Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications
Abstract
:1. Introduction
2. Overview of Electrospinning
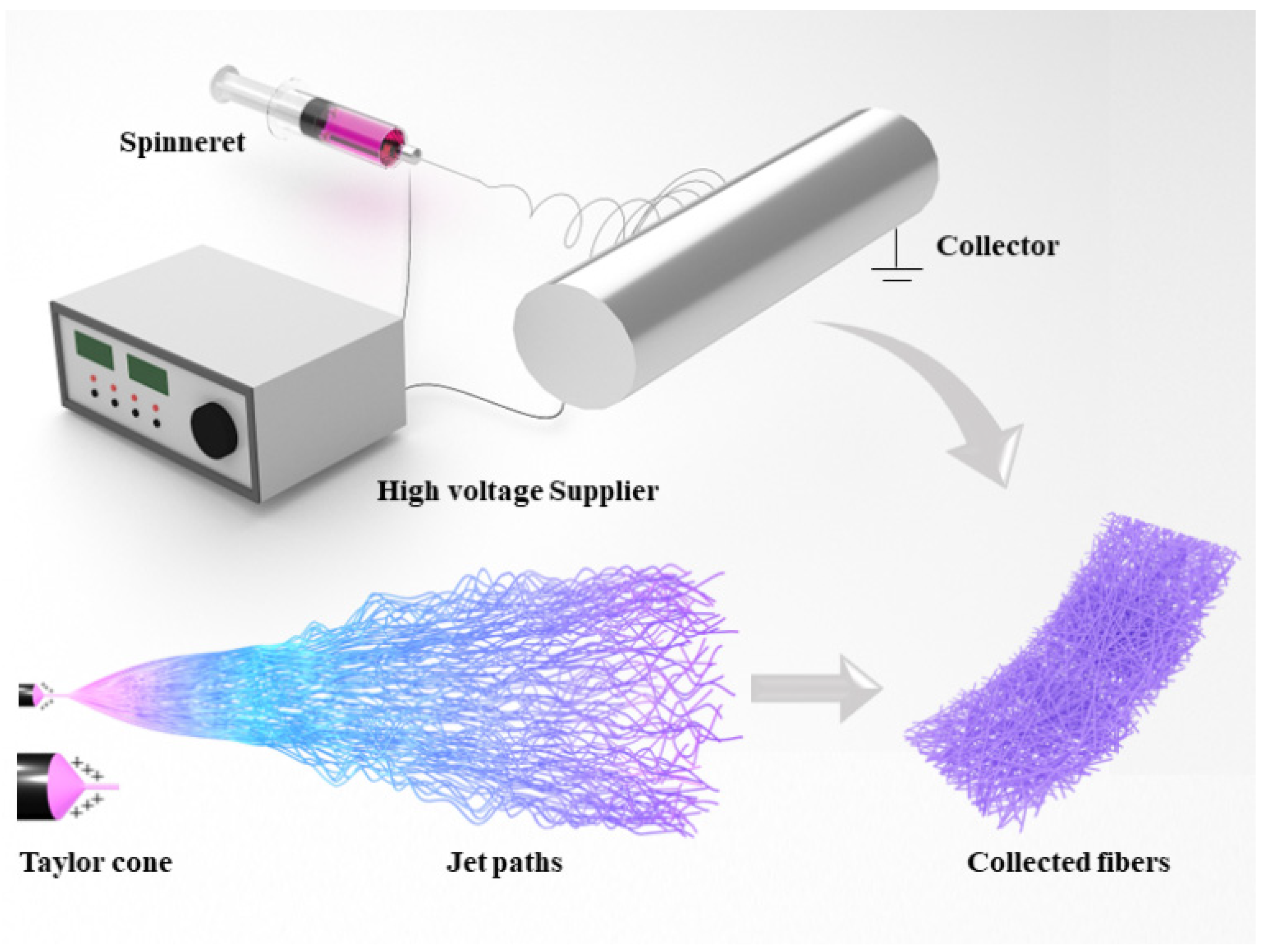
2.1. General Working Principle of Electrospinning
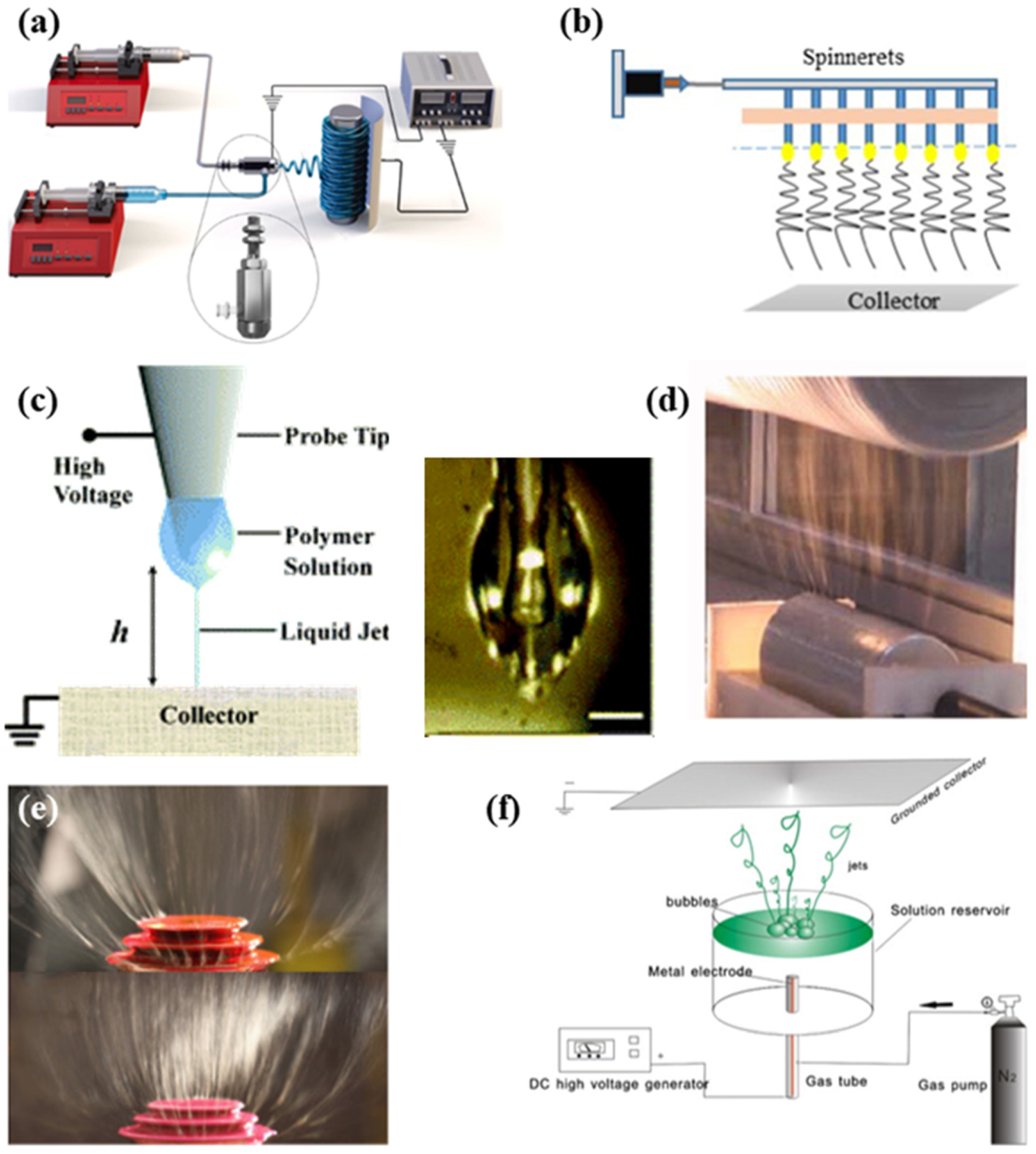
2.2. Different Electrospinning Approaches
2.2.1. Coaxial Electrospinning
2.2.2. Multi-Needle Electrospinning
2.2.3. Near-Field Electrospinning
2.2.4. Needleless Electrospinning
2.3. Electrospun Nanofibers
2.4. Diverse Biomaterials of Electrospinning
2.5. Typical Structures of Electrospun Nanofibers in the Biomedical Field
2.5.1. Core-Shell Fibers
2.5.2. Ribbon Fibers
2.5.3. Porous Fibers
2.5.4. Beaded Fibers
3. Recent Advances in Electrospun Nanofibers for Biosensors and Other Applications
3.1. Biosensors
3.1.1. Electrochemical Biosensors
3.1.2. Optical Biosensors
3.1.3. Thermometric Biosensors
3.2. Other Applications
3.2.1. Tissue Engineering
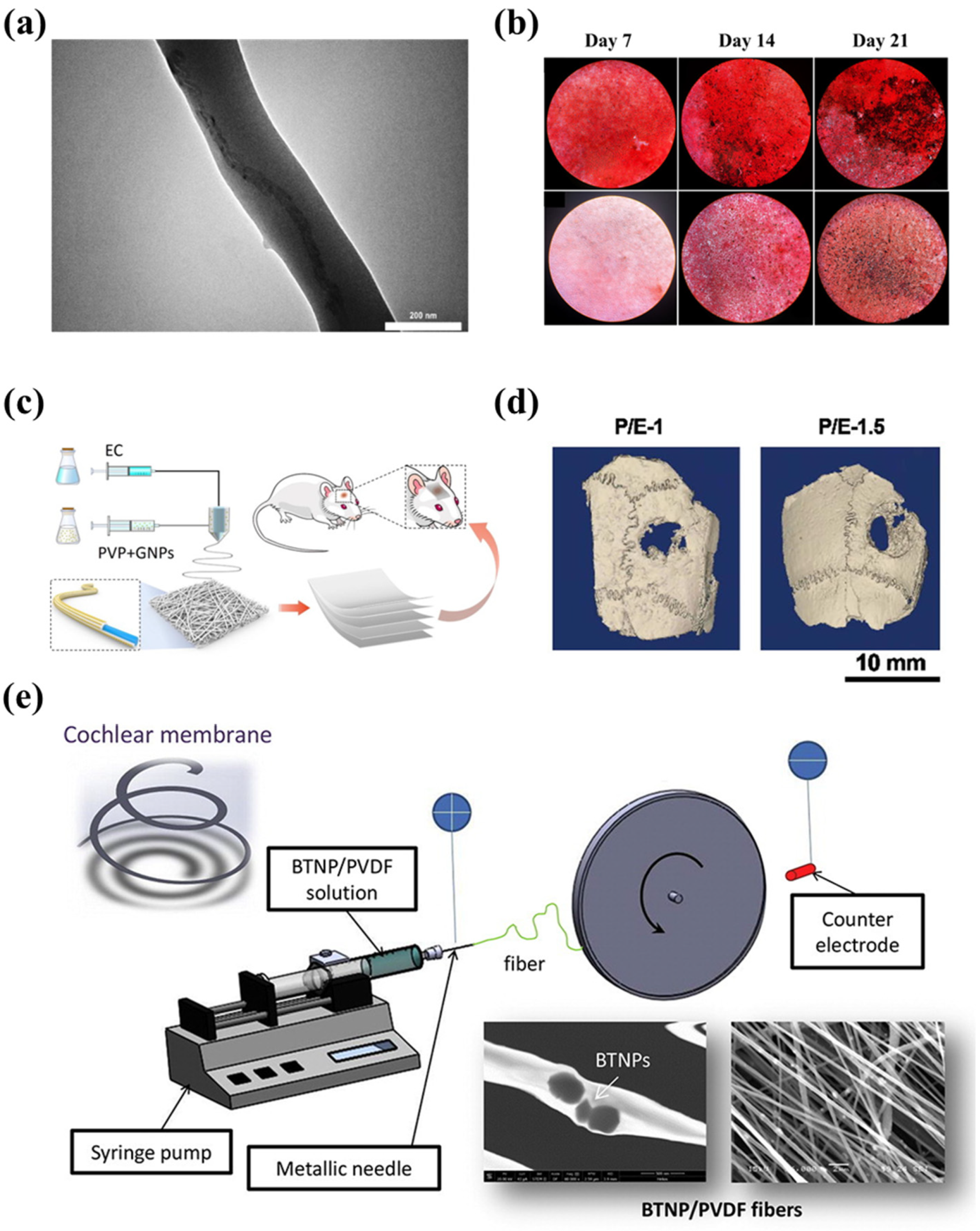
3.2.2. Drug Delivery
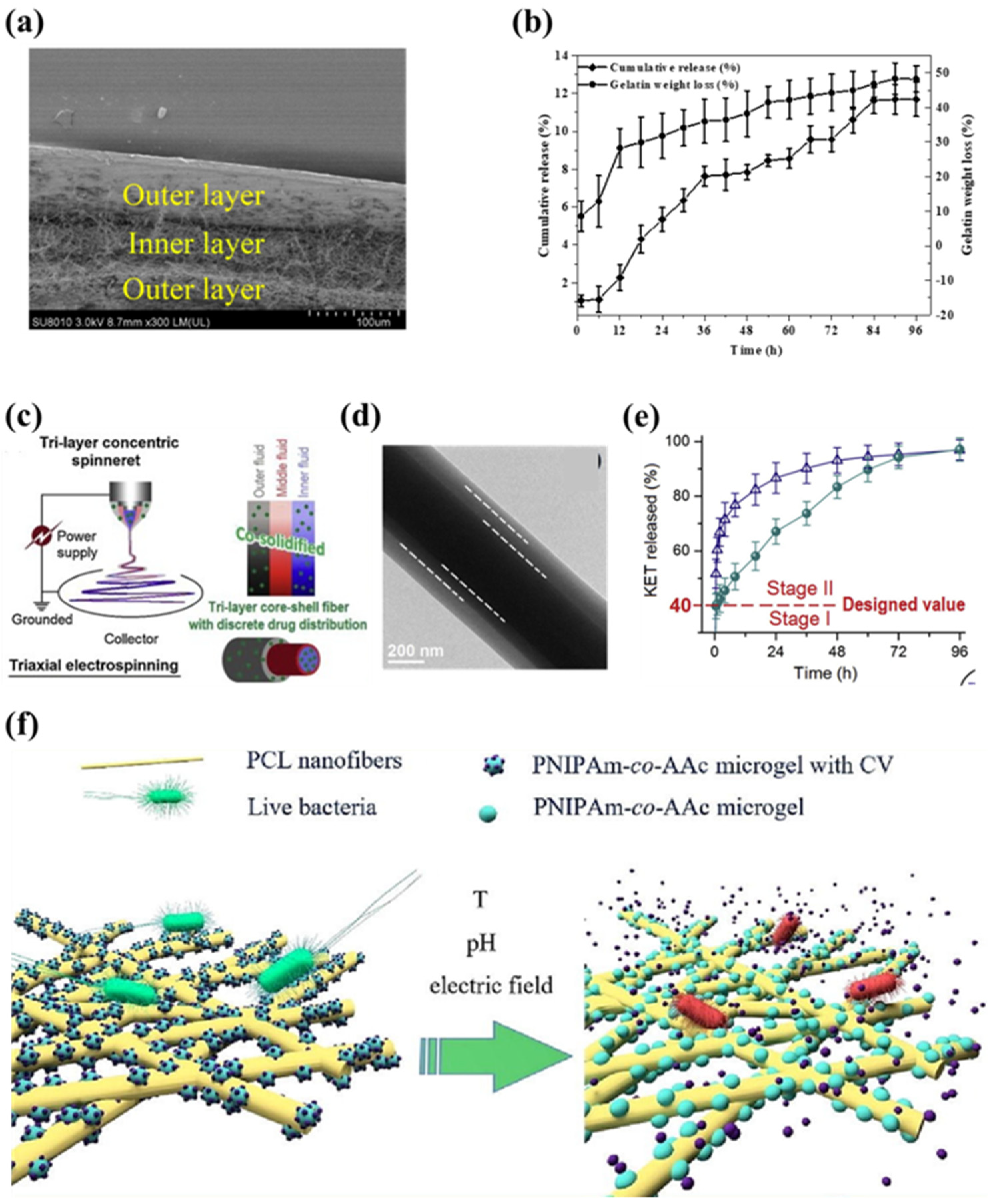
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, W.; Shin, H.; Choi, B.; Rhim, W.K.; Na, K.; Han, D.K. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Tyan, Y.C.; Yang, M.H.; Chang, C.C.; Chung, T.W. Biocompatibility of Materials for Biomedical Engineering. In Biomimicked Biomaterials: Advances in Tissue Engineering and Regenerative Medicine; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1250, pp. 125–140. [Google Scholar]
- Vanholme, B.; El Houari, I.; Boerjan, W. Bioactivity: Phenylpropanoids’ best kept secret. Curr. Opin. Biotechnol. 2019, 56, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; John, J.V.; McCarthy, A.; Xie, J. New forms of electrospun nanofiber materials for biomedical applications. J. Mater. Chem. B 2020, 8, 3733–3746. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Sill, T.J.; Recum, H. Electrospinning: Applications in drug delivery and tissue engineering—ScienceDirect. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Heikkil, P.; Taipale, A.; Lehtimki, M.; Harlin, A. Electrospinning of polyamides with different chain compositions for filtration application. Polym. Eng. Sci. 2010, 48, 1168–1176. [Google Scholar] [CrossRef]
- Li, S.F.; Chen, J.P.; Wu, W.T. Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. J. Mol. Catal. B Enzym. 2007, 47, 117–124. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Taylor, G. Electrically Driven Jets. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1969, 313, 453–475. [Google Scholar]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022, 15, 787–804. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core–Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1329–1932. [Google Scholar] [CrossRef]
- Yu, J.H.; Fridrikh, S.V.; Rutledge, G.C. Production of Submicrometer Diameter Fibers by Two-Fluid Electrospinning. Adv. Mater. 2004, 16, 1562–1566. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M. Electrospinning as a versatile method for fabricating coreshell, hollow and porous nanofibers. Sci. Iran. 2012, 19, 2029–2034. [Google Scholar] [CrossRef]
- Woo, Y.C.; Yao, M.; Shim, W.G.; Kim, Y.; Tijing, L.D.; Jung, B.; Kim, S.H.; Shon, H.K. Co-axially electrospun superhydrophobic nanofiber membranes with 3D-hierarchically structured surface for desalination by long-term membrane distillation. J. Membr. Sci. 2021, 623, 119028. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef]
- He, X.X.; Zheng, J.; Yu, G.F.; You, M.H.; Yu, M.; Ning, X.; Long, Y.Z. Near-field electrospinning: Progress and applications. J. Phys. Chem. C 2017, 121, 8663–8678. [Google Scholar] [CrossRef]
- Niu, H.; Lin, T.; Wang, X. Needleless electrospinning. I. A comparison of cylinder and disk nozzles. J. Appl. Polym. Sci. 2009, 114, 3524–3530. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, S.; Wang, Y.; Qin, X. An improved free surface electrospinning with micro-bubble solution system for massive production of nanofibers. Mater. Lett. 2015, 144, 22–25. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y.; Xu, L.; Yu, J.Y.; Sun, G. BioMimic fabrication of electrospun nanofibers with high-throughput. Chaos Solitons Fractals 2008, 37, 643–651. [Google Scholar] [CrossRef]
- Han, W.; Minhao, L.; Xin, C.; Junwei, Z.; Xindu, C.; Ziming, Z. Study of deposition characteristics of multi-nozzle near-field electrospinning in electric field crossover interference conditions. Aip Adv. 2015, 5, 78–88. [Google Scholar] [CrossRef]
- Sun, D.; Chang, C.; Li, S.; Lin, L. Near-Field Electrospinning. Nano Lett. 2006, 6, 839. [Google Scholar] [CrossRef]
- Williams, G.H.; Raimi-Abraham, B.T.; Luo, C.J. Nanofibres in Drug Delivery; UCL Press: London, UK, 2018. [Google Scholar]
- Miao, Y.; Dong, R.; Xu, Y.; Yu, G.; You, M.; Xin, N.; Long, Y. Recent Advances in Needleless Electrospinning of Ultrathin Fibers: From Academia to Industrial Production. Macromol. Mater. Eng. 2017, 302, 1700002. [Google Scholar]
- Salehhudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Afifi, A.M. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process. 2017, 33, 479–498. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; lvarez-Láinez, M.; Lagaron, J.M. The Influence of Electrospinning Parameters and Solvent Selection on the Morphology and Diameter of Polyimide Nanofibers. Mater. Today Commun. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Lxy, D.; Xia, Y.N. Electrospinning of Nanofibers: Reinventing the Wheel. Adv. Mater. 2004, 16, 1151–1170. [Google Scholar]
- Liu, W.; Lipner, J.; Xie, J.; Manning, C.N.; Thomopoulos, S.; Xia, Y. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl. Mater. Interfaces 2014, 6, 2842–2849. [Google Scholar] [CrossRef]
- Yang, T.; Zhan, L.; Huang, C.Z. Recent insights into functionalized electrospun nanofibrous films for chemo-/bio-sensors. Trac-Trends Anal. Chem. 2020, 124, 115813. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, S.H.; Liu, J.; Soker, S.; Atala, A.; Yoo, J.J. The use of thermal treatments to enhance the mechanical properties of electrospun poly (ε-caprolactone) scaffolds. Biomaterials 2008, 29, 1422–1430. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, R.; He, R.; Lei, T.; Misra, R.; Nie, H.; Ma, C.; Lin, N.; Wang, Z. Effects of integrated bioceramic and uniaxial drawing on mechanically-enhanced fibrogenesis for bionic periosteum engineering. Colloids Surf. B Biointerfaces 2022, 214, 112459. [Google Scholar] [CrossRef]
- Jordan, A.M.; Viswanath, V.; Kim, S.E.; Pokorski, J.K.; Korley, L.T.J. Processing and surface modification of polymer nanofibers for biological scaffolds: A review. J. Mater. Chem. B 2016, 4, 5958–5974. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, B.S.; Yoo, Y.C.; Khil, M.S.; Kim, H.Y. Enhanced mechanical properties of multilayer nano-coated electrospun nylon 6 fibers via a layer-by-layer self-assembly. J. Appl. Polym. Sci. 2008, 107, 2211–2216. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, B.T. The effect of cross-linking on the microstructure, mechanical properties and biocompatibility of electrospun polycaprolactone–gelatin/PLGA–gelatin/PLGA–chitosan hybrid composite. Sci. Technol. Adv. Mater. 2012, 13, 035002. [Google Scholar] [CrossRef]
- Catto, V.; Farè, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small diameter electrospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.C.; Ajji, A. Core-Shell Structured PEO-Chitosan Nanofibers by Coaxial Electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y.N. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Liu, L.G.; He, J.H. Solvent evaporation in a binary solvent system for controllable fabrication of porous fibers by electrospinning. Therm. Sci. 2017, 21, 1821–1825. [Google Scholar] [CrossRef]
- Han, W.; Rao, D.; Gao, H.; Yang, X.; Fan, H.; Li, C.; Dong, L.; Meng, H. Green-solvent-processable biodegradable poly (lactic acid) nanofibrous membranes with bead-on-string structure for effective air filtration: “Kill two birds with one stone”. Nano Energy 2022, 97, 107237. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Jiang, L. Bio-mimic multichannel microtubes by a facile method. J. Am. Chem. Soc. 2007, 129, 764–765. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 80, 371–378. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Zeng, Y. Morphology development of helical structure in bicomponent fibers during spinning process. Polymer 2020, 201, 122609. [Google Scholar] [CrossRef]
- Su, Y.; Fan, T.; Bai, H.; Guan, H.; Ning, X.; Yu, M.; Long, Y. Bioinspired superhydrophobic and superlipophilic nanofiber membrane with pine needle-like structure for efficient gravity-driven oil/water separation. Sep. Purif. Technol. 2021, 274, 119098. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, S.; Seuss, M.; Fery, A.; Lang, G.; Scheibel, T.; Agarwal, S. Two-in-One composite fibers with side-by-side arrangement of silk fibroin and poly(L-lactide) by electrospinning. Macromol. Mater. Eng. 2016, 301, 48–55. [Google Scholar] [CrossRef]
- Inozemtseva, O.A.; Salkovskiy, Y.E.; Severyukhina, A.N.; Vidyasheva, I.V.; Gorin, D.A. Electrospinning of functional materials for biomedicine and tissue engineering. Russ. Chem. Rev. 2015, 84, 251. [Google Scholar] [CrossRef]
- Mogo Anu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef]
- Soares, R.M.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef]
- Lesage, J.; Toncelli, C.; Fortunato, G.; Rossi, R.M.; Spano], F. Crosslinking dextran electrospun nanofibers via borate chemistry: Proof of concept for wound patches. Eur. Polym. J. 2019, 110, 276–282. [Google Scholar]
- Kyzio, A.; Michna, J.; Moreno, I.; Gamez, E.; Irusta, S. Preparation and characterization of electrospun alginate nanofibers loaded with ciprofloxacin hydrochloride. Eur. Polym. J. 2017, 96, 350–360. [Google Scholar] [CrossRef]
- Brenner, E.K.; Schiffman, J.D.; Thompson, E.A.; Toth, L.J.; Schauer, C.L. Electrospinning of hyaluronic acid nanofibers from aqueous ammonium solutions. Carbohydr. Polym. 2012, 87, 926–929. [Google Scholar] [CrossRef]
- Adhikary, P.; Jana, S.; Biswas, A.; Sencadas, V.; Gupta, S.D.; Tudu, B.; Mandal, D. Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring. Nano Energy 2017, 36, 166–175. [Google Scholar]
- Gümüşderelioğlu, M.; Dalkıranoğlu, S.; Aydın, R.S.T.; Çakmak, S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J. Biomed. Mater. Res. Part A 2011, 98, 461–472. [Google Scholar] [CrossRef]
- Yeb, A.; Mo, B.; Dk, C.; Nda, B. Development of PCL/PEO electrospun fibrous membranes blended with silane-modified halloysite nanotube as a curcumin release system—ScienceDirect. Appl. Clay Sci. 2020, 186, 105430. [Google Scholar]
- Lin, C.C.; Fu, S.J.; Lin, Y.C.; Yang, I.K.; Gu, Y. Chitosan-coated electrospun PLA fibers for rapid mineralization of calcium phosphate. Int. J. Biol. Macromol. 2014, 68, 39–47. [Google Scholar] [CrossRef]
- Qi, R.L.; Tian, X.J.; Guo, R.; Luo, Y.; Shen, M.W.; Yu, J.Y.; Shi, X.Y. Controlled release of doxorubicin from electrospun MWCNTs/PLGA hybrid nanofibers. Chin. J. Polym. Sci. 2016, 34, 1047–1059. [Google Scholar] [CrossRef]
- Porto, M.D.A.; Dos Santos, J.P.; Hackbart, H.; Bruni, G.P.; Fonseca, L.M.; da Rosa Zavareze, E.; Dias, A.R.G. Immobilization of α-amylase in ultrafine polyvinyl alcohol (PVA) fibers via electrospinning and their stability on different substrates. Int. J. Biol. Macromol. 2019, 126, 834–841. [Google Scholar] [CrossRef]
- Manikandan, A.; Mani, M.P.; Jaganathan, S.K.; Rajasekar, R.; Jagannath, M. Formation of functional nanofibrous electrospun polyurethane and murivenna oil with improved hemocompatibility for wound healing. Polym. Test. 2017, 61, 106–113. [Google Scholar] [CrossRef]
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Neisiany, R.E.; Khorasani, S.N.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135, 46265. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Rastegar, A.; Mahmoodi, M.; Mirjalili, M.; Nasirizadeh, N. Platelet-Rich Fibrin-Loaded PCL/Chitosan Core-Shell fibers Scaffold for Enhanced Osteogenic Differentiation of Mesenchymal Stem Cells. Carbohydr. Polym. 2021, 269, 118351. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Hui, Z. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Johnson, P.M.; Knewtson, K.E.; Hodge, J.G.; Lehtinen, J.M.; Trofimoff, A.S.; Fritz, D.J.; Robinson, J.L. Surfactant location and internal phase volume fraction dictate emulsion electrospun fiber morphology and modulate drug release and cell response. Biomater. Sci. 2021, 9, 1397–1408. [Google Scholar] [CrossRef]
- Zhan, F.; Yan, X.; Li, J.; Sheng, F.; Li, B. Encapsulation of tangeretin in PVA/PAA crosslinking electrospun fibers by emulsion-electrospinning: Morphology characterization, slow-release, and antioxidant activity assessment. Food Chem. 2020, 337, 127763. [Google Scholar] [CrossRef]
- Su, S.; Bedir, T.; Kalkandelen, C.; Baar, A.O.; Gunduz, O. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci. B Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zheng, M.; Dunne, F.; Liu, T.; Fu, X.; Kong, L.; Pa, N.S.; Zhong, W.H. Morphology engineering of protein fabrics for advanced and sustainable filtration. J. Mater. Chem. A 2018, 6, 21585–21595. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millan, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 119, 111602. [Google Scholar] [CrossRef] [PubMed]
- Pryadko, A.S.; Botvin, V.V.; Mukhortova, Y.R.; Pariy, I.; Wagner, D.V.; Laktionov, P.P.; Chernonosova, V.S.; Chelobanov, B.P.; Chernozem, R.V.; Surmeneva, M.A.; et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers 2022, 14, 14030529. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Thomas, N.L. Fabrication of porous fibers via electrospinning: Strategies and applications. Polym. Rev. 2020, 60, 595–647. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured fibers via electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Zhang, A.; Bai, H.; Li, L. Breath Figure: A Nature-Inspired Preparation Method for Ordered Porous Films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, G.; Li, Y.; Fan, G. Effects of the glycerophosphate-polylactic copolymer formation on electrospun fibers. Appl. Surf. Sci. 2018, 443, 236–243. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabricating porous poly(lactic acid) fibres via electrospinning. Eur. Polym. J. 2018, 99, 464–476. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Prasauskas, T.; Buivydiene, D.; Martuzevicius, D. The comparative study of aerosol filtration by electrospun polyamide, polyvinyl acetate, polyacrylonitrile and cellulose acetate nanofiber media. J. Aerosol Sci. 2016, 92, 27–37. [Google Scholar] [CrossRef]
- Korycka, P.; Mirek, A.; Kramek-Romanowska, K.; Grzeczkowicz, M.; Lewinska, D. Effect of electrospinning process variables on the size of polymer fibers and bead-on-string structures established with a 2(3) factorial design. Beilstein J. Nanotechnol. 2018, 9, 2466–2478. [Google Scholar] [CrossRef]
- Kadam, V.; Kyratzis, I.L.; Truong, Y.B.; Schutz, J.; Wang, L.; Padhye, R. Electrospun bilayer nanomembrane with hierarchical placement of bead-on-string and fibers for low resistance respiratory air filtration. Sep. Purif. Technol. 2019, 224, 247–254. [Google Scholar] [CrossRef]
- Zhan, N.; Li, Y.; Zhang, C.; Song, Y.; Wang, H.; Sun, L.; Yang, Q.; Hong, X. A novel multinozzle electrospinning process for preparing superhydrophobic PS films with controllable bead-on-string/microfiber morphology. J. Colloid Interface Sci. 2010, 345, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhu, M.; Yang, W.; Yu, H.; Chen, Y.; Zhang, Y. Experimental study on relationship between jet instability and formation of beaded fibers during electrospinning. Polym. Eng. Sci. 2005, 45, 704–709. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Song, W.-L.; Yu, D.-G.; Bligh, S.W.A. Electrospun Janus Beads-On-A-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biomolecules 2021, 11, 11050635. [Google Scholar] [CrossRef] [PubMed]
- Coulet, P.R.; Blum, L.J. Biosensor Principles and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and emerging healthcare and medicine possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huan, K.; Deng, D.; Yan, X.; Li, Y.; Luo, L. Coaxial electrospinning synthesis of size-tunable CuO/NiO hollow heterostructured nanofibers: Towards detection of glucose level in human serum. Colloids Surf. B Biointerfaces 2023, 222, 113047. [Google Scholar] [CrossRef]
- Su, J. Label-free biological and chemical sensing using whispering gallery mode optical resonators: Past, present, and future. Sensors 2017, 17, 540. [Google Scholar] [CrossRef]
- Hsieh, S.T.; Cheeney, J.E.; Ding, X.; Myung, N.V.; Haberer, E.D. Near-field electrospinning of polymer/phage whispering gallery mode microfiber resonators for label-free biosensing. Sens. Actuators B Chem. 2022, 367, 132062. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, H.; Kim, E.; Zhang, H.; Wu, K.; Zhang, H.; Shen, X.; Zheng, Q.; Yang, J.; Jeon, S. Flexible temperature sensors made of aligned electrospun carbon nanofiber films with outstanding sensitivity and selectivity towards temperature. Mater. Horiz. 2021, 8, 1488–1498. [Google Scholar] [CrossRef]
- Ghaffar, A.; Mehdi, M.; Hussain, S.; Pirzado, A.A.A.; Shah, S.A.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O. Plant extracted natural fluorescent protein C-phycocyanin doped in PVA nanofibers for advanced apparel application. Mater. Res. Express 2023, 10, 9. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Mo, T.; Wang, L.; Li, P. Immobilization of aminoacylase on electrospun nanofibrous membrane for the resolution of DL-theanine. J. Mol. Catal. B-Enzym 2015, 116, 24–28. [Google Scholar] [CrossRef]
- Atik, G.; Kilic, N.M.; Horzum, N.; Odaci, D.; Timur, S. Antibody-Conjugated Electrospun Nanofibers for Electrochemical Detection of Methamphetamine. Acs Appl. Mater. Interfaces 2023, 15, 24109–24119. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Bagheri, K.P.; Nadushan, R.M.; Adabi, M. Nanoarchitectonics of electrochemical aptasensor based on electrospun carbon nanofibers and gold nanoparticles for tetracycline detection in chicken ham. Appl. Phys. A-Mater. Sci. Process 2023, 129, 7. [Google Scholar] [CrossRef]
- Sapountzi, E.; Chateaux, J.F.; Lagarde, F. Combining Electrospinning and Vapor-Phase Polymerization for the Production of Polyacrylonitrile/Polypyrrole Core-Shell Nanofibers and Glucose Biosensor Application. Front. Chem 2023, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Jiang, K.R.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The detection of Escherichia coli with the pH sensitive hydrogel nanofiber-light addressable potentiometric sensor (NF-LAPS). Sens. Actuator B-Chem 2016, 226, 176–183. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mosallanejad, B.; Zare, Y.; Rhee, K.Y.; Park, S.J. CO3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors. Rev. Adv. Mater. Sci 2022, 61, 744–755. [Google Scholar] [CrossRef]
- Segundo, J.; de Moraes MO, S.; Brito, W.R.; Matos, R.S.; Salerno, M.; Barcelay, Y.R.; Segala, K.; da Fonseca, H.D.; d’Avila, M.A. Molecularly Imprinted Membrane Produced by Electrospinning for β-Caryophyllene Extraction. Materials 2022, 15, 16. [Google Scholar]
- Rao, C.; Bundhamcharoen, K.; Kelly, M.; Tangcharoensathien, V. Mortality estimates for WHO SEAR countries: Problems and prospects. BMJ Glob. Health 2021, 6, e007177. [Google Scholar] [CrossRef]
- Kivrak, E.; Ince-Yardimci, A.; Ilhan, R.; Kirmizibayrak, P.B.; Yilmaz, S.; Kara, P. Aptamer-based electrochemical biosensing strategy toward human non-small cell lung cancer using polyacrylonitrile/polypyrrole nanofibers. Anal. Bioanal. Chem. 2022, 412, 7851–7860. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Zhang, A.; Hu, J.; Wang, X.; Yang, Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomater. Sci. 2016, 4, 922–932. [Google Scholar] [CrossRef]
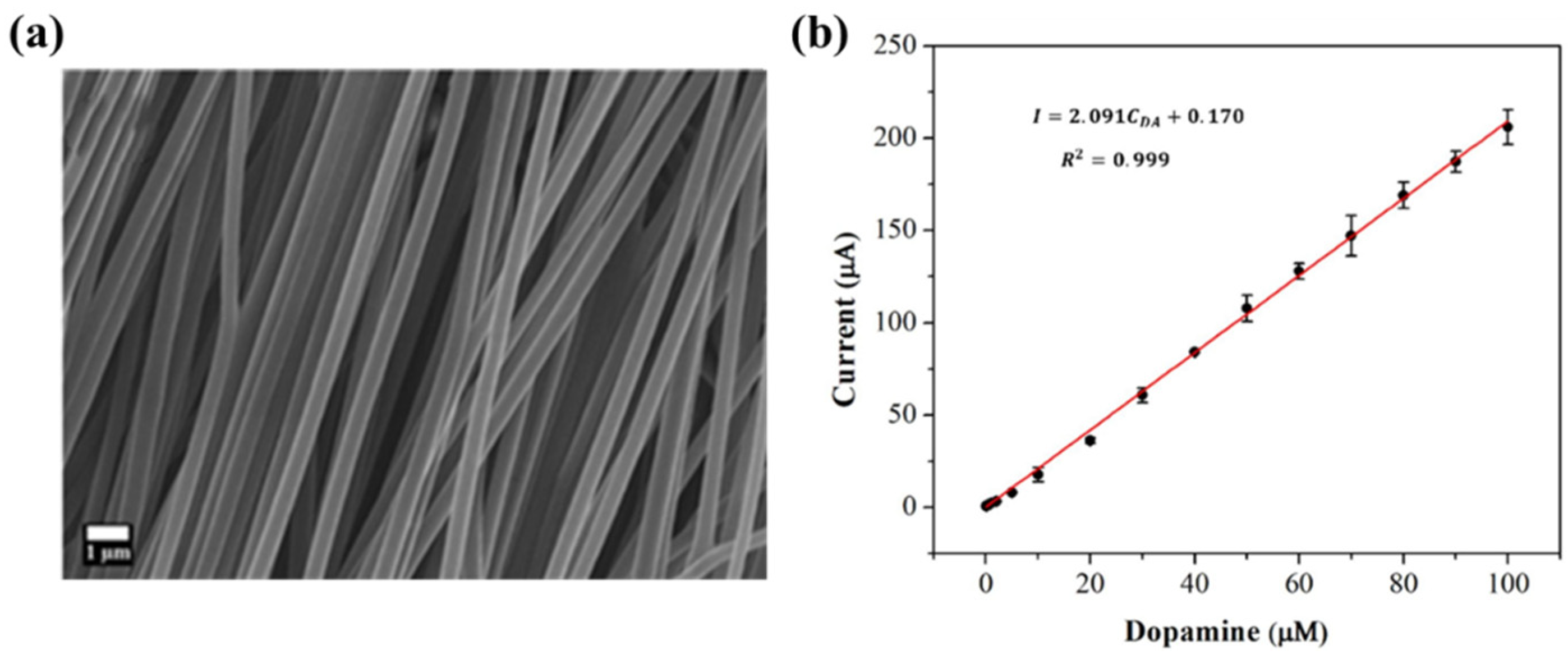
- Yin, Z.; Ji, Z.; PBloom, B.; Jayapalan, A.; Liu, M.; Zeng, X.; Waldeck, D.H. Manipulating cobalt oxide on N-doped aligned electrospun carbon nanofibers towards instant electrochemical detection of dopamine secreted by living cells. Appl. Surf. Sci. 2022, 577, 151912. [Google Scholar] [CrossRef]
- Soares, J.C.; Iwaki, L.E.; Soares, A.C.; Rodrigues, V.C.; Melendez, M.E.; Fregnani, J.H.T.; Reis, R.M.; Carvalho, A.L.; Correâ, D.S.; Oliveira, O.N., Jr. Immunosensor for pancreatic cancer based on electrospun nanofibers coated with carbon nanotubes or gold nanoparticles. ACS Omega 2017, 2, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Zhou, H.; Ma, K.; Yano, Y.; Li, S.; Yamahara, H. Electronic nose based on multiple electrospinning nanofibers sensor array and application in gas classification. Front. Sens. 2023, 4, 1170280. [Google Scholar] [CrossRef]
- Paimard, G.; Shahlaei, M.; Moradipour, P.; Akbari, H.; Jafari, M.; Arkan, E. An Impedimetric Immunosensor modified with electrospun core-shell nanofibers for determination of the carcinoma embryonic antigen. Sens. Actuators B Chem. 2020, 311, 127928. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, D.; Zhu, X.; Liu, S.; Zhu, Y.; Han, L.; Luo, L. Electrospun bimetallic Au-Ag/Co3O4 nanofibers for sensitive detection of hydrogen peroxide released from human cancer cells. Anal. Chim. Acta 2018, 1042, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Elmasry, M.R.; Sharipov, M.; Azizov, S.; Lee, C.H.; Lee, Y.-I. Dual emission nonionic molecular imprinting conjugated polythiophenes-based paper devices and their nanofibers for point-of-care biomarkers detection. Biosens. Bioelectron. 2020, 160, 112211. [Google Scholar] [CrossRef] [PubMed]
- Asmatulu, R.; Veisi, Z.; Uddin, M.; Mahapatro, A. Highly sensitive and reliable electrospun polyaniline nanofiber based biosensor as a robust platform for COX-2 enzyme detections. Fibers Polym. 2019, 20, 966–974. [Google Scholar] [CrossRef]
- Ali, M.A.; Mondal, K.; Singh, C.; Malhotra, B.D.; Sharma, A. Anti-epidermal growth factor receptor conjugated mesoporous zinc oxide nanofibers for breast cancer diagnostics. Nanoscale 2015, 7, 7234–7245. [Google Scholar] [CrossRef]
- Chavoshy, H.Z.; Ghasemi, R. Fabrication of a novel fluorescent polyacrylonitrile electrospun nanofiber for DNA-based optical biosensing of microRNA-21. Nano Express 2020, 1, 020031. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Vasita, R.; Katti, D.S. Growth factor-delivery systems for tissue engineering: A materials perspective. Expert Rev. Med. Devices 2006, 3, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2020, 117, 100721. [Google Scholar] [CrossRef]
- Jang, J.-H.; Castano, O.; Kim, H.-W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef]
- Vaz, C.; Van Tuijl, S.; Bouten, C.; Baaijens, F. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef]
- Asl, M.; Karbasi, A.; Beigi-Boroujeni, S.; Benisi, S.; Saeed, M. Polyhydroxybutyrate-starch/carbon nanotube electrospun nanocomposite: A highly potential scaffold for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 223, 524–542. [Google Scholar] [CrossRef]
- Jia, W.; Cui, D.; Liu, Y.; Ji, X.; Sun, M.; Cheng, Z.; Luo, Y.; Liu, G. Polyether-ether-ketone/poly(methyl methacrylate)/carbon fiber ternary composites prepared by electrospinning and hot pressing for bone implant applications. Mater. Des. 2021, 209, 109893. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef] [PubMed]
- Ueberschlag, P. PVDF piezoelectric polymer. Sens. Rev. 2001, 21, 118–126. [Google Scholar] [CrossRef]
- Mota, C.; Labardi, M.; Trombi, L.; Astolfi, L.; D’Acunto, M.; Puppi, D.; Gallone, G.; Chiellini, F.; Berrettini, S.; Bruschini, L. Design, fabrication and characterization of composite piezoelectric ultrafine fibers for cochlear stimulation. Mater. Des. 2017, 122, 206–219. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Ashinsky, B.G.; Gullbrand, S.E.; Bonnevie, E.D.; Wang, C.; Smith, H.E. Sacrificial Fibers Improve Matrix Distribution and Micromechanical Properties in a Tissue-Engineered Intervertebral Disc. Acta Biomater. 2020, 111, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Buschle-Diller, G.; Cooper, J.; Xie, Z.; Wu, Y.; Waldrup, J.; Ren, X. Release of antibiotics from electrospun bicomponent fibers. Cellulose 2007, 14, 553–562. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.; Kim, K.; Hsiao, B.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Gu, J.G.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.-G. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohydr. Polym. 2020, 243, 116477. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Xie, J. Smart Electrospun Nanofibers for Controlled Drug Release: Recent Advances and New Perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawlowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. Acs Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Puiggali-Jou, A.; Cejudo, A.; Del Valle, L.J.; Aleman, C. Smart Drug Delivery from Electrospun Fibers through Electroresponsive Polymeric Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1594–1605. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Sasikala, A.R.K.; Unnithan, A.R.; Thomas, R.G.; Jeong, Y.Y.; Vatankhah-Varnoosfaderani, M.; Stadler, F.J.; Park, C.H.; Kim, C.S. Mussel-Inspired Electrospun Smart Magnetic Nanofibers for Hyperthermic Chemotherapy. Adv. Funct. Mater. 2015, 25, 2867–2875. [Google Scholar] [CrossRef]
- Arafat, M.T.; Mahmud, M.M.; Wong, S.Y.; Li, X. PVA/PAA based electrospun nanofibers with pH-responsive color change using bromothymol blue and on-demand ciprofloxacin release properties. J. Drug Deliv. Sci. Technol. 2021, 61, 102297. [Google Scholar] [CrossRef]
- Khrystonko, O.; Rimpelova, S.; Burianova, T.; Svorcik, V.; Lyutakov, O.; Elashnikov, R. Smart multi stimuli-responsive electrospun nanofibers for on-demand drug release. J. Colloid Interface Sci. 2023, 648, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, Y.; Li, A.; Wei, L.; Wang, L.; Qin, X.; Yu, J. Mass production of high-quality nanofibers via constructing pre-Taylor cones with high curvature on needleless electrospinning. Mater. Des. 2021, 197, 109247. [Google Scholar] [CrossRef]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef]
| Polymer | Solvent | Fiber Diameter | Structure | Biomedical Application | Refs. | |
|---|---|---|---|---|---|---|
| Natural polymers | Chitosan/PEO | Acetic acid/dimethyl sulfoxide (10:1 w/w) | 181–395 nm |  | Periodontal regeneration | [54] |
| Dextran | Boric acid | 550–600 nm |  | Drug delivery | [55] | |
| Alginate/PEO | Water | 109–161 nm | 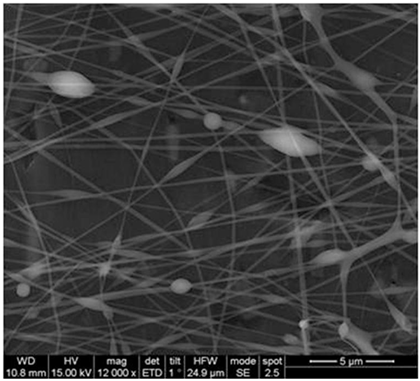 | Drug delivery | [56] | |
| Hyaluronic acid | Na4OH/N,N-Dimethylformamide (DMF) (4:1 w/w) | 27–51 nm |  | Ophthalmology; drug delivery; medical implants | [57] | |
| Gelatin | Detect 17α- Water | 400–1000 nm | 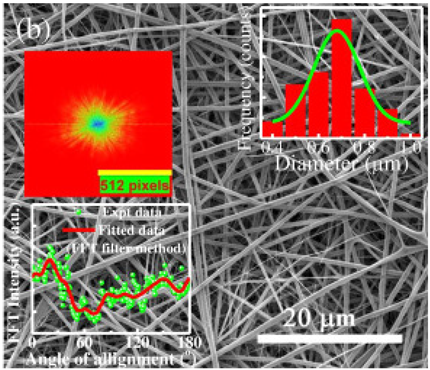 | Biosensor | [58] | |
| Synthetic polymers | PCL | DMF/dichloromethane (DCM) (1:1 w/w) | 236–332 nm | 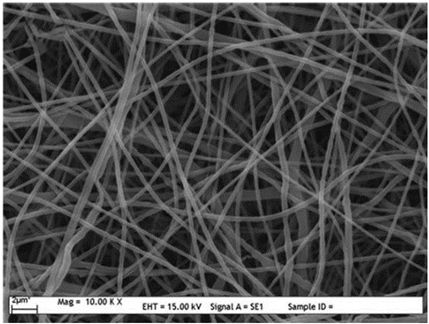 | Tissue engineering | [59] |
| PCL/PEO | DMF/Chloroform (1:9 w/w) | 541–753 nm | 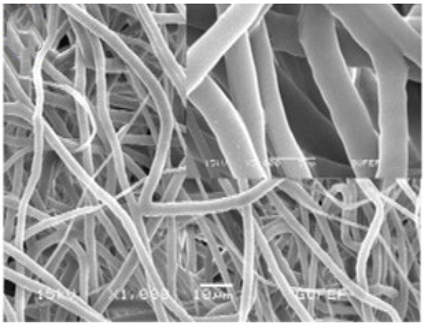 | Drug delivery | [60] | |
| PLA | Chloroform/dimethyl sulfoxide (DMSO) (75:25 w/w) | 232.4–498.3 nm | 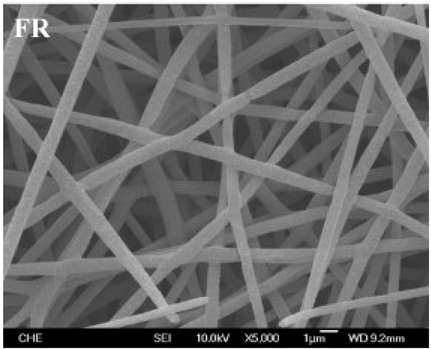 | Tissue engineering | [61] | |
| PLGA | Tetrahydrofuran (THF)/DMF (3:1 w/w) | 506–802 nm | 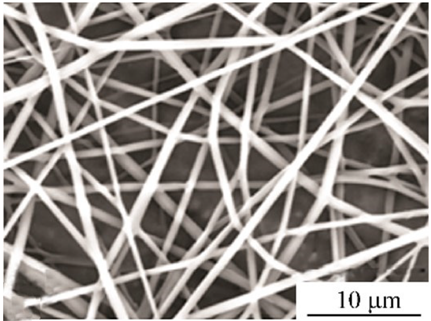 | Drug delivery | [62] | |
| PVA | Phosphate buffer | 187–282 nm | 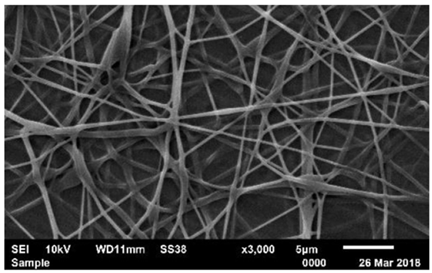 | Enzyme immobilization | [63] | |
| PU | DMF | 580–900 nm | 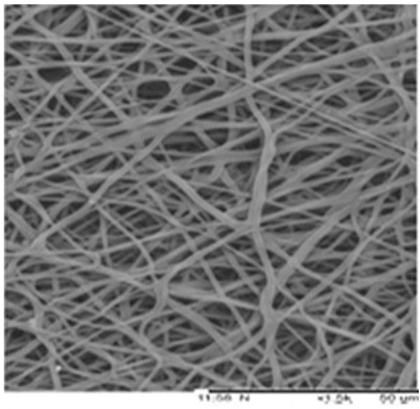 | Wound dressing | [64] |
| Polymer | Solvent | Fiber Diameter | Structure | Refs. |
|---|---|---|---|---|
| C-phycocyanin/PVA | water | 150–200 nm | 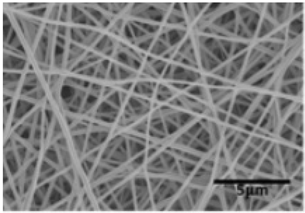 | [93] |
| Aminoacylase/PVA | water | 290–310 |  | [94] |
| PVDF-PEI/Anti-METH | DMF/Ac (2:8; v/v) | 382–408 nm |  | [95] |
| PAN | DMF | 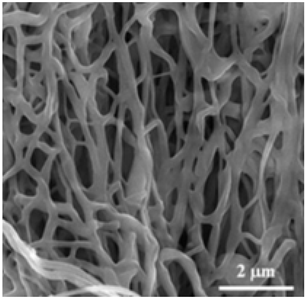 | [96] | |
| PAN | DMF | 657–697 nm | 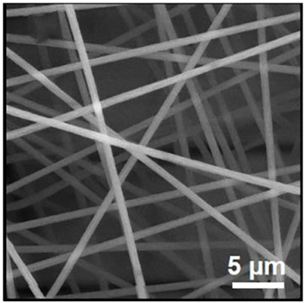 | [97] |
| PAA/PVA | water | 290–390 nm | 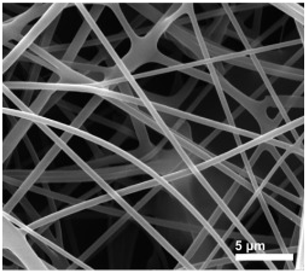 | [98] |
| CoAc/PAN | DMF | 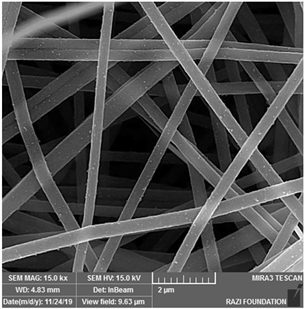 | [99] | |
| β-caryophyllene/PCL | Chloroform/acetone (1:1 wt) | 500–900 nm | 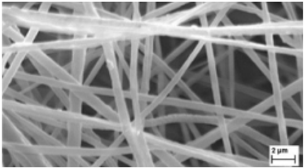 | [100] |
| Polymer Matrix | Sensor Type | Analytes | Cancer Diagnosed | Detection Method | Ref. |
|---|---|---|---|---|---|
| Polyamide 6 (PA6) and poly(allylamine) (PAH) | Immunosensor | Cancer antigen (CA19-9) | pancreatic cancer | Impedance spectroscopy | [105] |
| Polyvinylpyrrolidone (PVP) | Gas sensor | Ammonia, ethanol isoprene, acetaldehyde, isoprene and acetone | Lung cancer | Electrochemical | [106] |
| Poly(vinyl alcohol) (PVA) | Electrochemical immunosensor | Carcinomaembryonic antigen (CEA) | / | Electrochemical | [107] |
| Polyvinyl pyrrolidone (PVP) | Electrochemical sensor | Hydrogen peroxide (H2O2) | Breast cancer | Electrochemical | [108] |
| Polyacrylonitrile (PAN) | Fluorescent sensors | Cancer cells | Liver cancer | Molecular imprint; Enzyme-free signal amplification | [109] |
| Polyaniline | Electrochemical sensor | Cyclooxygenase-2 (COX-2) | / | Electrochemical | [110] |
| Polyacrylonitrile (PAN) | Immunosensor | Epidermal growth factor receptor (EGFR or ErbB2) | Breast cancer | Impedance spectroscopy | [111] |
| Polyacrylonitrile (PAN) | Fluorescent sensors | MicroRNA-21 (Mir-21) | Cholangiocarcinoma | Fluorescence | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Guan, M.; Bian, Y.; Yin, X. Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications. Biosensors 2024, 14, 13. https://doi.org/10.3390/bios14010013
Chen Z, Guan M, Bian Y, Yin X. Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications. Biosensors. 2024; 14(1):13. https://doi.org/10.3390/bios14010013
Chicago/Turabian StyleChen, Zhou, Mengdi Guan, Yi Bian, and Xichen Yin. 2024. "Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications" Biosensors 14, no. 1: 13. https://doi.org/10.3390/bios14010013
APA StyleChen, Z., Guan, M., Bian, Y., & Yin, X. (2024). Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications. Biosensors, 14(1), 13. https://doi.org/10.3390/bios14010013






