Post-Vaccination Detection of SARS-CoV-2 Antibody Response with Magnetic Nanoparticle-Based Electrochemical Biosensor System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Apparatus
2.2. Subjects and Study Design
2.3. Sample Collection
2.4. Biosensor Design
2.4.1. Optimization Studies
2.4.2. Sample Application
2.5. Confirmation Analysis: Immunoassay
2.6. Statistics
3. Results
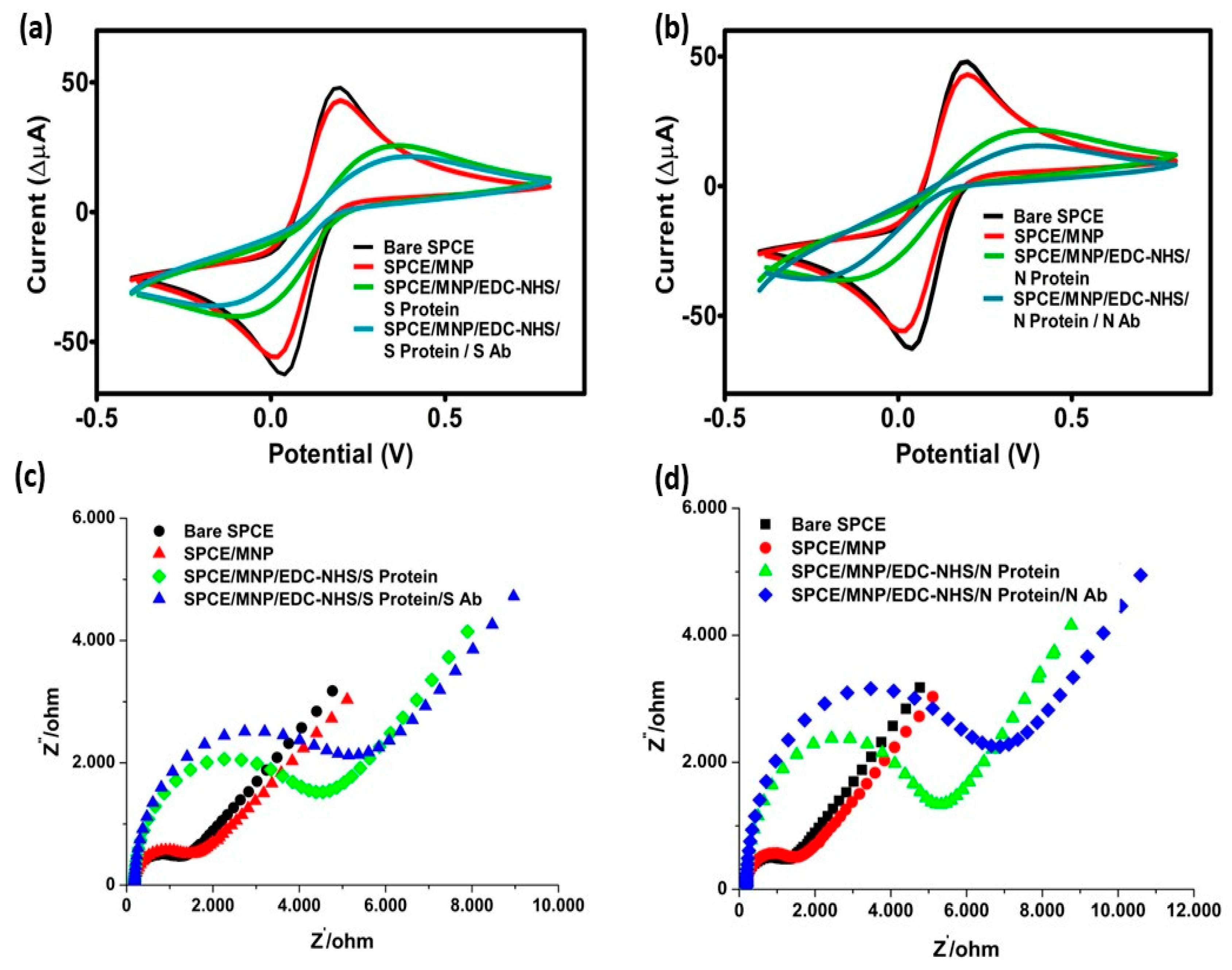
3.1. Surface Characterization
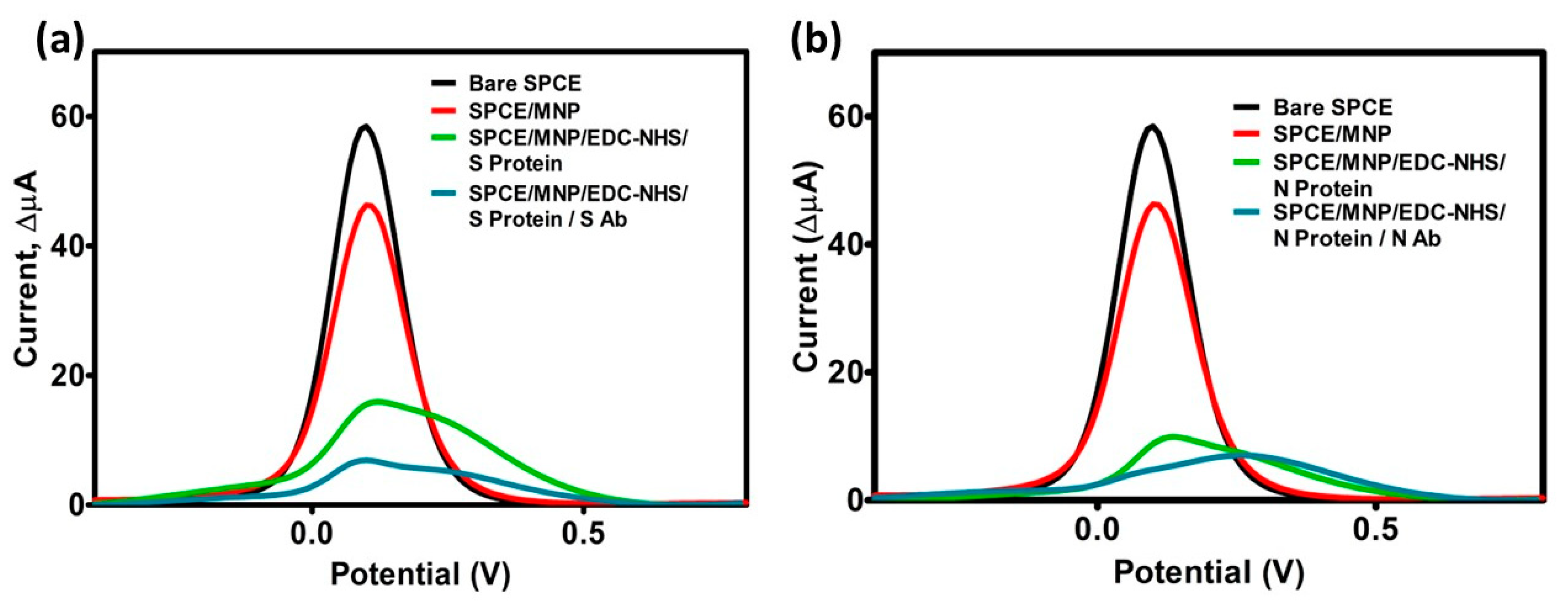
3.2. Optimization Results
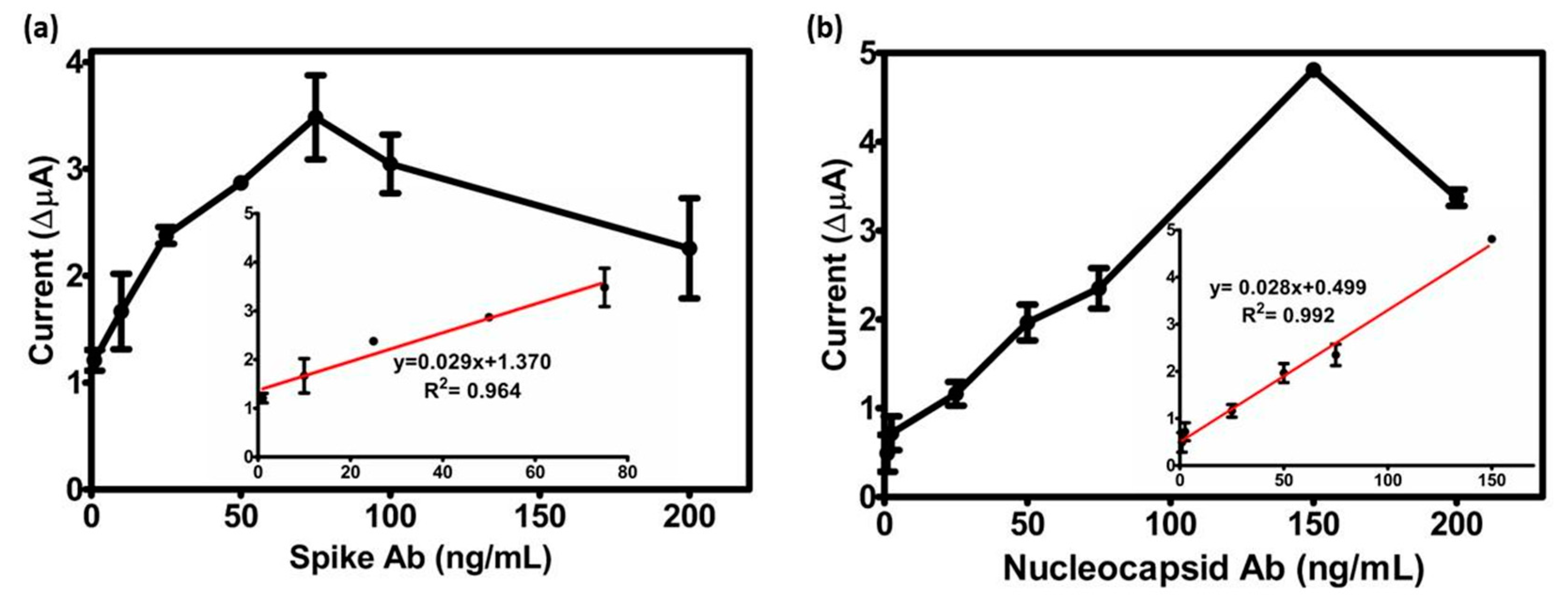
3.3. Analytical Parameters
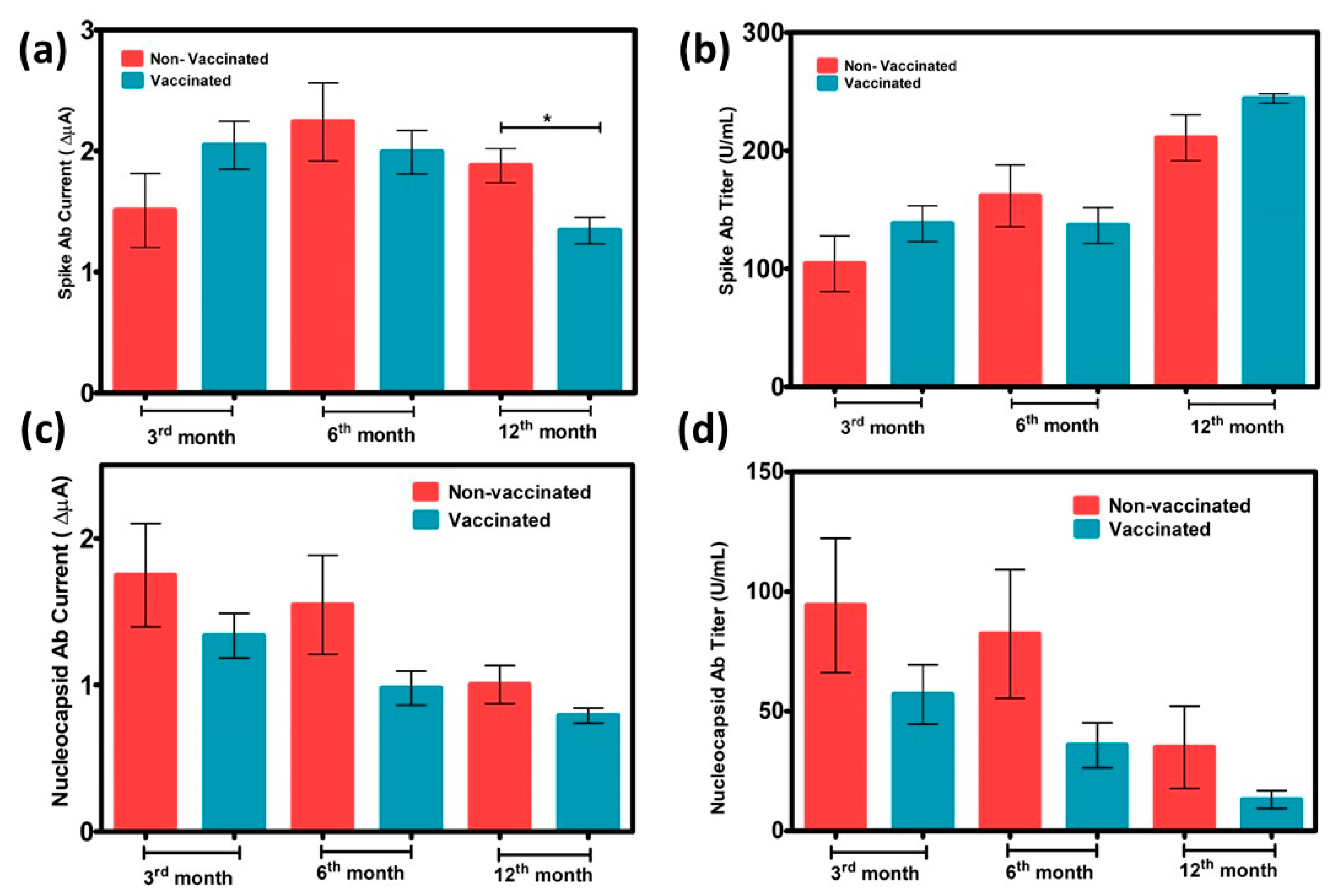
3.4. Interference Results and Sample Application
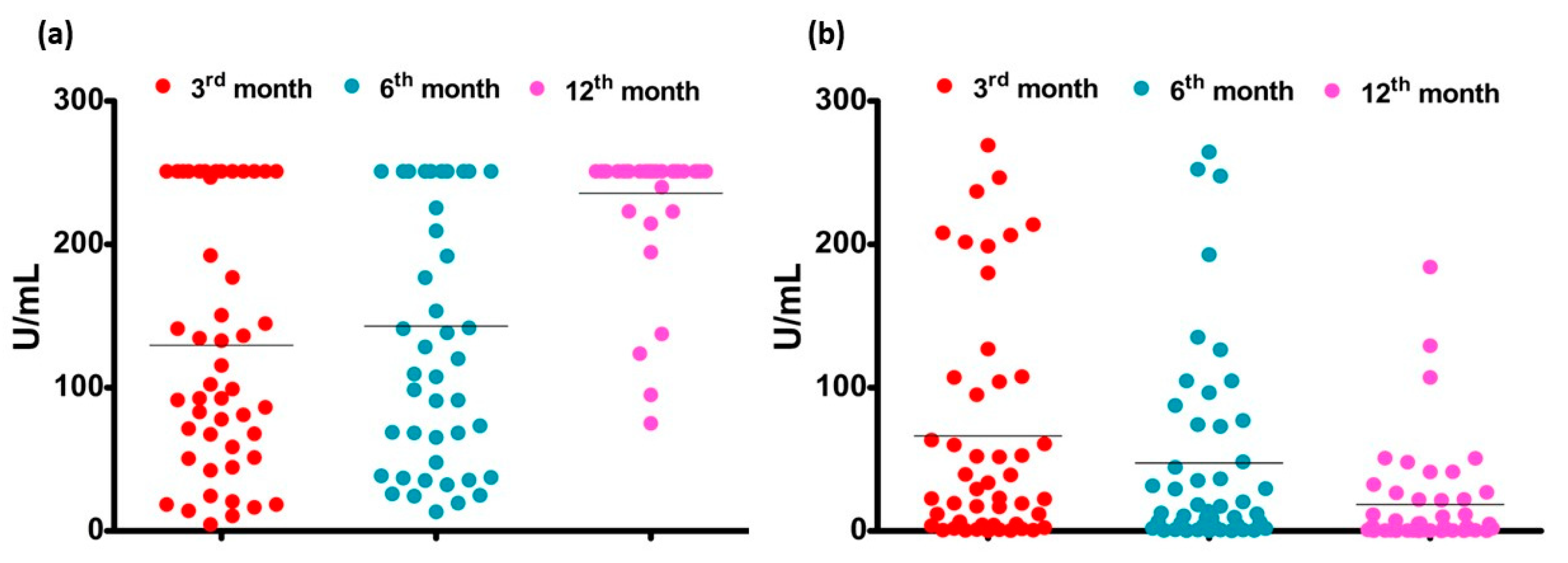
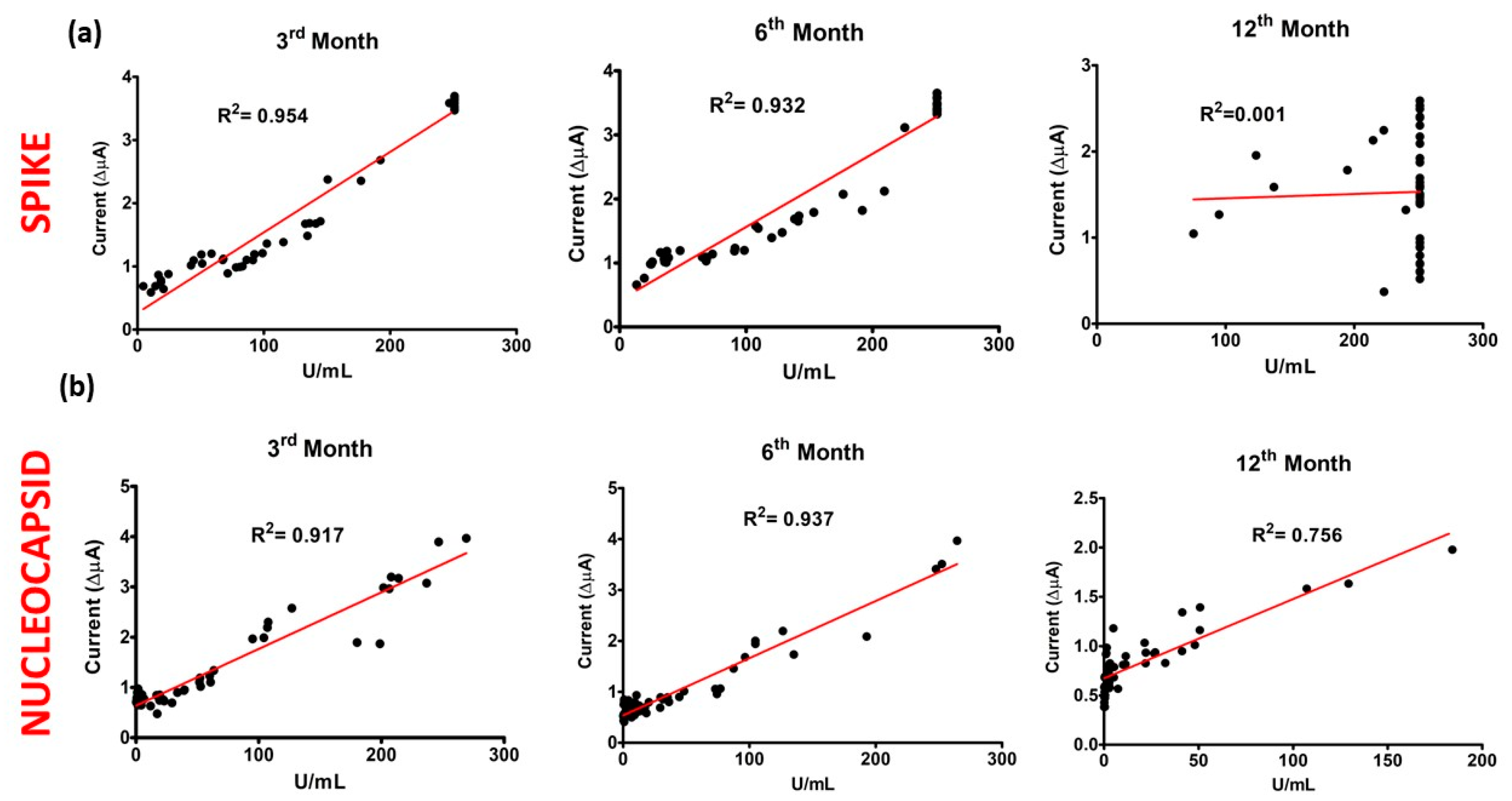
3.5. Confirmation and Comparison Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Healthy Adults Aged 18–59 Years: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; Andrews, J.R.; Boaventura, V.S.; Ranzani, O.T.; de Araújo Oliveira, V.; Paixão, E.S.; Júnior, J.B.; Machado, T.M.; Hitchings, M.D.T.; Dorion, M.; et al. Effectiveness of CoronaVac, ChAdOx1 NCoV-19, BNT162b2, and Ad26.COV2.S among Individuals with Previous SARS-CoV-2 Infection in Brazil: A Test-Negative, Case-Control Study. Lancet Infect. Dis. 2022, 22, 791–801. [Google Scholar] [CrossRef]
- Nam, M.; Seo, J.D.; Moon, H.-W.; Kim, H.; Hur, M.; Yun, Y.-M. Evaluation of Humoral Immune Response after SARS-CoV-2 Vaccination Using Two Binding Antibody Assays and a Neutralizing Antibody Assay. Microbiol. Spectr. 2021, 9, e01202-21. [Google Scholar] [CrossRef] [PubMed]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody Responses to SARS-CoV-2 Vaccines in 45,965 Adults from the General Population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to MRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef]
- Castrejón-Jiménez, N.S.; García-Pérez, B.E.; Reyes-Rodríguez, N.E.; Vega-Sánchez, V.; Martínez-Juárez, V.M.; Hernández-González, J.C. Challenges in the Detection of SARS-CoV-2: Evolution of the Lateral Flow Immunoassay as a Valuable Tool for Viral Diagnosis. Biosensors 2022, 12, 728. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Prasad, R.; Singh, J. Biological Biosensors for Monitoring and Diagnosis. Microb. Biotechnol. Basic Res. Appl. 2020, 317–335. [Google Scholar] [CrossRef]
- Qin, J.; Wang, W.; Gao, L.; Yao, S.Q. Emerging Biosensing and Transducing Techniques for Potential Applications in Point-of-Care Diagnostics. Chem. Sci. 2022, 13, 2857–2876. [Google Scholar] [CrossRef]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-Based Rapid Diagnosis for Point-of-Care Application. Microfluid. Nanofluid 2023, 27, 15. [Google Scholar] [CrossRef]
- Kim, J.H.; Suh, Y.J.; Park, D.; Yim, H.; Kim, H.; Kim, H.J.; Yoon, D.S.; Hwang, K.S. Technological Advances in Electrochemical Biosensors for the Detection of Disease Biomarkers. Biomed. Eng. Lett. 2021, 11, 309–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, N. Electrochemical Biosensors Based on Micro-Fabricated Devices for Point-of-Care Testing: A Review. Electroanalysis 2022, 34, 168–183. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic Nanoparticles: Preparation, Physical Properties, and Applications in Biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Man, E.; Harmanci, D.; Tozan Ruzgar, S.; Sanli, S.; Keles, N.A.; Ayden, A.; Tuna, B.G.; Duzgun, O.; Ozkan, O.F.; et al. Magnetic Nanoparticle-Based Electrochemical Sensing Platform Using Ferrocene-Labelled Peptide Nucleic Acid for the Early Diagnosis of Colorectal Cancer. Biosensors 2022, 12, 736. [Google Scholar] [CrossRef]
- Zhong, J.; Rösch, E.L.; Viereck, T.; Schilling, M.; Ludwig, F. Toward Rapid and Sensitive Detection of SARS-CoV-2 with Functionalized Magnetic Nanoparticles. ACS Sens. 2021, 6, 976–984. [Google Scholar] [CrossRef]
- Gholizadeh, O.; Yasamineh, S.; Amini, P.; Afkhami, H.; Delarampour, A.; Akbarzadeh, S.; Karimi Matloub, R.; Zahedi, M.; Hosseini, P.; Hajiesmaeili, M.; et al. Therapeutic and Diagnostic Applications of Nanoparticles in the Management of COVID-19: A Comprehensive Overview. Virol. J. 2022, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chugh, V.K.; Krishna, V.D.; di Girolamo, A.; Wang, Y.A.; Saha, R.; Liang, S.; Cheeran, M.C.-J.; Wang, J.-P. One-Step, Wash-Free, Nanoparticle Clustering-Based Magnetic Particle Spectroscopy Bioassay Method for Detection of SARS-CoV-2 Spike and Nucleocapsid Proteins in the Liquid Phase. ACS Appl. Mater. Interfaces 2021, 13, 44136–44146. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Ahsan, M.d.Z. Plausible Approach for Rapid Detection of SARS-CoV-2 Virus by Magnetic Nanoparticle Based Biosensors. Am. J. Nanosci. 2020, 6, 6–13. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Z.; Li, H.; Xiong, Y.; Cai, S.; Wang, C.; Chen, Y.; Han, N.; Yang, R. Magnet-Assisted Electrochemical Immunosensor Based on Surface-Clean Pd-Au Nanosheets for Sensitive Detection of SARS-CoV-2 Spike Protein. Electrochim. Acta 2022, 404, 139766. [Google Scholar] [CrossRef] [PubMed]
- Durmus, C.; Balaban Hanoglu, S.; Harmanci, D.; Moulahoum, H.; Tok, K.; Ghorbanizamani, F.; Sanli, S.; Zihnioglu, F.; Evran, S.; Cicek, C.; et al. Indiscriminate SARS-CoV-2 Multivariant Detection Using Magnetic Nanoparticle-Based Electrochemical Immunosensing. Talanta 2022, 243, 123356. [Google Scholar] [CrossRef]
- Singh, V.; Krishnan, S. An Electrochemical Mass Sensor for Diagnosing Diabetes in Human Serum. Analyst 2014, 139, 724–728. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 MRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Y.; Luo, C.; Chen, Y.; Touil, N.; Annaz, H.-E.; Zeng, S.; Dang, T.; Liang, J.; Hu, W.; et al. Novel Nanostructure-Coupled Biosensor Platform for One-Step High-Throughput Quantification of Serum Neutralizing Antibody after COVID-19 Vaccination. Biosens. Bioelectron. 2022, 199, 113868. [Google Scholar] [CrossRef]
- Zeng, J.; Duarte, P.A.; Ma, Y.; Savchenko, O.; Shoute, L.; Khaniani, Y.; Babiuk, S.; Zhuo, R.; Abdelrasoul, G.N.; Charlton, C.; et al. An Impedimetric Biosensor for COVID-19 Serology Test and Modification of Sensor Performance via Dielectrophoresis Force. Biosens. Bioelectron. 2022, 213, 114476. [Google Scholar] [CrossRef]
- Rajsri, K.S.; McRae, M.P.; Simmons, G.W.; Christodoulides, N.J.; Matz, H.; Dooley, H.; Koide, A.; Koide, S.; McDevitt, J.T. A Rapid and Sensitive Microfluidics-Based Tool for Seroprevalence Immunity Assessment of COVID-19 and Vaccination-Induced Humoral Antibody Response at the Point of Care. Biosensors 2022, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Racine-Brzostek, S.E.; Karbaschi, M.; Gaebler, C.; Klasse, P.J.; Yee, J.; Caskey, M.; Yang, H.S.; Hao, Y.; Sukhu, A.; Rand, S.; et al. TOP-Plus Is a Versatile Biosensor Platform for Monitoring SARS-CoV-2 Antibody Durability. Clin. Chem. 2021, 67, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, S.; Han, J.; Kim, T.G.; Bang, A.; Choi, H.W.; Min, G.E.; Shin, J.-H.; Moon, S.W.; Choi, S. An Excitation Wavelength-Optimized, Stable SERS Biosensing Nanoplatform for Analyzing Adenoviral and AstraZeneca COVID-19 Vaccination Efficacy Status Using Tear Samples of Vaccinated Individuals. Biosens. Bioelectron. 2022, 204, 114079. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Miller, B.L. Monitoring Serum Spike Protein with Disposable Photonic Biosensors Following SARS-CoV-2 Vaccination. Sensors 2021, 21, 5857. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Shang, M.; Sawan, M. Rapid Biosensing SARS-CoV-2 Antibodies in Vaccinated Healthy Donors. Biosens. Bioelectron. 2022, 204, 114054. [Google Scholar] [CrossRef]
- Cady, N.C.; Tokranova, N.; Minor, A.; Nikvand, N.; Strle, K.; Lee, W.T.; Page, W.; Guignon, E.; Pilar, A.; Gibson, G.N. Multiplexed Detection and Quantification of Human Antibody Response to COVID-19 Infection Using a Plasmon Enhanced Biosensor Platform. Biosens. Bioelectron. 2021, 171, 112679. [Google Scholar] [CrossRef] [PubMed]
- Muench, P.; Jochum, S.; Wenderoth, V.; Ofenloch-Haehnle, B.; Hombach, M.; Strobl, M.; Sadlowski, H.; Sachse, C.; Torriani, G.; Eckerle, I.; et al. Development and Validation of the Elecsys Anti-SARS-CoV-2 Immunoassay as a Highly Specific Tool for Determining Past Exposure to SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e01694-20. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Santano, R.; Jiménez, A.; Vidal, M.; Chi, J.; Rodrigo Melero, N.; Popovic, M.; López-Aladid, R.; Fernández-Barat, L.; Tortajada, M.; et al. Immunogenicity and Crossreactivity of Antibodies to the Nucleocapsid Protein of SARS-CoV-2: Utility and Limitations in Seroprevalence and Immunity Studies. Transl. Res. 2021, 232, 60–74. [Google Scholar] [CrossRef]





| Analytical Parameters | Values | |
|---|---|---|
| MNP/S Protein/S Ab | MNP/N Protein/ N Ab | |
| Linear Range (ng/mL) | 1.0–75 | 1.0–150 |
| Slope | 0.029 | 0.028 |
| Intercept | 1.370 | 0.499 |
| ±S.D. of Slope | 0.003 | 0.001 |
| ±S.D. of coefficient | 0.139 | 0.092 |
| Correlation coefficient | 0.964 | 0.992 |
| LOD (ng/mL) | 0.978 | 0.894 |
| Repeatability (±S.D.) | 0.04 | 0.061 |
| Coefficient of variation (%) | 2.330 | 3.460 |
| Sensor Type | Target | Biological Material | LOD | Ref |
|---|---|---|---|---|
| Nanostructure-coupled nanopore biosensor platform | Neutralizing antibody | Serum | 0.2 pM | [26] |
| Gold micro-interdigitated electrodes biosensor | IgG (anti-S Ab specific) | Serum | AuNp strategy: 200 ng/mLdielectrophoretic (DEP) approach: 2 μg/mL | [27] |
| Microfluidics-based tool | IgG | Serum | 47 ng/mL | [28] |
| Testing-on-a-probe plus panel | Total antibody (TAb) and surrogate neutralizing antibody (SNAb) | Serum | Various values | [29] |
| Label-free surface-enhanced Raman scattering biosensing platform | IgG | Tear | 1 × 10−14 M | [30] |
| Photonic assay platform | S1 + S2 | Serum | 4.2 μg/mL | [31] |
| Fiber optic-biolayer interferometry-based biosensor | Neutralizing antibody (Nabs), anti-receptor binding domain (anti-RBD) BAbs, and anti-extracellular domain of spike protein (anti-S-ECD) BAbs | Serum | Various values | [32] |
| Grating-coupled fluorescent plasmonics biosensor platform | RBD, spike S1 fragment, spike S1S2 extracellular domain | Serum | Various values | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmanci, D.; Balaban Hanoglu, S.; Akkus Kayali, G.; Durgunlu, E.; Ucar, N.; Cicek, C.; Timur, S. Post-Vaccination Detection of SARS-CoV-2 Antibody Response with Magnetic Nanoparticle-Based Electrochemical Biosensor System. Biosensors 2023, 13, 851. https://doi.org/10.3390/bios13090851
Harmanci D, Balaban Hanoglu S, Akkus Kayali G, Durgunlu E, Ucar N, Cicek C, Timur S. Post-Vaccination Detection of SARS-CoV-2 Antibody Response with Magnetic Nanoparticle-Based Electrochemical Biosensor System. Biosensors. 2023; 13(9):851. https://doi.org/10.3390/bios13090851
Chicago/Turabian StyleHarmanci, Duygu, Simge Balaban Hanoglu, Gozde Akkus Kayali, Evrim Durgunlu, Nursima Ucar, Candan Cicek, and Suna Timur. 2023. "Post-Vaccination Detection of SARS-CoV-2 Antibody Response with Magnetic Nanoparticle-Based Electrochemical Biosensor System" Biosensors 13, no. 9: 851. https://doi.org/10.3390/bios13090851
APA StyleHarmanci, D., Balaban Hanoglu, S., Akkus Kayali, G., Durgunlu, E., Ucar, N., Cicek, C., & Timur, S. (2023). Post-Vaccination Detection of SARS-CoV-2 Antibody Response with Magnetic Nanoparticle-Based Electrochemical Biosensor System. Biosensors, 13(9), 851. https://doi.org/10.3390/bios13090851





