 10−4 mg/L | 2 × 10−3~8 × 10−1 mg/L | Genuine mussels | [40] | |
| CAO-7 | 1.8 × 10−12 mol/L | 5.0 × 10−12~2.0 × 10−8 mol/L | Lake water | [43] | |
| CAO-9 | 1.1 × 10−11 mol/L | 3.0 × 10−11~4.0 × 10−8 mol/L | Lake water | [44] | |
| CAO-5 | 92 nM | 250~1 μM | Tap water | [25] | |
| CAO-10 | 1.1 × 10−4 ppb | 3.0 × 10−2~4.0 × 105 ppb | Traditional medicine | [45] | |
| Fluorescence detection | CAO-4 | 0.038 ng/mL | 0.10~100 µg/mL | Drinking water | [53] |
| CAO-11 | 0.34 μg/L | 1.12~224.82 μg/L | Drinking water | [55] | |
| CAO-12 | 2.15 nM | 7.19 nM~5.0 μM | Drinking water | [27] | |
| CAO-13 | 1.92 ng/mL | 5~140 ng/mL | Drinking water | [57] | |
| CAO-14 | 0.076 pM | 0.1~120 pM | Milk, coffee, and human blood serum | [6] | |
| CAO-1 | 0.36 nM | 0~10 nM | Pond water | [58] | |
| CAO-17 | 2.5 pM | 0~10 μM | Rice | [59] | |
| CAO-22 | 5 pM | 10 pM~100 μM | Human urine | [60] | |
| Naked eye observation | CAO-1 | 4.6 nM | 1~50 nM | Drinking water | [29] |
| CAO-2 | 1 ng/mL | 1~400 ng/mL | Rice and drinking water | [67] | |
| CAO-10 | 1.12 μg/L | 2~20 μg/L | Tap and drinking water | [10] | |
| CAO-10 | 0.7 ng/mL | 1~500 ng/mL | White wine | [69] | |
| CAO-25 | 2.4 μg/L | 5~100 μg/mL | Tap, river, lake, wastewater | [70] | |
| CAO-26 | 0.15 nM | 33.72~112.41 μg/L | Tap and pond water | [71] | |
| CAO-27 | 10 pM | 10 pM~1 μM | Tap, river, lake, wastewater | [72] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Liu, X.H.; Hussain, K.; Mushtaq, S.; Cabrera, J.; Zhang, P.P. The global research trend on cadmium in freshwater: A bibliometric review. Environ. Sci. Pollut. Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Makino, T.; Nakamura, K.; Katou, H.; Ishikawa, S.; Ito, M.; Honma, T.; Miyazaki, N.; Takehisa, K.; Sano, S.; Matsumoto, S.; et al. Simultaneous decrease of arsenic and cadmium in rice (Oryza sativa L.) plants cultivated under submerged field conditions by the application of iron-bearing materials. Soil Sci. Plant Nutr. 2016, 62, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Khoshbin, Z.; Moeenfard, M.; Zahraee, H.; Davoodian, N. A fluorescence imaging-supported aptasensor for sensitive monitoring of cadmium pollutant in diverse samples: A critical role of metal organic frameworks. Talanta 2022, 246, 123514. [Google Scholar] [CrossRef]
- Titov, V.I.; Goundobin, N.V.; Kotikov, V.N. Determination of Ruthenium in Heat-Resistant Nickel Alloys by Atomic Emission Spectrometry with Inductively Coupled Plasma. J. Appl. Spectrosc. 2013, 80, 477–481. [Google Scholar] [CrossRef]
- Ali, M.M.; Rizwani, H.M.; Yousef, A.F.; Zhi, C.; Chen, F.X. Analysis of toxic elements in leaves and fruits of loquat by inductively coupled plasma-mass spectrometry (ICP-MS). Acta Sci. Pol. Hortorum Cultus 2021, 20, 33–42. [Google Scholar] [CrossRef]
- Li, Y.; Ran, G.; Lu, G.; Ni, X.; Liu, D.; Sun, J.; Xie, C.; Yao, D.; Bai, W. Highly Sensitive Label-Free Electrochemical Aptasensor Based on Screen-Printed Electrode for Detection of Cadmium (II) Ions. J. Electrochem. Soc. 2019, 166, B449–B455. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, T.; Hu, Q.; Zhong, L.; Wang, X.; Wan, H.; Wang, P. In-situ detection of cadmium with aptamer functionalized gold nanoparticles based on smartphone-based colorimetric system. Talanta 2020, 208, 120231. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Niu, L.Y.; Yang, Q.Z.; Guan, Y.F.; Feng, L. An SPE-assisted BODIPY fluorometric paper sensor for the highly selective and sensitive determination of Cd2+ in complex sample: Rice. Analyst 2014, 139, 3146–3153. [Google Scholar] [CrossRef]
- Yuan, H.E.; Wu, X.X.; Ren, X.F.; Xue, B.; Qiu, W.J.; Nong, D.H.; Yang, T.; Xu, F. Mechanism of pH influence on aptamer binding with Cd2+ revealed by molecular dynamics simulation. New J. Chem. 2023, 47, 9239–9249. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Wang, S.; Yan, M.X.; Huang, J.S.; Yang, X.R. Label-Free and Regenerable Aptasensor for Real-Time Detection of Cadmium(II) by Dual Polarization Interferometry. Anal. Chem. 2020, 92, 10007–10015. [Google Scholar] [CrossRef] [PubMed]
- Park, C.R.; Park, S.J.; Lee, W.G.; Hwang, B.H. Biosensors Using Hybridization Chain Reaction—Design and Signal Amplification Strategies of Hybridization Chain Reaction. Biotechnol. Bioprocess Eng. 2018, 23, 355–370. [Google Scholar] [CrossRef]
- Dragan, A.I.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. Characterization of PicoGreen interaction with dsDNA and the origin of its fluorescence enhancement upon binding. Biophys. J. 2010, 99, 3010–3019. [Google Scholar] [CrossRef] [Green Version]
- Xiong, E.; Zhen, D.; Jiang, L. Homogeneous enzyme-free and entropy-driven isothermal fluorescent assay for nucleic acids based on a dual-signal output amplification strategy. Chem. Commun. 2018, 54, 12594–12597. [Google Scholar] [CrossRef]
- López Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoçi, A. All-integrated and highly sensitive paper based device with sample treatment platform for Cd2+ immunodetection in drinking/tap waters. Anal. Chem. 2013, 85, 3532–3538. [Google Scholar] [CrossRef] [PubMed]
- López_Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoçi, A. High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2+ detection in drinking waters. Biosens. Bioelectron. 2013, 47, 190–198. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Shao, H.; Wang, Z.; Wang, X.; Jiang, X. Label-Free Colorimetric Detection of Cadmium Ions in Rice Samples Using Gold Nanoparticles. Anal. Chem. 2014, 86, 8530–8534. [Google Scholar] [CrossRef]
- Xue, H.; Chen, Q.; Jiang, F.; Yuan, D.; Lv, G.; Liang, L.; Liu, L.; Hong, M. A regenerative metal–organic framework for reversible uptake of Cd(ii): From effective adsorption to in situ detection. Chem. Sci. 2016, 7, 5983–5988. [Google Scholar] [CrossRef] [Green Version]
- Knight, A.S.; Zhou, E.Y.; Francis, M.B. Development of peptoid-based ligands for the removal of cadmium from biological media. Chem. Sci. 2015, 6, 4042–4048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, Z.; Liu, H.; Mao, X.; Liu, W.; Zhang, S.; Nie, Z.; Lu, X. Ultratrace and robust visual sensor of Cd2+ ions based on the size-dependent optical properties of Au@g-CNQDs nanoparticles in mice models. Biosens. Bioelectron. 2018, 103, 87–93. [Google Scholar] [CrossRef]
- Kasprowicz, A.; Stokowa-Sołtys, K.; Wrzesiński, J.; Jeżowska-Bojczuk, M.; Ciesiołka, J. In vitro selection of deoxyribozymes active with Cd(2+) ions resulting in variants of DNAzyme 8-17. Dalton Trans. 2015, 44, 8138–8149. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.; Liu, J. Rational evolution of Cd2+−specific DNAzymes with phosphorothioate modified cleavage junction and Cd2+ sensing. Nucleic Acids Res. 2015, 43, 6125–6133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhad, H.; Torres, Y.M.R.; Lai, R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of Cd(II). J. Electroanal. Chem. 2017, 803, 89–94. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cheng, H.; Wang, J.; Xu, L.J.; Chen, H.X.; Pei, R.J. Selection and characterization of DNA aptamers for the development of light-up biosensor to detect Cd(II). Talanta 2016, 154, 498–503. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wang, Y.S.; Zhou, B.; Yu, J.H.; Peng, L.L.; Huang, Y.Q.; Li, X.J.; Chen, S.H.; Tang, X.; Wang, X.F. A multifunctional fluorescent aptamer probe for highly sensitive and selective detection of cadmium(II). Anal. Bioanal. Chem. 2017, 409, 4951–4958. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.G.; Zhan, S.S.; Wang, L.M.; Zhou, P. Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 2014, 139, 1550–1561. [Google Scholar] [CrossRef]
- Tang, W.; Wang, Z.; Yu, J.; Zhang, F.; He, P. Internal Calibration Potentiometric Aptasensors for Simultaneous Detection of Hg2+, Cd2+, and As3+ Based on a Screen-Printed Carbon Electrodes Array. Anal. Chem. 2018, 90, 8337–8344. [Google Scholar] [CrossRef]
- Nutiu, R.; Li, Y. In vitro selection of structure-switching signaling aptamers. Angew. Chem. Int. Ed. Engl. 2005, 44, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Liu, Y.; Deng, Y.; Chen, Z.; Chen, H.; Li, S.; He, N.Y. Selection of a High-Affinity DNA Aptamer for the Recognition of Cadmium Ions. J. Biomed. Nanotechnol. 2021, 17, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, B.; Yan, L.; Huang, H. An environmentally-friendly, highly efficient, gas pressure-assisted sample introduction system for ICP-MS and its application to detection of cadmium and lead in human plasma. Talanta 2017, 167, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, A.; Nakajima, T.; Takanashi, H.; Ohki, A.; Fujita, Y.; Yamashita, T. Determination of trace elements in coal and coal fly ash by joint-use of ICP-AES and atomic absorption spectrometry. Talanta 2007, 71, 251–257. [Google Scholar] [CrossRef]
- Yao, M.N.; Yang, X.J.; Cui, Z.D.; Zhu, S.L.; Li, Z.Y.; Liang, Y.Q. Detection of Cd2+ by Square Wave Anodic Stripping Voltammetry Using an Activated Bismuth-film Electrodes. J. Inorg. Mater. 2019, 34, 91–95. [Google Scholar]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Y.X.; Yang, G.J.; Tang, C.L.; Deng, Y.; Li, S.; Wang, Z.L. Cd-Aptamer Electrochemical Biosensor Based on AuNPs/CS Modified Glass Carbon Electrode. J. Biomed. Nanotechnol. 2017, 13, 1253–1259. [Google Scholar] [CrossRef]
- Fakude, C.T.; Arotiba, O.A.; Mabuba, N. Electrochemical aptasensing of cadmium (II) on a carbon black-gold nano-platform. J. Electroanal. Chem. 2020, 858, 113796. [Google Scholar] [CrossRef]
- Jin, H.; Gui, R.; Yu, J.; Lv, W.; Wang, Z. Fabrication strategies, sensing modes and analytical applications of ratiometric electrochemical biosensors. Biosens. Bioelectron. 2017, 91, 523–537. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, C.; Su, X.Y.; Zhang, W.; Zou, X.B. Signal on-off ratiometric electrochemical sensor based on semi-complementary aptamer couple for sensitive cadmium detection in mussel. Sens. Actuators B Chem. 2021, 346, 130506. [Google Scholar] [CrossRef]
- Swain, K.K.; Bhand, S. A colorimetric paper-based ATONP-ALP nanobiosensor for selective detection of Cd2+ ions in clams and mussels. Anal. Bioanal. Chem. 2021, 413, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Soo Oh, S.; Nie, J.; Stewart, R.; Eisenstein, M.; Chambers, J.; Marth, J.D.; Walker, F.; Thomson, J.A.; Soh, H.T. Quantitative selection and parallel characterization of aptamers. Proc. Natl. Acad. Sci. USA 2013, 110, 18460–18465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.Y.; Xie, H.; Luo, G.L.; Zhuang, Y.J.; Wu, X.Q.; Li, G.J.; Sun, W. ZnO-reduced graphene oxide composite based photoelectrochemical aptasensor for sensitive Cd(II) detection with methylene blue as sensitizer. Anal. Chim. Acta 2020, 1118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.Y.; Chen, Y.X.; Zhang, X.P.; Xie, H.; Luo, G.L.; Sun, W. Target-enhanced photoelectrochemical aptasensor for Cd(II) detection using graphite-like carbon nitride as sensitizer with high sensitivity. Microchem. J. 2021, 168, 106394. [Google Scholar] [CrossRef]
- Xu, H.F.; Zhang, S.Q.; Zhang, T.; Huang, W.H.; Dai, Y.T.; Zheng, R.X.; Wu, G.W. An electrochemiluminescence biosensor for cadmium ion based on target-induced strand displacement amplification and magnetic Fe3O4−GO nanosheets. Talanta 2022, 237, 122967. [Google Scholar] [CrossRef]
- Shim, S.; Tae, J. Rhodamine Cyclen-based Fluorescent Chemosensor for the Detection of Cd2+. Bull. Korean Chem. Soc. 2011, 32, 2928–2932. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.Z.; Pu, X.W.; Ma, G.L.; Wang, E.Q.; Kong, J.M.; Liu, Z.P.; Liu, Y.Z. Synthesis of a ratiometric fluorescent peptide sensor for the highly selective detection of Cd2+. Bioorganic Med. Chem. Lett. 2012, 22, 4014–4017. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, S.Q.; Qiu, S.Y.; Pu, S.Z. A novel fluorescent sensor based on a diarylethene containing a hydrazinylpyridine unit for Cd2+ and Zn(2+)with high selectivity. J. Photochem. Photobiol. A Chem. 2018, 367, 212–218. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Concilio, S.; Piotto, S.; Tuzi, A.; Panunzi, B. A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions. Dyes Pigment. 2018, 155, 249–257. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.; Shi, F.; Pu, S. A diarylethene based multi-functional sensor for fluorescent detection of Cd2+ and colorimetric detection of Cu2+. Dyes Pigment. 2019, 161, 34–43. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, X.Z.; Liu, X.Y.; Wang, M.; Liu, J.J.; Gao, G.; Zhang, X.Y.; Sun, R.Z.; Hou, S.C.; Wang, H.M.; et al. A highly sensitive multifunctional sensor based on phenylene-acetylene for colorimetric detection of Fe2+ and ratiometric fluorescent detection of Cd2+ and Zn2+. Sens. Actuators B Chem. 2018, 273, 1077–1084. [Google Scholar]
- Wang, Z.; Cui, S.; Qiu, S.; Pu, S. A new fluorescence probe based on diarylethene with a benzothiazine unit for selective detection of Cd2+. Tetrahedron 2018, 74, 7431–7437. [Google Scholar] [CrossRef]
- Luan, Y.; Lu, A.; Chen, J.; Fu, H.; Xu, L. A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection. Appl. Sci. 2016, 6, 432. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Dahiya, D.; Sharma, V.; Khan, N.; Chaurasia, D.; Nadda, A.K.; Varjani, S.; Pandey, A.; Bhargava, P.C. Advances from conventional to real time detection of heavy metal(loid)s for water monitoring: An overview of biosensing applications. Chemosphere 2022, 307 Pt 4, 136124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, X.Y.; Wang, Y.S.; Yi, J.C.; Zeng, Z.; Zhang, H.; Chen, Y.T.; Hu, X.J.; Suo, Q.L. Label-free fluorescent aptasensor of Cd2+ detection based on the conformational switching of aptamer probe and SYBR green I. Microchem. J. 2019, 144, 377–382. [Google Scholar] [CrossRef]
- Maghsoudi, A.S.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.W.; Ding, J.N.; Hayat, K.; Yang, X.J.; Zhan, X.J.; Zhang, D.; Lu, Y.T.; Zhou, P. A Facile Aptasensor for Instantaneous Determination of Cadmium Ions Based on Fluorescence Amplification Effect of MOPS on FAM-Labeled Aptamer. Biosensors 2021, 11, 133. [Google Scholar] [CrossRef]
- Xu, M.M.; Peng, Y.; Yang, H.L.; Zhou, Y. Highly sensitive biosensor based on aptamer and hybridization chain reaction for detection of cadmium ions. Luminescence 2022, 37, 665–671. [Google Scholar] [CrossRef]
- Chen, J.H.; Pan, J.F.; Liu, C.S. Versatile Sensing Platform for Cd2+ Detection in Rice Samples and Its Applications in Logic Gate Computation. Anal. Chem. 2020, 92, 6173–6180. [Google Scholar] [CrossRef]
- Pan, J.F.; Zeng, L.W.; Chen, J.H. An enzyme-free DNA circuit for the amplified detection of Cd2+ based on hairpin probe-mediated toehold binding and branch migration. Chem. Commun. 2019, 55, 11932–11935. [Google Scholar] [CrossRef]
- Xu, X.Y.; Daniel, W.L.; Wei, W.; Mirkin, C.A. Colorimetric Cu2+ Detection Using DNA-Modified Gold-Nanoparticle Aggregates as Probes and Click Chemistry. Small 2010, 6, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.S.; Liu, Y.Y.; Liu, D.B.; Wang, Z.; Jiang, X.Y. Copper-Mediated Amplification Allows Readout of Immunoassays by the Naked Eye. Angew. Chem. Int. Ed. 2011, 50, 3442–3445. [Google Scholar] [CrossRef]
- Qin, Y.K.; Cheng, C.L.; Geng, H.; Wang, C.; Hu, W.P.; Xu, W.; Shuai, Z.G.; Zhu, D.B. Efficient ambipolar transport properties in alternate stacking donor-acceptor complexes: From experiment to theory. Phys. Chem. Chem. Phys. 2016, 18, 14094–14103. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Miao, Y.Q.; Zhan, X.J.; Zhang, S.L.; Zhao, X.J. Colorimetric determination of cysteine by a paper-based assay system using aspartic acid modified gold nanoparticles. Microchim. Acta 2020, 187, 362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.L.; Li, L.; Zhao, Y.; Chen, Z.B. Colorimetric detection of DNA at the nanomolar level based on enzyme-induced gold nanoparticle de-aggregation. Microchim. Acta 2018, 185, 301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Chen, P.P.; Xu, Z.; Chen, M.M.; Ding, Y.A.; Yue, K.; Xu, J. A facile strategy to prepare porphyrin functionalized ZnS nanoparticles and their peroxidase-like catalytic activity for colorimetric sensor of hydrogen peroxide and glucose. Sens. Actuators B-Chem. 2017, 251, 339–348. [Google Scholar] [CrossRef]
- Xu, L.; Liang, J.; Wang, Y.H.; Ren, S.Y.; Wu, J.; Zhou, H.Y.; Gao, Z.X. Highly Selective, Aptamer-Based, Ultrasensitive Nanogold Colorimetric Smartphone Readout for Detection of Cd(II). Molecules 2019, 24, 2745. [Google Scholar] [CrossRef] [Green Version]
- Bafe Dilebo, W.; Desta Anchiso, M.; Tereke Kidane, T.; Eskezia Ayalew, M. Assessment of Selected Heavy Metals Concentration Level of Drinking Water in Gazer Town and Selected Kebele, South Ari District, Southern Ethiopia. Int. J. Anal. Chem. 2023, 2023, 1524850. [Google Scholar] [CrossRef]
- Tao, Z.; Wei, L.; Wu, S.; Duan, N.; Li, X.; Wang, Z. A colorimetric aptamer-based method for detection of cadmium using the enhanced peroxidase-like activity of Au-MoS(2) nanocomposites. Anal. Biochem. 2020, 608, 113844. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, J.L.; Zhou, P.; Tao, H.; Wang, X.L.; Wu, Y.G. Oligonucleotide-induced regulation of the oxidase-mimicking activity of octahedral Mn3O4 nanoparticles for colorimetric detection of heavy metals. Microchim. Acta 2020, 187, 99. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Y.T.; Yang, X.Y.; Wang, Y.S.; Hu, X.J.; Suo, Q.L. An Ultrasensitive Colorimetric Strategy for Detection of Cadmium Based on the Peroxidase-like Activity of G-Quadruplex-Cd(II) Specific Aptamer. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2019, 35, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.H.; Wu, W.; Li, Q.; Pan, J.F.; Chen, J.H. A label-free and enzyme-free aptasensor for visual Cd2+ detection based on split DNAzyme fragments. Anal. Methods 2019, 11, 3546–3551. [Google Scholar] [CrossRef]




| Probe | Sequences of Nucleotide |
|---|---|
| CAO-1 | GGACTGTTGTGGTATTATTTTTGGTTGTGC |
| CAO-2 | CTCAGGACGACGGGTTCACAGTCCGTTGTC |
| CAO-3 | TTAAGTTTGGGACAAGTTTAGCCTTTGCGTGGATGTGGCT |
| CAO-4 | ACCGACCGTGCTGGACTCTGGACTGTTGTGGTATTATTTTTGGTTGTGCAGTA TGAGCGAGCGTTGCG |
| CAO-5 | SH−(CH2)6GGACTGTTGTGGTATTATTTTTGGTTGTGCAGTATG |
| CAO-6 | SH-TTTTCGACGGGTTCACAGTCCGTTG |
| CAO-7 | SH-CATACTGCACAACCAAAAATAATACCACAACAGTCC |
| CAO-8 | GGACTGTTGTGGTATTATTTTTGGTTGTGCAGTATG |
| CAO-9 | SH−GGACTGTTGTGGTATTATTTTTGGTTGTGCAGTATG-NH2 |
| CAO-10 | ACCGACCGTGCTGGACTCTGGACTGTTGTGGTATTATTTTTGGTTGTGCAGTATG AGCGAGCGTTGCG |
| CAO-11 | GGGAGGGAACTGTTGTGGTATTATTTTTGGTTGTGCAGTAGGGCGGG |
| CAO-12 | GGGGACTGTTGTGGTATTATTTTTGGTTGTGCAGT |
| CAO-13 | GGACTGTTGTGGTATTATTTTTGGTTGTGCAGTCC |
| CAO-14 | ACTGTTGTGGTATTATTTTTGGTTGTGCAGTA |
| CAO-15 | CCAAAAATAATACCACAACAGTCCCAAAGTGGACTGTTGTGGTATTAT |
| CAO-16 | GGACTGTTGTGGTATTATTTTTGGATAATACCACAACAGTCCACTTTG |
| CAO-17 | CCCAACCAAATGACGATrAGGGTAGGGCGGGTTGGG |
| CAO-18 | GACGAGTCGCATATCGTCATTTGGTTGGGATGCGACTCGTCGATCACTAATGG |
| CAO-19 | GATCACTAATGGACTGTTGTGGTATTATTTTTTTTTGTGCAGTATGCGACTCGTC |
| CAO-20 | GACGAGTCGCATATTACACCCATGTTCGTCA |
| CAO-21 | CCATCCAGCGATTAATCCATTAGTGATC |
| CAO-22 | GGGTATGTTTGGGTAGGGCGGGGACTGTTGTGGTATTATTTTTGGTTGTGCAGT ATGAGGATGA |
| CAO-23 | GGGTATGTTTTCATCCTCCGCCCTACCCAAACATACCCGGAGGATGAGGGTAG GGCGGAGGGTATGTTTGGGTAGGGCGGGTTGGG |
| CAO-24 | AAACATACCCTCCGCCCTACCCTCATCCTCCGGGTATGTTTGGGTAGGGCGGAG GATGAGGGTAGGGCGGAGGATGA |
| CAO-25 | GCTTTCTTCTTTCTTCCCCCCTTGTTTGTTGTTTGC |
| CAO-26 | GGGCTGGGAGGGTTGGGGTATTATTTTTGGTTGTGCCCTATG |
| CAO-27 | ATGGGTCTCACTATGGGACTGTTGTGGTATTATTTTTGGTTGTGCAGTATGACTA |
| CAO-28 | TGGGTAGGGCGGGTTAGTCATCCCATAGTGAGACCCATATGGGTCAAGACATGG GTCTCACTATGGGT |
| CAO-29 | TGGGTAGGGCGGGTAGTGAGACCCATGTCTTGACCCATATGGGATGACTAATGG GTCAAGACATGGGT |
| CAO-30 | TGGGTAGGGCGGGTGTCTTGACCCATTAGTCATCCCATATGGGTCTCACTATGGG ATGACTAATGGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Wang, Y.; Wang, H.; Li, X.; Xu, Y.; Qiu, J. Recent Aptamer-Based Biosensors for Cd2+ Detection. Biosensors 2023, 13, 612. https://doi.org/10.3390/bios13060612
Gao Z, Wang Y, Wang H, Li X, Xu Y, Qiu J. Recent Aptamer-Based Biosensors for Cd2+ Detection. Biosensors. 2023; 13(6):612. https://doi.org/10.3390/bios13060612
Chicago/Turabian StyleGao, Zihan, Yin Wang, Haijian Wang, Xiangxiang Li, Youyang Xu, and Jieqiong Qiu. 2023. "Recent Aptamer-Based Biosensors for Cd2+ Detection" Biosensors 13, no. 6: 612. https://doi.org/10.3390/bios13060612





