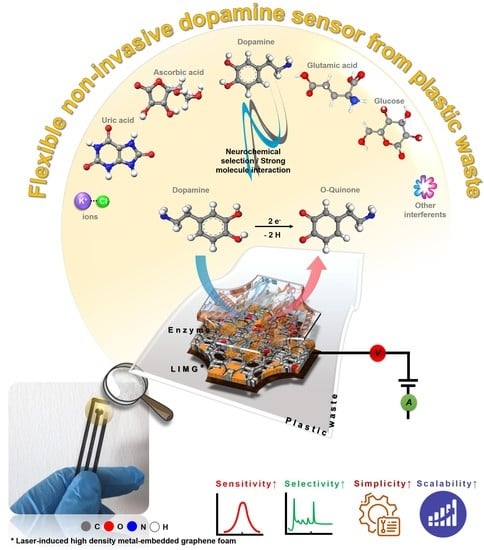
Laser Scribing Turns Plastic Waste into a Biosensor via the Restructuration of Nanocarbon Composites for Noninvasive Dopamine Detection
Abstract
:1. Introduction
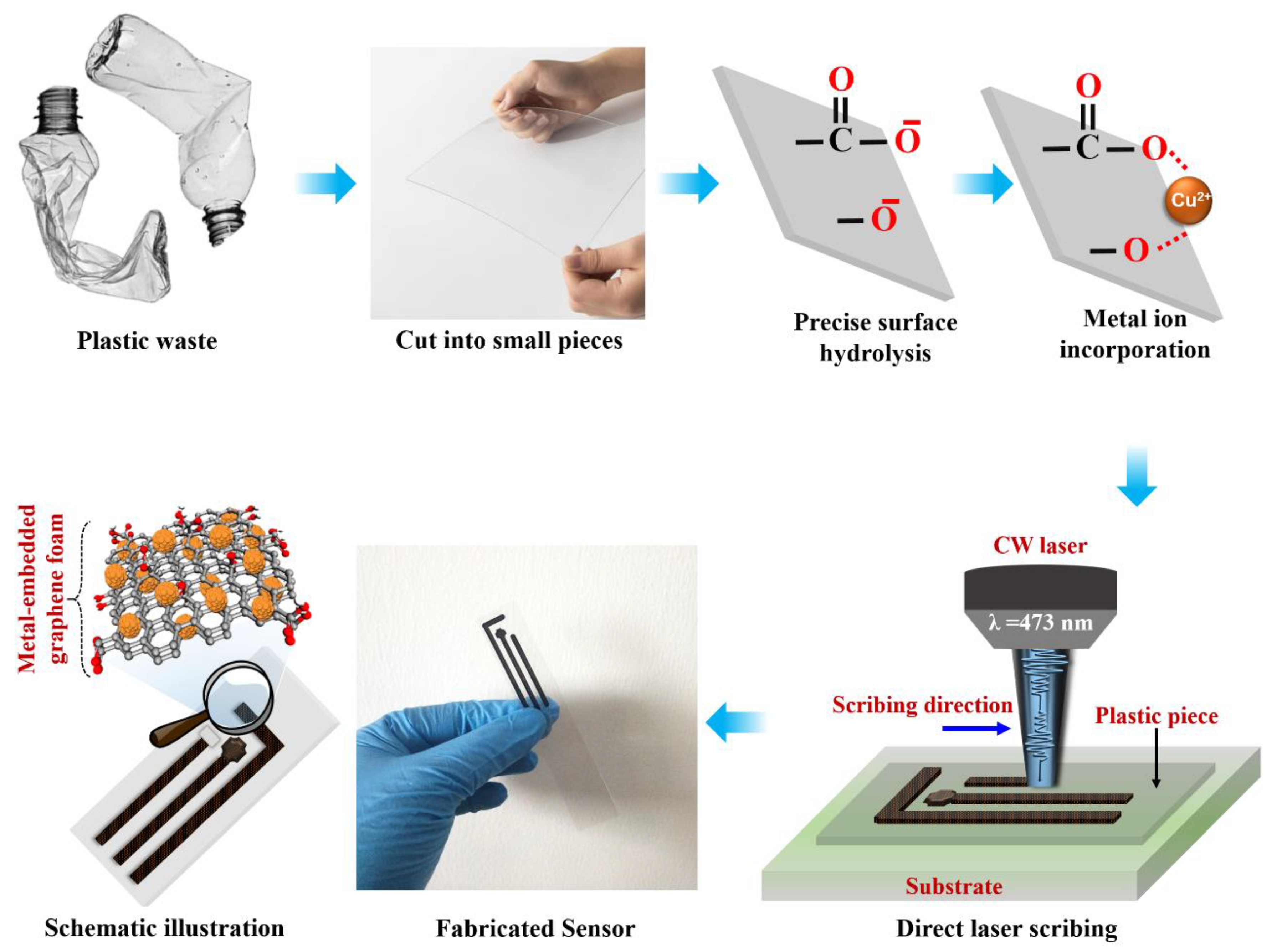
2. Methods
2.1. Sensor Fabrication
2.2. Fabrication of Flexible POC Device
3. Results and Discussion
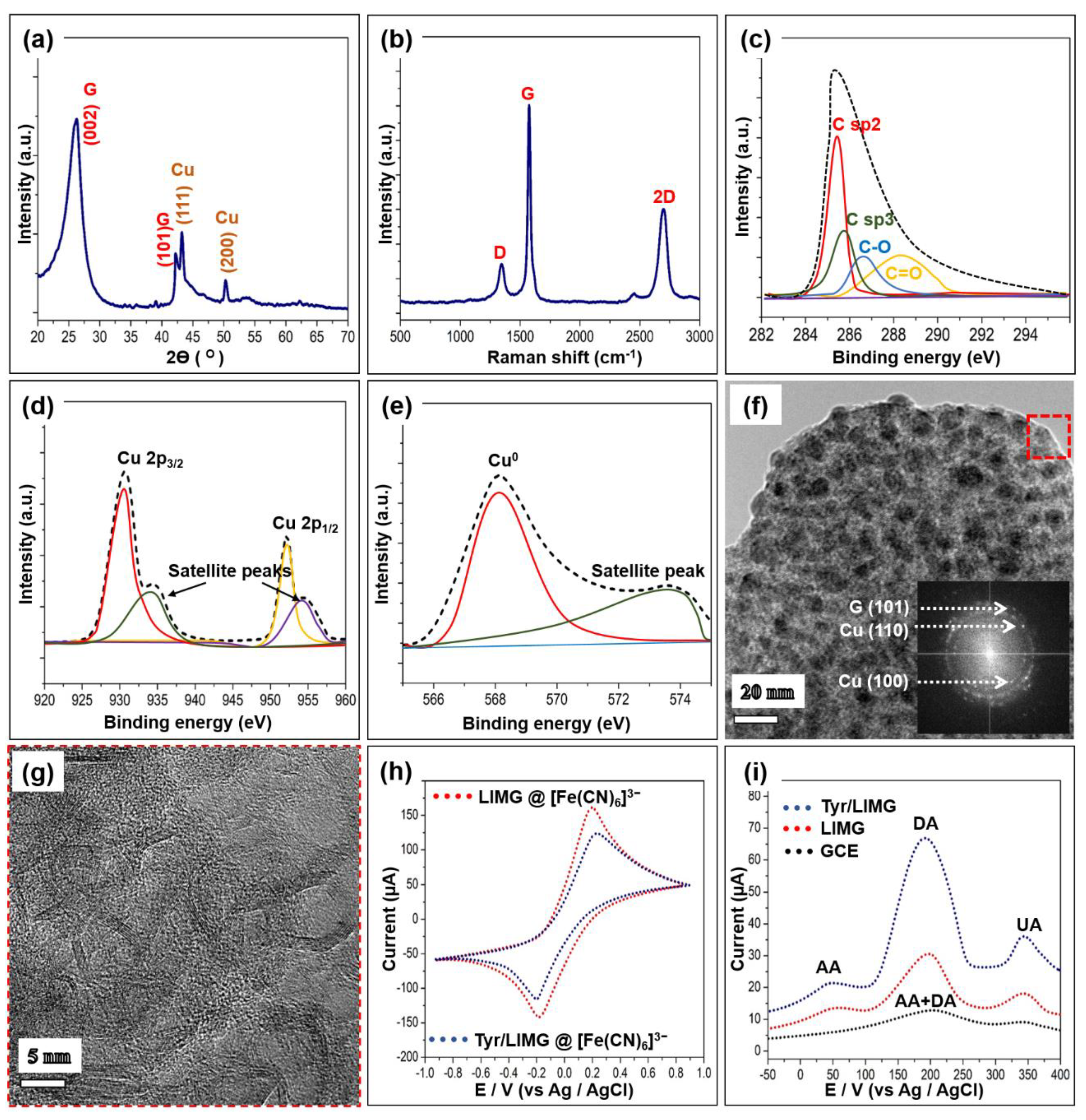
3.1. Morphological, Surface, and Structural Evaluation
3.2. Electrochemical Feature
- Dopamine + Tyr(ox) → Dopamine-O-quinone + 2H+ + Tyr(red);
- Tyr(red) + 2 [Fe(CN)6]3− ↔ Tyr(ox) + 2 [Fe(CN)6]4−;
- 2 [Fe(CN)6]4− ↔ 2 [Fe(CN)6]3− + 2e−.
3.3. Real-World Application Study
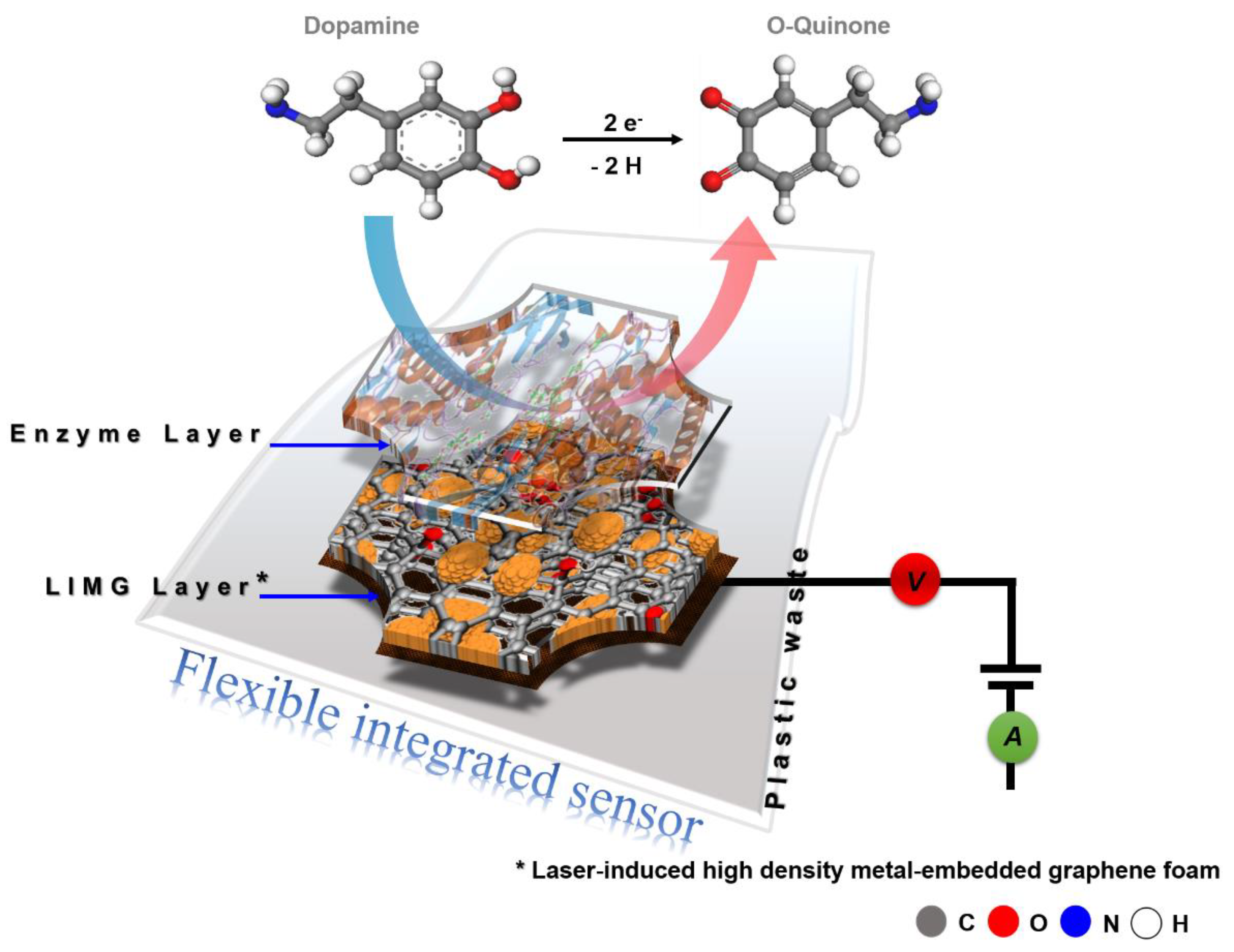
3.4. Material Formation and Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Papalini, S.; Beckers, T.; Vervliet, B. Dopamine: From prediction error to psychotherapy. Transl. Psychiatry 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- de la Mora, M.P.; Hernandez-Mondragon, C.; Crespo-Ramirez, M.; Rejon-Orantes, J.; Borroto-Escuela, D.O.; Fuxe, K. Conventional and novel pharmacological approaches to treat dopamine-related disorders: Focus on Parkinson’s disease and schizophrenia. Neuroscience 2019, 439, 309. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R. The Physiology, Signaling and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Volkow, N.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 9253, 354. [Google Scholar] [CrossRef]
- Michaelides, M.; Thanos, P.K.; Volkow, N.D.; Wang, G. Dopamine-related frontostriatal abnormalities in obesity and binge-eating disorder: Emerging evidence for developmental psychopathology. Int. Rev. Psychiatry 2012, 24, 211. [Google Scholar] [CrossRef]
- Dalirirad, S.; Steck, A.J. Lateral flow assay using aptamer-based sensing for on-site detection of dopamine in urine. Anal. Biochem. 2020, 596, 113637. [Google Scholar] [CrossRef]
- Verma, S.; Arya, P.; Singh, A.; Kaswan, J.; Shukla, A.; Kushwaha, H.R.; Gupta, S.; Singh, S.P. ZnO-rGO nanocomposite based bioelectrode for sensitive and ultrafast detection of dopamine in human serum. Biosens. Bioelectron. 2020, 165, 112347. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Bala, K.; Shan, L.; Wu, L.; Gupta, N. Molecular engineered carbon-based sensor for ultra-fast and specific detection of neurotransmitters. ACS Appl. Mater. Interfaces 2021, 13, 60878–60893. [Google Scholar] [CrossRef]
- Lei, Y.; Butler, D.; Lucking, M.C.; Zhang, F.; Xia, T.; Fujisawa, K.; Granzier-Nakajima, T.; Cruz-Silva, R.; Endo, M.; Terrones, H.; et al. Ebrahimi, Single-atom doping of MoS2 with manganese enables ultrasensitive detection of dopamine: Experimental and computational approach. Sci. Adv. 2020, 6, eabc4250. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprakash, J.; Gupta, N.; Wu, L.; Shan, L. Engineering of all solution/substrate processable biosensors for the detection of epinephrine as low as pM with rapid readout. Chem. Eng. J. 2022, 436, 135254. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Gupta, N.; Wu, L.; Shan, L. Immobilized Molecules’ Impact on the Efficacy of Nanocarbon Organic Sensors for Ultralow Dopamine Detection in Biofluids. Adv. Mater. Technol. 2022, 7, 2200099. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Wu, S.; Shen, J. Au nanoparticles decorated polypyrrole/reduced graphene oxide hybrid sheets for ultrasensitive dopamine detection. Sens. Actuators B Chem. 2014, 193, 759. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zheng, X.; Zheng, J. Synthesis of Au@Pt nanoflowers supported on graphene oxide for enhanced electrochemical sensing of dopamine. J. Electroanal. Chem. 2018, 817, 48. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Zuo, J.; Pang, H.; Tan, L.; Chen, T.; Ma, H. A dopamine electrochemical sensor based on Pd-Pt alloy nanoparticles decorated polyoxometalate and multiwalled carbon nanotubes. J. Electroanal. Chem. 2018, 827, 103. [Google Scholar] [CrossRef]
- Ivanova, M.N.; Grayfer, E.D.; Plotnikova, E.E.; Kibis, L.S.; Darabdhara, G.; Boruah, P.K.; Das, M.R.; Fedorov, V.E. Pt-decorated boron nitride nanosheets as artificial nanozyme for detection of dopamine. ACS Appl. Mater. Interfaces 2019, 11, 22102. [Google Scholar] [CrossRef]
- Bala, K.; Suriyaprakash, J.; Singh, P.; Chauhan, K.; Villae, A.; Gupta, N. Copper and cobalt nanoparticles embedded in naturally derived graphite electrodes for the sensing of the neurotransmitter epinephrine. New J. Chem. 2018, 42, 6604. [Google Scholar] [CrossRef]
- Bekmezci, M.; Ozturk, H.; Akin, M.; Bayat, R.; Sen, F.; Darabi, R. Karimi-Maleh, Bimetallic Biogenic Pt-Ag Nanoparticle and Their Application for Electrochemical Dopamine Sensor. Biosensors 2023, 13, 531. [Google Scholar] [CrossRef]
- Liu, S.; Xing, X.; Yu, J.; Lian, W.; Li, J.; Huang, M.C.J. A Novel Label-Free Electrochemical Aptasensor Based on Graphene-Polyaniline Composite Film for Dopamine Determination. Biosens. Bioelectron. 2012, 36, 186. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Davis, J.J.; Luo, X. Ultrasensitive and Selective Voltammetric Aptasensor for Dopamine Based on a Conducting Polymer Nanocomposite Doped with Graphene Oxide. Microchim. Acta 2015, 182, 1123. [Google Scholar] [CrossRef]
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K. Kaushik. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson’s Disease Diagnostics. Biosensors 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Fredj, Z.; Sawan, M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors 2023, 13, 211. [Google Scholar] [CrossRef]
- Butler, D.; Moore, D.; Glavin, N.R.; Robinson, J.A.; Ebrahimi, A. Facile post-deposition annealing of graphene ink enables ultrasensitive electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 2021, 13, 11185. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Fang, Y.; Zhang, D. Carbon-Based Electrochemical Sensors for In Vivo and In Vitro Neurotransmitter Detection. Crit. Rev. Anal. Chem. 2021, 53, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors 2022, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Demuru, S.; Nela, L.; Marchack, N.; Holmes, S.; Farmer, D.; Tulevski, G.; Lin, Q. Deligianni. Scalable Nanostructured Carbon Electrode Arrays for Enhanced Dopamine Detection. ACS Sens. 2018, 3, 799. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, G.; Li, Z.; Yuan, C.; Bai, Y.; Fu, D. Selective electrochemical detection of dopamine on polyoxometalate-based metal-organic framework and its composite with reduced graphene oxide. Adv. Mater. Interfaces 2017, 4, 1601241. [Google Scholar] [CrossRef]
- Li, Z.; Guyse, J.; Rosa, V.; Gorp, H.; Walke, P.; Rodríguez González, M.C.; Uji-i, H.; Hoogenboom, R.; Feyter, S.D.; Mertens, S.F.L. One-step covalent immobilization of β-cyclodextrin on sp2 carbon surfaces for selective trace amount probing of guests. Adv. Funct. Mater. 2019, 29, 1901488. [Google Scholar] [CrossRef]
- Shu, Y.; Lu, Q.; Yuan, F.; Tao, Q.; Jin, D.; Yao, H.; Xu, Q.; Hu, X. Stretchable electrochemical biosensing platform based on Ni-MOF composite/Au nanoparticle-coated carbon nanotubes for real-time monitoring of dopamine released from living cells. ACS Appl. Mater. Interfaces 2020, 12, 49480. [Google Scholar] [CrossRef]
- Ma, X.; Gao, F.; Dai, R.; Liu, G.; Zhang, Y.; Lu, L.; Yu, Y. Novel Electrochemical Sensing Platform Based on a Molecularly Imprinted Polymer-Decorated 3D-Multi-Walled Carbon Nanotube Intercalated Graphene Aerogel for Selective and Sensitive Detection of Dopamine. Anal. Methods 2020, 12, 1845. [Google Scholar] [CrossRef]
- Fu, Y.; Sheng, Q.; Zheng, J. The Novel Sulfonated Polyaniline Decorated Carbon Nanosphere Nanocomposites for Electrochemical Sensing of Dopamine. New J. Chem. 2017, 41, 15439. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, Q.; Li, X.; Wu, L.; Yu, A.; Lai, G.; Fu, L.; Wei, Q.; Dai, D.; Jiang, N.; et al. A Double-Deck Structure of Reduced Graphene Oxide Modified Porous Ti3C2Tx Electrode towards Ultrasensitive and Simultaneous Detection of Dopamine and Uric Acid. Biosensors 2021, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Cao, P. Electrochemical sensing of dopamine using a Ni-based metal-organic framework modified electrode. Ionics 2021, 27, 1339. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, D.; Liu, S.; Zhang, Y.; Leng, L.; Li, X.; Chen, N.; Zhang, Y. Metal-organic framework-based molecularly imprinted polymer as a high sensitive and selective hybrid for the determination of dopamine in injections and human serum samples. Biosens. Bioelec. 2018, 118, 129. [Google Scholar] [CrossRef]
- Nuh, S.; Numnuam, A.; Thavarungkul, P.; Phairatana, T. A Novel Microfluidic-Based OMC-PEDOT-PSS Composite Electrochemical Sensor for Continuous Dopamine Monitoring. Biosensors 2023, 13, 68. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing Conductive Metal–Organic Frameworks for Voltammetric Detection of Neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717. [Google Scholar] [CrossRef]
- Huang, X.; Shi, W.; Bao, N.; Yu, C.; Gu, H. Electrochemically Reduced Graphene Oxide and Gold Nanoparticles on an Indium Tin Oxide Electrode for Voltammetric Sensing of Dopamine. Microchim. Acta 2019, 186, 310. [Google Scholar] [CrossRef]
- Thirumalai, D.; Lee, S.; Kwon, M.; Paik, H.-J.; Lee, J.; Chang, S.-C. Disposable Voltammetric Sensor Modified with Block Copolymer-Dispersed Graphene for Simultaneous Determination of Dopamine and Ascorbic Acid in Ex Vivo Mouse Brain Tissue. Biosensors 2021, 11, 368. [Google Scholar] [CrossRef]
- Farajikhah, S.; Innis, P.; Paull, B.; Wallace, G.; Harris, A. Facile Development of a Fiber-Based Electrode for Highly Selective and Sensitive Detection of Dopamine. ACS Sens. 2019, 4, 2599. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Cai, J.; Yang, T.; Chen, J.; Hou, X. Electrochemical detection mechanism of dopamine and uric acid on titanium nitride-reduced graphene oxide composite with and without ascorbic acid. Sens. Actuators B Chem. 2019, 298, 126872. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, A.; Shen, Y.; Qiu, L.; Tian, X. Layer-by-layer inkjet printing of fabricating reduced graphene-polyoxometalate composite film for chemical sensors. Phys. Chem. Chem. Phys. 2012, 14, 12757. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Huang, Q.; Zhu, M.; Feng, D.; Li, X.; Wei, Y. A facile one-step electrochemical fabrication of reduced graphene oxide–mutilwall carbon nanotubes–phospotungstic acid composite for dopamine sensing. J. Electroanal. Chem. 2013, 693, 9. [Google Scholar] [CrossRef]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The Era of Digital Health: A Review of Portable and Wearable Affinity Biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Ye, R.; Peng, Z.; Wang, T.; Xu, Y.; Zhang, J.; Li, Y.; Nilewski, L.; Lin, J.; Tour, J. In Situ Formation of Metal Oxide Nanocrystals Embedded in Laser-Induced Graphene. ACS Nano 2015, 9, 9244. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X. Physical and Chemical Sensors on the Basis of Laser-Induced Graphene: Mechanisms, Applications, and Perspectives. ACS Nano 2021, 15, 18708. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, L.; Liu, Y.; Li, J.; Diao, Y.; Wang, C.; Guo, X.; Chen, D. Facile fabrication of laser induced versatile graphene-metal nanoparticles electrodes for the detection of hazardous molecules. Talanta 2023, 257, 124368. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Liu, X.; Yue, Z.; Chen, X.; Wan, Z. Doping of Laser-Induced Graphene and Its Applications. Adv. Mater. Technol. 2023, 2300244. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Liu, G.; Thi Dieu Thuy, N. A disposable and flexible electrochemical sensor for the sensitive detection of heavy metals based on a one-step laser-induced surface modification: A new strategy for the batch fabrication of sensors. Sens. Actuators B Chem. 2022, 350, 130834. [Google Scholar] [CrossRef]
- You, Z.; Qiu, Q.; Chen, H.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 111896. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Hong, Q.; Li, H.; Liu, C.; Li, F. Direct-Laser-Writing of Metal Sulfide-Graphene Nanocomposite Photoelectrode toward Sensitive Photoelectrochemical Sensing. Adv. Funct. Mater. 2019, 29, 1904000. [Google Scholar] [CrossRef]
- Scroccarello, A.; Álvarez-Diduk, R.; Della Pelle, F.; de Carvalho Castro e Silva, C.; Idili, A.; Parolo, C.; Compagnone, D.; Merkoçi, A. One-Step Laser Nanostructuration of Reduced Graphene Oxide Films Embedding Metal Nanoparticles for Sensing Applications. ACS Sens. 2023, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Sharifuzzaman, M.; Sharma, S.; Park, C.I.; Yoon, S.; Heum Kim, D.; Park, J.Y. A nanocomposite-decorated laser-induced graphene-based multi-functional hybrid sensor for simultaneous detection of water contaminants. Anal. Chim. Acta. 2022, 1209, 339872. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Basu, S.; Kumar Jha, S. Dopamine biosensor based on surface functionalized nanostructured nickel oxide platform. Biosens. Bioelectron. 2016, 84, 72. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Shan, L.; Gupta, N.; Wang, H.; Wu, L. Janus 2D-carbon nanocomposite-based ascorbic acid sensing device: Experimental and theoretical approaches. Comp. Part B Eng. 2022, 436, 135254. [Google Scholar] [CrossRef]
- Sharma, D.; Suriyaprakash, J.; Dogra, A.; Alijani, S.; Villa, A.; Gupta, N. Versatile carbon supported mono and bimetallic nanocomposites: Synthesis, characterization and their potential application for furfural reduction. Mater. Today Chem. 2020, 17, 100319. [Google Scholar] [CrossRef]
- Esparza, I.; Fan, J.; Michalik, J.M.; Rodríguez, L.; Ibarra, M.; Teresa, J. The nature of graphene–metal bonding probed by Raman spectroscopy: The special case of cobalt. J. Phys. D Appl. Phys. 2016, 49, 105301. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Q.; Zheng, J.; Lv, Z.; Wei, J.; Wang, A.; Feng, J. Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using Pt nanoparticles supported on reduced graphene oxide. Electrochim. Acta 2014, 115, 109. [Google Scholar] [CrossRef]
- Palanisamy, S.; Ku, S.; Chen, S. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim. Acta 2013, 180, 1037. [Google Scholar] [CrossRef]
- Min, K.; Yoo, Y. Amperometric detection of dopamine based on Tyrosinase-SWNTS-PPY composite electrode. Talanta 2009, 80, 1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, F.; Wang, C.; Yang, B.; Bin, D.; Zhang, K.; Du, Y. Simultaneous determination of dopamine, uric acid and ascorbic acid using a glassy carbon electrode modified with reduced graphene oxide. RSC Adv. 2014, 4, 26895. [Google Scholar] [CrossRef]
- Demuru, S.; Deligianni, H. Nafion coatings for enhanced dopamine, serotonin and adenosine sensing. J. Electrochem. Soc. 2017, 164, 129. [Google Scholar] [CrossRef]
- Chen, L.; Guo, C.; Pan, M.; Lai, C.; Wang, Y.; Liao, G.; Ma, Z.; Zhang, F.; Suriyaprakash, J.; Guo, L.; et al. Sub-40 nm Nanogratings Self-Organized in PVP-based Polymer Composite Film by Photoexcitation and Two Sequent Splitting under Femtosecond Laser Irradiation. Appl. Surf. Sci. 2023, 609, 155395. [Google Scholar] [CrossRef]
- Kogolev, D.; Semyonov, O.; Metalnikova, N.; Fatkullin, M.; Rodriguez, R.; Slepicka, P.; Guselnikova, O.; Boukherroube, R.; Postnikov, P. Waste PET upcycling to conductive carbon-based composite through laser-assisted carbonization of UiO-66. J. Mater. Chem. A 2023, 11, 1108. [Google Scholar] [CrossRef]
- Worku, B.G.; Wubieneh, T.A. Mechanical Properties of Composite Materials from Waste Poly(ethylene terephthalate) Reinforced with Glass Fibers and Waste Window Glass. Int. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef]

| Carbon/Polymer/Metal-Based Sensing Probe | Linear Range (µmol/L) | LOD (mol/L) | Real Sample Invasive/Noninvasive | Ref |
|---|---|---|---|---|
| rGO-PAP | 0.001–100 | 2.0 × 10−9 | Invasive | [10,13] |
| rGO-PAS | 0.001–100 | 1.1 × 10−10 | Invasive | [13] |
| rGO-PAB | 0.0001–100 | 1.0 × 10−11 | Invasive | [13] |
| Au/PPy/rGO | 0.001–5 | 1.8 × 10−11 | Invasive | [14] |
| Carbon NRs | 1–10 | 6.0 × 10−8 | Invasive | [27] |
| POMF-rGO | 1–200 | 8.0 × 10−8 | NA | [28] |
| HOPG-β-cyclodextrin | - | 1.0 × 10−7 | NA | [29] |
| NACP | 0.05–15 | 1.0 × 10−8 | Invasive | [30] |
| MIP/MWCNT/GAs/GCE | 0.005–20 | 1.6 × 10−6 | Invasive | [31] |
| SPANI/CNSs/GCE | 0.5–1780 | 1.5 × 10−8 | NA | [32] |
| Ni-MOF | 0.2–100 | 6.0 × 10−8 | Invasive | [34] |
| PPy/ZIF-67/Nafion | 0.08–100 | 3.0 × 10−8 | Invasive | [35] |
| Ni3HHTP2-MOF | 0.04–200 | 6.3 × 10−8 | NA | [37] |
| rGO-Au NPs/ITO | 0.02–200 | 1.5 × 10−8 | Invasive | [38] |
| rGO-SS | 1–1000 | 1.0 × 10−6 | NA | [40] |
| TiN-rGO | 5–175 | 1.59 × 10−7 | Noninvasive | [41] |
| rGO-PTA | 0.5–20 | - | NA | [42] |
| rGO-MWNT-PTA | 0.5–20 | 1.14 × 10−9 | NA | [43] |
| rGO-Pt | 10–170 | 2.50 × 10−7 | NA | [60] |
| rGO-Pd NPs | 1–150 | 2.33 × 10−7 | Invasive | [61] |
| 3D SWNT–PPy composite | 5–50 | 5.0 × 10−6 | NA | [62] |
| rGO-GC | 0.1–100 | 1.0 × 10−7 | NA | [63] |
| Carbon fiber | - | 4.1 × 10−8 | NA | [64] |
| Tyr/LIMG | 0.0001–100 | 1.0 × 10−10 | Noninvasive | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyaprakash, J.; Huang, Y.; Hu, Z.; Wang, H.; Zhan, Y.; Zhou, Y.; Thangavelu, I.; Wu, L. Laser Scribing Turns Plastic Waste into a Biosensor via the Restructuration of Nanocarbon Composites for Noninvasive Dopamine Detection. Biosensors 2023, 13, 810. https://doi.org/10.3390/bios13080810
Suriyaprakash J, Huang Y, Hu Z, Wang H, Zhan Y, Zhou Y, Thangavelu I, Wu L. Laser Scribing Turns Plastic Waste into a Biosensor via the Restructuration of Nanocarbon Composites for Noninvasive Dopamine Detection. Biosensors. 2023; 13(8):810. https://doi.org/10.3390/bios13080810
Chicago/Turabian StyleSuriyaprakash, Jagadeesh, Yang Huang, Zhifei Hu, Hao Wang, Yiyu Zhan, Yangtao Zhou, Indumathi Thangavelu, and Lijun Wu. 2023. "Laser Scribing Turns Plastic Waste into a Biosensor via the Restructuration of Nanocarbon Composites for Noninvasive Dopamine Detection" Biosensors 13, no. 8: 810. https://doi.org/10.3390/bios13080810
APA StyleSuriyaprakash, J., Huang, Y., Hu, Z., Wang, H., Zhan, Y., Zhou, Y., Thangavelu, I., & Wu, L. (2023). Laser Scribing Turns Plastic Waste into a Biosensor via the Restructuration of Nanocarbon Composites for Noninvasive Dopamine Detection. Biosensors, 13(8), 810. https://doi.org/10.3390/bios13080810