Green-Mediated Synthesis of NiCo2O4 Nanostructures Using Radish White Peel Extract for the Sensitive and Selective Enzyme-Free Detection of Uric Acid
Abstract
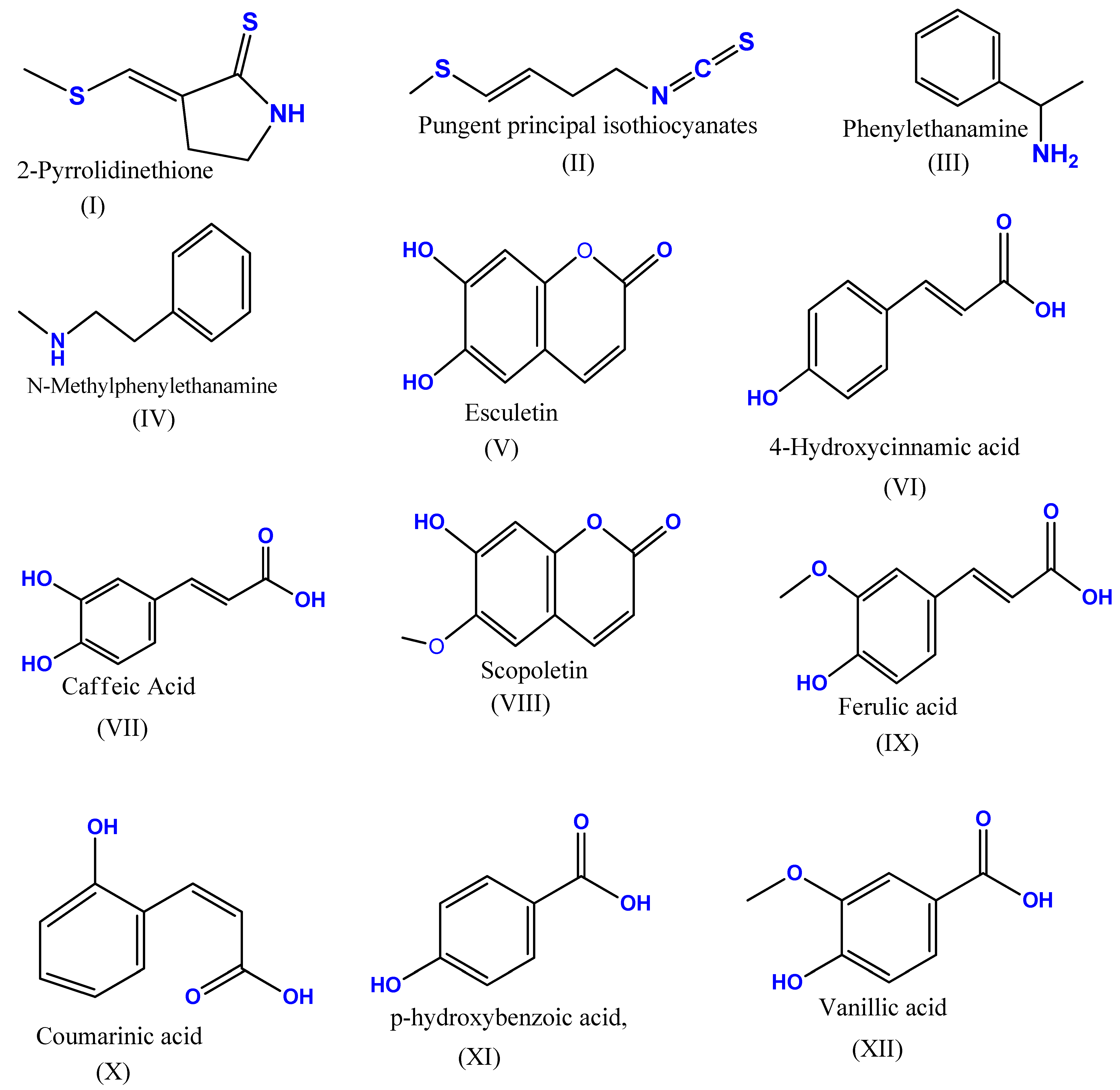
:1. Introduction
2. Experimental Section
2.1. Chemicals Used
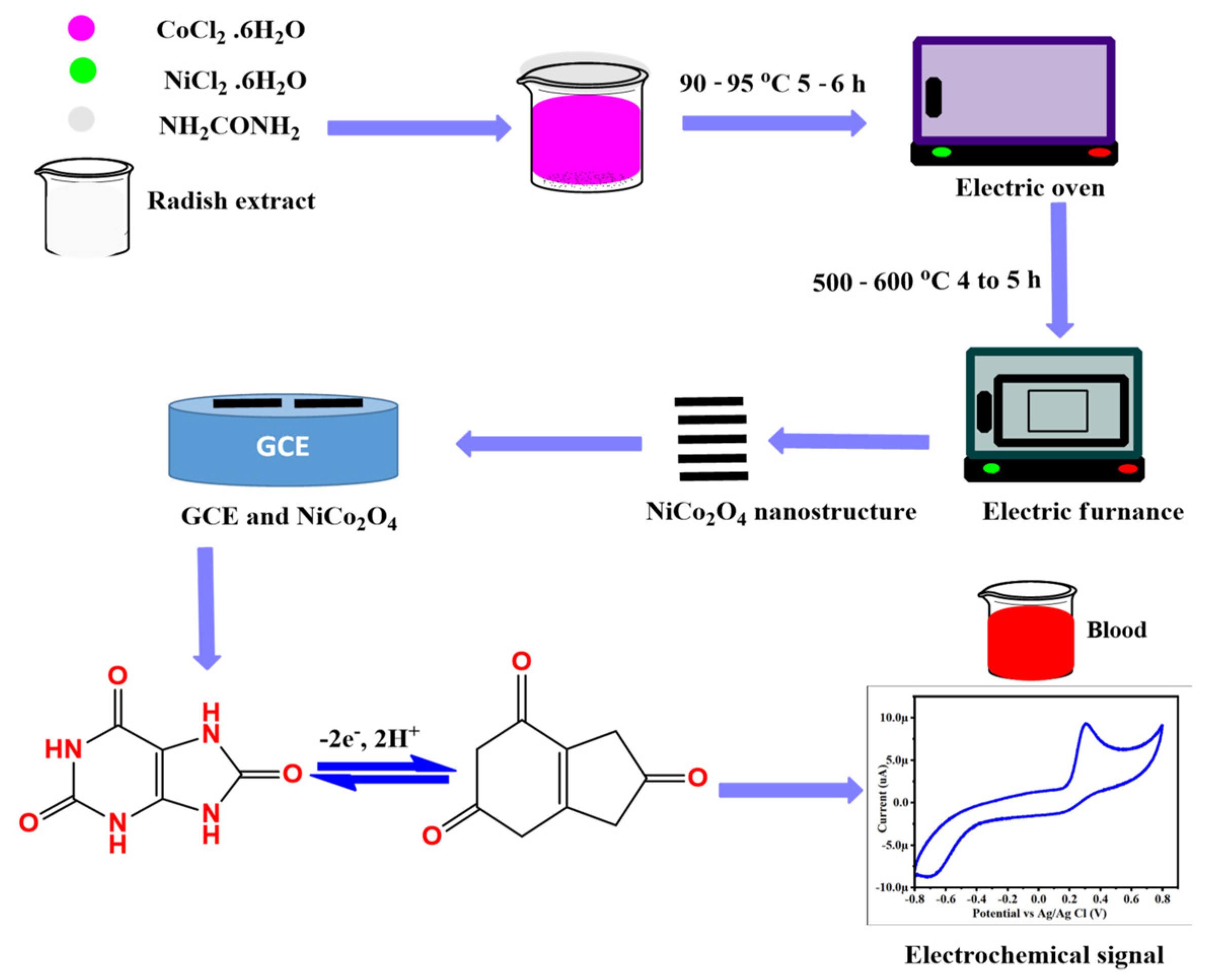
2.2. Green-Mediated Synthesis of NiCo2O4 Nanostructures Using Radish White Peel Extract
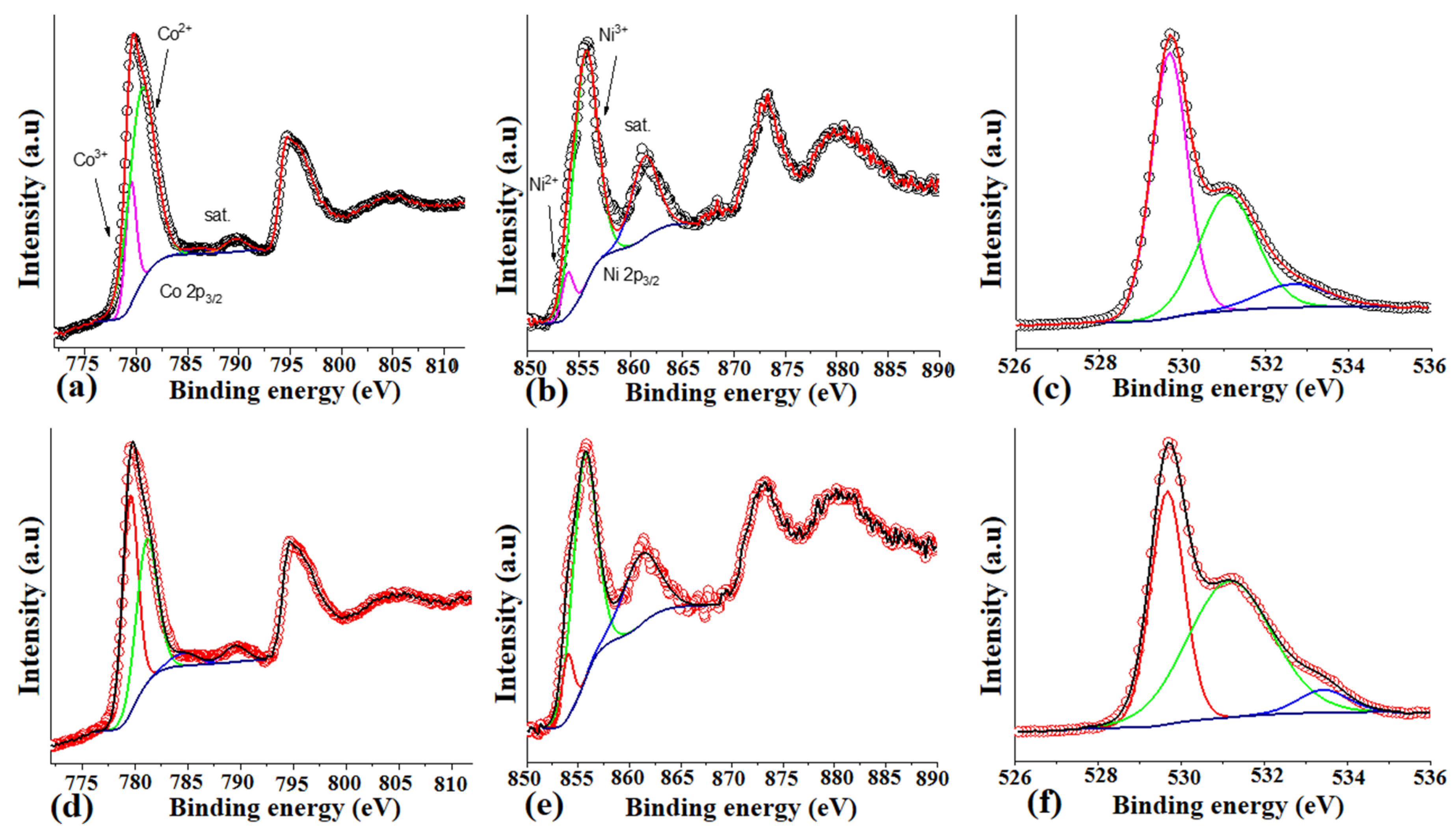
2.3. Physical Investigations on the Surface-Modified NiCo2O4 Nanostructures
2.4. Non-Enzymatic Sensing of UA onto Surface-Modified NiCo2O4 Nanostructures
3. Results and Discussion
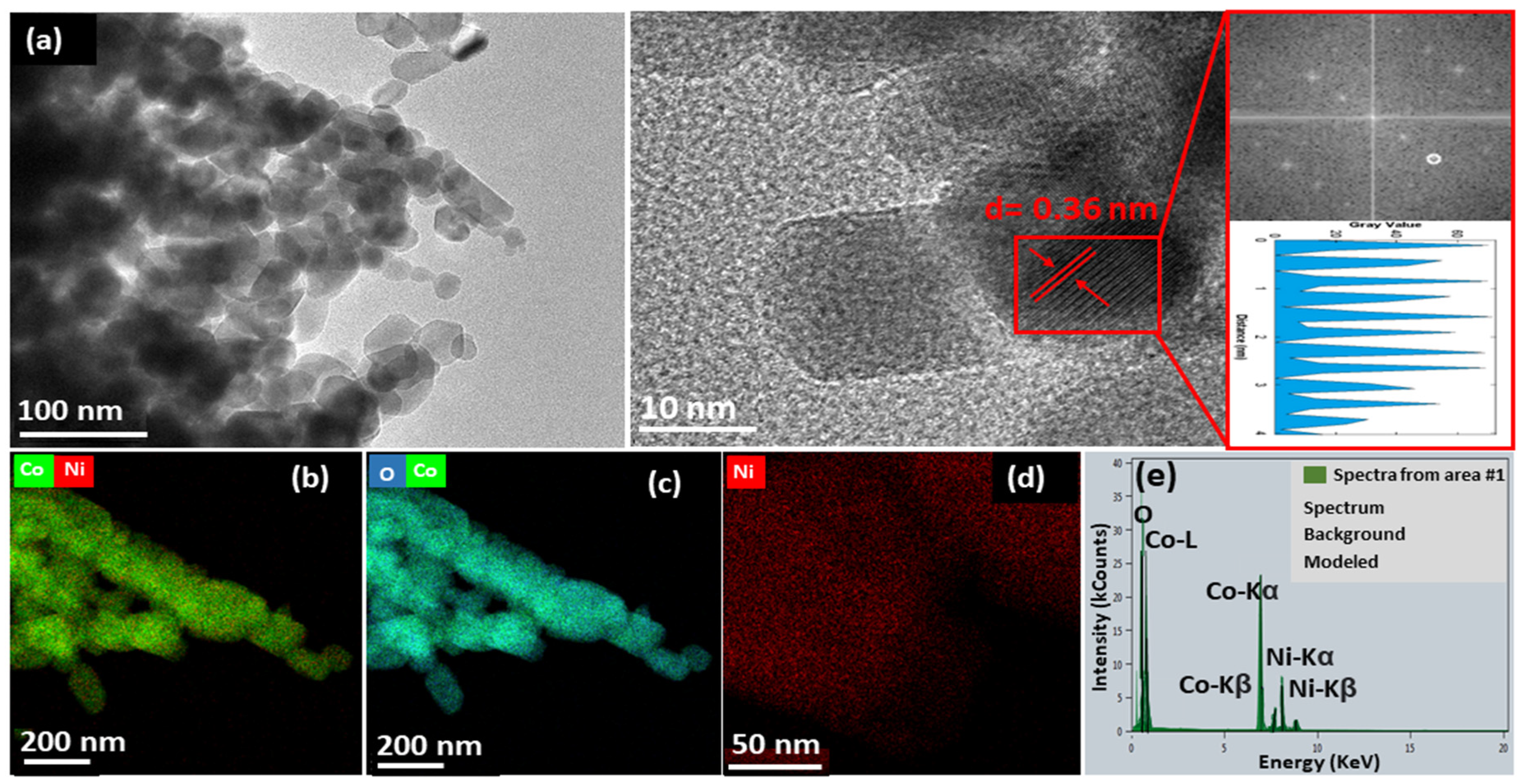
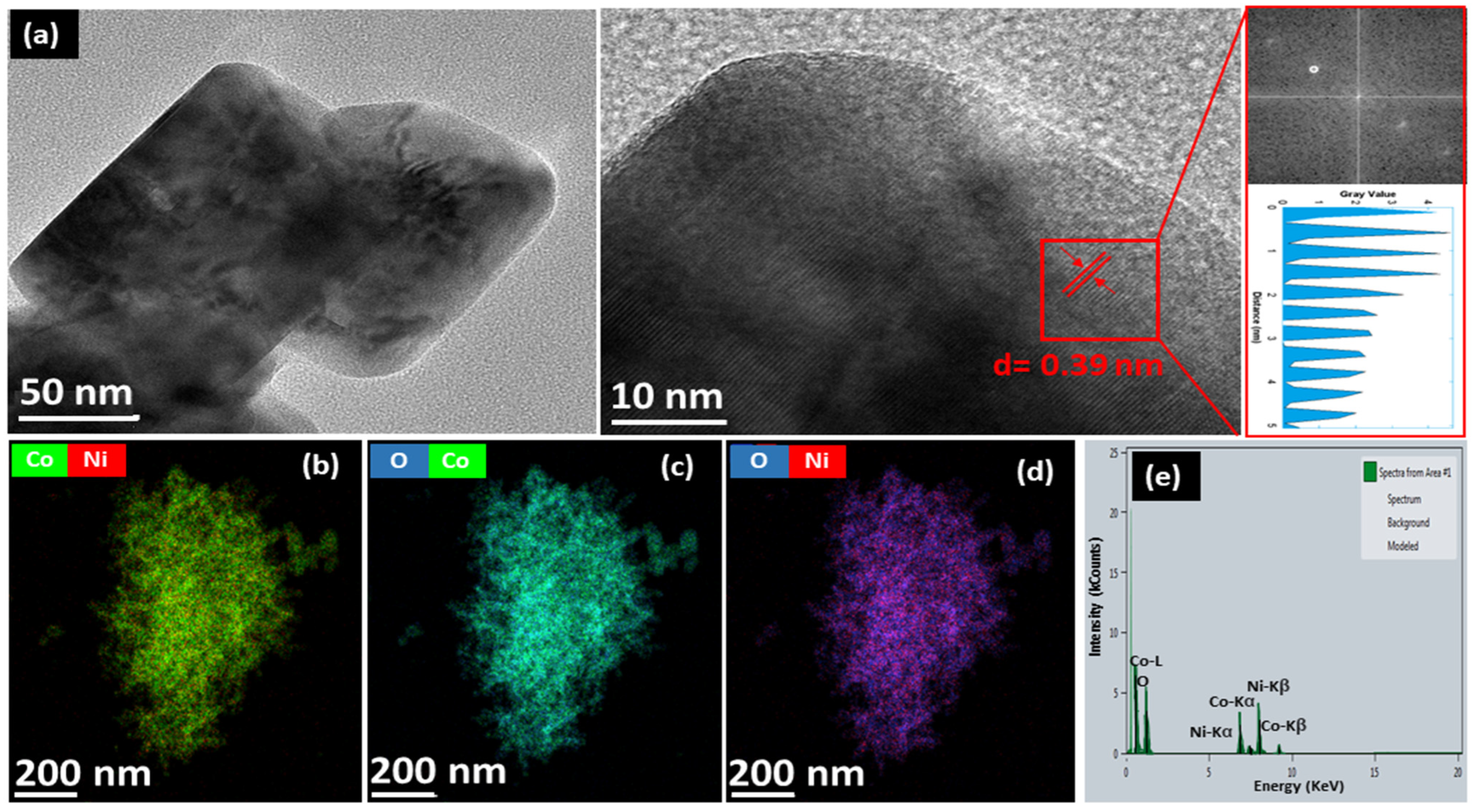
3.1. Structural and Morphological Investigations of Radish White Peel Extract-Assisted Synthesis of NiCo2O4 Nanostructures
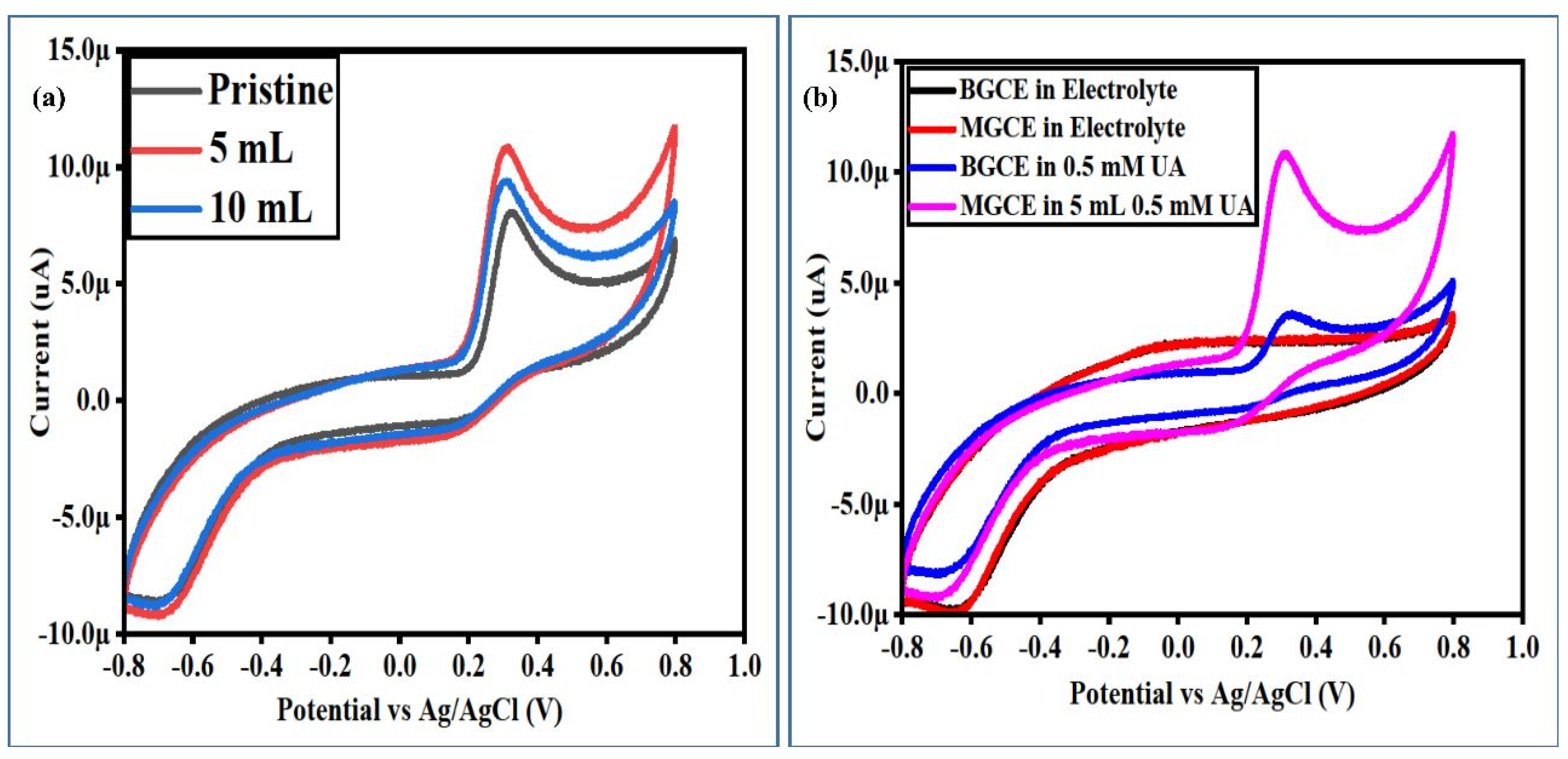
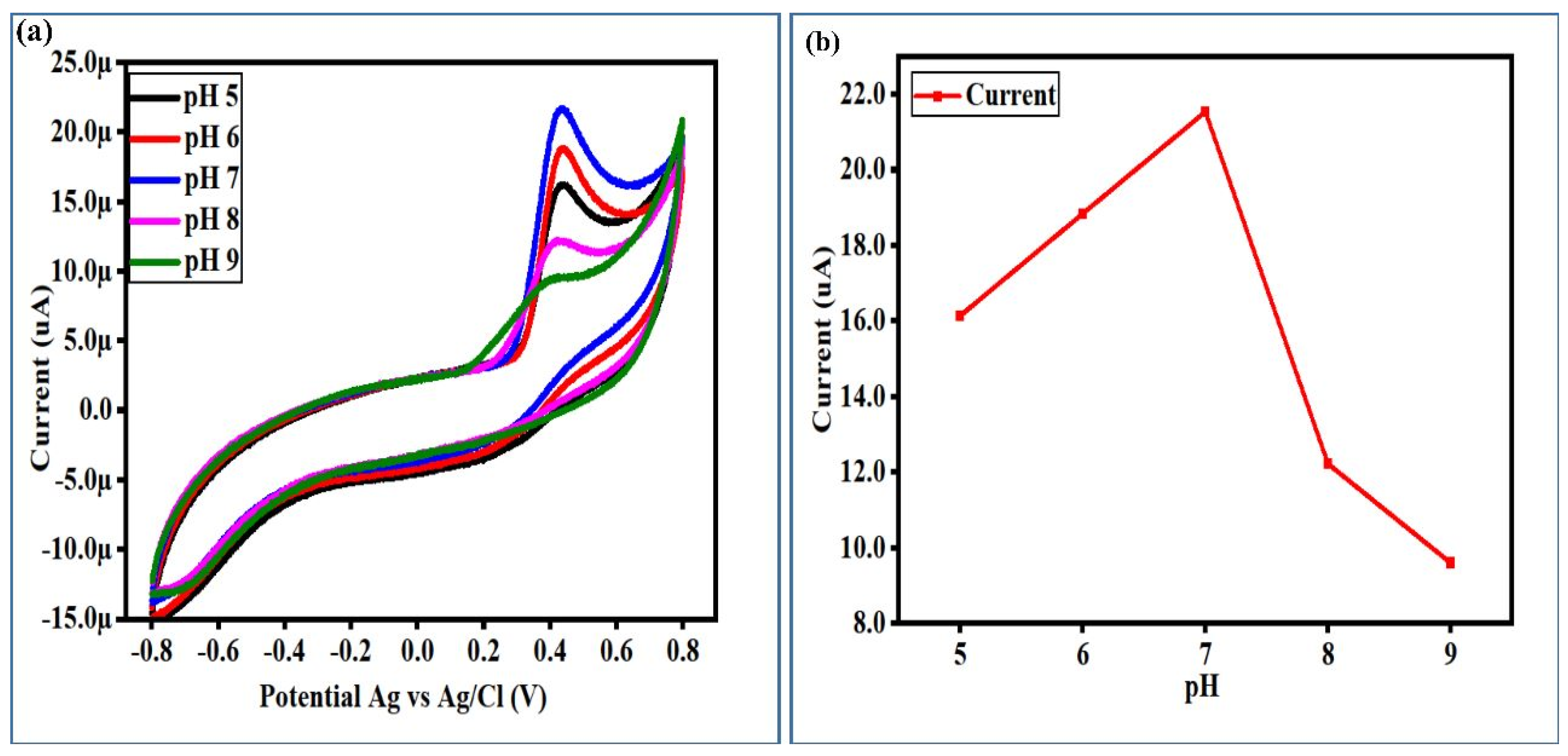
3.2. Non-Enzymatic Uric Acid (UA) Oxidation on the Surface-Modified NiCo2O4 Nanostructures with Radish White Peel Extract
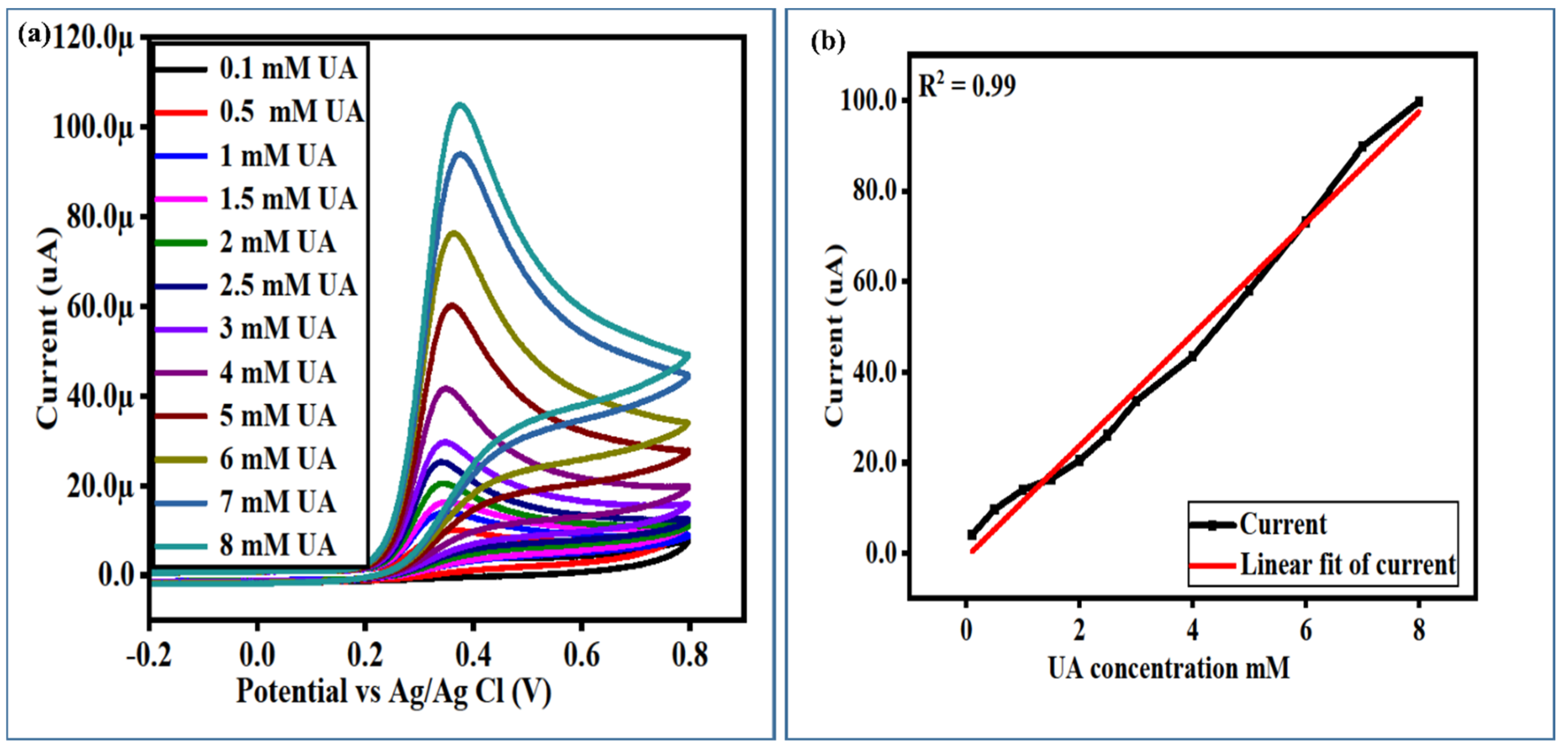
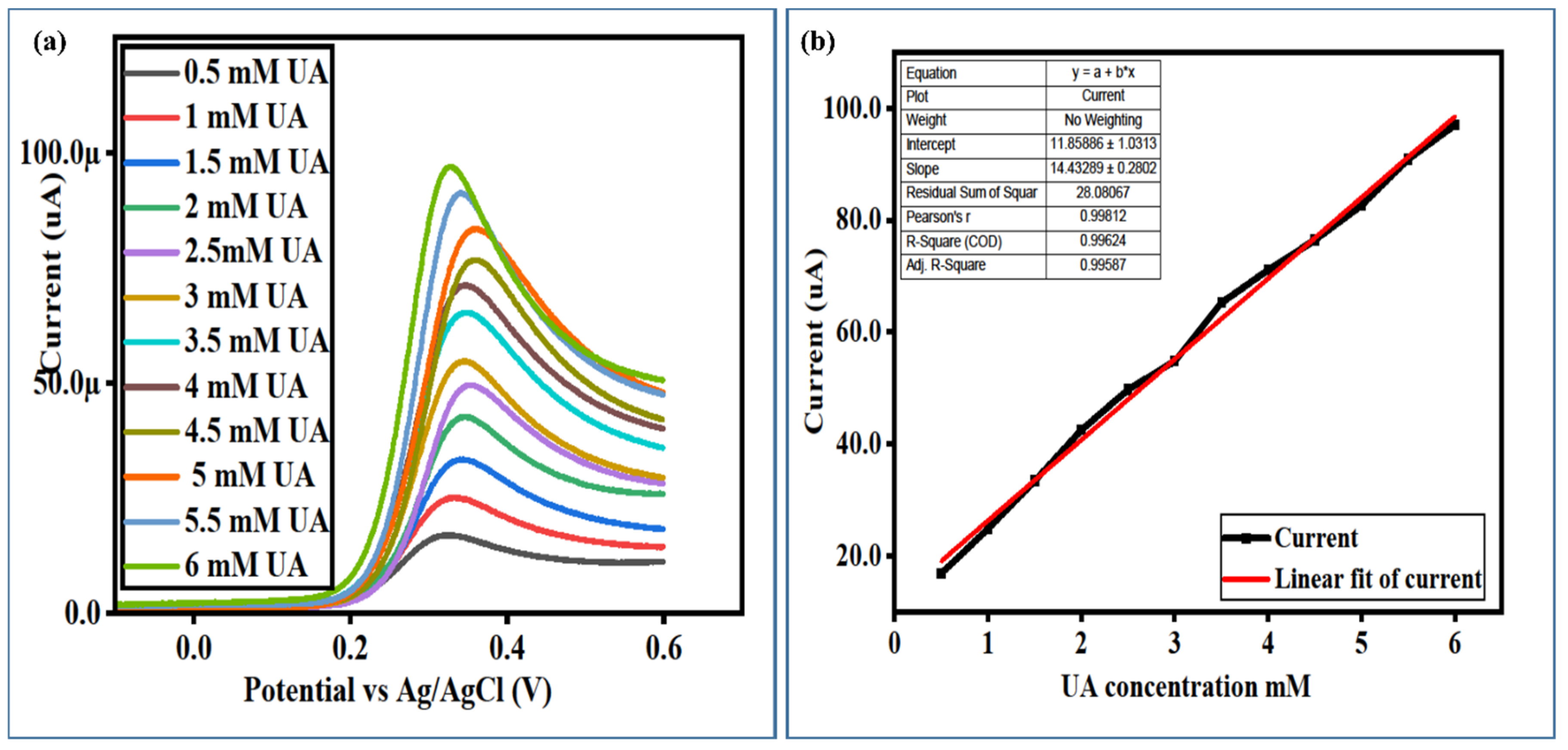
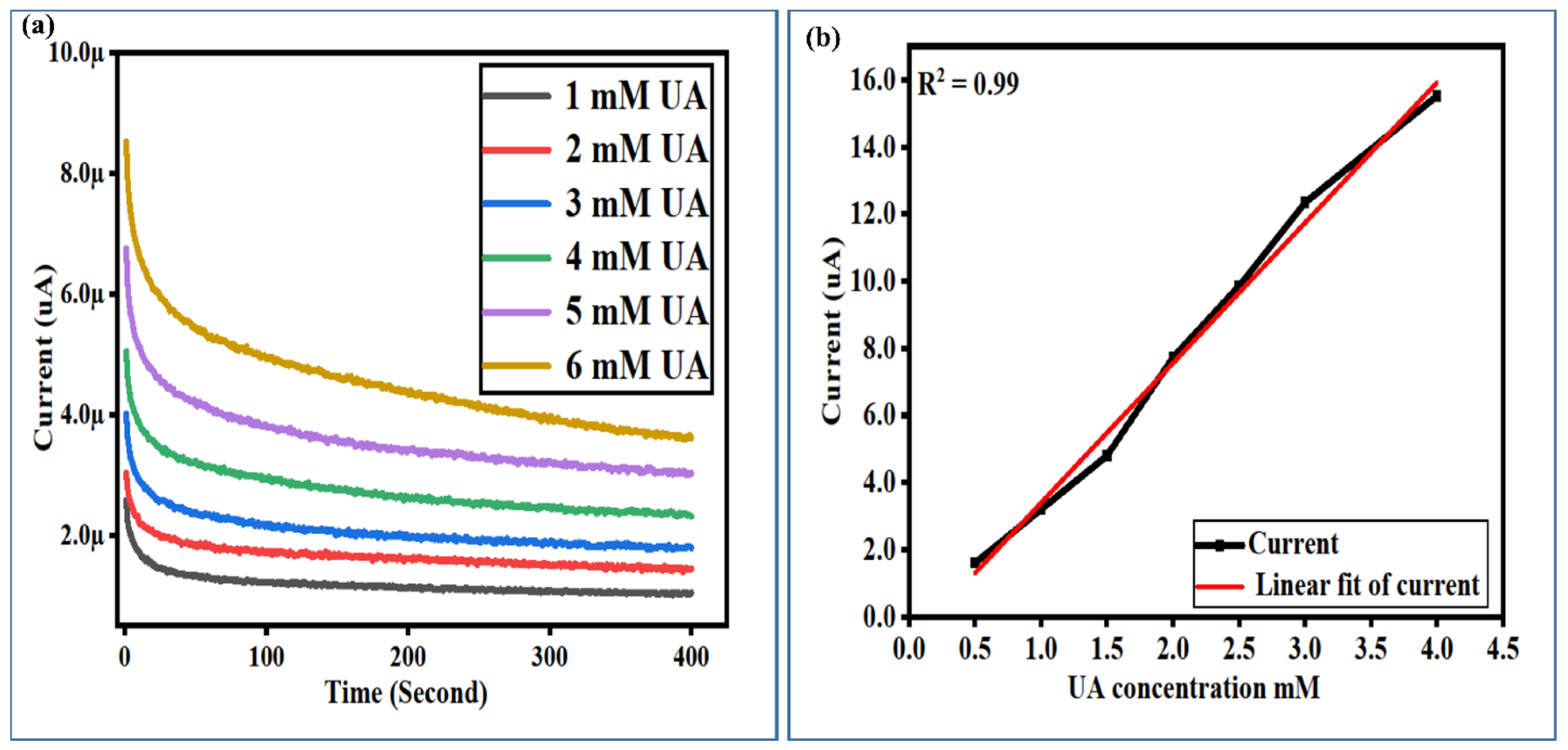
3.3. The Calibration Plots, Stability, Repeatability, and Selectivity Studies of a Newly Developed Non-Enzymatic UA Sensor Based on Surface-Modified NiCo2O4 Nanostructures
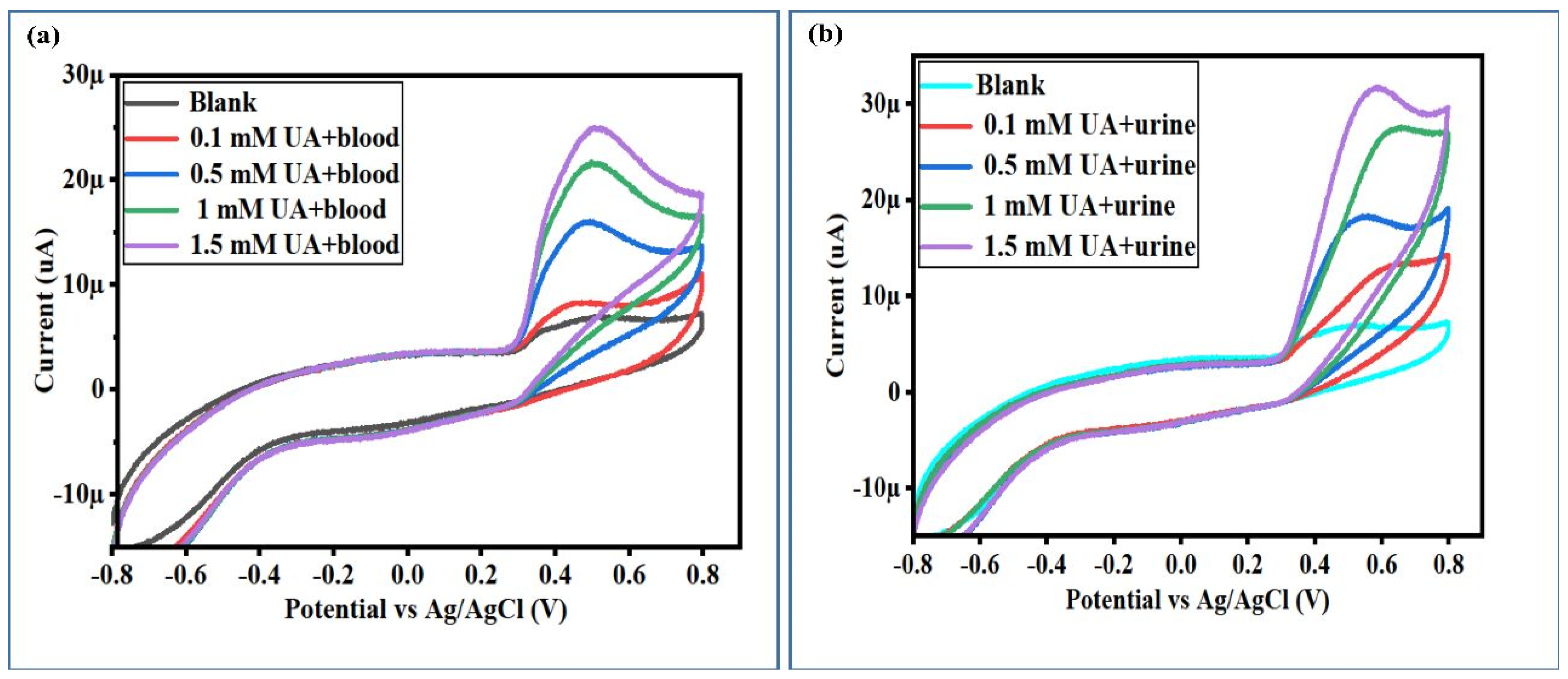
3.4. Human Blood Sample Analytical Applications of Proposed Non-Enzymatic UA Sensor-Based NiCo2O4 Nanostructures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinon, F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol. Rev. 2010, 233, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.X.; Hu, T.; Chen, S.; Wang, D.; Rao, Q.; Liu, Y.; Dai, F.; Guo, C.; Yang, H.B.; Li, C.M. Single-atom cobalt-based electrochemical biomimetic uric acid sensor with wide linear range and ultralow detection limit. Nano-Micro Lett. 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Li, Z.; Jia, Q. Detection of purine metabolite uric acid with picolinic-acid-functionalized metal–organic frameworks. ACS Appl. Mater. Interfaces 2019, 11, 34196–34202. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.-H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Palmer, I.; Schutte, A.; Huisman, H. Uric acid and the cardiovascular profile of African and Caucasian men. J. Hum. Hypertens. 2010, 24, 639–645. [Google Scholar]
- Roberts, J.M.; Gammill, H.S. Preeclampsia: Recent insights. Hypertension 2005, 46, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Dey, N.; Bhattacharya, S. Nanomolar level detection of uric acid in blood serum and pest-infested grain samples by an amphiphilic probe. Anal. Chem. 2017, 89, 10376–10383. [Google Scholar] [CrossRef]
- Talaat, K.M.; El-Sheikh, A.R. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am. J. Nephrol. 2007, 27, 435–440. [Google Scholar]
- Chen, X.; Chen, J.; Wang, F.; Xiang, X.; Luo, M.; Ji, X.; He, Z. Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosens. Bioelectron. 2012, 35, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Pormsila, W.; Krähenbühl, S.; Hauser, P.C. Capillary electrophoresis with contactless conductivity detection for uric acid determination in biological fluids. Anal. Chim. Acta 2009, 636, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Westley, C.; Xu, Y.; Thilaganathan, B.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Absolute quantification of uric acid in human urine using surface enhanced Raman scattering with the standard addition method. Anal. Chem. 2017, 89, 2472–2477. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, I.A.; Piedade, J.A.P.; Oliveira-Brett, A.M. Development of an HPLC method with electrochemical detection of femtomoles of 8-oxo-7, 8-dihydroguanine and 8-oxo-7, 8-dihydro-2′-deoxyguanosine in the presence of uric acid. Talanta 2004, 63, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yuan, R.; Chai, Y.; Chen, S.; Hu, F.; Zhang, M. Simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan on gold nanoparticles/overoxidized-polyimidazole composite modified glassy carbon electrode. Anal. Chim. Acta 2012, 741, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Yan, B. Phosphonate MOFs composite as off–on fluorescent sensor for detecting purine metabolite uric acid and diagnosing hyperuricuria. Inorg. Chem. 2017, 56, 6802–6808. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Vlessidis, A.G.; Evmiridis, N.P. Microdialysis sampling and monitoring of uric acid in vivo by a chemiluminescence reaction and an enzyme on immobilized chitosan support membrane. Anal. Chim. Acta 2003, 478, 23–30. [Google Scholar] [CrossRef]
- Yang, X.; Kirsch, J.; Simonian, A. Campylobacter spp. detection in the 21st century: A review of the recent achievements in biosensor development. J. Microbiol. Methods 2013, 95, 48–56. [Google Scholar] [CrossRef]
- Yang, X.; Kirsch, J.; Olsen, E.V.; Fergus, J.W.; Simonian, A.L. Anti-fouling PEDOT: PSS modification on glassy carbon electrodes for continuous monitoring of tricresyl phosphate. Sens. Actuators B Chem. 2013, 177, 659–667. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Shiraishi, Y.; Du, Y.; Yang, P. Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2017, 497, 172–180. [Google Scholar] [PubMed]
- Sha, R.; Puttapati, S.K.; Srikanth, V.V.; Badhulika, S. Ultra-sensitive non-enzymatic ethanol sensor based on reduced graphene oxide-zinc oxide composite modified electrode. IEEE Sens. J. 2017, 18, 1844–1848. [Google Scholar] [CrossRef]
- Sha, R.; Badhulika, S. Facile green synthesis of reduced graphene oxide/tin oxide composite for highly selective and ultra-sensitive detection of ascorbic acid. J. Electroanal. Chem. 2018, 816, 30–37. [Google Scholar] [CrossRef]
- Sha, R.; Komori, K.; Badhulika, S. Graphene–Polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim. Acta 2017, 233, 44–51. [Google Scholar] [CrossRef]
- Ndamanisha, J.C.; Guo, L. Electrochemical determination of uric acid at ordered mesoporous carbon functionalized with ferrocenecarboxylic acid-modified electrode. Biosens. Bioelectron. 2008, 23, 1680–1685. [Google Scholar] [CrossRef]
- Wu, L.; Feng, L.; Ren, J.; Qu, X. Electrochemical detection of dopamine using porphyrin-functionalized graphene. Biosens. Bioelectron. 2012, 34, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, T.; Gao, Z.; Xu, H.; Zhou, B.; Wang, C. Selective detection of uric acid in the presence of ascorbic acid at physiological pH by using a β-cyclodextrin modified copolymer of sulfanilic acid and N-acetylaniline. Biosens. Bioelectron. 2008, 23, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Chu, X.; Yang, Y.; Li, X.; Zhang, X.; Chen, J. Hollow nitrogen-doped carbon microspheres pyrolyzed from self-polymerized dopamine and its application in simultaneous electrochemical determination of uric acid, ascorbic acid and dopamine. Biosens. Bioelectron. 2011, 26, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Şahin, Y. Preparation of selective and sensitive electrochemically treated pencil graphite electrodes for the determination of uric acid in urine and blood serum. Biosens. Bioelectron. 2010, 25, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Wayu, M.B.; Schwarzmann, M.A.; Gillespie, S.D.; Leopold, M.C. Enzyme-free uric acid electrochemical sensors using β-cyclodextrin-modified carboxylic acid-functionalized carbon nanotubes. J. Mater. Sci. 2017, 52, 6050–6062. [Google Scholar] [CrossRef]
- Solangi, A.G.; Pirzada, T.; Shah, A.A.; Halepoto, I.A.; Chang, A.S.; Solangi, Z.A.; Solangi, M.Y.; Aftab, U.; Tonezzer, M.; Tahira, A. Phytochemicals of mustard (Brassica Campestris) leaves tuned the nickel-cobalt bimetallic oxide properties for enzyme-free sensing of glucose. J. Chin. Chem. Soc. 2022, 69, 1608–1618. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 2014, 6, 1369–1376. [Google Scholar] [CrossRef]
- Prathap, M.A.; Satpati, B.; Srivastava, R. Facile preparation of β-Ni(OH)2-NiCo2O4 hybrid nanostructure and its application in the electro-catalytic oxidation of methanol. Electrochim. Acta 2014, 130, 368–380. [Google Scholar] [CrossRef]
- Qian, L.; Gu, L.; Yang, L.; Yuan, H.; Xiao, D. Direct growth of NiCo2O4 nanostructures on conductive substrates with enhanced electrocatalytic activity and stability for methanol oxidation. Nanoscale 2013, 5, 7388–7396. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, Z.; Zheng, X.; Yao, S.; Yang, X.; Zhai, T. Core-shell structured Co3O4@NiCo2O4 electrodes grown on flexible carbon fibers with superior electrochemical properties. Nano Energy 2017, 31, 410–417. [Google Scholar] [CrossRef]
- Xu, K.; Yang, X.; Yang, J.; Hu, J. Synthesis of hierarchical Co3O4@NiCo2O4 core-shell nanosheets as electrode materials for supercapacitor application. J. Alloys Compd. 2017, 700, 247–251. [Google Scholar]
- Zhang, G.; Wang, T.; Yu, X.; Zhang, H.; Duan, H.; Lu, B. Nanoforest of hierarchical Co3O4@NiCo2O4 nanowire arrays for high-performance supercapacitors. Nano Energy 2013, 2, 586–594. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, L.; Gan, M.; Ye, M.; Li, X.; Zhai, Y.; Yan, F.; Cao, F. Monodisperse MnO2@NiCo2O4 core/shell nanospheres with highly opened structures as electrode materials for good-performance supercapacitors. Appl. Surf. Sci. 2018, 444, 1–9. [Google Scholar]
- Jokar, E.; Zad, A.I.; Shahrokhian, S. Synthesis and characterization of NiCo2O4 nanorods for preparation of supercapacitor electrodes. J. Solid State Electrochem. 2015, 19, 269–274. [Google Scholar]
- Chu, Q.; Yang, B.; Wang, W.; Tong, W.; Wang, X.; Liu, X.; Chen, J. Fabrication of a Stainless-Steel-Mesh-Supported Hierarchical Fe2O3@NiCo2O4 Core-Shell Tubular Array Anode for Lithium-Ion Battery. ChemistrySelect 2016, 1, 5569–5573. [Google Scholar]
- Huang, G.; Zhang, L.; Zhang, F.; Wang, L. Metal–organic framework derived Fe2O3@NiCo2O4 porous nanocages as anode materials for Li-ion batteries. Nanoscale 2014, 6, 5509–5515. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, R.M.P.; Perez, R.L. Raphanus sativus (Radish): Their chemistry and biology. Sci. World J. 2004, 4, 811. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [PubMed]
- Nawaz, H.; Shad, M.A.; Rauf, A. Optimization of extraction yield and antioxidant properties of Brassica oleracea Convar Capitata Var L. leaf extracts. Food Chem. 2018, 242, 182–187. [Google Scholar] [CrossRef]
- Amin, S.; Tahira, A.; Solangi, A.R.; Mazzaro, R.; Ibupoto, Z.H.; Fatima, A.; Vomiero, A. Functional nickel oxide nanostructures for ethanol oxidation in alkaline media. Electroanalysis 2020, 32, 1052–1059. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Tahira, A.; Shah, A.A.; Aftab, U.; Solangi, M.Y.; Leghari, J.A.; Samoon, A.H.; Bhatti, A.L.; Bhatti, M.A.; Mazzaro, R. NiCo2O4 nanostructures loaded onto pencil graphite rod: An advanced composite material for oxygen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 6650–6665. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B.; Lin, R.; Ho-Kimura, S.; He, G.; Zhou, X.; Hu, J.; Parkin, I.P. A dendritic nickel cobalt sulfide nanostructure for alkaline battery electrodes. Adv. Funct. Mater. 2018, 28, 1705937. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hang, T.; Chu, D.; Li, M. Three-dimensional hierarchical nanostructured Cu/Ni–Co coating electrode for hydrogen evolution reaction in alkaline media. Nano-Micro Lett. 2015, 7, 347–352. [Google Scholar]
- López-Tinoco, J.; Mendoza-Cruz, R.; Bazán-Díaz, L.; Karuturi, S.C.; Martinelli, M.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Jacobs, G. The preparation and characterization of Co–Ni nanoparticles and the testing of a heterogenized Co–Ni/alumina catalyst for CO hydrogenation. Catalysts 2019, 10, 18. [Google Scholar]
- Lei, Y.; Li, J.; Wang, Y.; Gu, L.; Chang, Y.; Yuan, H.; Xiao, D. Rapid microwave-assisted green synthesis of 3D hierarchical flower-shaped NiCo2O4 microsphere for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 1773–1780. [Google Scholar] [CrossRef]
- Marco, J.; Gancedo, J.; Gracia, M.; Gautier, J.; Ríos, E.; Berry, F. Characterization of the nickel cobaltite, NiCo2O4, prepared by several methods: An XRD, XANES, EXAFS, and XPS study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Feng, S.; Chen, S.-M.; Ding, Y.; Chen, C.; Hao, Q.; Yang, T.-H.; Lei, W. A novel electrochemical sensor for uric acid detection based on PCN/MWCNT. Ionics 2019, 25, 4437–4445. [Google Scholar] [CrossRef]
- Tsierkezos, N.G.; Ritter, U.; Thaha, Y.N.; Downing, C.; Szroeder, P.; Scharff, P. Multi-walled carbon nanotubes doped with boron as an electrode material for electrochemical studies on dopamine, uric acid, and ascorbic acid. Microchim. Acta 2016, 183, 35–47. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.e.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar]
- Ma, L.; Zhang, Q.; Wu, C.; Zhang, Y.; Zeng, L. PtNi bimetallic nanoparticles loaded MoS2 nanosheets: Preparation and electrochemical sensing application for the detection of dopamine and uric acid. Anal. Chim. Acta 2019, 1055, 17–25. [Google Scholar] [PubMed]
- Hassanvand, Z.; Jalali, F. Simultaneous determination of l-DOPA, l-tyrosine and uric acid by cysteic acid-modified glassy carbon electrode. Mater. Sci. Eng. C 2019, 98, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef]
- Chang, A.S.; Tahira, A.; Chang, F.; Memon, N.N.; Nafady, A.; Kasry, A.; Ibupoto, Z.H. Silky Co3O4 nanostructures for the selective and sensitive enzyme free sensing of uric acid. RSC Adv. 2021, 11, 5156–5162. [Google Scholar] [CrossRef]
- Guan, H.; Peng, B.; Gong, D.; Han, B.; Zhang, N. Electrochemical Enhanced Detection of Uric Acid Based on Peroxidase-like Activity of Fe3O4@Au. Electroanalysis 2021, 33, 1736–1745. [Google Scholar]
- Balwinder, K.; Biswarup, S.; Rajendra, S. Synthesis of NiCo2O4/Nano-ZSM-5 nanocomposite material with enhanced electrochemical properties for the simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. New. J. Chem. 2015, 39, 1115–1124. [Google Scholar]
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold Nanoparticle Decorated Polypyrrole/Graphene Oxide Nanosheets as a Modified Electrode for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. New. J. Chem. 2020, 12, 4916–4926. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ye, D.; Luo, J.; Su, B.; Zhang, S.; Kong, J. An electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan based on MWNTs bridged mesocellular graphene foam nanocomposite. Talanta 2014, 127, 255–261. [Google Scholar]
- Mobili, R.; Preda, G.; La Cognata, S.; Toma, L.; Pasini, D.; Amendola, V. Chiroptical sensing of perrhenate in aqueous media by a chiral organic cage. Chem. Commun. 2022, 58, 3897–3900. [Google Scholar] [CrossRef] [PubMed]





| Sensing Electrode Material | Linear Range (ụM) | Detection of Limit (ụM) | References |
|---|---|---|---|
| PCN a/MWCNT b | 0.2–20 | 0.139 | [50] |
| B-MWCNTS c | 62–250 | 0.65 | [51] |
| Pd/RGO d | 6–469.5 | 1.6 | [18] |
| Au/RGO d | 8.8–53 | 1.8 | [52] |
| PtNi@MoS2 e | 0.5–600 | 0.1 | [53] |
| Cysteic acid | 1.0–19 | 0.36 | [54] |
| Co3O4 | 500–3500 | 100 | [56] |
| Fe2O3 @Au | 100–10,000 | 0.087 | [57] |
| NiCo2O4 (30%)/Nano-ZSM-5 | 0.9–1000 | 0.7 | [58] |
| AuNPs@GO/PPy/CFP | 2–360 | 1.68 | [59] |
| MWNTs/MGF | 300–1000 | 0.93 | [60] |
| NiCo2O4 NPs | 100–8000 | 0.005 | This work |
| Sample | Added (mM) | Found (mM) | %Recovery | % RSD |
|---|---|---|---|---|
| Blood 1 | - | 0.1 | - | |
| - | 0.5 | 0.612 ± 0.002 | 101.66 | 0.52 |
| - | 1 | 1.123 ± 0.0023 | 101.81 | 0.53 |
| - | 2 | 2.084 ± 0.0017 | 99 | 0.49 |
| - | 2.5 | 2.621 ± 0.0013 | 100.77 | 0.54 |
| Blood 2 | - | 1.00 | - | - |
| - | 1.5 | 2.533 ± 0.0022 | 101.2 | 0.48 |
| - | 2 | 3.082 ± 0.0019 | 102.66 | 0.56 |
| - | 2.5 | 3.514 ± 0.0024 | 100.28 | 0.50 |
| - | 3 | 4.063 ± 0.0018 | 101.5 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solangi, A.G.; Tahira, A.; Waryani, B.; Chang, A.S.; Pirzada, T.; Nafady, A.; Dawi, E.A.; Saleem, L.M.A.; Padervand, M.; Haj Ismail, A.A.K.; et al. Green-Mediated Synthesis of NiCo2O4 Nanostructures Using Radish White Peel Extract for the Sensitive and Selective Enzyme-Free Detection of Uric Acid. Biosensors 2023, 13, 780. https://doi.org/10.3390/bios13080780
Solangi AG, Tahira A, Waryani B, Chang AS, Pirzada T, Nafady A, Dawi EA, Saleem LMA, Padervand M, Haj Ismail AAK, et al. Green-Mediated Synthesis of NiCo2O4 Nanostructures Using Radish White Peel Extract for the Sensitive and Selective Enzyme-Free Detection of Uric Acid. Biosensors. 2023; 13(8):780. https://doi.org/10.3390/bios13080780
Chicago/Turabian StyleSolangi, Abdul Ghaffar, Aneela Tahira, Baradi Waryani, Abdul Sattar Chang, Tajnees Pirzada, Ayman Nafady, Elmuez A. Dawi, Lama M. A. Saleem, Mohsen Padervand, Abd Al Karim Haj Ismail, and et al. 2023. "Green-Mediated Synthesis of NiCo2O4 Nanostructures Using Radish White Peel Extract for the Sensitive and Selective Enzyme-Free Detection of Uric Acid" Biosensors 13, no. 8: 780. https://doi.org/10.3390/bios13080780
APA StyleSolangi, A. G., Tahira, A., Waryani, B., Chang, A. S., Pirzada, T., Nafady, A., Dawi, E. A., Saleem, L. M. A., Padervand, M., Haj Ismail, A. A. K., Lv, K., Vigolo, B., & Ibupoto, Z. H. (2023). Green-Mediated Synthesis of NiCo2O4 Nanostructures Using Radish White Peel Extract for the Sensitive and Selective Enzyme-Free Detection of Uric Acid. Biosensors, 13(8), 780. https://doi.org/10.3390/bios13080780









