Chromogenic Mechanisms of Colorimetric Sensors Based on Gold Nanoparticles
Abstract
:1. Introduction
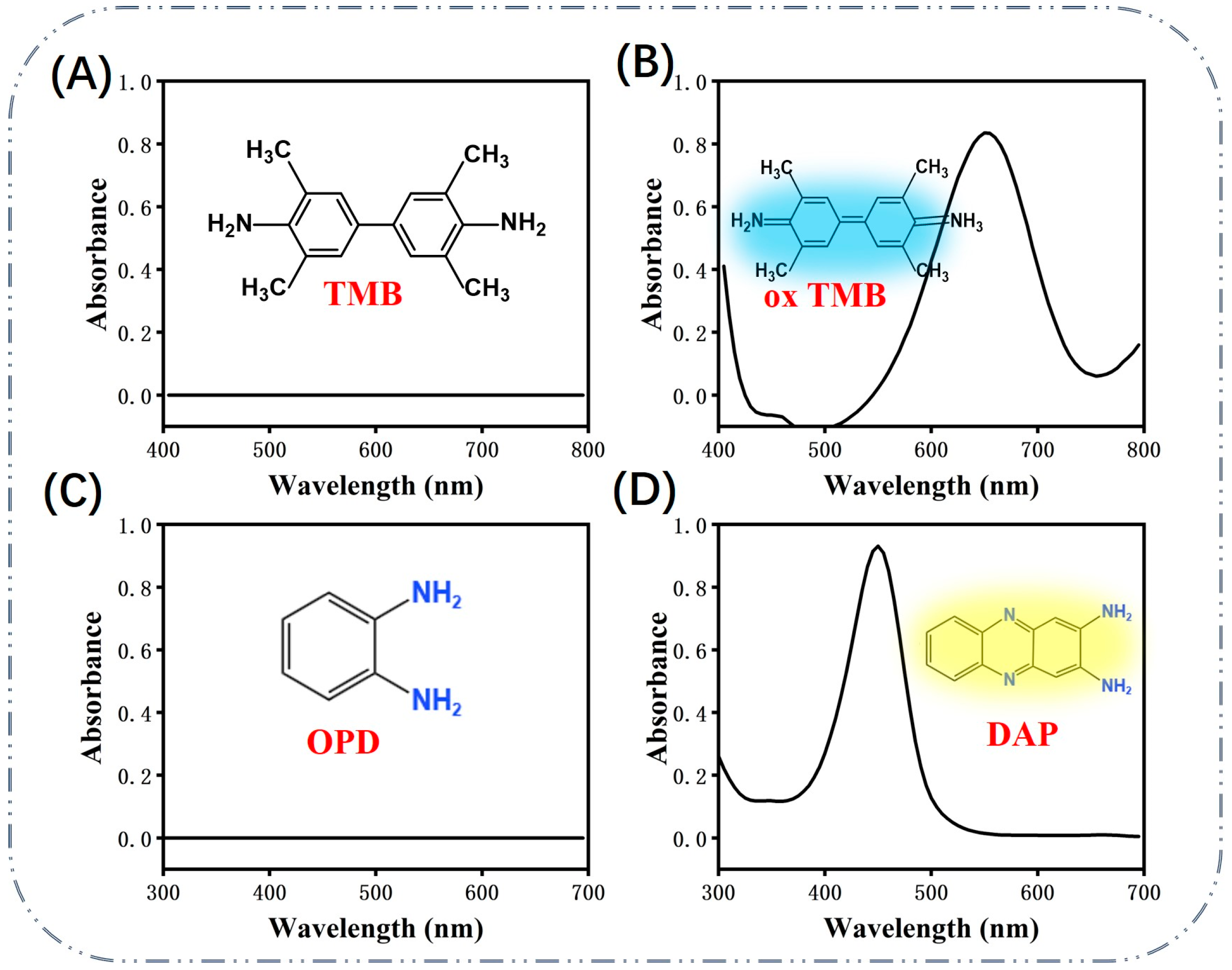
2. Sensing Strategy Based on the Activity of Au NP-Like Enzymes
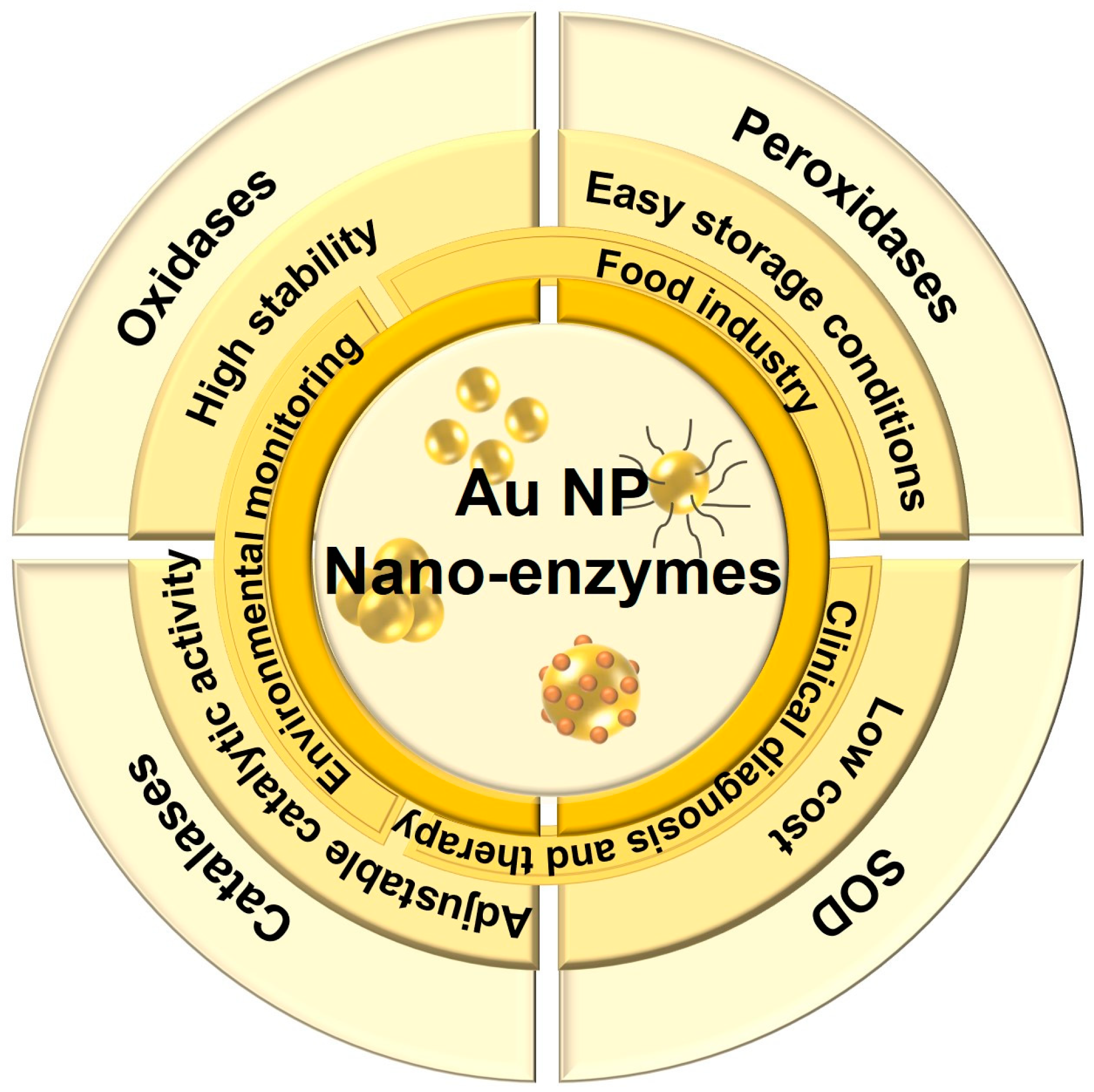
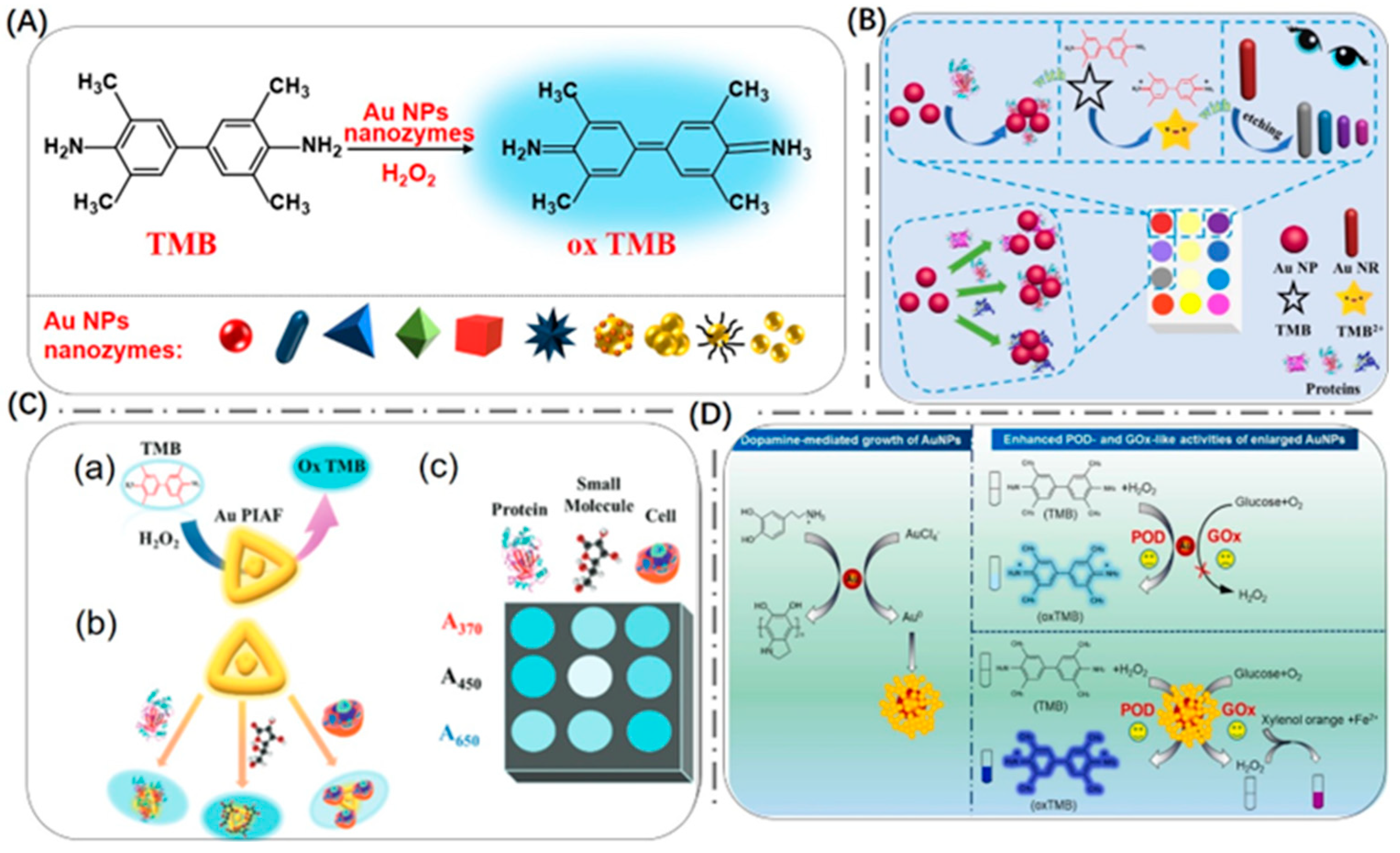
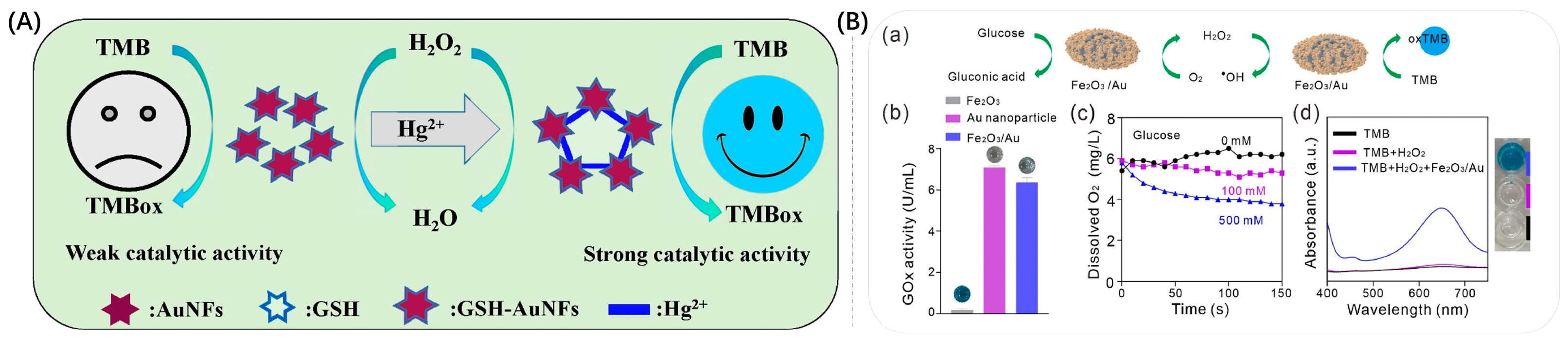
2.1. Gold Nano-Enzymes
2.2. Composite Nano-Enzymes
2.2.1. Surface Modification
2.2.2. Hybridization
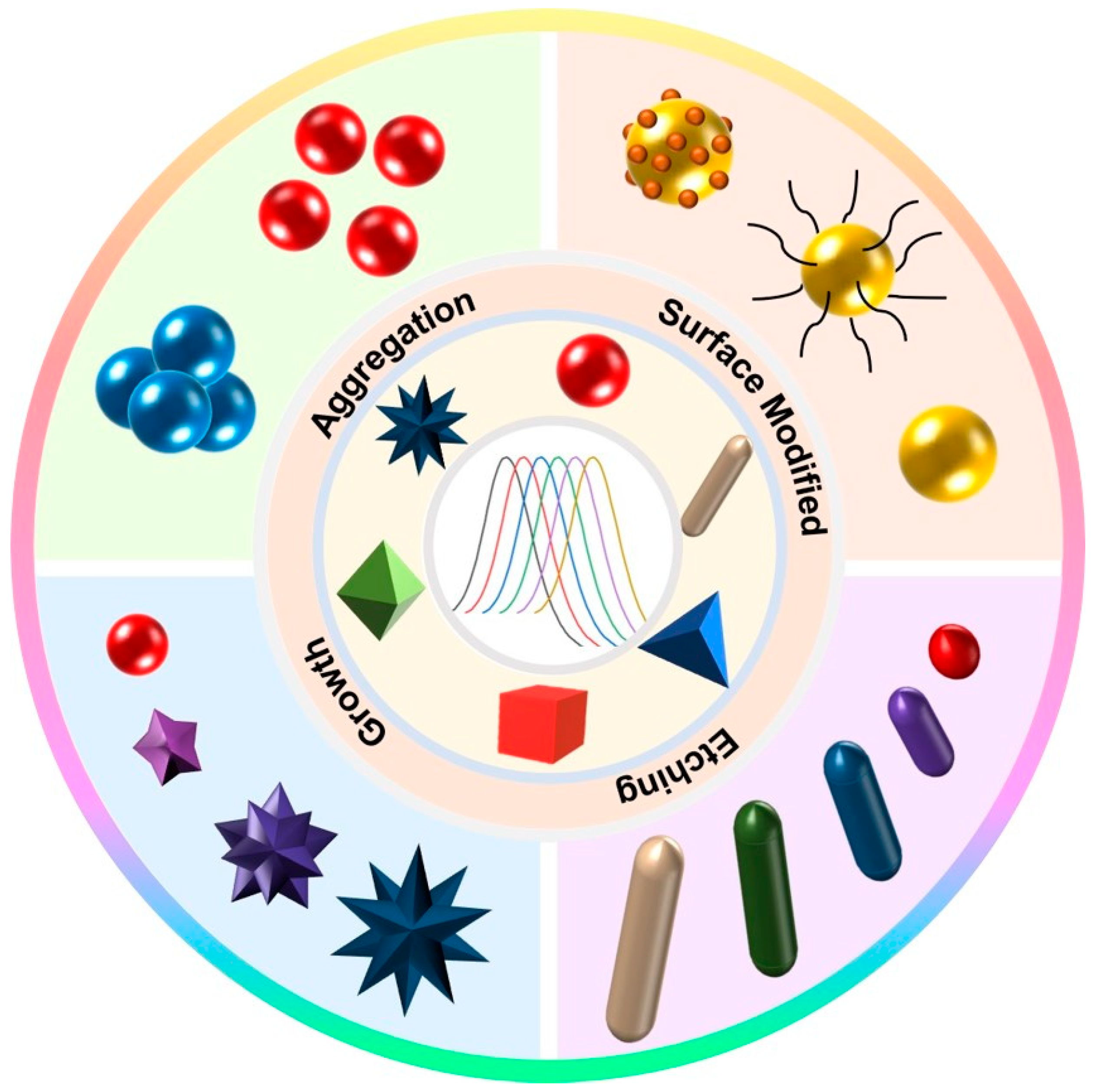
3. Sensing Strategy Based on the LSPR Properties of Au NPs
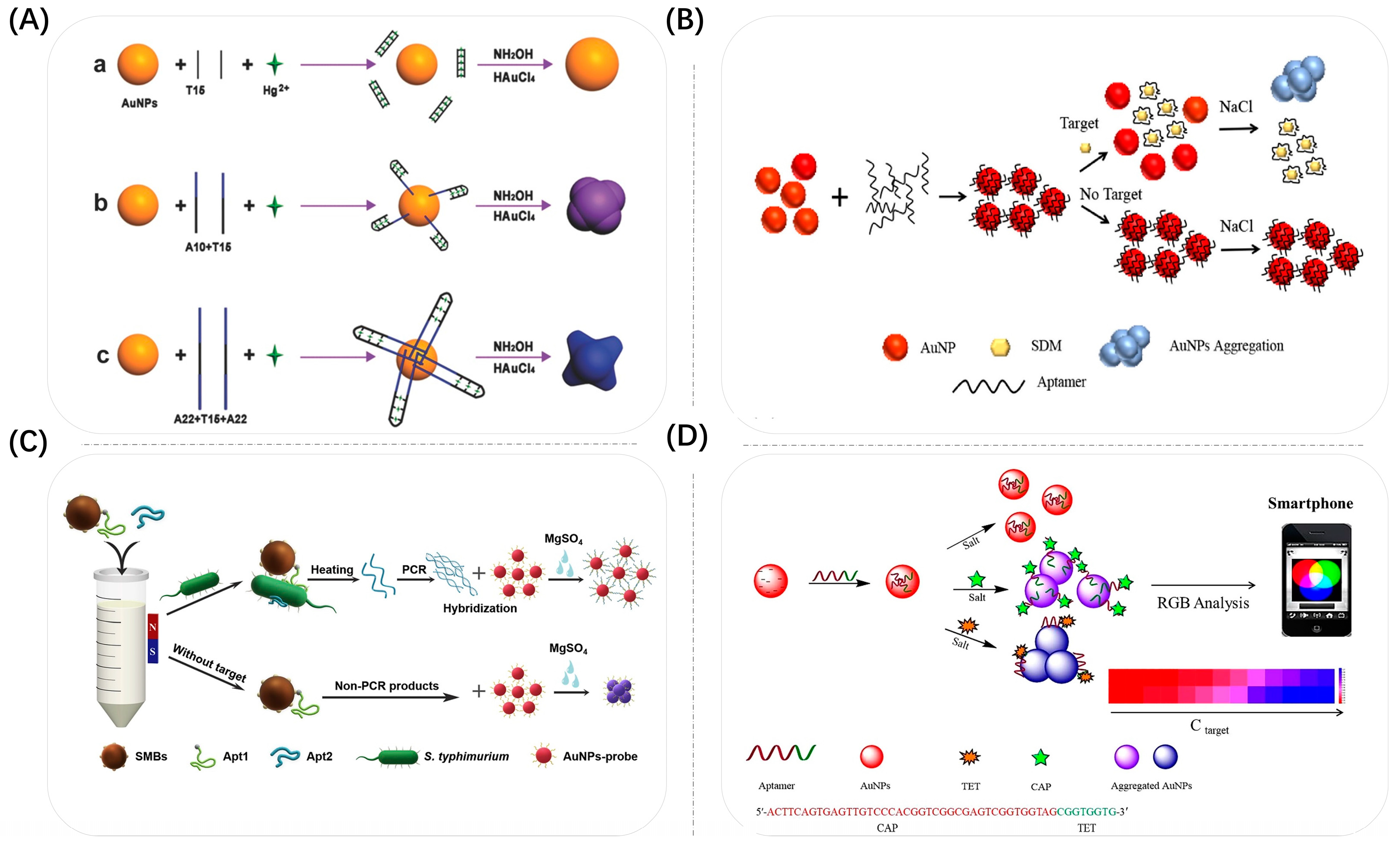
3.1. Aggregation
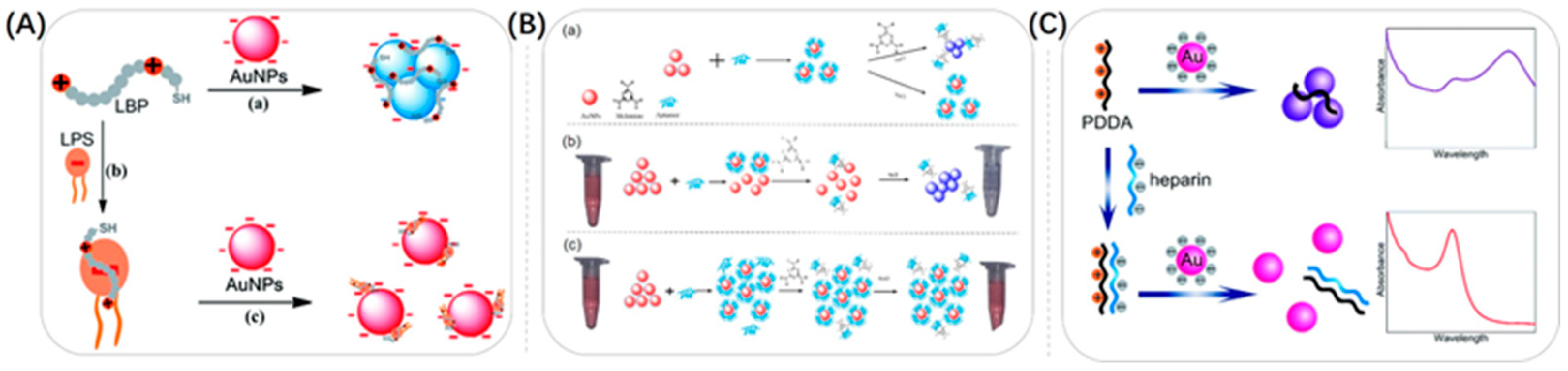
3.1.1. Bio-Specificity
3.1.2. Electrostatic Interactions
3.1.3. Covalent Bonding
3.2. Surface Modification
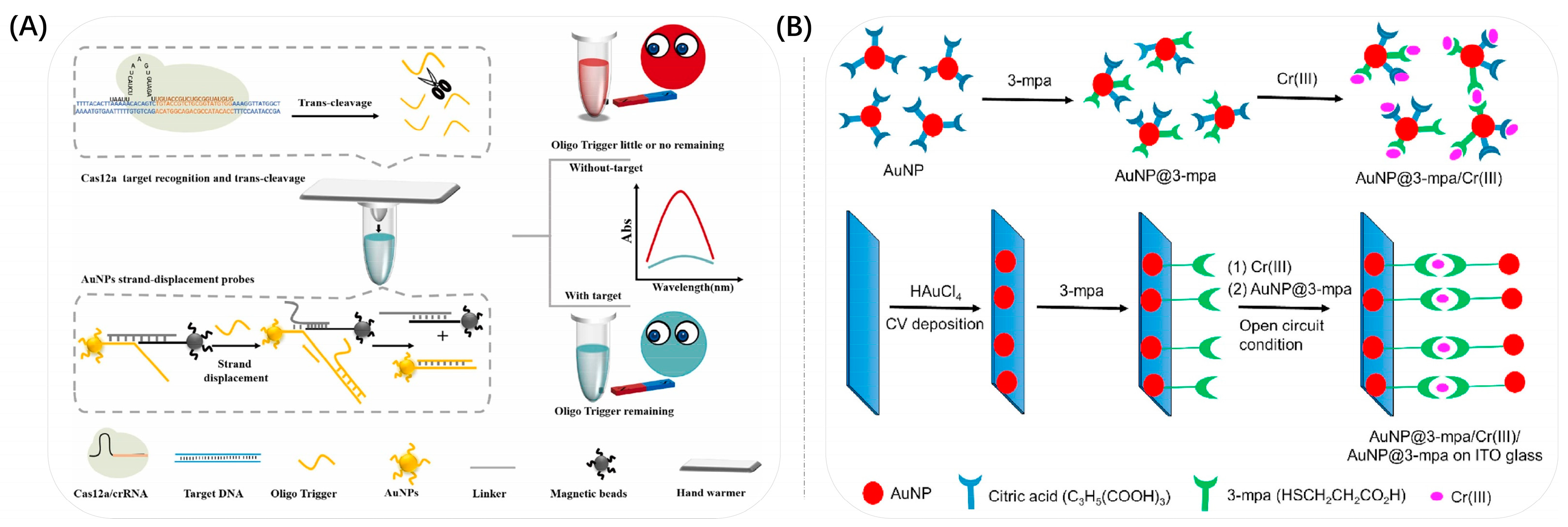
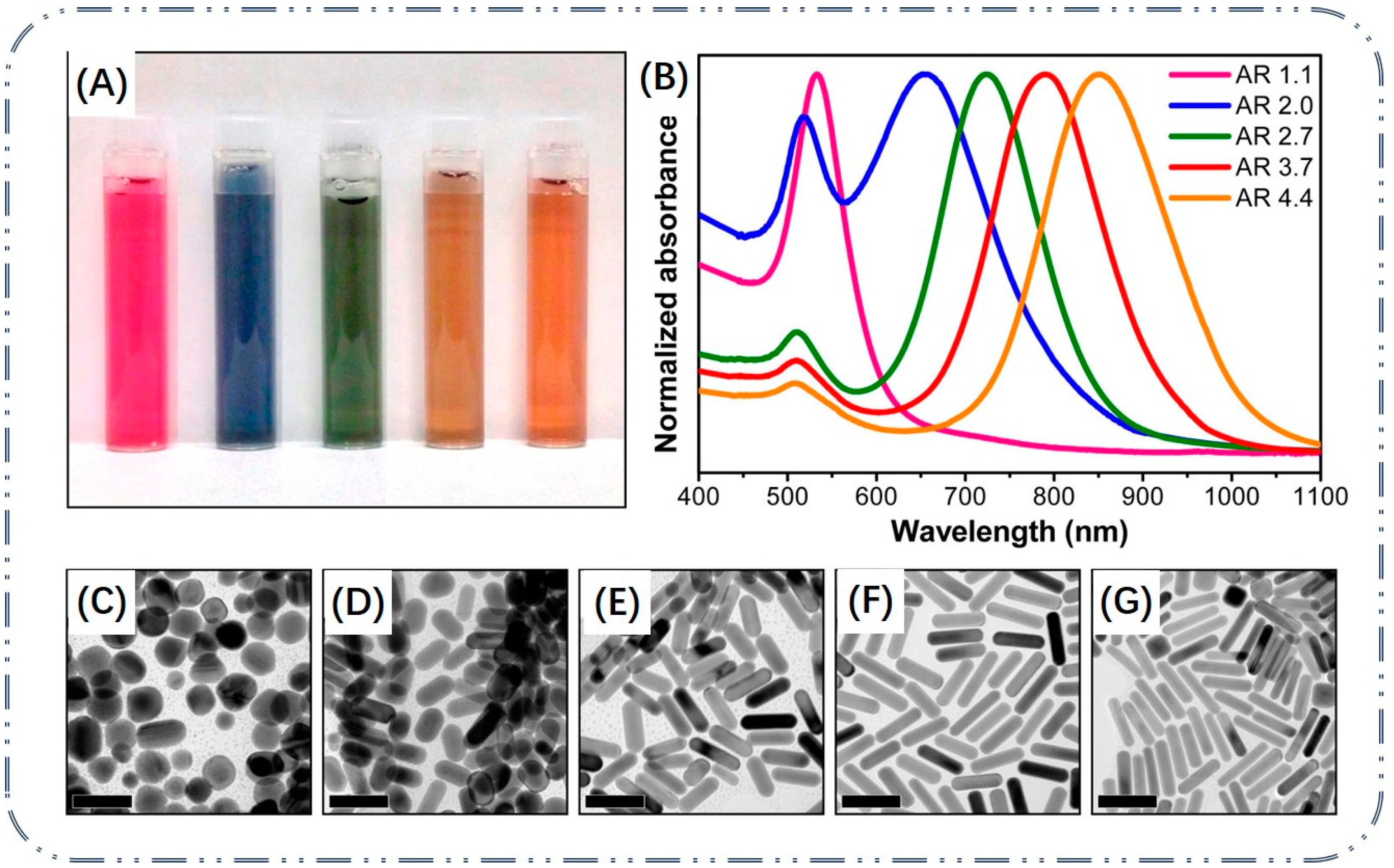
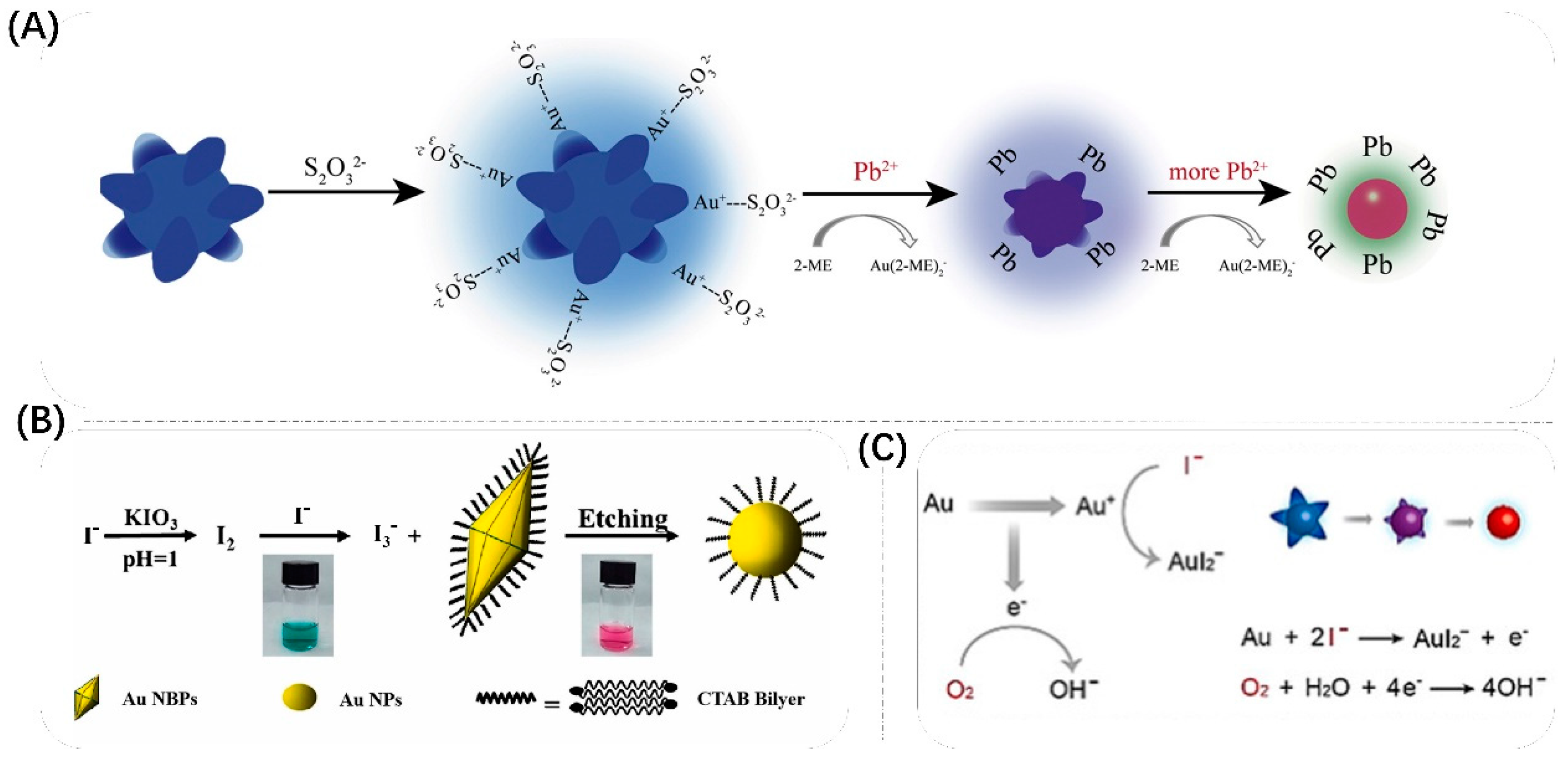
3.3. Growth
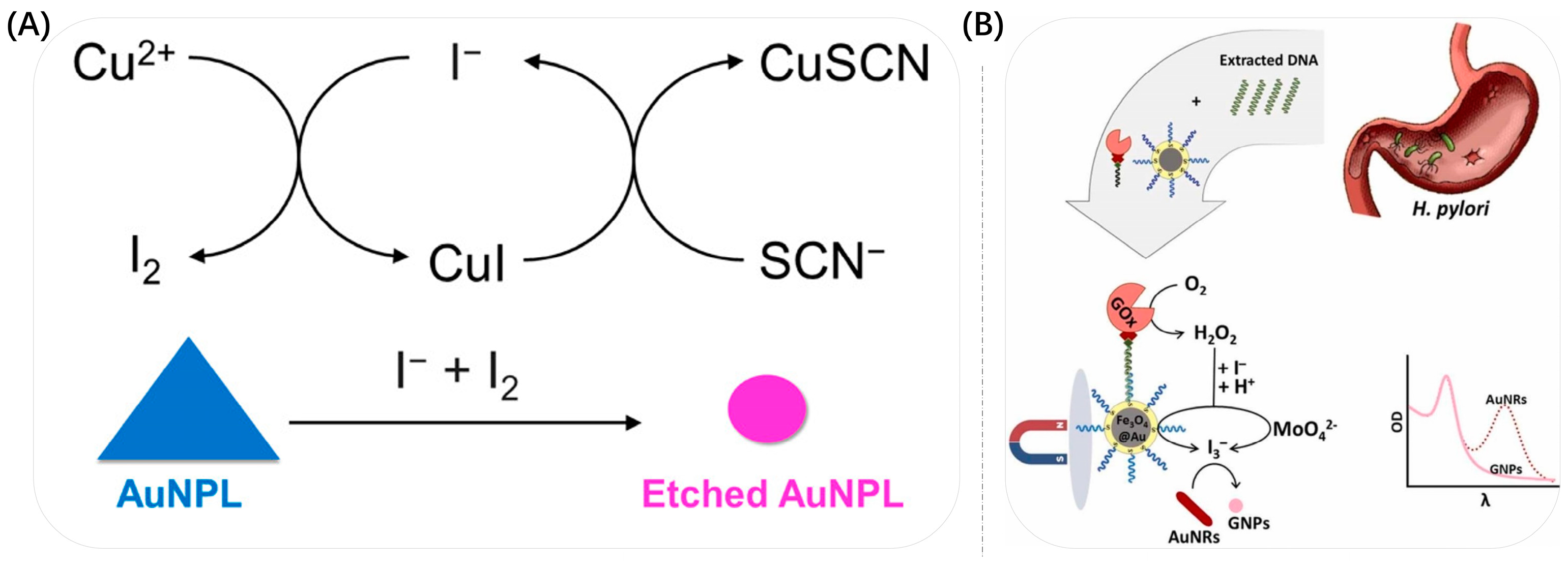
3.4. Etching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, L.Q.; Hong, F.; Zeng, L.W.; Chen, Y.P. “Three-in-one” Zr-MOF multifunctional carrier-mediated fluorescent and colorimetric dual-signal readout biosensing platform to enhance analytical performance. ACS Appl. Mater. Interfaces 2022, 14, 51234–51243. [Google Scholar] [CrossRef]
- Dou, L.N.; Luo, L.P.; Zhang, X.; Zhang, W.T.; Liu, L.Z.; Yin, X.C.; Liu, S.J.; Sun, J.; Zhang, D.H.; Wang, J.L. Biomimetic cell model for fluorometric and smartphone colorimetric dual-signal readout detection of bacterial toxin. Sens. Actuators B Chem. 2020, 312, 127956. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.W.; Liu, Q.Y.; Chen, Z.B. Colorimetric differentiation of multiple oxidizing anions based on two core-shell Au@Ag nanoparticles with different morphologies as array recognition elements. Anal. Chem. 2020, 92, 7123–7129. [Google Scholar] [CrossRef]
- Jing, W.J.; Cui, X.K.; Kong, F.B.; Wei, W.; Li, Y.C.; Fan, L.Z.; Li, X.H. Fe-N/C single-atom nanozyme-based colorimetric sensor array for discriminating multiple biological antioxidants. Analyst 2021, 146, 207–212. [Google Scholar] [CrossRef]
- Wang, H.Q.; Rao, H.H.; Xue, X.; An, P.L.; Gao, M.; Luo, M.Y.; Liu, X.H.; Xue, Z.H. Target-mediated surface chemistry of gold nanorods for breaking the low color resolution limitation of monocolorimetric sensor. Anal. Chim. Acta 2020, 1097, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Tawa, K. Plasmonic coloration of silver nanodome arrays for a smartphone-based plasmonic biosensor. Nanoscale Adv. 2019, 1, 3699–3708. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yuan, C.L.; Geng, G.X.; Shi, R.; Cheng, S.Q.; Wang, Y.L. Enzyme-free colorimetric determination of uric acid based on inhibition of gold nanorods etching. Sens. Actuators B Chem. 2021, 333, 129638. [Google Scholar] [CrossRef]
- Zhang, C.H.; Jiang, X.K.; Yu, F.H.; Liu, Y.; Yue, Q.; Yang, P.; Liu, Y.C. Antagonistic action regulated anti-etching colorimetric detection of thiram residue in soil based on triangular silver nanoplates. Sens. Actuators B Chem. 2021, 344, 130304. [Google Scholar] [CrossRef]
- Yao, L.; Li, X.; Li, H.; Liao, Z.B.; Xie, C.C.; Ning, G.; Wu, Y.H.; Wang, Y.H. Detection of pyrophosphate and alkaline phosphatase activity based on polyT single stranded DNA-copper nanoclusters. J. Fluoresc. 2022, 32, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Yang, Z.L.; Tang, F.L.; Wang, D.; Feng, M.; Liao, X.J.; Yang, X.P. Fe3O4/Carbon nanodot hybrid nanoparticles for the indirect colorimetric detection of glutathione. ACS Appl. Mater. Interfaces 2019, 2, 3951–3959. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, J.R.; Hu, K.L.; He, Y.; Huang, K.; Chen, P.P. Enzymatic reaction modulated gold nanoparticle aggregation-induced photothermal and smartphone readable colorimetry dual-mode biosensing platform for trypsin detection in clinical samples. Sens. Actuators B Chem. 2023, 374, 132841. [Google Scholar] [CrossRef]
- Luo, Q.; Tian, M.J.; Luo, F.; Zhao, M.; Lin, C.Y.; Qiu, B.; Wang, J.; Lin, Z.Y. Multicolor biosensor for trypsin detection based on the regulation of the peroxidase activity of bovine serum albumin-coated gold nanoclusters and etching of gold nanobipyramids. Anal. Chem. 2023, 95, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Zhao, C.X.; Lin, L.L. Plasma-based instant synthesis of functionalized gold nanoparticles for colorimetric detection of lead ions. Chem. Eng. Sci. 2022, 260, 117849. [Google Scholar] [CrossRef]
- Creyer, M.N.; Jin, Z.C.; Retout, M.; Yim, W.; Zhou, J.J.; Jokerst, J.V. Gold-silver core-shell nanoparticle crosslinking mediated by protease activity for colorimetric enzyme detection. Langmuir 2022, 38, 14200–14207. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, J.; Luo, Z.; Kim, J.; Kim, J.H.; Hyeon, T.; Mehraeen, S.; Park, S.; Park, J. Shape transformation mechanism of gold nanoplates. ACS Nano 2023, 17, 2007–2018. [Google Scholar] [CrossRef]
- Liang, D.W.; Wang, Y.W.; Ma, L.R.; Liu, Y.L.; Fu, R.J.; Liu, H.R.; Peng, Y.L.; Zhang, Y.H.; Wang, C.Q.; Jiao, B.N.; et al. Controlled growth of gold nanobipyramids using thiocholine for plasmonic colorimetric detection of organophosphorus pesticides. ACS Appl. Mater. Interfaces 2022, 5, 16978–16986. [Google Scholar] [CrossRef]
- Dandu, S.S.; Joshi, D.J.; Park, T.J.; Kailasa, S.K. Functionalization of gold nanostars with melamine for colorimetric detection of uric acid. Appl. Spectrosc. 2023, 77, 360–370. [Google Scholar] [CrossRef]
- Thakkar, S.; Liu, J.; Dumee, L.F.; Singh, B.R.; Shukla, S.; Yang, W.R. Arsenic ion assisted core-satellites nano-assembly of gold nanoparticles for its colorimetric determination in water. J. Water Process. Eng. 2022, 48, 102833. [Google Scholar] [CrossRef]
- Tian, Q.; Cao, S.; He, G.; Long, Y.; Zhou, X.; Zhang, J.; Xie, J.; Zhao, X. Plasmonic Au-Ag alloy nanostars based high sensitivity surface enhanced Raman spectroscopy fiber probes. J. Alloys Compd. 2022, 900, 163345. [Google Scholar] [CrossRef]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, functionalization, modification, and applications of nanostructured gold: A critical review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Mat’atkova, O.; Michailidu, J.; Miskovska, A.; Kolouchova, I.; Masak, J.; Cejkova, A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.K.; Chen, K.C.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.Q.; Jiao, L.; Tang, Y.J.; Chen, Y.F.; Gu, W.L.; Zhu, C.Z. Defect engineering in nanozymes. Mater. Today 2022, 52, 327–347. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yan, X.; Fan, K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, G.M.; Xu, P.; Lai, C.; Tang, L. How do enzymes ‘meet’ nanoparticles and nanomaterials? Trends Biochem. Sci. 2017, 42, 914–930. [Google Scholar] [CrossRef]
- Perwez, M.; Lau, S.Y.; Hussain, D.; Anboo, S.; Arshad, M.; Thakur, P. Nanozymes and nanoflower: Physiochemical properties, mechanism and biomedical applications. Colloids Surf. B 2023, 225, 113241. [Google Scholar] [CrossRef]
- Fu, M.L.; Li, L.; Yang, D.Y.; Tu, Y.F.; Yan, J.L. Colorimetric detections of iodide and mercuric ions based on a regulation of an Enzyme-Like activity from gold nanoclusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121450. [Google Scholar] [CrossRef]
- Ni, P.J.; Liu, S.Y.; Wang, B.; Chen, C.X.; Jiang, Y.Y.; Zhang, C.H.; Chen, J.B.; Lu, Y.Z. Light-responsive Au nanoclusters with oxidase-like activity for fluorescent detection of total antioxidant capacity. J. Hazard. Mater. 2021, 411, 125106. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, X.; Han, L.; Li, F. “Non-naked” gold with glucose oxidase-like activity: A nanozyme for tandem catalysis. Small 2018, 14, 1803256. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, N.; Wen, J.; Yang, D.; Chen, H.; Long, Y.; Zheng, H. Detecting uric acid base on the dual inner filter effect using BSA@Au nanoclusters as both peroxidase mimics and fluorescent reporters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122504. [Google Scholar] [CrossRef]
- Tang, Z.; Ali, I.; Hou, Y.; Akakuru, O.U.; Zhang, Q.; Mushtaq, A.; Zhang, H.; Lu, Y.; Ma, X.; Ge, J.; et al. pH-Responsive Au@Pd bimetallic core-shell nanorods for enhanced synergistic targeted photothermal-augmented nanocatalytic therapy in the second near-infrared window. J. Mater. Chem. B 2022, 10, 6532–6545. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Tang, S.; Guo, S.; Li, H.; Shao, N. In situ synthesis of red fluorescent gold nanoclusters with enzyme-like activity for oxidative stress amplification in chemodynamic therapy. Chin. Chem. Lett. 2022, 33, 1331–1336. [Google Scholar] [CrossRef]
- Yang, F.; Chen, T.M.; Wu, X.J.; Chen, Y.; Yang, G.W. A hybrid gold-carbyne nanocrystals platform for light-induced crossover of redox enzyme-like activities. Chem. Eng. J. 2021, 408, 127244. [Google Scholar] [CrossRef]
- Hong, C.Y.; Meng, X.Q.; He, J.Y.; Fan, K.L.; Yan, X.Y. Nanozyme: A promising tool from clinical diagnosis and environmental monitoring to wastewater treatment. Particuology 2022, 71, 90–107. [Google Scholar] [CrossRef]
- Heo, J.H.; Sung, M.; Trung, T.Q.; Lee, Y.; Jung, D.H.; Kim, H.; Kaushal, S.; Lee, N.E.; Kim, J.W.; Lee, J.H.; et al. Sensor design strategy for environmental and biological monitoring. Ecomat 2023, 5, e12332–e12358. [Google Scholar] [CrossRef]
- Balasurya, S.; Okla, M.K.; Al-ghamdi, A.A.; Al-amri, S.A.; Alatar, A.A.; Abdel-Maksoud, M.A.; Aufy, M.; Khan, S.S. A sensitive, fast, selective, and reusable enzyme-free simultaneous determination of glucose and environmental monitoring of phosphorus sensor based on Ag@Li dual shell hallow nanospheres. J. Clust. Sci. 2023, 34, 2487–2496. [Google Scholar] [CrossRef]
- Song, Y.F.; Huang, C.Z.; Li, Y.F. Nanozymes from Cu(II) metal-organic gel and melamine for highly active peroxidase-like activity to detect alkaline phosphatase. ACS Appl. Mater. Interfaces 2023, 6, 1369–1378. [Google Scholar] [CrossRef]
- Song, E.H.; Li, Y.X.; Chen, L.L.; Lan, X.P.; Hou, C.S.; Liu, C.L.; Liu, C.Z. An amino acid-based supramolecular nanozyme by coordination self-assembly for cascade catalysis and enhanced chemodynamic therapy towards biomedical applications. Nanoscale Adv. 2021, 3, 6482–6489. [Google Scholar] [CrossRef]
- Ding, X.T.; Zhao, Z.; Zhang, Y.F.; Duan, M.L.; Liu, C.Z.; Xu, Y.H. Activity regulating strategies of nanozymes for biomedical applications. Small 2023, 19, 2207142. [Google Scholar] [CrossRef]
- Chi, C.; Zhang, W.; Luo, M.; Zhang, M.; Chen, G. A potential application of a novel synergistic catalytic nanocluster system composed of biological enzymes and nanozyme in glycerol bioconversion. Chem. Eng. J. 2023, 458, 141321. [Google Scholar] [CrossRef]
- Cao, L.; Wang, P.; Chen, L.; Wu, Y.; Di, J. A photoelectrochemical glucose sensor based on gold nanoparticles as a mimic enzyme of glucose oxidase. Sci. Adv. 2019, 9, 15307–15313. [Google Scholar] [CrossRef]
- Hafez, M.E.; Ma, H.; Ma, W.; Long, Y.-T. Unveiling the intrinsic catalytic activities of single-gold-nanoparticle-based enzyme mimetics. Angew. Chem. Int. Ed. 2019, 58, 6327–6332. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, I.; Lee, S.; Yang, Y.I.; Kang, T.; Yi, J. Sensitive and colorimetric detection of the structural evolution of superoxide dismutase with gold nanoparticles. Anal. Chem. 2009, 81, 1378–1382. [Google Scholar] [CrossRef]
- Dashtestani, F.; Ghourchian, H.; Eskandari, K.; Rafiee-Pour, H.-A. A superoxide dismutase mimic nanocomposite for amperometric sensing of superoxide anions. Mikrochim. Acta 2015, 182, 1045–1053. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Wu, Y. A simple and rapid chemosensor for colorimetric detection of dimethoate pesticide based on the peroxidase-mimicking catalytic activity of gold nanoparticles. Anal. Methods 2019, 11, 5337–5347. [Google Scholar] [CrossRef]
- Wang, F.; Na, N.; Ouyang, J. A catalytic-regulated gold nanorods etching process as a receptor with multiple readouts for protein detection. Sens. Actuators B Chem. 2020, 318, 128215. [Google Scholar] [CrossRef]
- Wang, F.Y.; Na, N.; Ouyang, J. Particle-in-a-frame gold nanomaterials with an interior nanogap-based sensor array for versatile analyte detection. Chem. Commun. 2021, 57, 4520–4523. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, D.; Xu, W.; He, L.; Liu, S. Accelerating the peroxidase- and glucose oxidase-like activity of Au nanoparticles by seeded growth strategy and their applications for colorimetric detection of dopamine and glucose. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130555. [Google Scholar] [CrossRef]
- Zuo, L.; Ren, K.; Guo, X.; Pokhrel, P.; Pokhrel, B.; Hossain, M.A.; Chen, Z.-X.; Mao, H.; Shen, H. Amalgamation of DNAzymes and nanozymes in a coronazyme. J. Am. Chem. Soc. 2023, 145, 5750–5758. [Google Scholar] [CrossRef]
- Suo, Z.; Hou, X.; Liu, Y.; Xing, F.; Chen, Y.; Feng, L. β-Lactoglobulin amyloid fibril-templated gold nanoclusters for cellular multicolor fluorescence imaging and colorimetric blood glucose assay. Analyst 2020, 145, 6919–6927. [Google Scholar] [CrossRef]
- McVey, C.; Logan, N.; Thanh, N.T.K.; Elliott, C.; Cao, C. Unusual switchable peroxidase-mimicking nanozyme for the determination of proteolytic biomarker. Nano Res. 2019, 12, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Bai, D.H.; Xue, Z.Y.; Guo, P.Q.; Qiu, M.Z.; Lei, X.F.; Li, Y.J.; Ma, C.J.; Zhang, D.X.; Zhou, X.B. Ultrasensitive colorimetric detection of Hg2+ based on glutathione-modified Au nanoflowers. Microchem. J. 2022, 181, 107766. [Google Scholar] [CrossRef]
- Cheng, C.; Qiao, J.; Zhao, Z.W.; Zhang, H.Y.; Qi, L. Poly(N-2-hydroxypropylmethacrylamide)-capped gold nanoparticles as nanozymes with peroxidase-mimicking performance for the colorimetric monitoring of serum homocysteine. Anal. Bioanal. Chem. 2023, 415, 953–960. [Google Scholar] [CrossRef]
- Ma, Q.; Qiao, J.; Liu, Y.; Qi, L. Colorimetric monitoring of serum dopamine with promotion activity of gold nanocluster-based nanozymes. Analyst 2021, 146, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.M.; Ruan, Y.H.; Chen, Q.; Yan, S.Q.; Huang, W. Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chem. Eng. J. 2023, 452, 138422. [Google Scholar] [CrossRef]
- Tang, R.; Xia, X.; Zhang, X.; Jiang, H.; Wang, B.; Zhang, P.; Zhang, Y.; Tang, Y.; Zhou, Y. Synergistic function of Au NPs/GeO2 nanozymes with enhanced peroxidase-like activity and SERS effect to detect choline iodide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 266, 120467. [Google Scholar] [CrossRef] [PubMed]
- Qixin, L.; Ping, T.; Xinyue, X.; Wendai, C.; Shengde, L.; Xiaoxu, L.; Liyun, Z. Colorimetry /SERS dual-sensor of H2O2 constructed via TMB-Fe3O4@ AuNPs. Talanta 2022, 240, 123118. [Google Scholar]
- Huang, W.; Xu, Y.; Wang, Z.P.; Liao, K.; Zhang, Y.; Sun, Y.M. Dual nanozyme based on ultrathin 2D conductive MOF nanosheets intergraded with gold nanoparticles for electrochemical biosensing of H2O2 in cancer cells. Talanta 2022, 249, 123612. [Google Scholar] [CrossRef]
- Asen, P.; Esfandiar, A.; Kazemi, M. Nonenzymatic sweat-based glucose sensing by flower-like Au nanostructures/graphene oxide. ACS Appl. Mater. Interfaces 2022, 5, 13361–13372. [Google Scholar] [CrossRef]
- Zhao, X.P.; Yang, T.T.; Wang, D.Q.; Zhang, N.; Yang, H.B.; Jing, X.A.; Niu, R.X.; Yang, Z.W.; Xie, Y.C.; Meng, L.J. Gold nanorods/metal-organic framework hybrids: Photo-enhanced peroxidase-like activity and SERS performance for organic dyestuff degradation and detection. Anal. Chem. 2022, 94, 4484–4494. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Sun, Y.J.; Yang, M.; Jin, H.; Gui, R.J. A petal-shaped MOF assembled with a gold nanocage and urate oxidase used as an artificial enzyme nanohybrid for tandem catalysis and dual-channel biosensing. Nanoscale 2021, 13, 13014–13023. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; He, S.; Qiu, B.; Luo, F.; Guo, L.H.; Lin, Z.Y. Noble metal nanoparticle-based multicolor immunoassays: An approach toward visual quantification of the analytes with the naked eye. ACS Sens. 2019, 4, 782–791. [Google Scholar] [CrossRef]
- Do, P.Q.T.; Huong, V.T.; Phuong, N.T.T.; Nguyen, T.-H.; Ta, H.K.T.; Ju, H.; Phan, T.B.; Phung, V.-D.; Trinh, K.T.L.; Tran, N.H.T. The highly sensitive determination of serotonin by using gold nanoparticles (Au NPs) with a localized surface plasmon resonance (LSPR) absorption wavelength in the visible region. RSC Adv. 2020, 10, 30858–30869. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Brennan, M.R.; Wilson, W.L.; Murphy, C.J. Distance and plasmon wavelength dependent fluorescence of molecules bound to silica-coated gold nanorods: A study of plasmon-induced luminescence enhancement. J. Am. Chem. Soc. 2014, 8, 248. [Google Scholar]
- Zhang, L.; Wang, J.; Zhang, J.; Liu, Y.; Wu, L.; Shen, J.; Zhang, Y.; Hu, Y.; Fan, Q.; Huang, W.; et al. Individual Au-Nanocube Based Plasmonic Nanoprobe for Cancer Relevant MicroRNA Biomarker Detection. ACS Sens. 2017, 2, 1435–1440. [Google Scholar] [CrossRef]
- Thiele, M.; Knauer, A.; Malsch, D.; Csaki, A.; Henkel, T.; Koehler, J.M.; Fritzsche, W. Combination of microfluidic high-throughput production and parameter screening for efficient shaping of gold nanocubes using Dean-flow mixing. Lab Chip 2017, 17, 1487–1495. [Google Scholar] [CrossRef]
- Ou, J.M.; Zhou, Z.D.; Chen, Z.; Tan, H.J. Optical diagnostic based on functionalized gold nanoparticles. Int. J. Mol. Sci. 2019, 20, 4346. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Sun, J.; Warden, A.R.; Ding, X. Colorimetric and photographic detection of bacteria in drinking water by using 4-mercaptophenylboronic acid functionalized AuNPs. Food Control 2020, 108, 106885. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Huang, P.C.; Wu, F.Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020, 304, 125377. [Google Scholar] [CrossRef]
- Lei, F.J.; Ye, Z.Y.; Dong, Z.; Zhang, X.F.; Wu, P. Thioflavine T-induced charge neutralization assembly of AuNPs for colorimetric sensing of thallium. Sens. Actuators B Chem. 2022, 370, 132437. [Google Scholar] [CrossRef]
- Alizadeh, A.; Abdi, G.; Khodaei, M.M. Colorimetric and visual detection of silver(I) using gold nanoparticles modified with furfuryl alcohol. Mikrochim. Acta 2016, 183, 1995–2003. [Google Scholar] [CrossRef]
- Orouji, A.; Abbasi-Moayed, S.; Ghasemi, F.; Hormozi-Nezhad, M.R. A wide-range pH indicator based on colorimetric patterns of gold@silver nanorods. Sens. Actuators B Chem. 2022, 358, 131479. [Google Scholar] [CrossRef]
- Wang, H.; Fang, T.; Liu, H.; Wei, T.; Dai, Z. Gold nanostar-based sensitive catechol plasmonic colorimetric sensing platform with ultra-wide detection range. Chemosensors 2022, 10, 439. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Peng, L.J.; Tian, T.; Zhang, W.Y.; Chen, G.Y.; Zhang, H.; Yang, F.Q. Multicolor colorimetric assay for copper ion detection based on the etching of gold nanorods. Mikrochim. Acta 2022, 189, 420. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, T.Y.; Mao, W.; Zhong, Y.; Dai, S.S.; Zeng, X.M.; Liu, C.; Tang, S.; Qiao, F.; Shi, E.; et al. Three-dimensional DNA/Ni-Fe layered double oxide frame networks-induced “cusp-exposure” of Au@Ag nanostars for ultrasensitive determination of kanamycin. Sens. Actuators B Chem. 2021, 343, 130082. [Google Scholar] [CrossRef]
- Xu, S.H.; Jiang, L.P.; Liu, Y.Y.; Liu, P.P.; Wang, W.; Luo, X.L. A morphology-based ultrasensitive multicolor colorimetric assay for detection of blood glucose by enzymatic etching of plasmonic gold nanobipyramids. Anal. Chim. Acta 2019, 1071, 53–58. [Google Scholar] [CrossRef]
- Chang, C.C.; Wang, G.Q.; Takarada, T.; Maeda, M. Iodine-mediated etching of triangular gold nanoplates for colorimetric sensing of copper ion and aptasensing of chloramphenicol. ACS Appl. Mater. Interfaces 2017, 9, 34518–34525. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Pang, B.; Wang, X.; Shi, Y.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Mikrochim. Acta 2020, 187, 504–512. [Google Scholar] [CrossRef]
- Luo, X.; Xie, X.; Meng, Y.; Sun, T.; Ding, J.; Zhou, W. Ligands dissociation induced gold nanoparticles aggregation for colorimetric Al3+ detection. Anal. Chim. Acta 2019, 1087, 76–85. [Google Scholar] [CrossRef]
- Montaño-Priede, J.L.; Sanromán-Iglesias, M.; Zabala, N.; Grzelczak, M.; Aizpurua, J. Robust Rules for Optimal Colorimetric Sensing Based on Gold Nanoparticle Aggregation. ACS Sens. 2023, 8, 1827–1834. [Google Scholar] [CrossRef]
- Mauriz, E. Clinical applications of visual plasmonic colorimetric sensing. Sensors 2020, 20, 6214. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.L.; Chen, Z.B.; Zhang, C.; Wei, X.C.; Lou, T.H.; Zhao, Y. Colorimetric detection of Hg2+ based on the growth of aptamer-coated AuNPs: The effect of prolonging aptamer strands. Small 2017, 13, 1603370. [Google Scholar] [CrossRef]
- Memon, A.G.; Xing, Y.P.; Zhou, X.H.; Wang, R.Y.; Liu, L.H.; Zeng, S.Y.; He, M.; Ma, M. Ultrasensitive colorimetric aptasensor for Hg2+ detection using Exo-III assisted target recycling amplification and unmodified AuNPs as indicators. J. Hazard. Mater. 2020, 384, 120948. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, L.; Li, X.C.; Li, X.J. AuNP aggregation-induced quantitative colorimetric aptasensing of sulfadimethoxine with a smartphone. Chin. Chem. Lett. 2022, 33, 3078–3082. [Google Scholar] [CrossRef]
- Chen, S.H.; Yang, X.Y.; Fu, S.Q.; Qin, X.; Yang, T.; Man, C.X.; Jiang, Y.J. A novel AuNPs colorimetric sensor for sensitively detecting viable salmonella typhimurium based on dual aptamers. Food Control 2020, 115, 107281. [Google Scholar] [CrossRef]
- Kaushal, S.; Pinnaka, A.K.; Soni, S.; Singhal, N.K. Antibody assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near infrared light enhanced antibacterial activity. Sens. Actuators B Chem. 2021, 329, 129141. [Google Scholar] [CrossRef]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Chandra Ray, P. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, G.; La, M.; Liu, L. Colorimetric and electrochemical methods for the detection of SARS-CoV-2 main protease by peptide-triggered assembly of gold nanoparticles. Molecules 2022, 27, 615. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Qiao, Z.; Fu, Y.; Li, Y. Colorimetric detection of lipopolysaccharides based on a lipopolysaccharide-binding peptide and AuNPs. Anal. Methods 2016, 8, 8079–8083. [Google Scholar] [CrossRef] [Green Version]
- Li, D.X.; Wang, S.; Wang, L.; Zhang, H.; Hu, J.D. A simple colorimetric probe based on anti-aggregation of AuNPs for rapid and sensitive detection of malathion in environmental samples. Anal. Bioanal. Chem. 2019, 411, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Wang, J.; Lao, S. Dual-mode detection of organophosphate pesticides in pear and Chinese cabbage based on fluorescence and AuNPs colorimetric assays. Food Chem. 2021, 364, 130326. [Google Scholar] [CrossRef]
- Hu, X.R.; Chang, K.K.; Wang, S.; Sun, X.Q.; Hu, J.D.; Jiang, M. Aptamer-functionalized AuNPs for the high-sensitivity colorimetric detection of melamine in milk samples. PLoS ONE 2018, 13, 0201626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.Y.; Kou, X.Y.; Xu, Y.Y.; Yang, D.W.; Miao, P. Colorimetric sensing strategy for heparin assay based on PDDA-induced aggregation of gold nanoparticles. Nanoscale Adv. 2019, 1, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Chu, S.H.; Zhan, X.J.; Wang, L.M.; Zhou, P.; Zhang, D. Dual-mode colorimetric determination of As(III) based on negatively-charged aptamer-mediated aggregation of positively-charged AuNPs. Anal. Chim. Acta 2022, 1221, 340111. [Google Scholar] [CrossRef]
- Kim, Y.J.; Johnson, R.C.; Hupp, J.T. Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Lin, S.Y.; Liu, S.W.; Lin, C.M.; Chen, C.H. Recognition of potassium ion in water by 15-crown-5 functionalized gold nanoparticles. Anal. Chem. 2002, 74, 330–335. [Google Scholar] [CrossRef]
- Tang, J.; Wu, P.; Hou, X.; Xu, K. Modification-free and N-acetyl-L-cysteine-induced colorimetric response of AuNPs: A mechanistic study and sensitive Hg2+ detection. Talanta 2016, 159, 87–92. [Google Scholar] [CrossRef]
- Liu, S.T.; Xie, T.; Pei, X.J.; Li, S.J.; He, Y.F.; Tong, Y.G.; Liu, G.Q. CRISPR-Cas12a coupled with universal gold nanoparticle strand-displacement probe for rapid and sensitive visual SARS-CoV-2 detection. Sens. Actuators B Chem. 2023, 377, 133009. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.J.; Cao, H.X.; Liang, G.X. Ultrasensitive colorimetric miRNA detection based on magnetic 3D DNA walker and unmodified AuNPs. Sens. Actuators B Chem. 2021, 337, 129813. [Google Scholar] [CrossRef]
- Shahdordizadeh, M.; Yazdian-Robati, R.; Ansari, N.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. An aptamer-based colorimetric lead(II) assay based on the use of gold nanoparticles modified with dsDNA and exonuclease I. Mikrochim. Acta 2018, 185, 151–157. [Google Scholar] [CrossRef]
- Xu, C.N.; Ying, Y.B.; Ping, J.F. Colorimetric aggregation assay for kanamycin using gold nanoparticles modified with hairpin DNA probes and hybridization chain reaction-assisted amplification. Mikrochim. Acta 2019, 186, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.Y.; Liu, T.; Yang, X.B.; Tang, Y.G.; Gong, X.; Miao, P. Role of tripodal DNA modified gold nanoparticles in colorimetric aptasensing. Colloids Interface Sci. Commun. 2017, 21, 19–21. [Google Scholar] [CrossRef]
- Ejeta, S.Y.; Imae, T. Selective colorimetric and electrochemical detections of Cr(III) pollutant in water on 3-mercaptopropionic acid-functionalized gold plasmon nanoparticles. Anal. Chim. Acta 2021, 1152, 338272. [Google Scholar] [CrossRef]
- Shi, X.B.; Lu, D.; Wang, Z.M.; Zhang, D.; Gao, W.L.; Zhang, C.Y.; Deng, J.H.; Guo, S.Y. Colorimetric and visual determination of acrylamide via acrylamide-mediated polymerization of acrylamide-functionalized gold nanoparticles. Mikrochim. Acta 2018, 185, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Li, J.F.; Liu, X.; Wu, F.Y. Colorimetric determination of aluminum(III) based on the aggregation of Schiff base-functionalized gold nanoparticles. Mikrochim. Acta 2016, 183, 863–869. [Google Scholar] [CrossRef]
- Liu, L.; Deng, D.; Wang, Y.; Song, K.; Shang, Z.; Wang, Q.; Xia, N.; Zhang, B. A colorimetric strategy for assay of protease activity based on gold nanoparticle growth controlled by ascorbic acid and Cu(II)-coordinated peptide. Sens. Actuators B Chem. 2018, 266, 246–254. [Google Scholar] [CrossRef]
- Edachana, R.P.; Kumaresan, A.; Balasubramanian, V.; Thiagarajan, R.; Nair, B.G.; Gopalakrishnan, S.B.T. Paper-based device for the colorimetric assay of bilirubin based on in-situ formation of gold nanoparticles. Microchimica Acta 2020, 187, 60–69. [Google Scholar] [CrossRef]
- Munyayi, T.A.; Vorster, B.C.; Mulder, D.W. The effect of capping agents on gold nanostar stability, functionalization, and colorimetric biosensing capability. Nanomaterials 2022, 12, 2470. [Google Scholar] [CrossRef]
- Su, L.; Hu, H.; Tian, Y.; Jia, C.; Wang, L.; Zhang, H.; Wang, J.; Zhang, D. Highly sensitive colorimetric/surface-enhanced raman spectroscopy immunoassay relying on a metallic core-shell Au/Au nanostar with clenbuterol as a target analyte. Anal. Chem. 2021, 93, 8362–8369. [Google Scholar] [CrossRef]
- Duan, N.; Yao, T.; Li, C.; Wang, Z.; Wu, S. Surface-enhanced raman spectroscopy relying on bimetallic Au-Ag nanourchins for the detection of the food allergen beta-lactoglobulin. Talanta 2022, 245, 123445. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, S.Z.; Jia, B.Z.; Zhang, W.F.; Wang, H.; Wei, X.Q.; Shen, Y.D.; Xu, Z.L.; Yang, J.Y. A high-resolution colorimetric immunoassay for tyramine detection based on enzyme-enabled growth of gold nanostar coupled with smartphone readout. Food Chem. 2022, 396, 133729. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Y.; Zhang, Q.; Zha, Y.; Lu, S.; Yang, Y.; Li, P.; Zhou, Y. Colorimetric immunoassay via smartphone based on Mn2+-mediated aggregation of AuNPs for convenient detection of fumonisin B1. Food Control 2022, 132, 108481. [Google Scholar] [CrossRef]
- Allen, A.C.; Efrem, M.; Mahalingam, U.; Guarino-Hotz, M.; Foley, A.R.; Raskatov, J.A.; Song, C.Y.; Lindley, S.A.; Li, J.; Chen, B.; et al. Hollow gold nanosphere templated synthesis of PEGylated hollow gold nanostars and use for SERS detection of amyloid beta in solution. J. Phys. Chem. B 2021, 125, 12344–12352. [Google Scholar] [CrossRef]
- Kermanshahian, K.; Yadegar, A.; Moghimi, H.; Ghourchian, H. The synergy of Fe3O4@Au and molybdate as HRP-mimetic catalysts for gold nanorods etching: Development of an ultrasensitive genosensor for detection of Helicobacter pylori. Sens. Actuators B Chem. 2022, 371, 132600. [Google Scholar] [CrossRef]
- Wang, S.Y.; Huang, X.H.; An, Q.X.; Zhou, R.J.; Xu, W.Z.; Xu, D.; Lin, Q.L.; Cao, X. Gold nanostar as an ultrasensitive colorimetric probe for picomolar detection of lead ion. Anal. Chim. Acta 2021, 1160, 338380. [Google Scholar] [CrossRef]
- Liu, M.; Fu, X.J.; Lu, M.J.; Liu, J.J.; Xie, H.H.; Wei, P.; Zhang, W.D.; Xie, Y.H.; Qi, Y. Colorimetric and visual determination of iodide ions via morphology transition of gold nanobipyramids. Anal. Bioanal. Chem. 2023, 666, 115077. [Google Scholar] [CrossRef]
- Luo, Q.; Lin, Y.S.; Cai, Q.H.; Luo, F.; Lin, C.Y.; Wang, J.; Qiu, B.; Lin, Z.Y. A multicolor biosensor for alkaline phosphatase activity detection based on the peroxidase activity of copper nanoclusters and etching of gold nanorods. Analyst 2022, 147, 2749–2756. [Google Scholar] [CrossRef]
- Zhong, Q.M.; Qin, X.; Yuan, C.L.; Shi, R.; Wang, Y.L. Colorimetric determination of sarcosine in human urine with enzyme-like reaction mediated Au nanorods etching. Microchem. J. 2021, 165, 106120. [Google Scholar] [CrossRef]
- Xianyu, Y.L.; Lin, Y.Y.; Chen, Q.; Belessiotis-Richards, A.; Stevens, M.M.; Thomas, M.R. Iodide-mediated rapid and sensitive surface etching of gold nanostars for biosensing. Angew. Chem. Int. Ed. 2021, 60, 9891–9896. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Li, D.D.; Cao, S.Y.; Huang, X.; Yang, H.F.; Yang, D.; Huang, Z.Y.; Cai, R.; Tan, W.H. Sensitive and multicolor detection of nitrite based on iodide-mediated etching of gold nanostars. Chem. Commun. 2022, 58, 12983–12986. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.Q.; Wang, S.; Chen, H.Y.; Ren, L.X.; Liang, K.; Wei, L.A.; Long, W.J.; Yang, J.; Guo, L.P.; Han, X.L.; et al. Digital image colorimetry in combination with chemometrics for the detection of carbaryl based on the peroxidase-like activity of nanoporphyrins and the etching process of gold nanoparticles. Food Chem. 2022, 394, 133495. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; He, W.; Li, Y.F.; Gao, P.F.; Huang, C.Z. Telomerase activity assay via 3,3′,5,5′-tetramethylbenzidine dilation etching of gold nanorods. ACS Appl. Mater. Interfaces 2022, 5, 1484–1490. [Google Scholar] [CrossRef]
- He, Z.; Zhu, J.; Weng, G.J.; Li, J.J.; Zhao, J.W. Detection of ferrous ion by etching-based multi-colorimetric sensing of gold nanobipyramids. Nanotechnology 2020, 31, 335505. [Google Scholar] [CrossRef]
- Wu, R.F.; Li, K.; Shen, C.Y.; Huang, J.; Gao, Z.Y.; Tang, K.R.; Leng, Y.M.; Chen, Z.B. Antioxidant recognition by colorimetric sensor array based on differential etching of gold nanorods and gold nanobypyramids. ACS Appl. Mater. Interfaces 2021, 4, 8482–8490. [Google Scholar] [CrossRef]


| Modification Method | Type of Au NPs | Type of Target | Time of Color Change | The Limit of Detection | Reference |
|---|---|---|---|---|---|
| Aggregation | Au NSPs | Gram-positive and Gram-negative strains | Within 20 min | 104 CFU/mL | [69] |
| Aggregation | Au NSPs | Tetracycline and chloramphenicol | 16 min | 32.9 nM & 7.0 nM | [70] |
| Aggregation | Au NSPs | Tl | 2 min | 3.2 nM | [71] |
| Surface modification | Au NSPs | Ag(I) | 10 min | 12 nM | [72] |
| Growth | Au NRs | pH | 3 min | pH 2.0 ~ 12.0 | [73] |
| Growth | Au NSs | Catechol | 2 h | 1 nM | [74] |
| Etching | Au NRs | Cu2+ | 15 min | 0.034 μM | [75] |
| Etching | Au NSs | Kanamycin | 3 min | 3 aM | [76] |
| Etching | Au NBPs | Blood glucose | 30 min | 0.02 mM | [77] |
| Etching | Au NPLs | Cu2+ and chloramphenicol | 20 min | 10 uM & 5 uM | [78] |
| Etching | Au NSPs | S. aureus | 65 min | 10 CFU/mL | [79] |
| Ways Based on Etching | Type of Au NPs | Targets | Color Change | The Limit of Detection | Reference |
|---|---|---|---|---|---|
| direct etching of the target | Au NPLs | Chloramphenicol | from blue to red | 10 μM & 1 μM | [78] |
| direct etching of the target | Au NRs | Helicobacter pylori | from blue to purple to red | 40–2040 aM & 31.8 aM | [116] |
| reaction formation complex-induced etching | Au NSs | Pb2+ | from blue-green to blue, to purple to red, and finally to colorless. | 1.5 pM | [117] |
| reaction formation complex-induced etching | Au NBPs | I− | from blue-green to red | 4 μM & 0.2 μM | [118] |
| oxidant-mediated etching | Au NBPs | Glucose | from light brown to blue to pink | 0.02 μM | [77] |
| oxidant-mediated etching | Au NRs | Cu2+ | rainbow colors | 0.034 μM | [75] |
| inhibitor-mediated etching | Au NRs | ALP | from light brown to green to blue to pink | 4.6 UL−1 | [119] |
| inhibitor-mediated etching | Au NRs | UA | from light brown to blue to pink | 0.76 μM | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhao, J.; Li, H. Chromogenic Mechanisms of Colorimetric Sensors Based on Gold Nanoparticles. Biosensors 2023, 13, 801. https://doi.org/10.3390/bios13080801
Cui Y, Zhao J, Li H. Chromogenic Mechanisms of Colorimetric Sensors Based on Gold Nanoparticles. Biosensors. 2023; 13(8):801. https://doi.org/10.3390/bios13080801
Chicago/Turabian StyleCui, Yanyun, Jun Zhao, and Huidan Li. 2023. "Chromogenic Mechanisms of Colorimetric Sensors Based on Gold Nanoparticles" Biosensors 13, no. 8: 801. https://doi.org/10.3390/bios13080801





