Handheld Biosensor System Based on a Gradient Grating Period Guided-Mode Resonance Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the GGP-GMR Sensor
2.2. Fabrication of an Optofluidic Chip
2.3. Assay Protocol
2.4. Detection Principle and Handheld Device
3. Results
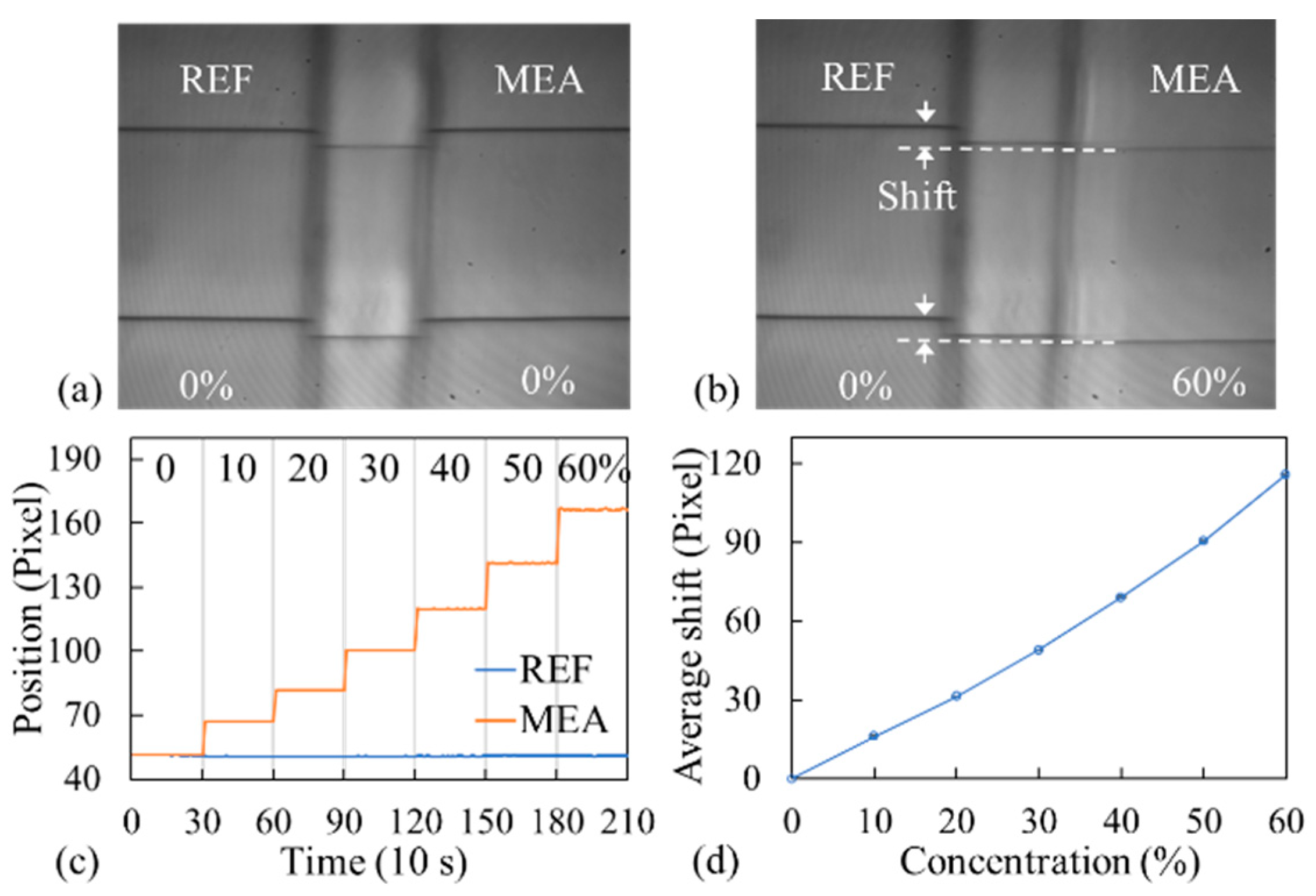
3.1. Bulk Solution Measurement
3.2. Optimization of the Optical Setup and GGP-GMR Sensor
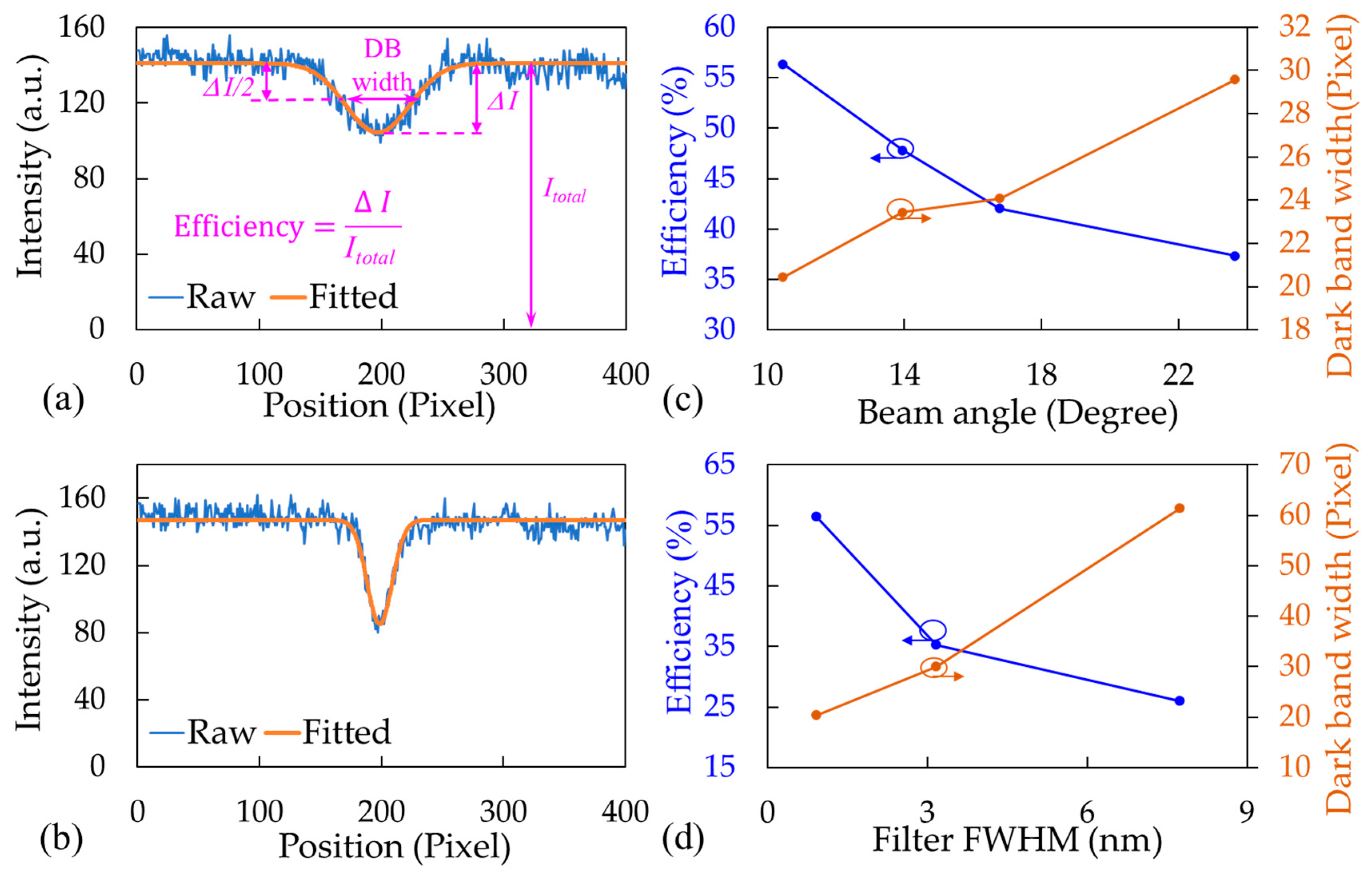
3.2.1. Effects of LED BA and BF Bandwidth
3.2.2. Substrate RI and Waveguide Thickness
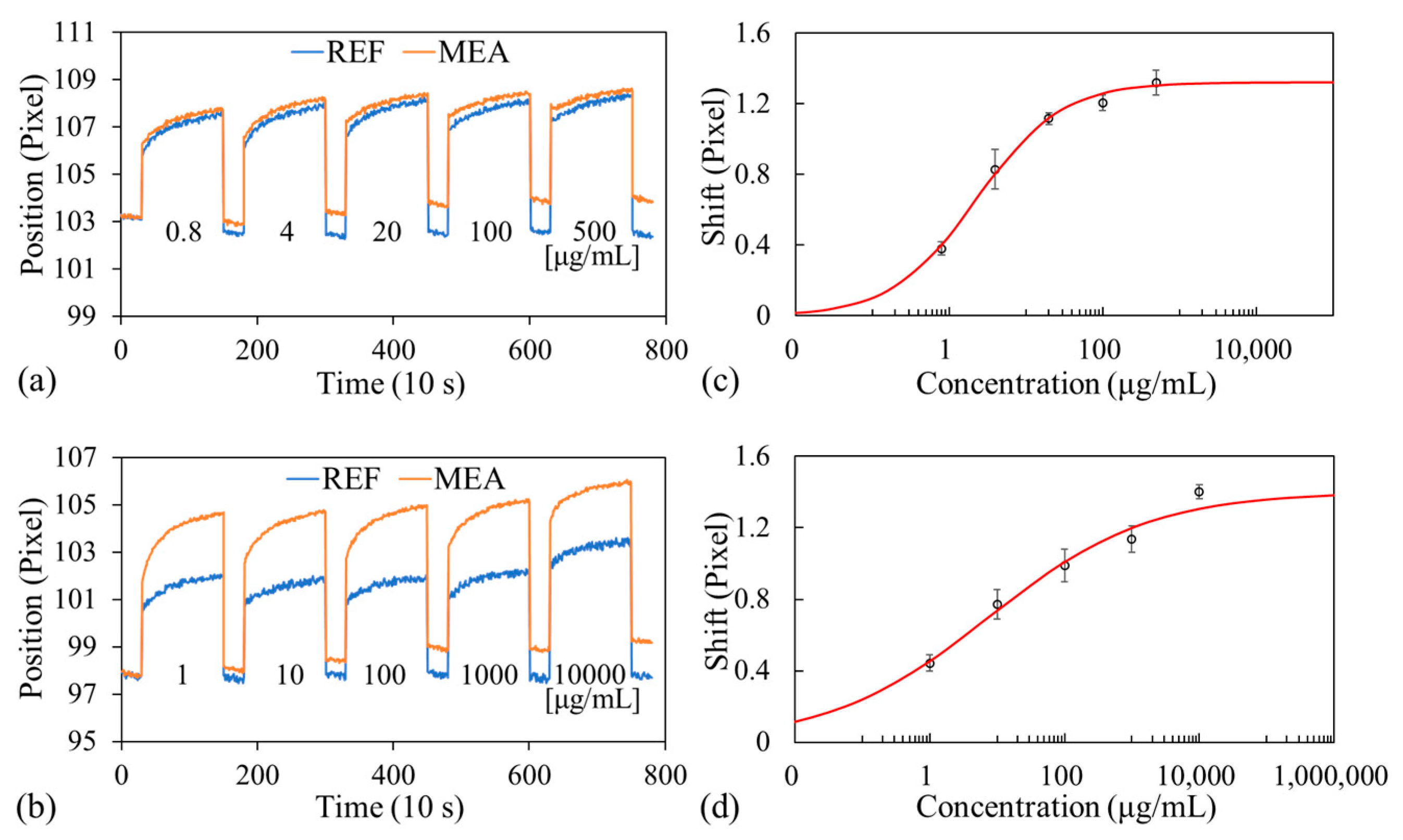
3.3. Biomolecule Detection
3.4. Specificity Demonstration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, B.T. Label-free optical biosensors: An introduction. In Label-Free Biosensors: Techniques and Applications; Cooper, M.A., Ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 1–28. [Google Scholar]
- Fan, X.D.; White, I.M.; Shopova, S.I.; Zhu, H.Y.; Suter, J.D.; Sun, Y.Z. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Ciminelli, C.; Campanella, C.M.; Dell’Olio, F.; Campanella, C.E.; Armenise, M.N. Label-free optical resonant sensors for biochemical applications. Prog. Quantum Electron. 2013, 37, 51–107. [Google Scholar] [CrossRef]
- O’Malley, S. Recent advances in label-free biosensors applications in protein biosynthesis and HTS screening. In Protein Biosynthesis; Esterhouse, T.E., Petrinos, L.B., Eds.; Nova Science: New York, NY, USA, 2009; pp. 163–194. [Google Scholar]
- Gauglitz, G. Direct optical detection in bioanalysis: An update. Anal. Bioanal. Chem. 2010, 398, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lee, Y.C.; Lai, Y.H.; Lim, J.C.; Huang, N.T.; Lin, C.T.; Huang, J.J. Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Kenaan, A.; Li, K.Z.; Barth, I.; Johnson, S.; Song, J.; Krauss, T.F. Guided mode resonance sensor for the parallel detection of multiple protein biomarkers in human urine with high sensitivity. Biosens. Bioelectron. 2020, 153, 112047. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.T.; Zhang, M.; Zhuo, Y.; Kwon, L.; Race, C. Recent Advances in Biosensing with Photonic Crystal Surfaces: A Review. IEEE Sens. J. 2016, 16, 3349–3366. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Optical biosensors: Where next and how soon? Drug Discov. Today 2006, 11, 1061–1067. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.W.; Guo, Z.H.; Wu, X. Guided Mode Resonance Sensors with Optimized Figure of Merit. Nanomaterials 2019, 9, 837. [Google Scholar] [CrossRef]
- Triggs, G.J.; Wang, Y.; Reardon, C.P.; Fischer, M.; Evans, G.J.O.; Krauss, T.F. Chirped guided-mode resonance biosensor. Optica 2017, 4, 229–234. [Google Scholar] [CrossRef]
- Lin, H.A.; Huang, C.S. Linear variable filter based on a gradient grating period guided-mode resonance filter. IEEE Photonics Technol. Lett. 2016, 28, 1042–1045. [Google Scholar] [CrossRef]
- Hsiung, C.T.; Huang, C.S. Refractive Index Sensor Based on a Gradient Grating Period Guided-Mode Resonance. IEEE Photonics Technol. Lett. 2019, 31, 253–256. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, Y.C.; Yang, J.M.; Huang, C.S.; Kuo, S.H.; Li, B.R. Gradient Grating Period Guided-Mode Resonance for Potential Biosensing Applications. IEEE Sens. J. 2021, 21, 4184–4189. [Google Scholar] [CrossRef]
- Avrutsky, A.I.; Sychugov, V.A. Reflection of a beam of finite size from a corrugated waveguide. J. Mod. Opt. 1989, 36, 1527–1539. [Google Scholar] [CrossRef]
- Wang, S.S.; Magnusson, R. Theory and applications of guided-mode resonance filters. Appl. Opt. 1993, 32, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, D.; Sharon, A.; Friesem, A.A. Resonant grating waveguide structures. IEEE J. Quantum Electron. 1997, 33, 2038–2059. [Google Scholar] [CrossRef]
- Guo, T.B.; Evans, J.; Wang, N.; Jin, Y.; He, J.L.; Sun, Y.R. Guided Mode Resonance in a Low-Index Waveguide Layer. Appl. Sci. 2021, 11, 3312. [Google Scholar] [CrossRef]
- Sun, G.Y.; Wang, Q. Electrically tunable polarization-independent visible transmission guided-mode resonance filter based on polymer-dispersed liquid crystals. Microw. Opt. Technol. Lett. 2020, 62, 3727–3732. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Z.H.; Zhou, W.J.; Li, S.; Liu, Z.R.; Zhao, X.Y.; Wu, X. High-Q guided mode resonance sensors based on shallow sub-wavelength grating structures. Nanotechnology 2020, 31, 325501. [Google Scholar] [CrossRef]
- Zhang, C.R.; Zhou, Y.; Mi, L.; Ma, J.; Wu, X.; Fei, Y.Y. High Performance of a Metal Layer-Assisted Guided-Mode Resonance Biosensor Modulated by Double-Grating. Biosensors 2021, 11, 221. [Google Scholar] [CrossRef]
- Huang, L.J.; Jin, R.; Zhou, C.B.; Li, G.H.; Xu, L.; Overvig, A.; Deng, F.; Chen, X.S.; Lu, W.; Alu, A.; et al. Ultrahigh-Q guided mode resonances in an All-dielectric metasurface. Nat. Commun. 2023, 14, 3433. [Google Scholar] [CrossRef]
- Wang, Y.; Ali, M.A.; Chow, E.K.C.; Dong, L.; Lu, M. An optofluidic metasurface for lateral flow-through detection of breast cancer biomarker. Biosens. Bioelectron. 2018, 107, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Yuan, W.; Kimber, M.; Lu, M.; Dong, L. Rapid Differentiation of Host and Parasitic Exosome Vesicles Using Microfluidic Photonic Crystal Biosensor. ACS Sens. 2018, 3, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Yuan, W.; Zhang, Q.M.; Wang, Y.X.; Kimber, M.; Dong, L.; Lu, M. Exosome microarray based on label-free imaging biosensor. In Proceedings of the 18th IEEE Sensors Conference, Montreal, QC, Canada, 27–30 October 2019. [Google Scholar]
- Joseph, S.; Rajpal, S.; Kar, D.; Devinder, S.; Pandey, S.; Mishra, P.; Joseph, J. Guided mode resonance immunosensor for label-free detection of pathogenic bacteria Pseudomonas aeruginosa. Biosens. Bioelectron. 2023, 241, 115695. [Google Scholar] [CrossRef]
- Magnusson, R.; Ding, Y.; Lee, K.J.; Shin, D.; Priambodo, P.S.; Young, P.P.; Maldonado, T.A. Photonic devices enabled by waveguide-mode resonance effects in periodically modulated films. In Nano-and Micro-Optics for Information Systems, Proceedings of the Society of Photo-Optical Instrumentation Engineers (Spie), San Diego, CA, USA, 3–8 August 2003; Eldada, L.A., Ed.; SPIE: Bellingham, WA, USA, 2003; Volume 5225, pp. 20–34. [Google Scholar]
- Block, I.D.; Ganesh, N.; Lu, M.; Cunningham, B.T. Sensitivity model for predicting photonic crystal biosensor performance. IEEE Sens. J. 2008, 8, 274–280. [Google Scholar] [CrossRef]
- Gao, J.J.; Chiu, C.W.; Wen, K.H.; Huang, C.S. A Compact Detection Platform Based on Gradient Guided-Mode Resonance for Colorimetric and Fluorescence Liquid Assay Detection. Sensors 2021, 21, 2797. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, C.S. Flexible Linear and Angular Displacement Sensor Based on a Gradient Guided- Mode Resonance Filter. IEEE Sens. J. 2018, 18, 9925–9930. [Google Scholar] [CrossRef]
- Lin, H.A.; Hsu, H.Y.; Chang, C.W.; Huang, C.S. Compact spectrometer system based on a gradient grating period guided-mode resonance filter. Opt. Express 2016, 24, 10972–10979. [Google Scholar] [CrossRef]
- Mattix, H.J.; Hsu, C.Y.; Shaykevich, S.; Curhan, G. Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J. Am. Soc. Nephrol. 2002, 13, 1034–1039. [Google Scholar] [CrossRef]
- Price, C.P.; Newall, R.G.; Boyd, J.C. Use of protein: Creatinine ratio measurements on random urine samples for prediction of significant proteinuria: A systematic review. Clin. Chem. 2005, 51, 1577–1586. [Google Scholar] [CrossRef]
- International Commission for Uniform Methods of Sugar Analysis. Report of the Proceedings of the Ninth Session. Analyst 1937, 62, 4. [Google Scholar]
- Ju, J.; Han, Y.-a.; Kim, S.-m. Design Optimization of Structural Parameters for Highly Sensitive Photonic Crystal Label-Free Biosensors. Sensors 2013, 13, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.S.; Li, S.; Guo, Z.H.; Zeng, P.; He, J.B.; Wang, D.C.; Zhang, R.J.; Lu, M.; Zhang, S.Y.; et al. Symmetric guided-mode resonance sensors in aqueous media with ultrahigh figure of merit. Opt. Express 2019, 27, 34788–34802. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, M.; Kang, X.B.; Liu, W.X.; Dong, C.; Zhang, J.; Xia, S.Q.; Zhang, X.Z. Improving the sensitivity of compound waveguide grating biosensor via modulated wavevector. Appl. Phys. Express 2018, 11, 4. [Google Scholar] [CrossRef]
- Wan, Y.H.; Krueger, N.A.; Ocier, C.R.; Su, P.; Braun, P.V.; Cunningham, B.T. Resonant Mode Engineering of Photonic Crystal Sensors Clad with Ultralow Refractive Index Porous Silicon Dioxide. Adv. Opt. Mater. 2017, 5, 1700605. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.C.; Tseng, W.-C.; Tsai, W.-T.; Huang, C.-S. Handheld Biosensor System Based on a Gradient Grating Period Guided-Mode Resonance Device. Biosensors 2024, 14, 21. https://doi.org/10.3390/bios14010021
Chiang CC, Tseng W-C, Tsai W-T, Huang C-S. Handheld Biosensor System Based on a Gradient Grating Period Guided-Mode Resonance Device. Biosensors. 2024; 14(1):21. https://doi.org/10.3390/bios14010021
Chicago/Turabian StyleChiang, Chien Chieh, Wen-Chun Tseng, Wen-Tsung Tsai, and Cheng-Sheng Huang. 2024. "Handheld Biosensor System Based on a Gradient Grating Period Guided-Mode Resonance Device" Biosensors 14, no. 1: 21. https://doi.org/10.3390/bios14010021
APA StyleChiang, C. C., Tseng, W.-C., Tsai, W.-T., & Huang, C.-S. (2024). Handheld Biosensor System Based on a Gradient Grating Period Guided-Mode Resonance Device. Biosensors, 14(1), 21. https://doi.org/10.3390/bios14010021



