Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics
Abstract
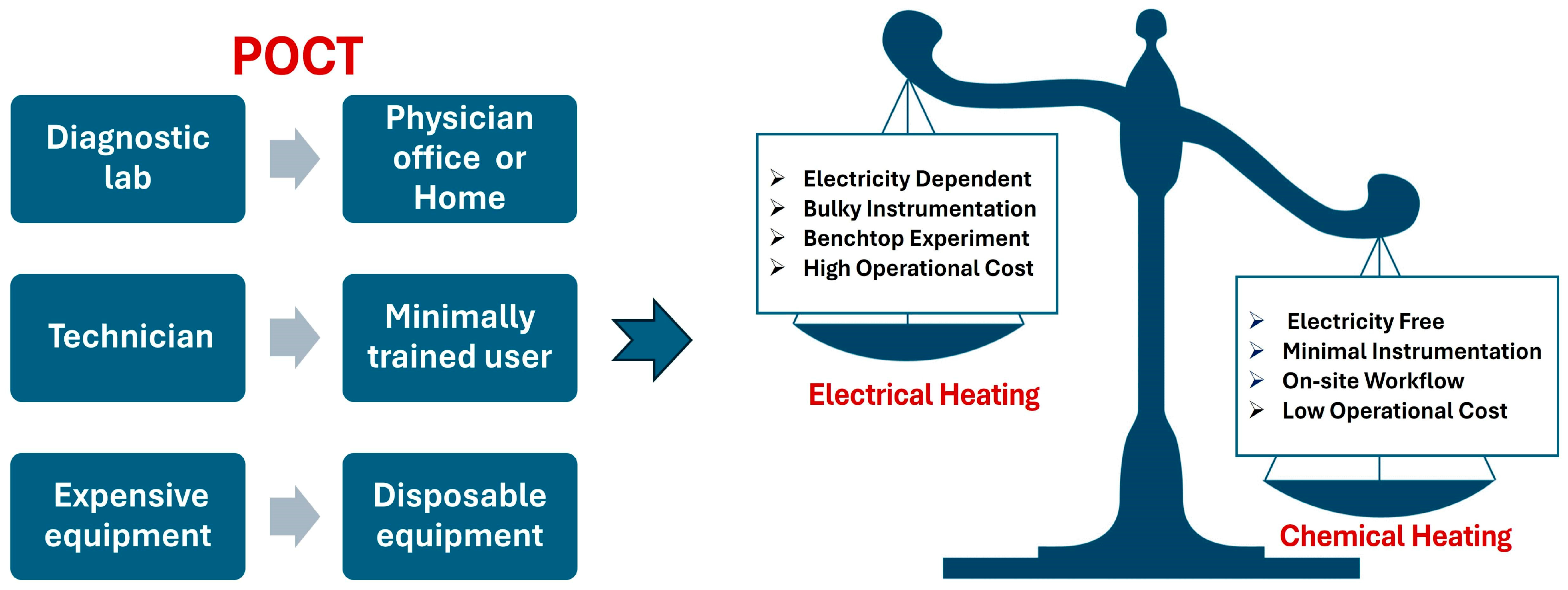
:1. Introduction
2. Chemical Heating Reactions
3. Phase-Change Materials (PCMs)
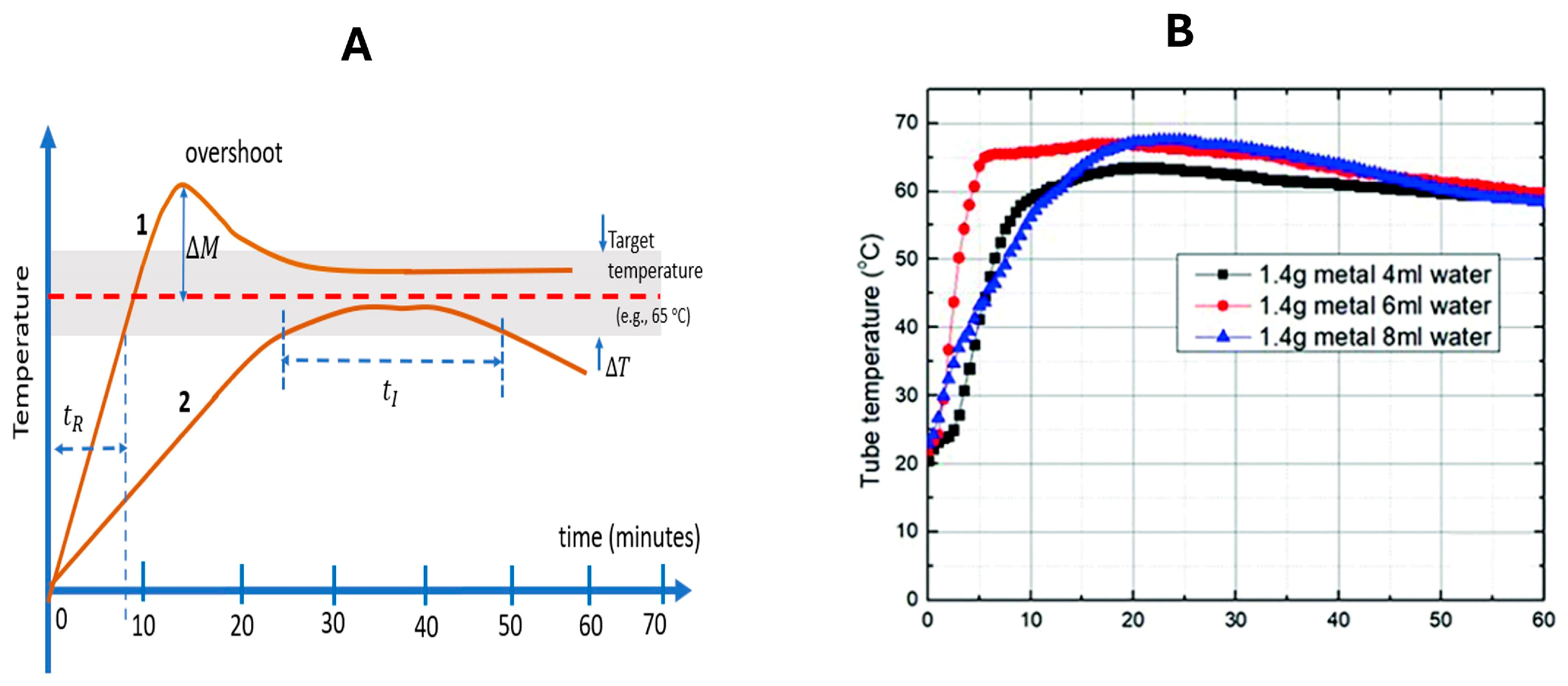
4. Estimations
5. Performance
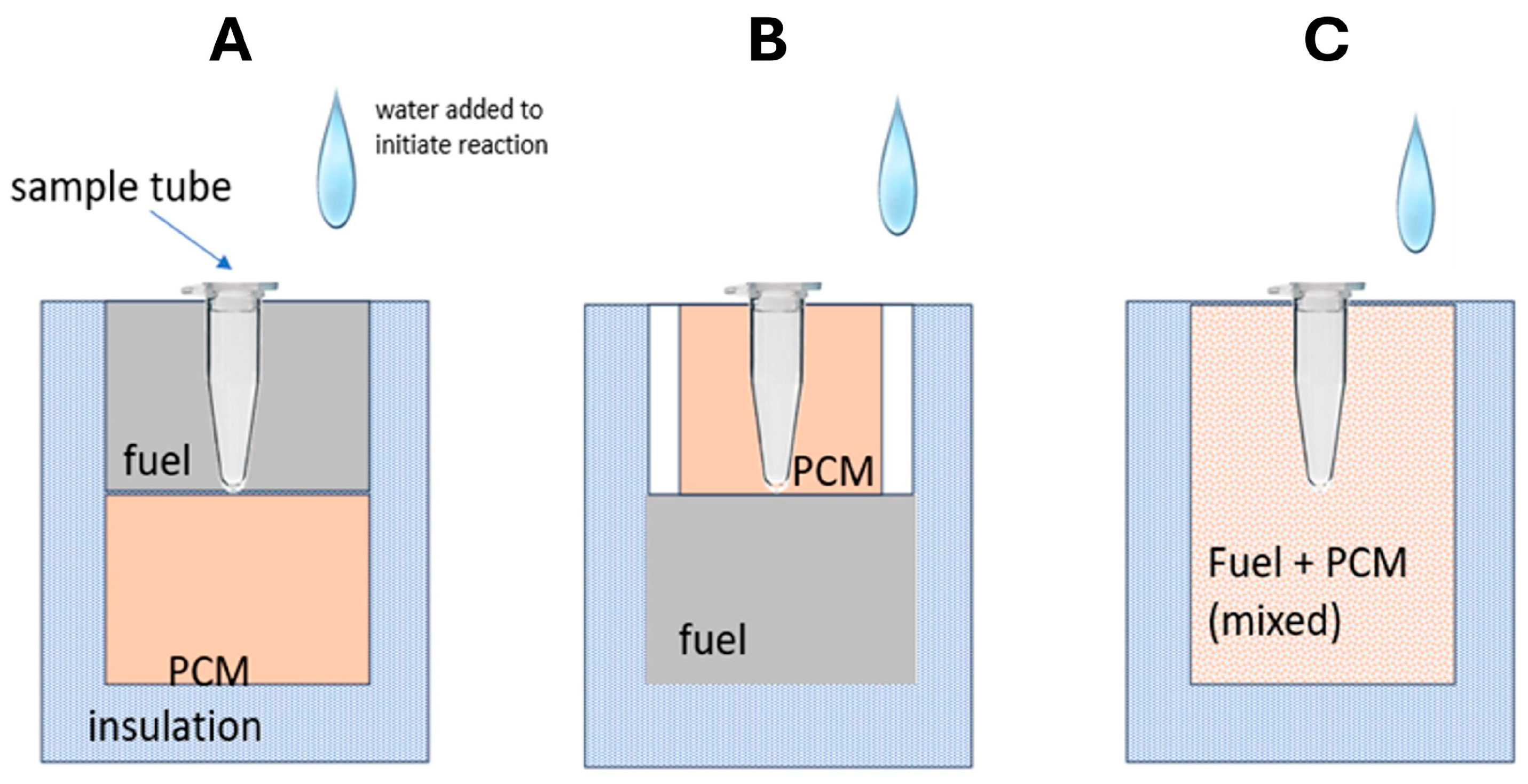
6. Chemical Heating Formats
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miralles, V.; Huerre, A.; Malloggi, F.; Jullien, M.C. A review of heating and temperature control in microfluidic systems: Techniques and application. Diagnostics 2013, 3, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Hema, M.; Charan, K.N. Recent developments in detection and diagnosis of plant viruses. In Recent Developments in Applied Microbiology and Biochemistry; Viswanath, B., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 2, pp. 163–180. [Google Scholar]
- Scott, D.; Meadows, R. Hot Meals. ChemMatters 1992, 10, 12–13. [Google Scholar]
- Sands, W.A.; Kimmel, W.L.; Wurtz, B.R.; Stone, M.H.; McNeal, J.R. Comparison of commercially available disposable chemical hand and foot warmers. Wilderness Environ. Med. 2009, 20, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Alp, F.B.; Balköse, D. Hand warmer types, silica and zeolite 4A as potential hand warmers. Sigma J. Eng. Nat. Sci. 2024, 42, 356–365. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, D. Isothermal nucleic acid amplification and its uses in modern diagnostic technologies. 3 Biotech 2023, 13, 200. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchai, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Euler, M.; Wang, Y.; Otto, P.; Tomaso, H.; Escudero, R.; Anda, P.; Hufert, F.T.; Weidmann, M. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J. Clin. Microbiol. 2012, 50, 2234–2238. [Google Scholar] [CrossRef]
- World Energy Outlook 2019; International Energy Agency (IEA) IEA: Paris, France, 2019.
- Song, J.; Pandian, V.; Mauk, M.G.; Bau, H.H.; Cherry, S.; Tisi, L.C.; Liu, C. Smartphone-based mobile detection platform for molecular diagnostics and spatiotemporal disease mapping. Anal. Chem. 2018, 90, 4823–4831. [Google Scholar] [CrossRef]
- Song, J.; Mauk, M.G.; Hackett, B.A.; Cherry, S.; Bau, H.H.; Liu, C. Instrument-Free Point-of-Care Molecular De-tection of Zika Virus. Anal. Chem. 2016, 88, 7289–7294. [Google Scholar] [CrossRef]
- Babaev, B.D. Principles of heat accumulation and heat-accumulating materials in use. High Temp. 2014, 52, 736–751. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Q.; Sun, J. Thermal analysis of nickel cobalt lithium manganese with varying nickel content used for lithium ion batteries. Thermochim. Acta 2017, 655, 176–180. [Google Scholar] [CrossRef]
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; Von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. 2019, 144, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Özay, B.; McCalla, S.E. A review of reaction enhancement strategies for isothermal nucleic acid amplification reactions. Sens. Actuators Rep. 2021, 3, 100033. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Bau, H.H. Molecular Detection of Respiratory Tract Viruses in Chickens at the Point of Need by Loop-Mediated Isothermal Amplification (LAMP). Viruses 2024, 16, 1248. [Google Scholar] [CrossRef]
- Moon, Y.-J.; Lee, S.-Y.; Oh, S.-W. A Review of Isothermal Amplification Methods and Food-Origin Inhibitors against Detecting Food-Borne Pathogens. Foods 2022, 11, 322. [Google Scholar] [CrossRef]
- LaBarre, P.; Gerlach, J.; Wilmoth, J.; Beddoe, A.; Singleton, J.; Weigl, B. Non-instrumented nucleic acid amplification (NINA): Instrument-free molecular malaria diagnostics for low-resource settings. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1097–1099. [Google Scholar]
- LaBarre, P.; Hawkins, K.R.; Gerlach, J.; Wilmoth, J.; Beddoe, A.; Singleton, J.; Boyle, D.; Weigl, B. A simple, inexpensive device for nucleic acid amplification without electricity-toward instrument-free molecular diagnostics in low-resource settings. PLoS ONE 2011, 6, e19738. [Google Scholar] [CrossRef]
- Singleton, J.; Zentner, C.; Buser, J.; Yager, P.; LaBarre, P.; Weigl, B.H. Instrument-free exothermic heating with phase change temperature control for paper microfluidic devices. Proc. SPIE Int. Soc. Opt. Eng. 2013, 8615, 86150R. [Google Scholar]
- Singleton, J.; Osborn, J.L.; Lillis, L.; Hawkins, K.; Guelig, D.; Price, W.; Johns, R.; Ebels, K.; Boyle, D.; Weigl, B.; et al. Electricity-free amplification and detection for molecular point-of-care diagnosis of HIV-1. PLoS ONE 2014, 9, e113693. [Google Scholar] [CrossRef]
- Curtis, K.A.; Rudolph, D.L.; Morrison, D.; Guelig, D.; Diesburg, S.; McAdams, D.; Burton, R.A.; LaBarre, P.; Owen, M. Single-use, electricity-free amplification device for detection of HIV-1. J. Virol. Methods. 2016, 237, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Lillis, L.; Lehman, D.; Singhal, M.C.; Cantera, J.; Singleton, J.; Labarre, P.; Toyama, A.; Piepenburg, O.; Parker, M.; Wood, R.; et al. Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PLoS ONE 2014, 9, e108189. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.B.; Li, Z.; Alhassan, A.; Guelig, D.; Diesburg, S.; Tanner, N.A.; Zhang, Y.; Evans, T.C., Jr.; LaBarre, P.; Wanji, S.; et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using non-instrumented nucleic acid loop-mediated isothermal amplification (NINA-LAMP). PLoS ONE 2017, 12, e0169011. [Google Scholar] [CrossRef] [PubMed]
- Buser, J.R.; Diesburg, S.; Singleton, J.; Guelig, D.; Bishop, J.D.; Zentner, C.; Burton, R.; LaBarre, P.; Yager, P.; Weigl, B.H. Precision chemical heating for diagnostic devices. Lab Chip. 2015, 15, 4423–4432. [Google Scholar] [CrossRef]
- Sergev, S.S.; Black, S.A.; Jenkins, J.F. Supercorroding Galvanic Cell Alloys for Generation of Heat and Gas. US Patent 4264362, 28 April 1981. [Google Scholar]
- Huang, S.; Do, J.; Mahalanabis, M.; Fan, A.; Zhao, L.; Jepeal, L.; Singh, S.K.; Klapperich, C.M. Low cost extraction and isothermal amplification of DNA for infectious diarrhea diagnosis. PLoS ONE 2013, 8, e60059. [Google Scholar] [CrossRef]
- Shaw, S.A.; Balasubramanian, B.; Bonacorsi, S.; Cortes, J.C.; Cao, K.; Chen, B.C.; Dai, J.; Decicco, C.; Goswami, A.; Guo, Z.; et al. Synthesis of Biologically Active Piperidine Metabolites of Clopidogrel: Determination of Structure and Analyte Development. J. Org. Chem. 2015, 80, 7019–7032. [Google Scholar] [CrossRef]
- Li, Z.; Ding, X.; Yin, K.; Avery, L.; Ballesteros, E.; Liu, C. Instrument-free, CRISPR-based diagnostics of SARS-CoV-2 using self-contained microfluidic system. Biosens. Bioelectron. 2022, 199, 113865. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Chan, W.C.W. Tunable and precise miniature lithium heater for point-of-care applications. Proc. Natl. Acad. Sci. USA 2020, 117, 4632–4641. [Google Scholar] [CrossRef]
- Feng, F.; Yuan, Y.; Fu, Q.; Cao, F.; Kong, R.; Ji, D.; Liu, H. An integrated self-heating recombinase polymerase amplification lateral flow strip biosensor for quantification of enterohemorrhagic Escherichia coli O157: H7. Microchem. J. 2024, 199, 109979. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, W.Q.; Guo, J.L.; Peng, C.; Chen, X.Y.; Xu, X.L.; Wei, W.; Yang, L.; Ca, J.; Xu, J.F. A Fast, Visual, and Instrument-free Platform Involving Rapid DNA Extraction, Chemical Heating, and Recombinase Aided Amplification for On-Site Nucleic Acid Detection. Front Bioeng. Biotechnol. 2021, 9, 764306. [Google Scholar] [CrossRef]
- Vloemans, D.; Dal Dosso, F.; Verboven, P.; Nicolai, B.; Lammertyn, J. Exploiting phase change materials in tunable passive heating system for low-resource point-of-care diagnostics. Appl. Therm. Eng. 2020, 173, 115269. [Google Scholar] [CrossRef]
- Liu, C.; Mauk, M.G.; Hart, R.; Qiu, X.; Bau, H.H. A self-heating cartridge for molecular diagnostics. Lab Chip 2011, 11, 2686. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Mauk, M.G.; Seok, Y.; Bau, H.H. Electricity-free chemical heater for isothermal nucleic acid amplification with applications in COVID-19 home testing. Analyst 2021, 146, 4212–4218. [Google Scholar]
- Oscorbin, I.; Filipenko, M. Bst polymerase—A humble relative of Taq polymerase. Comput. Struct. Biotechnol. J. 2023, 21, 4519–4535. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-mediated isothermal amplification (LAMP)–review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Chen, X.; Song, X.; Tang, K. Experimental Study of an Enhanced Phase Change Material of Paraffin/Expanded Graphite/Nano-Metal Particles for a Personal Cooling System. Materials 2020, 13, 980. [Google Scholar] [CrossRef]
- Liao, S.-C.; Peng, J.; Mauk, M.G.; Awasthi, S.; Song, J.; Friedman, H.; Bau, H.H.; Liu, C. Smart cup: A minimally-instrumented, smartphone-based point-of-care molecular diagnostic device. Sens. Actuators B Chem. 2016, 229, 232–238. [Google Scholar] [CrossRef]
- Goertz, J.P.; Colvin, K.M.; Lippe, A.B.; Daristotle, J.L.; Kofinas, P.; White, I.M. Multistage Chemical Heating for Instrument-Free Biosensing. ACS Appl. Mater. Interfaces. 2018, 10, 33043–33048. [Google Scholar] [CrossRef]
- Fu, S.; Jiang, Y.; Qin, X.; Yang, T.; Chen, S.; Yang, X.; Zhang, W.; Qu, Y.; Man, C. Electricity-free amplification and visual detection of Cronobacter species in powdered infant formula. J. Dairy Sci. 2020, 103, 6882–6893. [Google Scholar] [CrossRef]
- Curtis, K.A.; Rudolph, D.L.; Nejad, I.; Singleton, J.; Beddoe, A.; Weigl, B.; LaBarre, P.; Owen, S.M. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PLoS ONE. 2012, 7, e31432. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Mauk, M.G.; Rankin, S.C.; Lok, J.B.; Greenberg, R.M.; Bau, H.H. Two-Stage Isothermal Enzymatic Amplification for Concurrent Multiplex Molecular Detection. Clin. Chem. 2017, 63, 714–722. [Google Scholar] [CrossRef] [PubMed]
- El-Tholoth, M.; Anis, E.; Bau, H.H. Two stage, nested isothermal amplification in a single tube. Analyst 2021, 146, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.; Yin, Q.; Bai, H.; Bau, H.H. Sensitive, Single-Pot, Two-Stage Assay for Hepatitis Viruses. Anal. Chem. 2022, 94, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
| Isothermal Amplification Assay | Pathogen (s) | Fuel | Reactants | Time to Chemical Heating | Amplification Time | Amplified Product Detection | Analytical Sensitivity | The Used Device | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1—LAMP | Zika virus | Magnesium Iron | Mg-Fe + H2O | 10 min | 60 min | Bioluminescence | 5 PFU/sample | 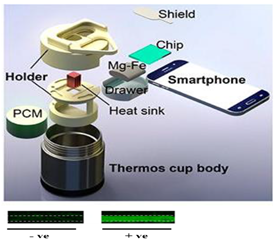 | [11] |
| 2—LAMP | Zika virus | Magnesium Iron | Mg-Fe + H2O | 10 min | 40 min | Colorimetric detection—intercalating dye Leco Crystal Violet (LCV) | 5 PFU/sample |  | [12] |
| 3—LAMP | Plasmodium falciparum malaria | Calcium oxide | CaO + H2O | 15 min | 45 min | Turbid visual detection and fluorescent (Calcein) detection | 50 copies/µL |  | [20] |
| 4—LAMP | Plasmodium falciparum malaria | Calcium oxide | CaO + H2O | 15 min | 60 min | Turbid visual detection and fluorescent (Calcein) detection | 50 copies/µL |  | [21] |
| 5—LAMP | HIV-1 | Magnesium Iron | Mg-Fe + H2O | 12 min | 60 min | Nucleic acid lateral flow detection | 75 copies/reaction |  | [23] |
| 6—LAMP | HIV-1 | Magnesium Iron | Mg-Fe + H2O | 11 min | 60 min | BHQ1-labeled quencher probe | 3115 copies/mL | 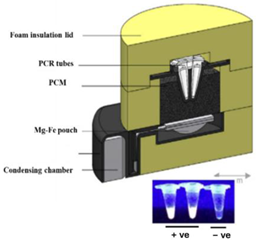 | [24] |
| 7—RPA | HIV-1 | Sodium acetate | Na+ + C2H3O2 | 1 min | 20–30 min | Nucleic acid lateral flow detection | 10 copies/µL |  | [25] |
| 8—LAMP | Filarial parasites (B. malayi, O. volvulus, and W. bancrofti) | Magnesium Iron | Mg-Fe + H2O | 15 min | 40–70 min | Colorimetric detection—proton detection (pH-sensitive indicator dyes) | B. malayi 1.0 pg DNA/reaction, O. volvulus 0.01 ng DNA/reaction, and W. bancrofti 0.1 pg DNA/reaction |  | [26] |
| 9—HDA | Clostridium difficile (C. difficile) | Iron filings | Fe + 3 O2 | 22 min | 30 min | Gel electrophoresis analysis | 1.25 × 10−2 pg/reaction |  | [29] |
| 10—RPA/CRISPR | SARS-CoV-2 | Iron filings | Fe + 3 O2 | 2 min | 15 min | Nucleic acid lateral flow detection | 100 copies/reaction |  | [31] |
| 11—RPA | T4 bacteriophage | Lithium | Li + H2O | 1 min | 15 min | - Fluorescent test (Taqman probe) - Gel electrophoresis | NA |  | [32] |
| 12—RPA | Enterohemorrhagic Escherichia coli O157:H7 | Calcium oxide | CaO + H2O | 9 min | 15 min | Nucleic acid lateral flow detection | 36.23 CFU/mL | 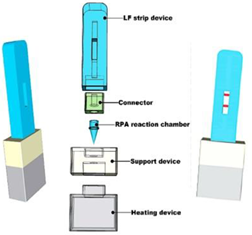 | [33] |
| 13—LAMP | Cronobacter species | Calcium oxide | CaO + H2O | 7 min | 60 min | HNB (Hydroxy naphthol blue)—Metal indicator | 4.2 × 102 cfu/g |  | [43] |
| 14—LAMP | Herpes simplex virus type 2 (HSV-2) | Magnesium Iron | Mg-Fe + H2O | 10 min | 60 min | Fluorescent intercalating dyes | 4.2 PFU/sample | 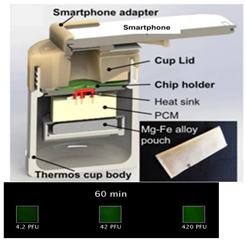 | [41] |
| 15—LAMP | SARS-CoV-2 | Magnesium Iron | Mg-Fe + H2O | 3 min | 30 min | - pH-sensitive indicator dyes (Phenol red) - Fluorescent intercalating dyes | 10 copies/reaction | 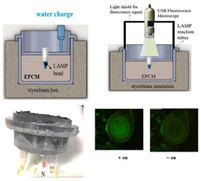 | [37] |
| 16—LAMP | HIV-1 | Calcium oxide | CaO + H2O | 10–15 min | 60 min | Fluorescence detection using labeled primer along with a quencher probe | HIV- 1 DNA: 10 copies/reaction HIV-1 RNA: 140 copies/reaction | 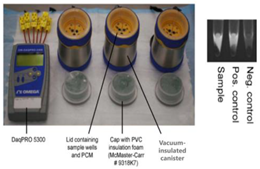 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauk, M.G.; Ansah, F.; El-Tholoth, M. Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics. Biosensors 2024, 14, 554. https://doi.org/10.3390/bios14110554
Mauk MG, Ansah F, El-Tholoth M. Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics. Biosensors. 2024; 14(11):554. https://doi.org/10.3390/bios14110554
Chicago/Turabian StyleMauk, Michael G., Felix Ansah, and Mohamed El-Tholoth. 2024. "Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics" Biosensors 14, no. 11: 554. https://doi.org/10.3390/bios14110554
APA StyleMauk, M. G., Ansah, F., & El-Tholoth, M. (2024). Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics. Biosensors, 14(11), 554. https://doi.org/10.3390/bios14110554





