Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers
Abstract
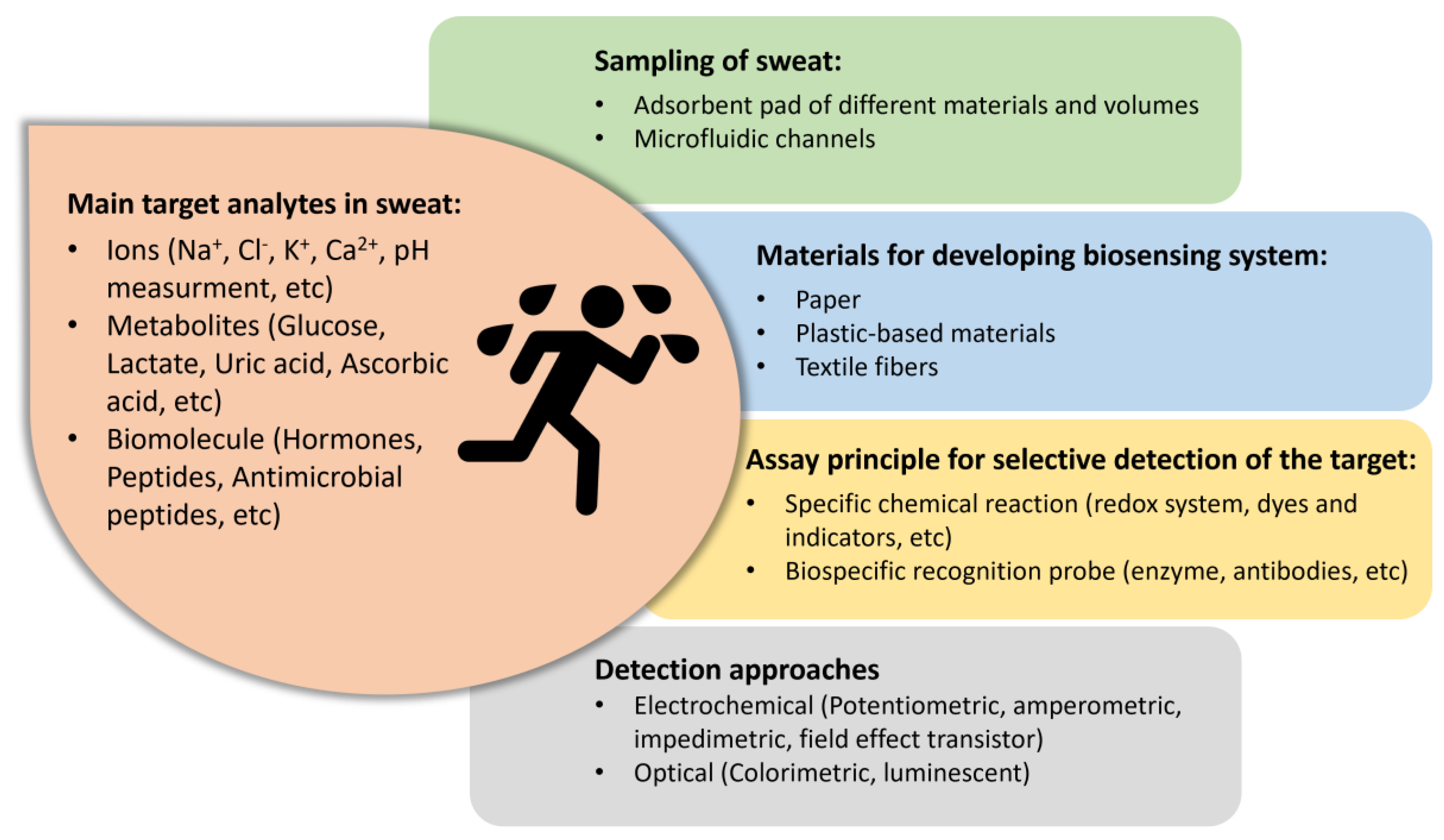
1. Introduction
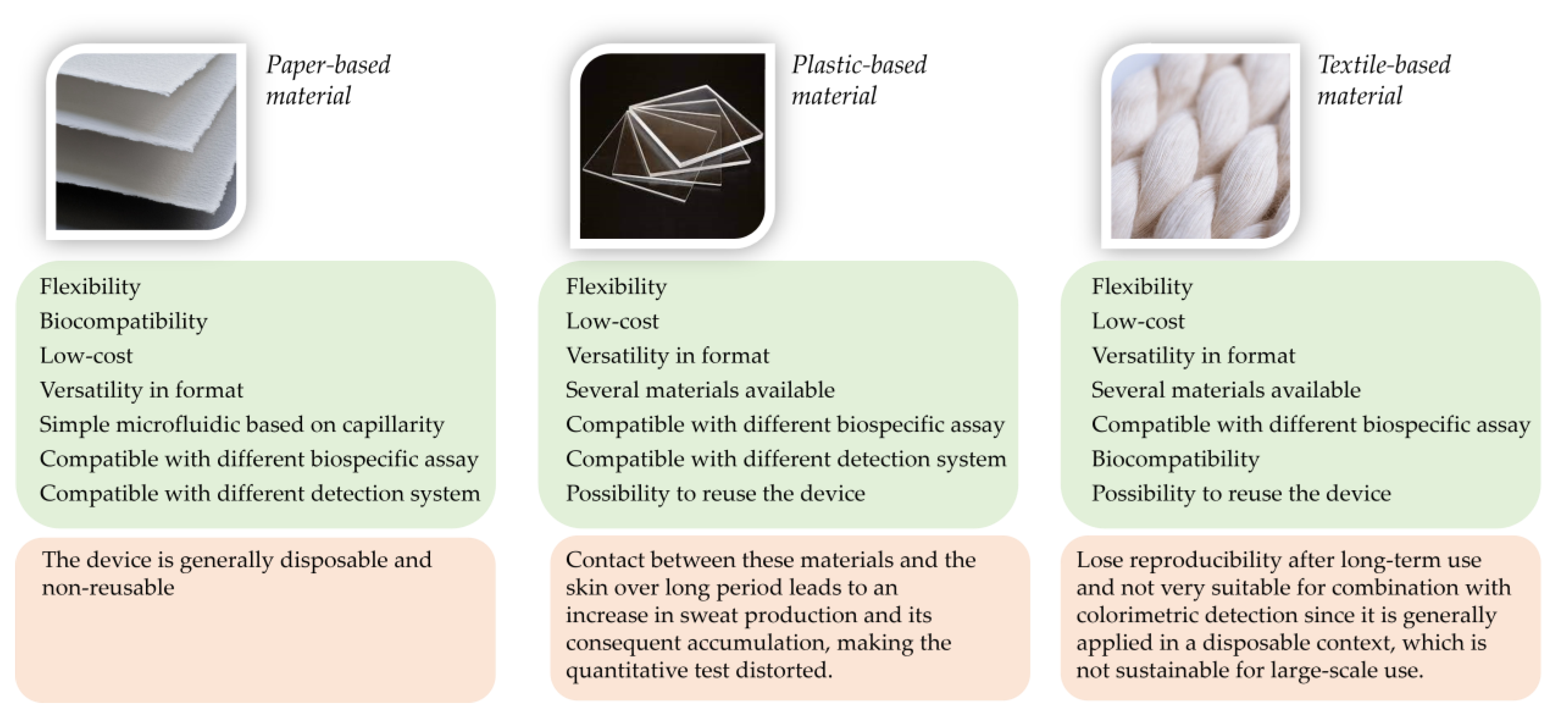
2. Paper-Based Microfluidic
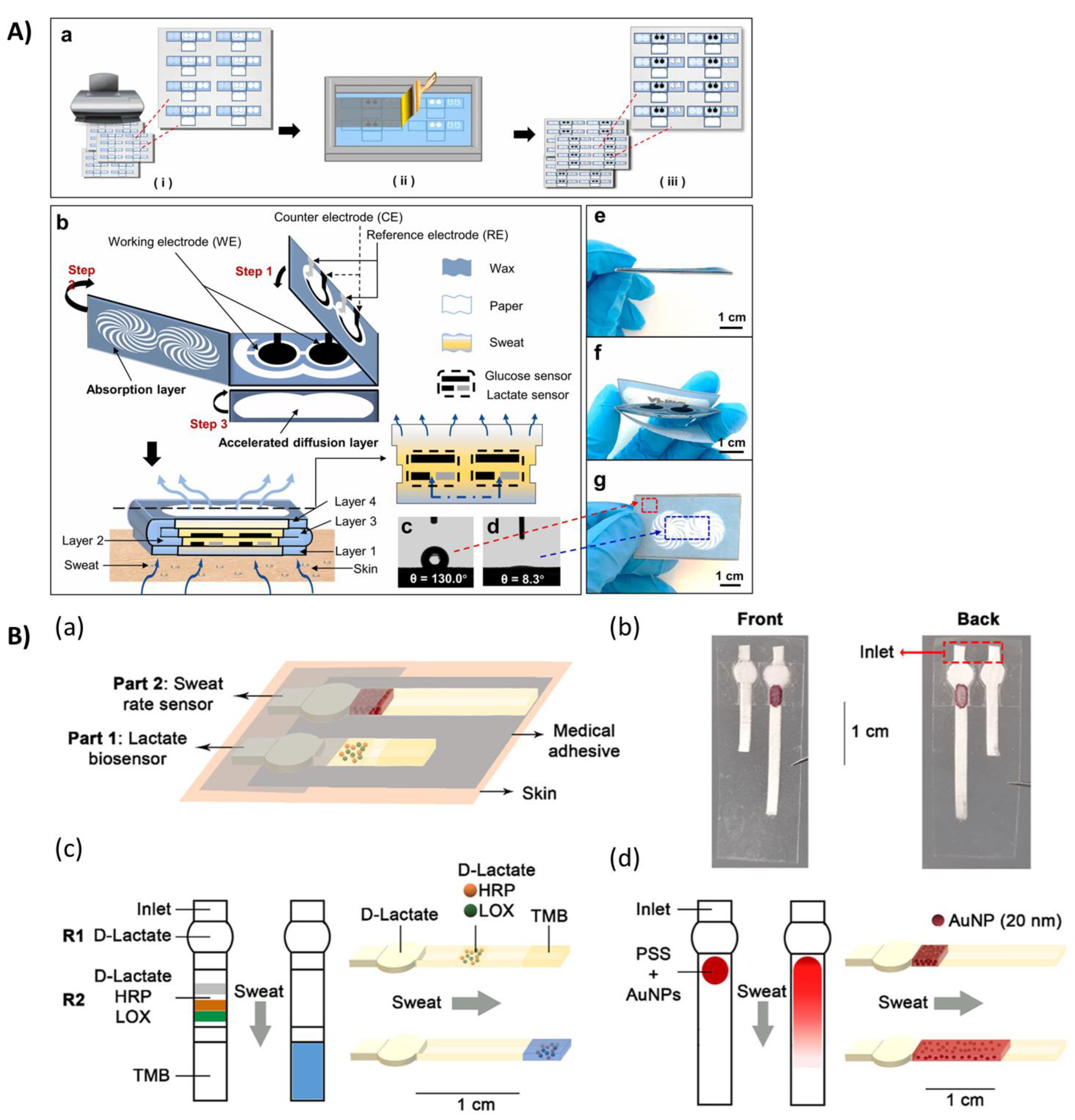
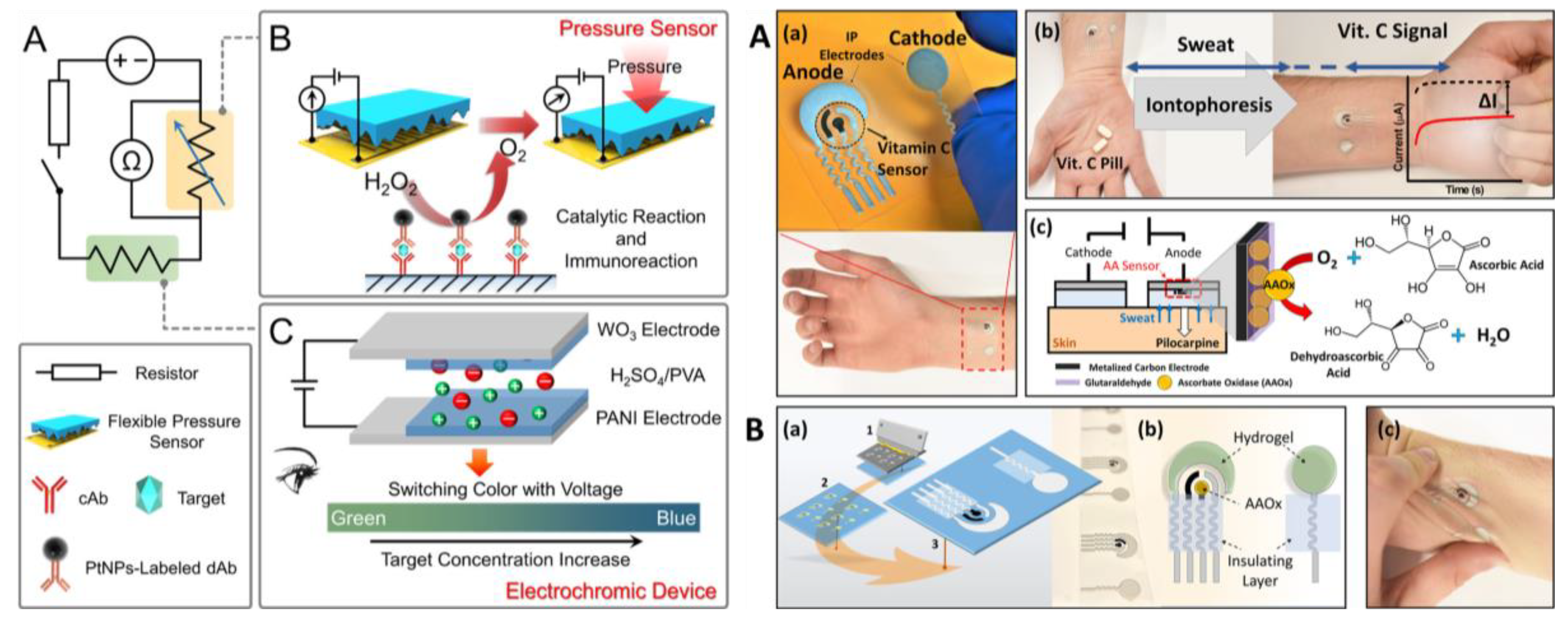
3. Wearable Biosensors Based on Highly Flexible Plastic Substrate
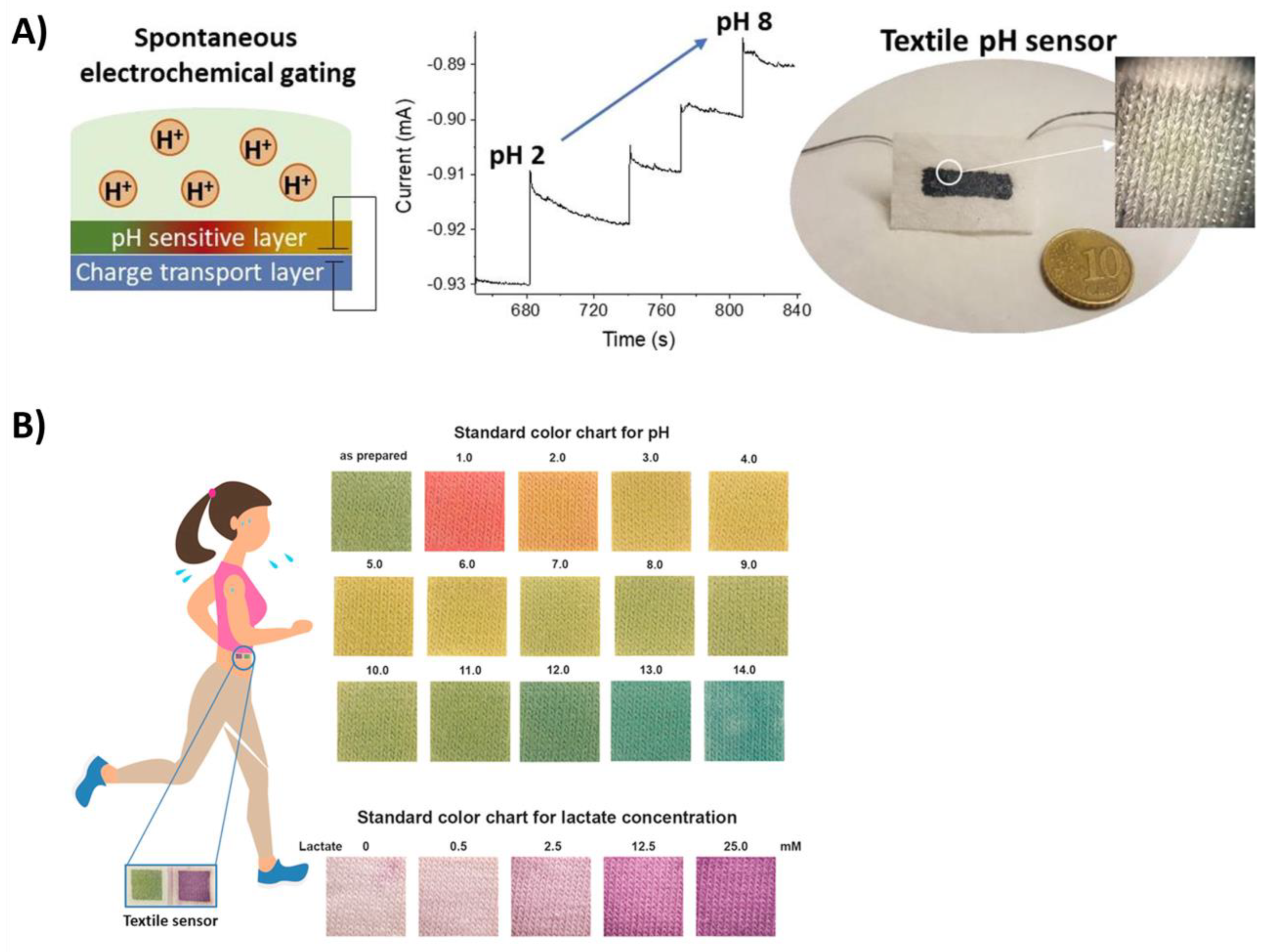
4. Textile Materials for Developing Sweat-Real-Time Monitoring Biosensors
5. Outlook and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable chemical sensors: Present challenges and future prospects. ACS Sens. 2016, 5, 464–482. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Matzeu, G.; Florea, L.; Diamond, D. Advances in wearable chemical sensor design for monitoring biological fluids. Sens. Actuators B Chem. 2015, 211, 403–418. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef]
- Padash, M.; Enz, C.; Carrara, S. Microfluidics by additive manufacturing for wearable biosensors: A review. Sensors 2020, 20, 4236. [Google Scholar] [CrossRef]
- Li, G.; Mo, X.; Law, W.-C.; Chan, K.C. Wearable Fluid Capture Devices for Electrochemical Sensing of Sweat. ACS Appl. Mater. Interfaces 2018, 11, 238–243. [Google Scholar] [CrossRef]
- Jo, S.; Sung, D.; Kim, S.; Koo, J. A review of wearable biosensors for sweat analysis. Biomed. Eng. Lett. 2021, 11, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID Sensor Patch for Monitoring of Sweat Electrolytes. IEEE Trans. Biomed. Eng. 2014, 62, 1457–1465. [Google Scholar]
- Lim, C.T. Emergence of microfluidic wearable technologies. Lab Chip 2016, 16, 4082–4090. [Google Scholar]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, T.; Wang, D.; Zhang, X. The role of sampling in wearable sweat sensors. Talanta 2020, 212, 120801. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.; Tai, L.C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Reeder, J.T.; Choi, J.; Xue, Y.; Gutruf, P.; Hanson, J.; Liu, M.; Ray, T.; Bandodkar, A.J.; Avila, R.; Xia, W.; et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 2019, 5, eaau6356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Nyein, H.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef]
- Kaya, T.; Liu, G.; Ho, J.; Yelamarthi, K.; Miller, K.; Edwards, J.; Stannard, A.B. Wearable Sweat Sensors: Background and Current Trends. Electroanalysis 2018, 31, 411–421. [Google Scholar] [CrossRef]
- Choi, J.; Kang, D.; Han, S.; Kim, S.B.; Rogers, J.A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 2017, 6, 1601355. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Zhang, Y.; Won, S.M.; Bandodkar, A.J.; Sekine, Y.; Xue, Y.; Koo, J.; Harshman, S.W.; Martin, J.A.; Park, J.M.; et al. Super-absorbent polymer valves and colorimetric chemistries for time-sequenced discrete sampling and chloride analysis of sweat via skin-mounted soft microfluidics. Small 2018, 14, 1703334. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tan, J.; Zhu, J.; Lin, S.; Zhao, Y.; Yu, W.; Hojaiji, H.; Wang, B.; Yang, S.; Cheng, X.; et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 2020, 11, 4405. [Google Scholar] [CrossRef] [PubMed]
- ShariatiPour, S.R.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Electrochemical vs. Optical Biosensors for Point-of-Care Applications: A Critical Review. Chemosensors 2023, 11, 546. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in medical wearable biosensors: Design, fabrication and materials strategies in healthcare monitoring. Molecules 2021, 27, 165. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Ghosh, R.; Gopalakrishnan, S.; Savitha, R.; Renganathan, T.; Pushpavanam, S. Fabrication of laser printed microfluidic paper-based analytical devices (LP-µPADs) for point-of-care applications. Sci. Rep. 2019, 9, 7896. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, X.; Jin, D.; Zhang, B.; Gu, Y.; An, Y. based immunosensors: Current trends in the types and applied detection techniques. TrAC Trends Anal. Chem. 2019, 111, 100–117. [Google Scholar] [CrossRef]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in paper-based analytical devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-based immunosensors with bio-chemiluminescence detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Kung, C.T.; Chen, R.F.; Tsai, M.H.; Chao, H.R.; Wang, Y.N.; Fu, L.M. Recent advances in microfluidic paper-based assay devices for diagnosis of human diseases using saliva, tears and sweat samples. Sens. Actuators B Chem. 2021, 342, 130078. [Google Scholar] [CrossRef]
- Ye, Z.; Yuan, Y.; Zhan, S.; Liu, W.; Fang, L.; Li, T. Paper-based microfluidics in sweat detection: From design to application. Analyst 2023, 148, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Guardigli, M.; Calabria, D.; Calabretta, M.M.; Cevenini, L.; Michelini, E. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 2014, 139, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, J.; Wang, C.; Feng, L.; Song, Z.; Zhao, W.; Wang, Q.; Liu, C. The application of wearable glucose sensors in point-of-care testing. Front. Bioeng. Biotechnol. 2021, 9, 774210. [Google Scholar] [CrossRef] [PubMed]
- Deroco, P.B.; Wachholz Junior, D.; Kubota, L.T. Paper-based Wearable Electrochemical Sensors: A New Generation of Analytical Devices. Electroanalysis 2023, 35, e202200177. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 2019, 9, 5674–5681. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef]
- Vaquer, A.; Barón, E.; de la Rica, R. Dissolvable polymer valves for sweat chrono-sampling in wearable paper-based analytical devices. ACS Sens. 2022, 7, 488–494. [Google Scholar] [CrossRef]
- Vaquer, A.; Barón, E.; de la Rica, R. Wearable analytical platform with enzyme-modulated dynamic range for the simultaneous colorimetric detection of sweat volume and sweat biomarkers. ACS Sens. 2020, 6, 130–136. [Google Scholar] [CrossRef]
- Yokus, M.A.; Saha, T.; Fang, J.; Dickey, M.D.; Velev, O.D.; Daniele, M.A. Towards wearable electrochemical lactate sensing using osmotic-capillary microfluidic pumping. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Liu, D.; Liu, Z.; Feng, S.; Gao, Z.; Chen, R.; Cai, G.; Bian, S. Wearable Microfluidic Sweat Chip for Detection of Sweat Glucose and pH in Long-Distance Running Exercise. Biosensors 2023, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Mohammadifar, M.; Choi, S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Azizi, M.; Lee, M.; Davidowsky, P.; Lawrence, P.; Abbaspourrad, A. A versatile, cost-effective, and flexible wearable biosensor for in situ and ex situ sweat analysis, and personalized nutrition assessment. Lab Chip 2019, 19, 3448–3460. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Cao, Q.; Mao, X.; Pan, W.; Tu, T.; Fang, L.; Ye, X. An integrated paper-based microfluidic device for real-time sweat potassium monitoring. IEEE Sens. J. 2020, 21, 9642–9648. [Google Scholar] [CrossRef]
- Gao, Y.; Choi, S. Micropatternable Janus Paper as a Wearable Skin Patch for Sweat Collection and Analysis. Adv. Mater. Technol. 2023, 8, 2300396. [Google Scholar] [CrossRef]
- Xiao, G.; He, J.; Qiao, Y.; Wang, F.; Xia, Q.; Wang, X.; Yu, L.; Lu, Z.; Li, C.M. Facile and low-cost fabrication of a thread/paper-based wearable system for simultaneous detection of lactate and pH in human sweat. Adv. Fiber Mater. 2020, 2, 265–278. [Google Scholar] [CrossRef]
- Shi, H.; Cao, Y.; Xie, Z.; Zhao, Y.; Zhang, C.; Chen, Z. Multi-parameter photoelectric data fitting for microfluidic sweat colorimetric analysis. Sens. Actuators B Chem. 2022, 372, 132644. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, S.; Ning, Q.; Li, T.; Xu, H.; Sun, Q.; Cui, D.; Wang, K. Dual-signal readout paper-based wearable biosensor with a 3D origami structure for multiplexed analyte detection in sweat. Microsyst. Nanoeng. 2023, 9, 36. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G.; et al. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B Chem. 2023, 379, 133258. [Google Scholar] [CrossRef]
- de Brito Ayres, L.; Brooks, J.; Whitehead, K.; Garcia, C.D. Rapid detection of Staphylococcus aureus using paper-derived electrochemical biosensors. Anal. Chem. 2022, 94, 16847–16854. [Google Scholar] [CrossRef]
- Ardalan, S.; Hosseinifard, M.; Vosough, M.; Golmohammadi, H. Towards smart personalized perspiration analysis: An IoT-integrated cellulose-based microfluidic wearable patch for smartphone fluorimetric multi-sensing of sweat biomarkers. Biosens. Bioelectron. 2020, 168, 112450. [Google Scholar] [CrossRef] [PubMed]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Sempionatto, J.R.; Kim, J.; Jeong, Y.; Gu, J.; Wang, J.; Park, I. Microscale biosensor array based on flexible polymeric platform toward lab-on-a-needle: Real-time multiparameter biomedical assays on curved needle surfaces. ACS Sens. 2020, 5, 1363–1373. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, C.; Liu, Z.; Yuan, W.; Wu, X.; Lu, Y.; Low, S.S.; Liu, J.; Zhu, L.; Ji, D.; et al. Battery-free and wireless epidermal electrochemical system with all-printed stretchable electrode array for multiplexed In situ sweat analysis. Adv. Mater. Technol. 2019, 4, 1800658. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, G.; Liu, X.; Tang, D. Pressure-based biosensor integrated with a flexible pressure sensor and an electrochromic device for visual detection. Anal. Chem. 2021, 93, 2916–2925. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; De Loyola e Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal enzymatic biosensors for sweat vitamin C: Toward personalized nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Hung, V.W.; Jia, W.; Valdés-Ramírez, G.; Windmiller, J.R.; Martinez, A.G.; Ramírez, J.; Chan, G.; Kerman, K.; Wang, J. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 2013, 138, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixao, T.R.; Wang, J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-based wearable electrochemical devices: A review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Curto, V.F.; Fay, C.; Coyle, S.; Byrne, R.; O’Toole, C.; Barry, C.; Hughes, S.; Moyna, N.; Diamond, D.; Benito-Lopez, F. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 2012, 171, 1327–1334. [Google Scholar] [CrossRef]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Z.; Huang, Q.; Lin, Y.; Zheng, Z. Wearable sweat biosensors on textiles for health monitoring. J. Semicond. 2023, 44, 021601. [Google Scholar] [CrossRef]
- Yin, J.; Li, J.; Reddy, V.S.; Ji, D.; Ramakrishna, S.; Xu, L. Flexible textile-based sweat sensors for wearable applications. Biosensors 2023, 13, 127. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shan, S.S.; Liao, Y.T.; Liao, Y.C. Bio-inspired fractal textile device for rapid sweat collection and monitoring. Lab Chip 2021, 21, 2524–2533. [Google Scholar] [CrossRef]
- Promphet, N.; Hinestroza, J.P.; Rattanawaleedirojn, P.; Soatthiyanon, N.; Siralertmukul, K.; Potiyaraj, P.; Rodthongkum, N. Cotton thread-based wearable sensor for non-invasive simultaneous diagnosis of diabetes and kidney failure. Sens. Actuators B Chem. 2020, 321, 128549. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Lin, Z.; Meng, Z.; Shi, C.; Xu, Z.; Yang, L.; Liu, X.Y. Coupling of silk fibroin nanofibrils enzymatic membrane with ultra-thin PtNPs/graphene film to acquire long and stable on-skin sweat glucose and lactate sensing. Small Methods 2021, 5, 2000926. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, X.; Lu, Q.; Hu, X.; Uppal, N.; Omenetto, F.G.; Kaplan, D.L. Stabilization of enzymes in silk films. Biomacromolecules 2009, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Gu, K.; Yao, J.; Shao, Z.; Chen, X. Silk-based electrochemical sensor for the detection of glucose in sweat. Biomacromolecules 2022, 23, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Winder, M.; Hossain, G. Modified graphene-based nanocomposite material for smart textile biosensor to detect lactate from human sweat. Biosens. Bioelectron. 2022, 10, 100103. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Sun, C.; Zhao, X.; Jiao, C.; Du, A.; Wang, Q.; Mao, Y.; Liu, B. Wearable biosensors for real-time sweat analysis and body motion capture based on stretchable fiber-based triboelectric nanogenerators. Biosens. Bioelectron. 2022, 205, 114115. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile organic electrochemical transistors as a platform for wearable biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Possanzini, L.; Scavetta, E.; Fraboni, B. Textile chemical sensors based on conductive polymers for the analysis of sweat. Polymers 2021, 13, 894. [Google Scholar] [CrossRef]
- Possanzini, L.; Decataldo, F.; Mariani, F.; Gualandi, I.; Tessarolo, M.; Scavetta, E.; Fraboni, B. Textile sensors platform for the selective and simultaneous detection of chloride ion and pH in sweat. Sci. Rep. 2020, 10, 17180. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced wound dressing for real-time pH monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Mariani, F.; Gualandi, I.; Tonelli, D.; Decataldo, F.; Possanzini, L.; Fraboni, B.; Scavetta, E. Design of an electrochemically gated organic semiconductor for pH sensing. Electrochem. Commun. 2020, 116, 106763. [Google Scholar] [CrossRef]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B Chem. 2016, 222, 213–220. [Google Scholar] [CrossRef]
- Morris, D.; Coyle, S.; Wu, Y.; Lau, K.T.; Wallace, G.; Diamond, D. Bio-sensing textile based patch with integrated optical detection system for sweat monitoring. Sens. Actuators B Chem. 2009, 139, 231–236. [Google Scholar] [CrossRef]
- Yapor, J.P.; Alharby, A.; Gentry-Weeks, C.; Reynolds, M.M.; Alam, A.M.; Li, Y.V. Polydiacetylene nanofiber composites as a colorimetric sensor responding to Escherichia coli and pH. ACS Omega 2017, 2, 7334–7342. [Google Scholar] [CrossRef] [PubMed]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Q.; Chen, L.; Zhao, Y.; Gong, J.; Li, Z.; Zhang, J. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring. Lab Chip 2021, 21, 916–932. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Ding, X.R.; Poon, C.C.Y.; Lo, B.P.L.; Zhang, H.; Zhou, X.L.; Yang, G.Z.; Zhao, N.; Zhang, Y.T. Unobtrusive sensing and wearable devices for health informatics. IEEE Trans. Biomed. Eng. 2014, 61, 1538–1554. [Google Scholar] [CrossRef]
- Andreu-Perez, J.; Poon, C.C.Y.; Merrifield, R.D.; Wong, S.T.C.; Yang, G.Z. Big data for health. IEEE J. Biomed. Health Inform. 2015, 19, 1193–1208. [Google Scholar] [CrossRef]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef]

| Target Analyte | Detection Principle | Limit of Detection | Ref |
|---|---|---|---|
| Glucose | Wax screen-printing patterns on cellulose paper are folded to form five stacked layers: the sweat collector, vertical channel, transverse channel, electrode layer, and sweat evaporator. A screen-printed glucose sensor based on a glucose oxidase enzymatic reaction was developed on a polyethylene terephthalate substrate. | 5 μM | [37] |
| Glucose and lactate | Printed paper folded into a multi-layer structure was designed to incorporate into the same platform conducting electrodes, specific enzymes, and MXene/methylene blue. The sweat diffusion path was established by connecting the hydrophilic regions of each layer along the vertical direction of the folded paper. | Glucose 17.05 μM Lactate 3.73 μM | [38] |
| pH and urea | Dried polymers were used as closed valves that deflect the flow of liquids to different transducers in a multisensor. As time passes, the polymer dissolves, and the valve opens. The sequential opening of the valves results in a succession of measurements that reveal fluctuations in the concentration of the target analyte. The colorimetric detection was performed using dyes for pH detection and urease for the enzymatic reaction involving urea. | Urea 5 mM | [39] |
| Lactate | The detection platform consists of two adjacent sensors (one for detecting lactate and one related to the sweat volume) made entirely of paper whose inlets are in direct contact with the skin. Lactate was detected through the colorimetric reaction involving lactate oxidase/HRP/TMB. | 0.06 mM | [40] |
| Lactate | The patch consisted of a hydrogel and paper-based microfluidic device for combined osmotic and capillary pumping of sweat, coupled with screen-printed electrodes for analysis of lactate concentration by exploiting an enzymatic reaction based on lactate oxidase. | 2 mM | [41] |
| Glucose and pH | A PDMS-based chip was modified with nonionic surfactants for the automatic collection of sweat combined with a paper-based sensor based on colorimetric measurement of pH and enzymatic reaction for detecting glucose using a smartphone for image acquisition. | 0.05 mM | [42] |
| Glucose | The paper-based device wicks sweat by using capillary forces in a reservoir where glucose is detected electrochemically by exploiting catalytic reactions. The device used a 3D paper-based fuel cell configuration, an electrically conducting microfluidic reservoir for a high anode surface area and efficient mass transfer, and a direct electron transfer between glucose oxidase and anodes for enhanced electron discharge properties. | 0.02 mg/mL | [43] |
| pH, glucose, and lactate | The channels/patterns were designed and printed on the paper using a wax printer. These channels directed the sweat to the collection and detection zones of the device, where the intake, storage, and evaporation of sweat were designed to maximize the efficiency of the colorimetric catalytic reactions. The measurement was performed using a smartphone. | Glucose 50 μM Lactate 5 mM | [44] |
| Potassium | The paper-based microfluidic pad was fabricated by printing wax patterns on cellulose paper and then folding the pre-patterned paper to form a five-layer stacked structure: sweat collector, vertical channel, transverse channel, electrode layer, and sweat evaporator. | 1 mM | [45] |
| pH, chloride, sodium, and glucose | A single sheet of paper was double-sided wax-printed and heat-treated for asymmetric wettability, allowing unidirectional sweat transport and delivering the sweat to the sensing areas for multiplexed colorimetric assays. | Chloride 20 mM Sodium 40 mM Glucose 0.1 mM | [46] |
| Lactate, pH | Hydrophilic silk thread serves as the micro-channel to guide the liquid flow, while filter papers were functionalized to prepare colorimetric sensors for lactate and pH measurements performed by smartphone cameras. | 0.98 mM | [47] |
| Lactate, glucose, and pH | A paper-based analytical sensor was integrated into a PDMS microfluidic chamber prepared through 3D printing. A photoelectric sensor with an LED light source was used for acquiring the color information (including R, G, B, Lux, and color temperature value), and the data were transmitted wirelessly with Bluetooth. | Glucose 50 μM Lactate 10 mM | [48] |
| Glucose, lactate, uric acid, magnesium ions, and pH (colorimetric detection) Cortisol (electrochemical detection) | The entire chip is composed of hydrophilically and hydrophobically treated filter paper, and 3D microfluidic channels are constructed by using folding paper. The thread-based channels formed after the hydrophilic and hydrophobic modifications are used to control the rate of sweat flow, which in turn can be used to control the sequence of reactions in the differently developing colored regions to ensure that signals of the best color can be captured simultaneously by a smartphone camera. Cortisol was detected electrochemically using molecular imprinting polymers as probes for the recognition reaction. | Glucose 10 μM Lactate 2 mM Uric acid 2 μM Magnesium ion 0.5 mM Cortisol 100 ppm | [49] |
| Cortisol | The paper-based microfluidic pattern was made using wax printing and laser-cutter techniques for the delivery of capillary-driven microfluidics. The presence of magnetic beads functionalized with monoclonal antibodies for the recognition of the cortisol in the reaction zone allows a competitive reaction between the target cortisol and the labeled cortisol with the acetylcholinesterase enzyme, giving an electrochemical response by simply folding the pad loaded with the enzymatic substrate. | 10 ng/mL | [50] |
| Staphylococcus aureus through the detection of K4Fe[CN]6 | Paper-derived carbon electrodes were modified with a thin layer of sputtered gold and with chitosan. The resulting material was laser-patterned and applied for the development of an electrochemical biosensor wirelessly controlled by a custom-built, portable potentiostat. | 8.6 μM | [51] |
| Glucose, lactate, pH, and chloride | The sensor comprised fluorescent sensing probes embedded in paper substrates, and microfluidic channels consisted of cotton threads to harvest sweat from the skin surface and transport it to the paper-based sensing probes. The imaging module was fabricated by a 3D printer equipped with UV-LED lamps and an optical filter to provide the in situ capability of capturing digital images of the sensors via a smartphone. | Glucose 7 μM Lactate 0.4 mM Chloride 5 mM | [52] |
| Uric acid | Plasmonic paper-based microfluidics based on label-free surface-enhanced Raman spectroscopy (SERS) for providing “fingerprint” information for analyte identification. | 20 μM | [53] |
| Platform | Detection Principle | Limit of Detection | Ref |
|---|---|---|---|
| Paper-based | Electrochemical | 5 µM | [37] |
| Paper-based | Electrochemical | 17.05 μM | [38] |
| Paper-based | Colorimetric | 0.05 mM | [42] |
| Paper-based | Electrochemical | 0.02 mg/mL | [43] |
| Paper-based | Colorimetric | 50 µM | [44] |
| Paper-based | Colorimetric | 50 µM | [48] |
| Paper based | Fluorescent | 10 µM | [52] |
| PET | Electrochemical | 50 µM | [56] |
| Polyamide | Electrochemical | 0.5 mM | [57] |
| PDMS | Electrochemical | 100 µM | [58] |
| Ecoflex fiber | Electrochemical | 0.0056 mmol/L | [79] |
| SilkNCT | Electrochemical | 5 µM | [80] |
| SilkRGO | Electrochemical | 300 nM | [77] |
| Cotton and regenerated cellulose yarn | Colorimetric | 10 μM | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pour, S.R.S.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers. Biosensors 2024, 14, 29. https://doi.org/10.3390/bios14010029
Pour SRS, Calabria D, Emamiamin A, Lazzarini E, Pace A, Guardigli M, Zangheri M, Mirasoli M. Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers. Biosensors. 2024; 14(1):29. https://doi.org/10.3390/bios14010029
Chicago/Turabian StylePour, Seyedeh Rojin Shariati, Donato Calabria, Afsaneh Emamiamin, Elisa Lazzarini, Andrea Pace, Massimo Guardigli, Martina Zangheri, and Mara Mirasoli. 2024. "Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers" Biosensors 14, no. 1: 29. https://doi.org/10.3390/bios14010029
APA StylePour, S. R. S., Calabria, D., Emamiamin, A., Lazzarini, E., Pace, A., Guardigli, M., Zangheri, M., & Mirasoli, M. (2024). Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers. Biosensors, 14(1), 29. https://doi.org/10.3390/bios14010029







