A Novel Salivary Sensor with Integrated Au Electrodes and Conductivity Meters for Screening of Diabetes
Abstract
:1. Introduction
2. Materials and Methods
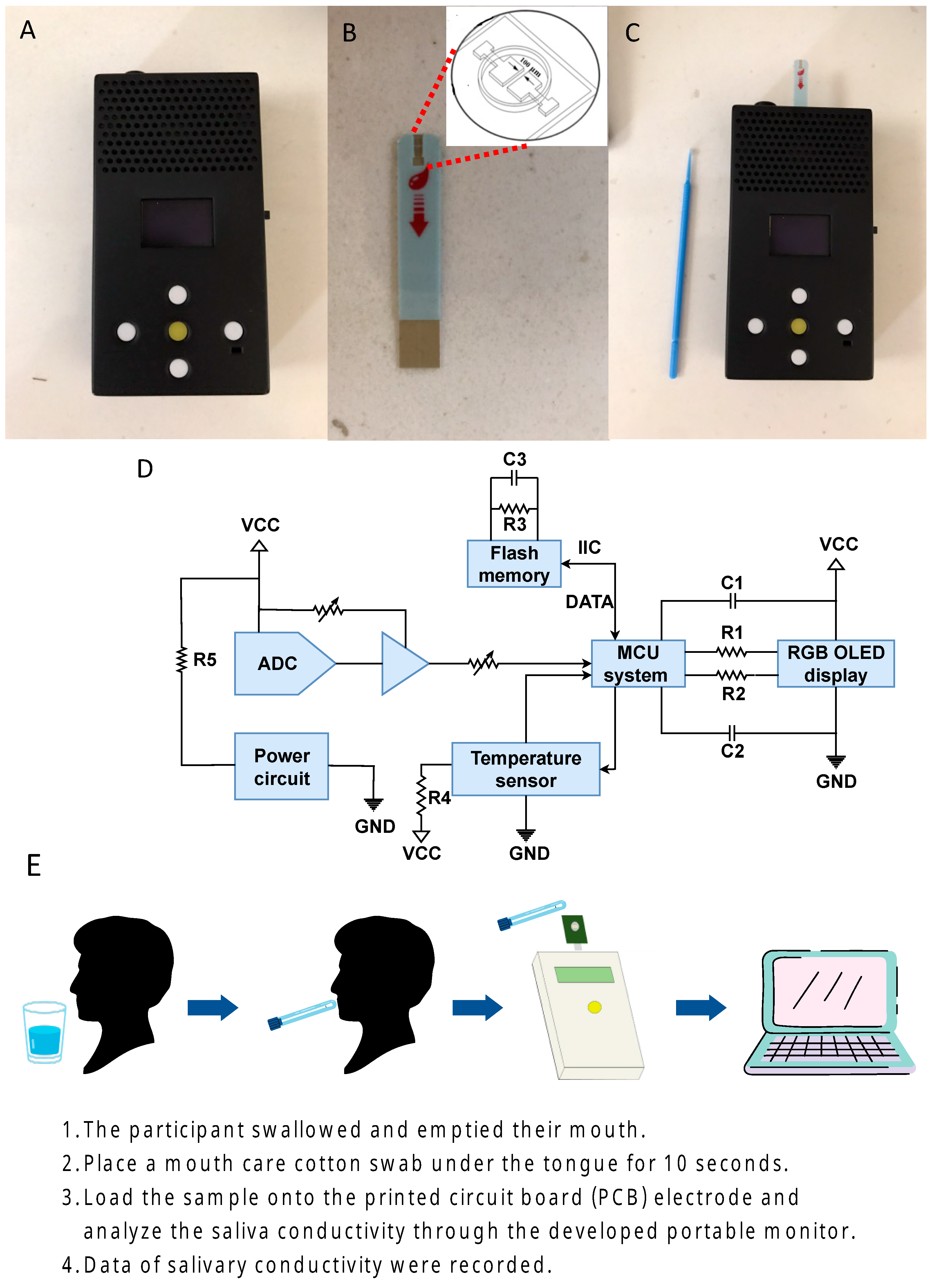
2.1. The Sensing Device
2.2. Saliva Collection and Analysis
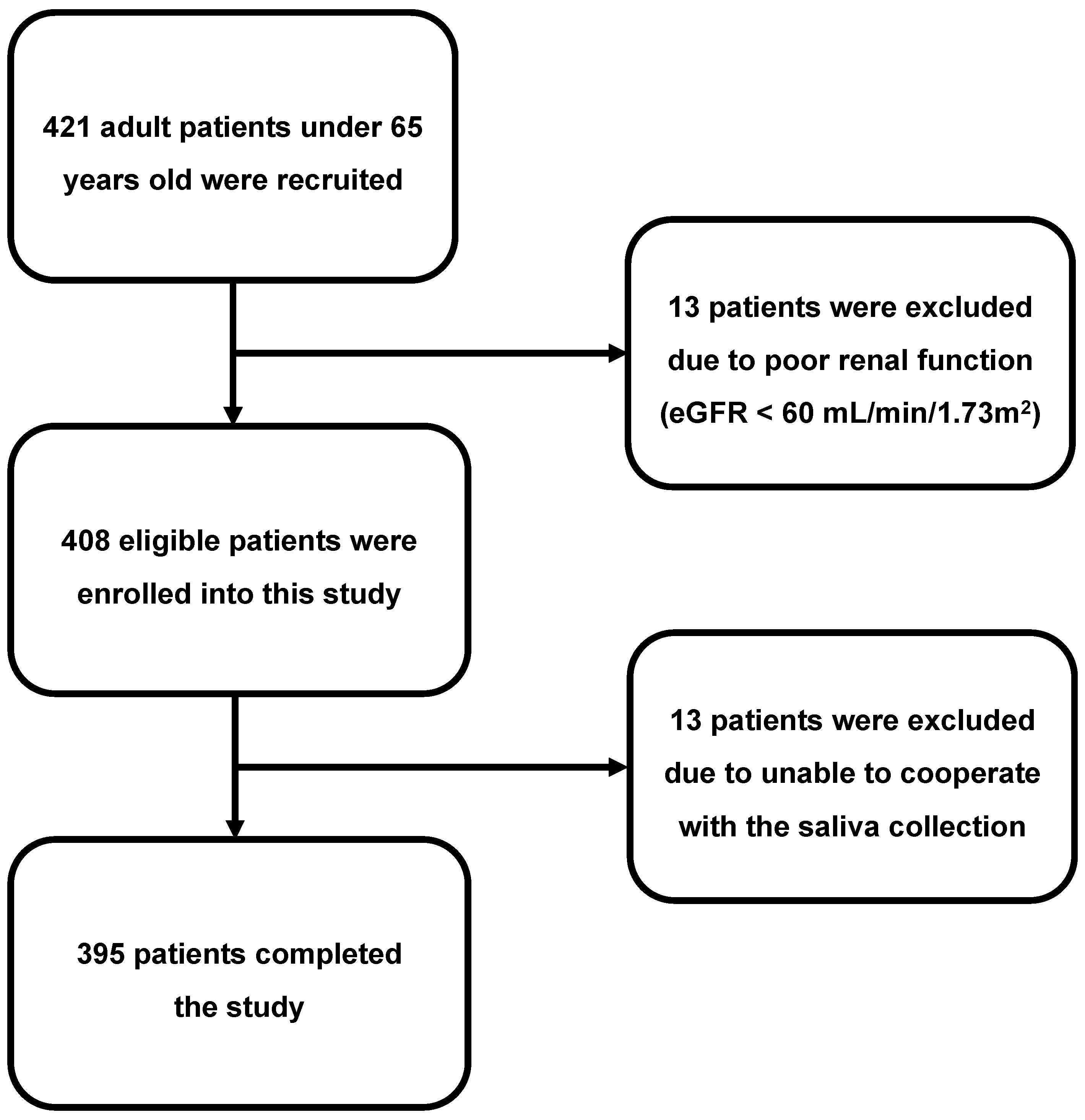
2.3. Study Participants
2.4. Clinical Study Design
2.5. Definition of Diabetes
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Group
3.2. Comparison of DM versus Non-DM Study Group
3.3. Relationship between Salivary Conductivity and Diabetes Mellitus
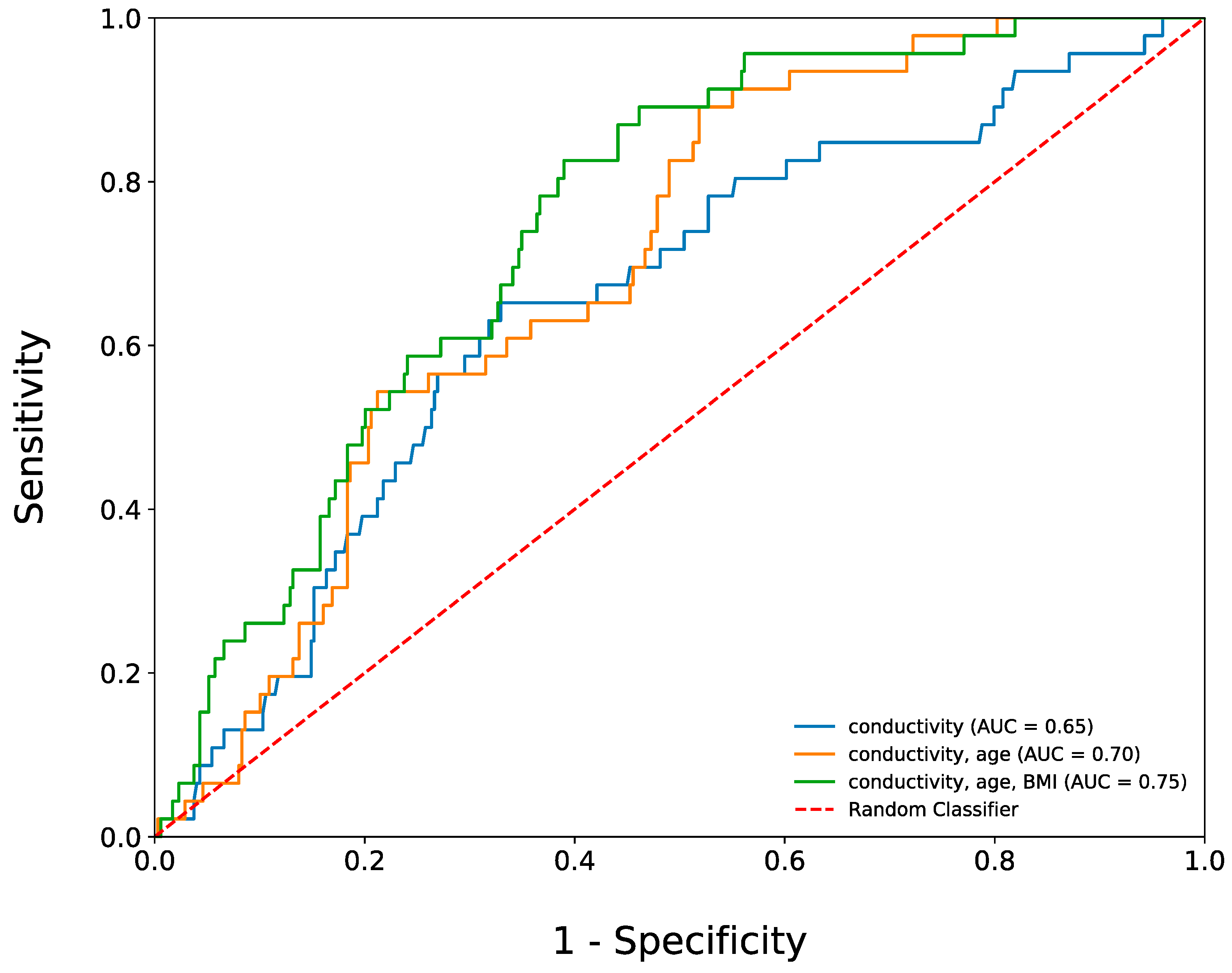
3.4. The Use of Salivary Conductivity to Diagnose Participants with Diabetes Mellitus
3.5. Comparison of Low versus High Salivary Conductivity Study Group
3.6. Associations between Salivary Conductivity and the Risk of Diabetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Assanangkornchai, S.; Taneepanichskul, S.; Putwatana, P. Prevalence of diabetes and relationship with socioeconomic status in the Thai population: National Health Examination Survey, 2004–2014. J. Diabetes Res. 2018, 2018, 1654530. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Uddin, S.; Srinivasan, U. Comorbidity network for chronic disease: A novel approach to understand type 2 diabetes progression. Int. J. Med. Inform. 2018, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.J.; Keyserling, T.C.; Ammerman, A.S.; Berkowitz, S.A. Diet quality trends among adults with diabetes by socioeconomic status in the US: 1999–2014. BMC Endocr. Disord. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Volaco, A.; Cavalcanti, A.M.; Précoma, D.B. Socioeconomic status: The missing link between obesity and diabetes mellitus? Curr. Diabetes Rev. 2018, 14, 321–326. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Jung, C.-H.; Son, J.W.; Kang, S.; Kim, W.J.; Kim, H.-S.; Kim, H.S.; Seo, M.; Shin, H.-J.; Lee, S.-S.; Jeong, S.J. Diabetes fact sheets in Korea, 2020: An appraisal of current status. Diabetes Metab. J. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 81. [Google Scholar] [CrossRef]
- Association, A.D. Introduction: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45, S1–S2. [Google Scholar] [CrossRef]
- Celec, P.; Tóthová, Ľ.; Šebeková, K.; Podracká, Ľ.; Boor, P. Salivary markers of kidney function—Potentials and limitations. Clin. Chim. Acta 2016, 453, 28–37. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Fisher, L.; Hessler, D.; Edelman, S.V. What is so tough about self-monitoring of blood glucose? Perceived obstacles among patients with Type 2 diabetes. Diabet. Med. 2014, 31, 40–46. [Google Scholar] [CrossRef]
- Chojnowska, S.; Baran, T.; Wilińska, I.; Sienicka, P.; Cabaj-Wiater, I.; Knaś, M. Human saliva as a diagnostic material. Adv. Med. Sci. 2018, 63, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Czumbel, L.M.; Kiss, S.; Farkas, N.; Mandel, I.; Hegyi, A.; Nagy, Á.; Lohinai, Z.; Szakács, Z.; Hegyi, P.; Steward, M.C. Saliva as a candidate for COVID-19 diagnostic testing: A meta-analysis. Front. Med. 2020, 7, 465. [Google Scholar] [CrossRef]
- Gleerup, H.S.; Hasselbalch, S.G.; Simonsen, A.H. Biomarkers for Alzheimer’s disease in saliva: A systematic review. Dis. Mrk. 2019, 2019, 4761054. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.N.; Attavar, S.H.; Shetty, N.; Hegde, N.D.; Hegde, N.N. Saliva as a biomarker for dental caries: A systematic review. J. Conserv. Dent. 2019, 22, 2. [Google Scholar] [PubMed]
- Wang, J.; Zhao, Y.; Ren, J.; Xu, Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 671–678. [Google Scholar] [CrossRef]
- Kułak-Bejda, A.; Waszkiewicz, N.; Bejda, G.; Zalewska, A.; Maciejczyk, M. Diagnostic value of salivary markers in neuropsychiatric disorders. Dis. Mrk. 2019, 2019, 4360612. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Bielas, M.; Zalewska, A.; Gerreth, K. Salivary biomarkers of oxidative stress and inflammation in stroke patients: From basic research to clinical practice. Oxid. Med. Cell. Longev. 2021, 2021, 5545330. [Google Scholar] [CrossRef]
- Matczuk, J.; Zalewska, A.; Łukaszuk, B.; Knaś, M.; Maciejczyk, M.; Garbowska, M.; Ziembicka, D.M.; Waszkiel, D.; Chabowski, A.; Żendzian-Piotrowska, M.; et al. Insulin Resistance and Obesity Affect Lipid Profile in the Salivary Glands. J. Diabetes Res. 2016, 2016, 8163474. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Trivedi, A.; Gajdhar, S.K.; Bhagwat, H.; Khutwad, G.K.; Mall, P.E.; Kulkarni, D. Correlation of blood glucose levels, salivary glucose levels and oral colony forming units of Candida albicans in type 2 diabetes mellitus patients. J. Contemp. Dent. Pr. 2019, 20, 494–498. [Google Scholar]
- Mohammadnejad, P.; Asl, S.S.; Aminzadeh, S.; Haghbeen, K. A new sensitive spectrophotometric method for determination of saliva and blood glucose. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117897. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Karthigadevi, G.; Duraisamy, R.; Vishnu Priya, V.; Santhosh Kumar, M. Analysis of salivary electrolytes in diabetic and non-diabetic patients. Drug Invent. Today 2018, 10, 1801–1805. [Google Scholar]
- Kolahdouz-Mohammadi, R.; Soltani, S.; Clayton, Z.S.; Salehi-Abargouei, A. Sodium status is associated with type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2021, 60, 3543–3565. [Google Scholar] [CrossRef] [PubMed]
- Mrag, M.; Kassab, A.; Omezzine, A.; Belkacem, C.R.; Ben, F.I.F.; Douki, N.; Laouani, K.C.; Bouslema, A.; Ben, A.F. Saliva diagnostic utility in patients with type 2 diabetes: Future standard method. J. Med. Biochem. 2020, 39, 140. [Google Scholar] [CrossRef]
- Onyemaechi, E.V.; Peggy, O.E.; Elias, D.T.M.; Ogheneyoma, O.O.; Chukwuebuka, N.B.; Ojugbeli, E.T.; Osuagwu, U.L.; Kingsley, N.E. Comparative Analysis of Fasting Blood Glucose and Salivary Electrolytes Concentrations in Type II Diabetic Individuals: A Randomized Controlled Hospital Based Study. Toxicol. Rep. 2022, 9, 1268–1272. [Google Scholar]
- Lu, Y.-P.; Huang, J.-W.; Lee, I.; Weng, R.-C.; Lin, M.-Y.; Yang, J.-T.; Lin, C.-T. A portable system to monitor saliva conductivity for dehydration diagnosis and kidney healthcare. Sci. Rep. 2019, 9, 14771. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Lu, Y.-P.; Lee, A.-T.; Tung, C.-W.; Tsai, Y.-H.; Tsay, H.-P.; Lin, C.-T.; Yang, J.-T. A Portable Biodevice to Monitor Salivary Conductivity for the Rapid Assessment of Fluid Status. J. Pers. Med. 2021, 11, 577. [Google Scholar] [CrossRef]
- Lee, A.-T.; Lu, Y.-P.; Chen, C.-H.; Chang, C.-H.; Tsai, Y.-H.; Tung, C.-W.; Yang, J.-T. The Association of Salivary Conductivity with Cardiomegaly in Hemodialysis Patients. Appl. Sci. 2021, 11, 7405. [Google Scholar] [CrossRef]
- Lin, C.-W.; Tsai, Y.-H.; Lu, Y.-P.; Yang, J.-T.; Chen, M.-Y.; Huang, T.-J.; Weng, R.-C.; Tung, C.-W. Application of a Novel Biosensor for Salivary Conductivity in Detecting Chronic Kidney Disease. Biosensors 2022, 12, 178. [Google Scholar] [CrossRef]
- Sheen, Y.-J.; Hsu, C.-C.; Jiang, Y.-D.; Huang, C.-N.; Liu, J.-S.; Sheu, W.H.-H. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J. Formos. Med. Assoc. 2019, 118, S66–S73. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary biomarkers for Alzheimer’s disease and related disorders. Neurol. Ther. 2019, 8, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labat, C.; Thul, S.; Pirault, J.; Temmar, M.; Thornton, S.N.; Benetos, A.; Bäck, M. Differential associations for salivary sodium, potassium, calcium, and phosphate levels with carotid intima media thickness, heart rate, and arterial stiffness. Dis. Mrk. 2018, 2018, 3152146. [Google Scholar] [CrossRef]
- Tomás, I.; Marinho, J.; Limeres, J.; Santos, M.; Araújo, L.; Diz, P. Changes in salivary composition in patients with renal failure. Arch. Oral Biol. 2008, 53, 528–532. [Google Scholar] [CrossRef]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, D.G.; Jung, D.; Kong, S.H. A lab-on-a-chip-based non-invasive optical sensor for measuring glucose in saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, N.; Kuwabara, A.; Holt, T. Non-invasive and minimally invasive glucose monitoring devices: A systematic review and meta-analysis on diagnostic accuracy of hypoglycaemia detection. Syst. Rev. 2021, 10, 145. [Google Scholar] [CrossRef]
- Lerdsri, J.; Chananchana, W.; Upan, J.; Sridara, T.; Jakmunee, J. Label-free colorimetric aptasensor for rapid detection of aflatoxin B1 by utilizing cationic perylene probe and localized surface plasmon resonance of gold nanoparticles. Sens. Actuators B Chem. 2020, 320, 128356. [Google Scholar] [CrossRef]
- Menon, P.S.; Said, F.A.; Mei, G.S.; Berhanuddin, D.D.; Umar, A.A.; Shaari, S.; Majlis, B.Y. Urea and creatinine detection on nano-laminated gold thin film using Kretschmann-based surface plasmon resonance biosensor. PLoS ONE 2018, 13, e0201228. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.K. Wavelength modulation based surface plasmon resonance sensor for detection of cardiac marker proteins troponin I and troponin T. Sens. Actuators A Phys. 2021, 332, 113104. [Google Scholar] [CrossRef]

| All (N = 395) | DM (N = 46) | Non-DM (N = 349) | p-Value | |
|---|---|---|---|---|
| Salivary conductivity, ms/cm | 5.58 ± 1.61 | 6.29 ± 1.58 | 5.48 ± 1.59 | <0.01 |
| Demographics | ||||
| Age, years | 51.78 ± 11.31 | 56.96 ± 6.78 | 51.10 ± 11.61 | <0.01 |
| Gender (male), n (%) | 126 (31.9) | 15 (32.7) | 111 (31.8) | 0.91 |
| Body weight, kg | 64.43 ± 12.53 | 70.41 ± 12.90 | 63.65 ± 12.29 | <0.01 |
| Body height, cm | 160.65 ± 7.84 | 160.66 ± 7.94 | 160.65 ± 7.83 | 0.91 |
| Body mass index, kg/m2 | 24.90 ± 4.15 | 27.19 ± 4.01 | 24.60 ± 4.08 | <0.01 |
| Systolic blood pressure, mmHg | 128.36 ± 21.15 | 138.87 ± 24.84 | 126.97 ± 20.26 | <0.01 |
| Diastolic blood pressure, mmHg | 78.72 ± 12.61 | 82.24 ± 12.61 | 78.26 ± 12.55 | 0.04 |
| Comorbid conditions, n (%) @ | ||||
| Known history of DM | 46 (11.6) | 26 (56.5) | 20 (5.7) | <0.01 |
| Hypertension | 95 (24.1) | 19 (41.3) | 76 (21.8) | <0.01 |
| Dyslipidemia | 47 (11.9) | 10 (21.7) | 37 (10.6) | 0.03 |
| Gout | 11 (2.8) | 1 (2.2) | 10 (2.9) | 1.00 |
| Laboratory parameters | ||||
| BUN, mg/dL | 13.97 ± 4.11 | 14.85 ± 4.65 | 13.86 ± 4.03 | 0.11 |
| Creatinine, mg/dL | 0.74 ± 0.16 | 0.73 ± 0.16 | 0.74 ± 0.16 | 0.44 |
| eGFR, mL/min/1.73 m2 | 101.54 ± 21.18 | 101.97 ± 22.20 | 101.49 ± 21.08 | 0.86 |
| Serum osmolality, mOsm/kgH2O | 291.41 ± 6.63 | 294.91 ± 5.94 | 290.95 ± 6.58 | <0.01 |
| Fasting glucose, mg/dL | 108.40 ± 35.69 | 170.15 ± 56.35 | 100.26 ± 21.47 | <0.01 |
| Hemoglobin A1c, % | 5.88 ± 0.93 | 7.80 ± 1.51 | 5.63 ± 0.35 | <0.01 |
| Low Salivary Conductivity Group * (N = 251) | High Salivary Conductivity Group (N = 144) | p-Value | |
|---|---|---|---|
| Salivary conductivity, ms/cm | 4.57 ± 0.81 | 7.33 ± 1.06 | <0.01 # |
| Demographics | |||
| Age, years | 50.57 ± 11.35 | 53.90 ± 10.95 | <0.01 # |
| Gender (male), n (%) | 79 (31.5) | 47 (32.6) | 0.81 |
| Body weight, kg | 62.47 ± 11.32 | 67.85 ± 13.79 | <0.01 # |
| Body height, cm | 160.37 ± 7.44 | 161.15 ± 8.49 | 0.61 |
| Body mass index, kg/m2 | 24.23 ± 3.67 | 26.07 ± 4.65 | <0.01 # |
| Systolic blood pressure, mmHg | 125.81 ± 20.06 | 132.80 ± 22.32 | <0.01 # |
| Diastolic blood pressure, mmHg | 77.76 ± 12.46 | 80.40 ± 12.73 | 0.05 # |
| Comorbid conditions, n (%) | |||
| Known history of DM | 17 (6.8) | 29 (20.1) | <0.01 # |
| Hypertension | 51 (20.3) | 44 (30.6) | 0.03 # |
| Dyslipidemia | 23 (9.2) | 24 (16.7) | 0.03 # |
| Gout | 7 (2.8) | 4 (2.8) | 1.00 |
| Laboratory parameters | |||
| BUN, mg/dL | 13.71 ± 4.20 | 14.44 ± 3.93 | 0.02 # |
| Creatinine, mg/dL | 0.73 ± 0.16 | 0.76 ± 0.16 | 0.05 # |
| eGFR, mL/min/1.73 m2 | 103.20 ± 20.48 | 98.65 ± 22.14 | 0.01 # |
| Serum osmolality, mOsm/kgH2O | 291.09 ± 6.83 | 291.97 ± 6.23 | 0.12 |
| Fasting glucose, mg/dL | 105.02 ± 33.83 | 114.30 ± 38.12 | <0.01 # |
| Hemoglobin A1c, % | 5.77 ± 0.88 | 6.08 ± 1.00 | <0.01 # |
| Model | Odds Ratio | (95% CI) | p-Value |
|---|---|---|---|
| Crude | 3.82 | 1.44–5.56 | <0.01 |
| Model 1 * | 3.35 | 1.74–6.46 | <0.01 |
| Model 2 # | 2.69 | 1.36–5.32 | <0.01 |
| Parameters | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Crude | Model 1 * | Model 2 # | ||||
| Odds Ratio | p-Value | Odds Ratio | p-Value | Odds Ratio | p-Value | |
| Age | 1.06 | <0.01 | 1.05 | <0.01 | 1.05 | 0.02 |
| Gender | 1.04 | 0.91 | 1.02 | 0.95 | 0.98 | 0.97 |
| BMI | 1.15 | <0.01 | 1.11 | <0.01 | ||
| SBP | 1.03 | <0.01 | 1.02 | 0.05 | ||
| DBP | 1.02 | 0.05 | 0.99 | 0.46 | ||
| eGFR | 1.00 | 0.88 | 1.01 | 0.22 | ||
| Salivary conductivity | 3.82 | <0.01 | 3.35 | <0.01 | 2.65 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Tsai, Y.-H.; Peng, Y.-S.; Yang, J.-T.; Lu, Y.-P.; Chen, M.-Y.; Tung, C.-W. A Novel Salivary Sensor with Integrated Au Electrodes and Conductivity Meters for Screening of Diabetes. Biosensors 2023, 13, 702. https://doi.org/10.3390/bios13070702
Lin C-W, Tsai Y-H, Peng Y-S, Yang J-T, Lu Y-P, Chen M-Y, Tung C-W. A Novel Salivary Sensor with Integrated Au Electrodes and Conductivity Meters for Screening of Diabetes. Biosensors. 2023; 13(7):702. https://doi.org/10.3390/bios13070702
Chicago/Turabian StyleLin, Chen-Wei, Yuan-Hsiung Tsai, Yun-Shing Peng, Jen-Tsung Yang, Yen-Pei Lu, Mei-Yen Chen, and Chun-Wu Tung. 2023. "A Novel Salivary Sensor with Integrated Au Electrodes and Conductivity Meters for Screening of Diabetes" Biosensors 13, no. 7: 702. https://doi.org/10.3390/bios13070702
APA StyleLin, C.-W., Tsai, Y.-H., Peng, Y.-S., Yang, J.-T., Lu, Y.-P., Chen, M.-Y., & Tung, C.-W. (2023). A Novel Salivary Sensor with Integrated Au Electrodes and Conductivity Meters for Screening of Diabetes. Biosensors, 13(7), 702. https://doi.org/10.3390/bios13070702





