Massive Screening of Food Extracts for Quality Assessment and Standardization of Allergenic Activity
Abstract
1. Introduction
2. Materials and Methods
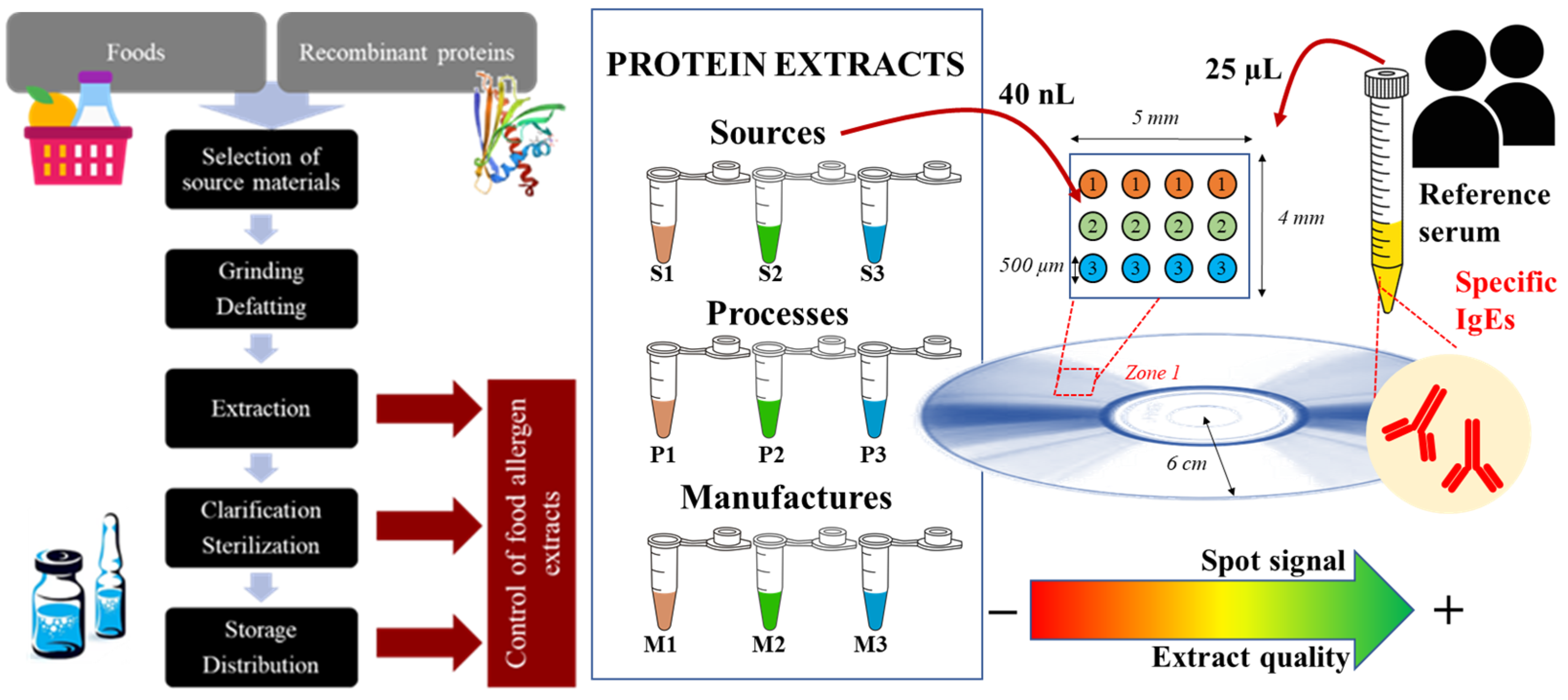
2.1. Protein Extracts
2.2. Chip Preparation
2.3. Preparation of Patient Standard Solutions and Buffers
2.4. Assay of IgE Reactivity
2.5. Detection and Image Processing
3. Results
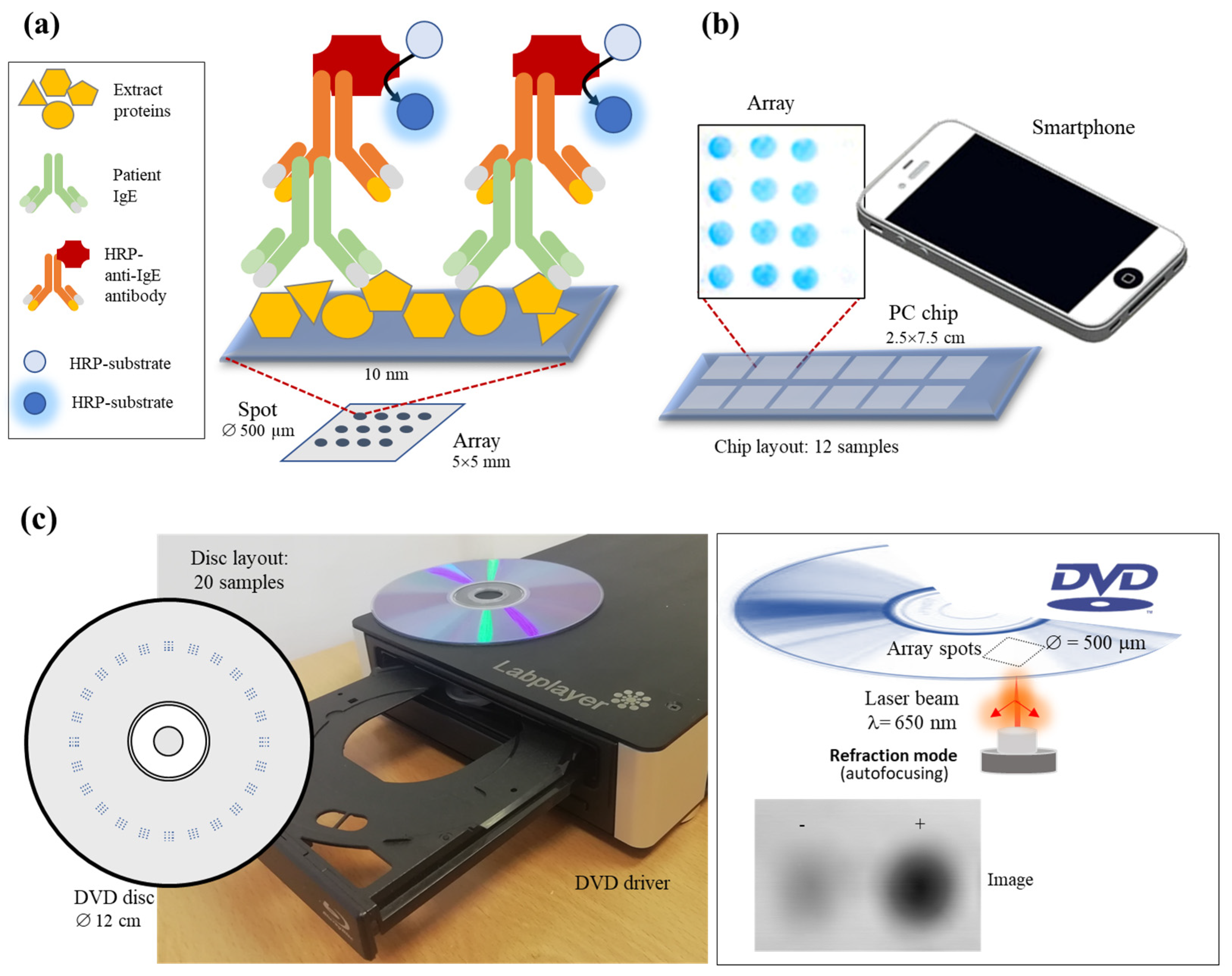
3.1. Assay Principle
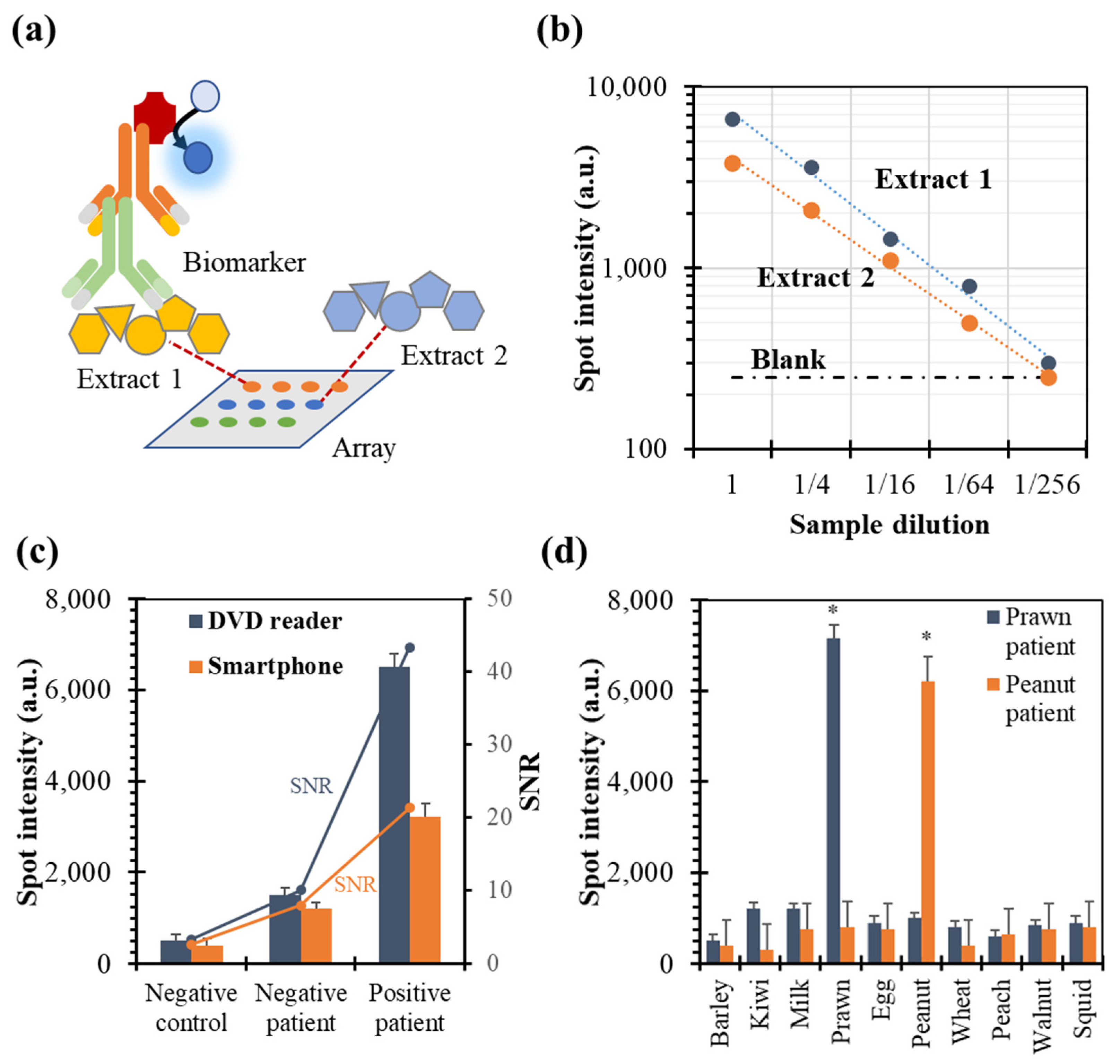
3.2. Setup of Recognition Event
3.3. High-Throughput Assay Performances
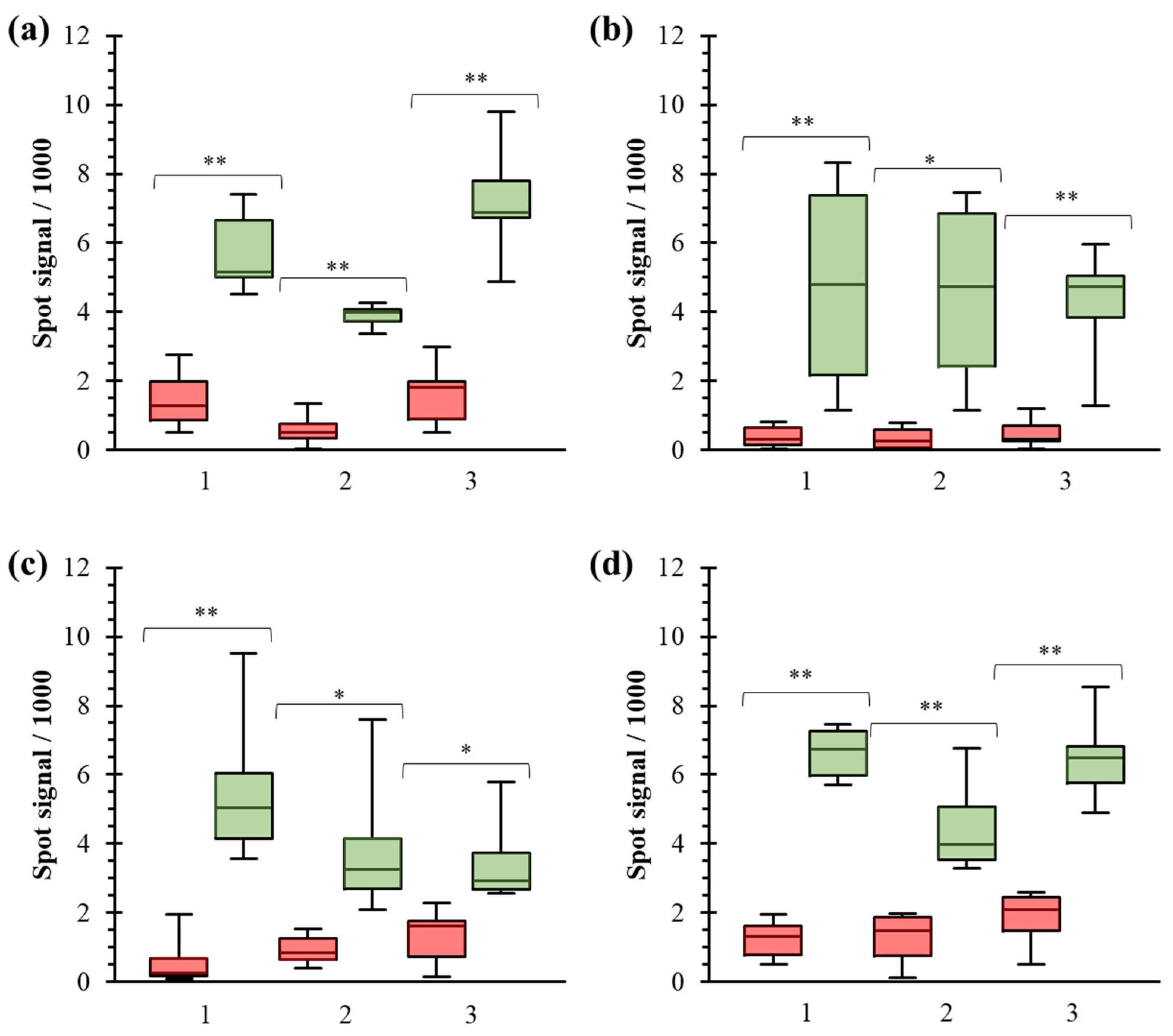
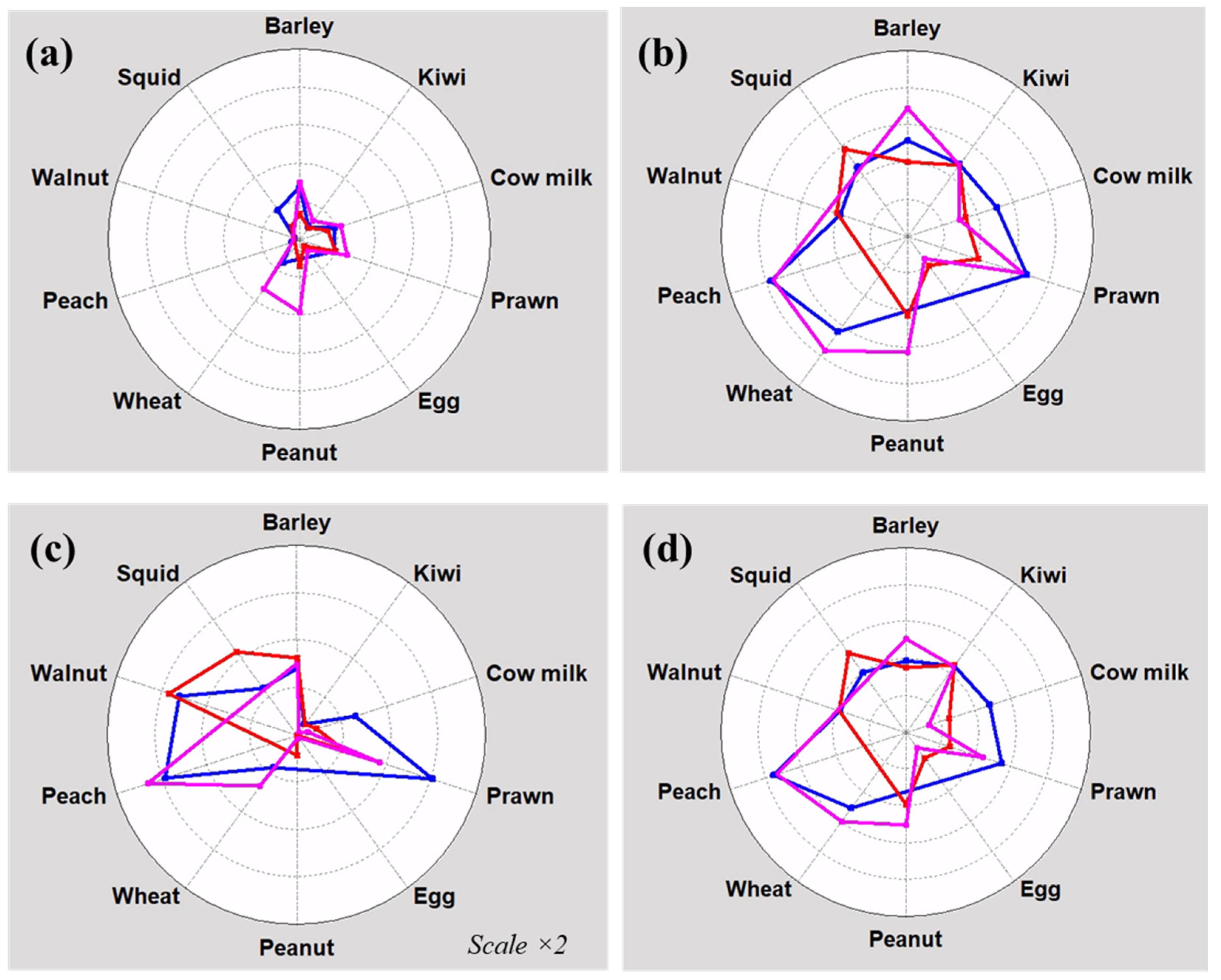
3.4. Comparison of Manufacturers
3.5. Quality Control Application
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-throughput screening: Today’s biochemical and cell-based approaches. Drug Discov. Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Wang, S. Application of microfluidic chip technology in pharmaceutical analysis: A review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org. Process. Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
- Larsen, J.N.; Houghton, C.G.; Vega, M.L.; Nolte, H.; Løwenstein, H. Manufacturing and standardizing allergen extracts in Europe. In Allergens and Allergen Immunotherapy, 6th ed.; Lockey, R.F., Ledford, D.K., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 363–380. [Google Scholar]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Alvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K.; et al. EAACI guidelines on the diagnosis of IgE-mediated food allergy. Allergy 2023, 78, 3057–3076. [Google Scholar] [CrossRef]
- Bonertz, A.; Mahler, V.; Vieths, S. Manufacturing and quality assessment of allergenic extracts for immunotherapy: State of the art. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 640–645. [Google Scholar] [CrossRef]
- Durham, S.R.; Shamji, M.H. Allergen immunotherapy: Past, present and future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Zimmer, J.; Vieths, S.; Kaul, S. Standardization and regulation of allergen products in the European Union. Curr. Allergy Asthma Rep. 2016, 16, 21. [Google Scholar] [CrossRef]
- Englert, L.; Mahler, V.; Bonertz, A. Regulatory requirements for the quality of allergen products for allergen immunotherapy of food allergy. Curr. Allergy Asthma Rep. 2021, 21, 32. [Google Scholar] [CrossRef]
- David, N.A.; Penumarti, A.; Burks, A.W.; Slater, J.E. Food allergen extracts to diagnose food-induced allergic diseases: How they are made. Ann. Allergy Asthma Immunol. 2017, 119, 101–107. [Google Scholar] [CrossRef]
- Goodman, R.E.; Chapman, M.D.; Slater, J.E. The allergen: Sources, extracts, and molecules for diagnosis of allergic disease. J. Allergy Clin. Immunol. Pract. 2020, 8, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.S. Standardized allergen extracts for allergen immunotherapy. Allergy Asthma Proc. 2022, 43, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Zhernov, Y.; Elisyutina, O.; Campana, R.; Focke-Tejkl, M.; Curin, M.; Namazova-Baranova, L.; Wang, J.Y.; et al. Allergen extracts for in vivo diagnosis and treatment of allergy: Is there a future? J. Allergy Clin. Immunol. Pract. 2018, 6, 1845–1855. [Google Scholar] [CrossRef]
- Larsen, J.M.; Bang-Berthelsen, C.H.; Qvortrup, K.; Sancho, A.I.; Hansen, A.H.; Andersen, K.I.H.; Thacker, S.S.N.; Eiwegger, T.; Upton, J.; Bøgh, K.L. Production of allergen-specific immunotherapeutic agents for the treatment of food allergy. Crit. Rev. Biotechnol. 2020, 40, 881–894. [Google Scholar] [CrossRef]
- Kaur, H.; Beckman, J.; Zhang, Y.; Li, Z.J.; Szigeti, M.; Guttman, A. Capillary electrophoresis and the biopharmaceutical industry: Therapeutic protein analysis and characterization. TrAC Trends Anal. Chem. 2021, 144, 116407. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Gagaoua, M.; Franco, D. Current trends in proteomic advances for food allergen analysis. Biology 2020, 9, 247. [Google Scholar] [CrossRef]
- Singh, J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J. Pharmacol. Pharmacother. 2015, 6, 185–187. [Google Scholar] [CrossRef]
- Soares, J.R.A.; e Silva, A.P.; Oliveira, A.L.d.S.; Guimarães, I.M.; Faccini, C.R.J.d.N.; Mattos, E.B.d.A.; Rodrigues, S.K.P.M.; Marmello, B.O.; Teixeira, G.A.P.B. Allergen extraction: Factors influencing immunogenicity and sensitivity of immunoassays. J. Immunol. Methods 2021, 498, 113125. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Khan, M.U.; Gao, X.; Yu, M.; Gao, H.; Li, Y.; Zhang, H.; Dasabnayaka, B.P.; Lin, H. Extraction of total wheat (Triticum aestivum) protein fractions and cross-reactivity of wheat allergens with other cereals. Food Chem. 2021, 347, 129064. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Jarad, N.A.; Didar, T.F. The fabrication and bonding of thermoplastic microfluidics: A review. Materials 2022, 15, 6478. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ou, L.M.; Yu, H.Z. DNA detection on plastic: Surface activation protocol to convert polycarbonate substrates to biochip platforms. Anal. Chem. 2007, 79, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, A.; Maquieira, Á.; Tortajada-Genaro, L.A. Discrimination of Single-Nucleotide Variants Based on an Allele-Specific Hybridization Chain Reaction and Smartphone Detection. ACS Sens. 2022, 7, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Tortajada-Genaro, L.A.; Yamanaka, E.S.; Maquieira, Á. Consumer electronics devices for DNA genotyping based on loop-mediated isothermal amplification and array hybridisation. Talanta 2019, 198, 424–431. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Casañ-Raga, N.; Mas, S.; Ibañez-Echevarria, E.; Morais, S.; Maquieira, Á. Reversed-phase allergen microarrays on optical discs for multiplexed diagnostics of food allergies. Microchim. Acta 2023, 190, 166. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Tortajada-Genaro, L.A.; Maquieira, A.; González-Martinez, M.A. Biosensors for food allergy detection according to specific IgE levels in serum. TrAC Trends Anal. Chem. 2020, 127, 115904. [Google Scholar] [CrossRef]
- Grudpan, K.; Kolev, S.D.; Lapanantnopakhun, S.; McKelvie, I.D.; Wongwilai, W. Applications of everyday IT and communications devices in modern analytical chemistry: A review. Talanta 2015, 136, 84–94. [Google Scholar] [CrossRef]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical relevance of cross-reactivity in food allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Mafra, I. Sesame as a source of food allergens: Clinical relevance, molecular characterization, cross-reactivity, stability toward processing and detection strategies. Crit. Rev. Food Sci. Nutr. 2022, 64, 4746–4762. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Quintero-Campos, P.; Juárez, M.J.; Ibañez-Echevarria, E.; Chiriac, A.M.; Fernández, E.; Morais, S.; Maquieira, Á. Development and validation study of compact biophotonic platform for detection of serum biomarkers. Talanta 2024, 278, 126511. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Liu, G.; Fu, L. Food allergomics based on high-throughput and bioinformatics technologies. Food Res. Int. 2020, 130, 108942. [Google Scholar] [CrossRef]
- Welch, C.J. High throughput analysis enables high throughput experimentation in pharmaceutical process research. React. Chem. Eng. 2019, 4, 1895–1911. [Google Scholar] [CrossRef]
- Aquino, A.; Conte-Junior, C.A. A systematic review of food allergy: Nanobiosensor and food allergen detection. Biosensors 2020, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.A.; Hayes, J.P.; Thissen, H. Protein patterning in polycarbonate microfluidic channels. SPIE 2004, 5275, 161–167. [Google Scholar]
- Bora, U.; Sharma, P.; Kumar, S.; Kannan, K.; Nahar, P. Photochemical activation of a polycarbonate surface for covalent immobilization of a protein ligand. Talanta 2006, 70, 624–629. [Google Scholar] [CrossRef]
- Muck, A.; Svatoš, A. Chemical modification of polymeric microchip devices. Talanta 2007, 74, 333–341. [Google Scholar] [CrossRef]
- Bañuls, M.J.; García-Piñón, F.; Puchades, R.; Maquieira, Á. Chemical derivatization of compact disc polycarbonate surfaces for SNPs detection. Bioconjugate Chem. 2008, 19, 665–672. [Google Scholar] [CrossRef]
- Tamarit-Lopez, J.; Morais, S.; Bañuls, M.J.; Puchades, R.; Maquieira, A. Development of hapten-linked microimmunoassays on polycarbonate discs. Anal. Chem. 2010, 82, 1954–1963. [Google Scholar] [CrossRef]
- Ogończyk, D.; Jankowski, P.; Garstecki, P. Functionalization of polycarbonate with proteins; open-tubular enzymatic microreactors. Lab A Chip 2012, 12, 2743–2748. [Google Scholar] [CrossRef]
- Morais, S.; Tortajada-Genaro, L.; Maquieira, Á. Array-on-a-disk? How Blu-ray technology can be applied to molecular diagnostics. Expert Rev. Mol. Diagn. 2014, 14, 773–775. [Google Scholar] [CrossRef][Green Version]
- Bañuls, M.J.; Morais, S.B.; Tortajada-Genaro, L.A.; Maquieira, Á. Microarray developed on plastic substrates. Microarray Technol. Methods Appl. 2016, 1368, 37–51. [Google Scholar]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluidics 2021, 25, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Stedtfeld, R.D.; Tourlousse, D.M.; Seyrig, G.; Stedtfeld, T.M.; Kronlein, M.; Price, S.; Ahmad, F.; Gulari, E.; Tiedje, J.M.; Hashsham, S.A. Gene-Z: A device for point of care genetic testing using a smartphone. Lab A Chip 2012, 12, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; O'Dell, D.; Jiang, L.; Oncescu, V.; Gumus, A.; Lee, S.; Mancuso, M.; Mehta, S. Smartphone technology can be transformative to the deployment of lab-on-chip diagnostics. Lab A Chip 2014, 14, 3159–3164. [Google Scholar] [CrossRef] [PubMed]
- Christodouleas, D.C.; Nemiroski, A.; Kumar, A.A.; Whitesides, G.M. Broadly available imaging devices enable high-quality low-cost photometry. Anal. Chem. 2015, 87, 9170–9178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Yamanaka, E.S.; Tortajada-Genaro, L.A.; Pastor, N.; Maquieira, Á. Polymorphism genotyping based on loop-mediated isothermal amplification and smartphone detection. Biosens. Bioelectron. 2018, 109, 177–183. [Google Scholar] [CrossRef]
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Bisetty, K. Smartphone based bioanalytical and diagnosis applications: A review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef]
- Zong, H.; Zhang, Y.; Liu, X.; Xu, Z.; Ye, J.; Lu, S.; Guo, X.; Yang, Z.; Zhang, X.; Chai, M.; et al. Recent trends in smartphone-based optical imaging biosensors for genetic testing: A review. View 2023, 4, 20220062. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Lucío, M.I.; Maquieira, Á. Fast DNA biosensing based on isothermal amplification, unmodified gold nanoparticles, and smartphone detection. Food Control 2022, 137, 108943. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Awazu, K.; Fons, P.; Tominaga, J.; Kumar, P.K. A sensitive multilayered structure suitable for biosensing on the BioDVD platform. Anal. Chem. 2009, 81, 4963–4970. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Rodrigo, A.; Hevia, E.; Mena, S.; Niñoles, R.; Maquieira, Á. Microarray on digital versatile disc for identification and genotyping of Salmonella and Campylobacter in meat products. Anal. Bioanal. Chem. 2015, 407, 7285–7294. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Felipe, S.; Tortajada-Genaro, L.A.; Carrascosa, J.; Puchades, R.; Maquieira, Á. Real-time loop-mediated isothermal DNA amplification in compact disc micro-reactors. Biosens. Bioelectron. 2016, 79, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Hwu, E.E.T.; Boisen, A. Hacking CD/DVD/Blu-ray for biosensing. ACS Sens. 2018, 3, 1222–1232. [Google Scholar] [CrossRef] [PubMed]

| Techniques | Nature | Advantages | Drawbacks |
|---|---|---|---|
| Skin test | In vivo | Real information about reactivity | Ethics restriction, discomfort for individuals, risk of adverse reactions. |
| Basophil activation test | Ex vivo | Valuable insights into the allergic mechanism | Complexity, high cost, more time consumption, requirement of specialized equipment and expertise, and limited standardization across laboratories |
| ELISA | In vitro | Possible automation, high sensitivity, specificity, and ability to quantify accurately | Limited working capability, specialized equipment and reagents |
| Electrophoretic techniques | In vitro | Easy visualization of protein profile | Limited information about reactivity, qualitative data, low working capability |
| Array technology | In vitro | Miniaturization, multiplexing, quantitative data, high throughput, low consumption of extract | Needs correct immobilization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortajada-Genaro, L.A. Massive Screening of Food Extracts for Quality Assessment and Standardization of Allergenic Activity. Biosensors 2024, 14, 615. https://doi.org/10.3390/bios14120615
Tortajada-Genaro LA. Massive Screening of Food Extracts for Quality Assessment and Standardization of Allergenic Activity. Biosensors. 2024; 14(12):615. https://doi.org/10.3390/bios14120615
Chicago/Turabian StyleTortajada-Genaro, Luis Antonio. 2024. "Massive Screening of Food Extracts for Quality Assessment and Standardization of Allergenic Activity" Biosensors 14, no. 12: 615. https://doi.org/10.3390/bios14120615
APA StyleTortajada-Genaro, L. A. (2024). Massive Screening of Food Extracts for Quality Assessment and Standardization of Allergenic Activity. Biosensors, 14(12), 615. https://doi.org/10.3390/bios14120615






