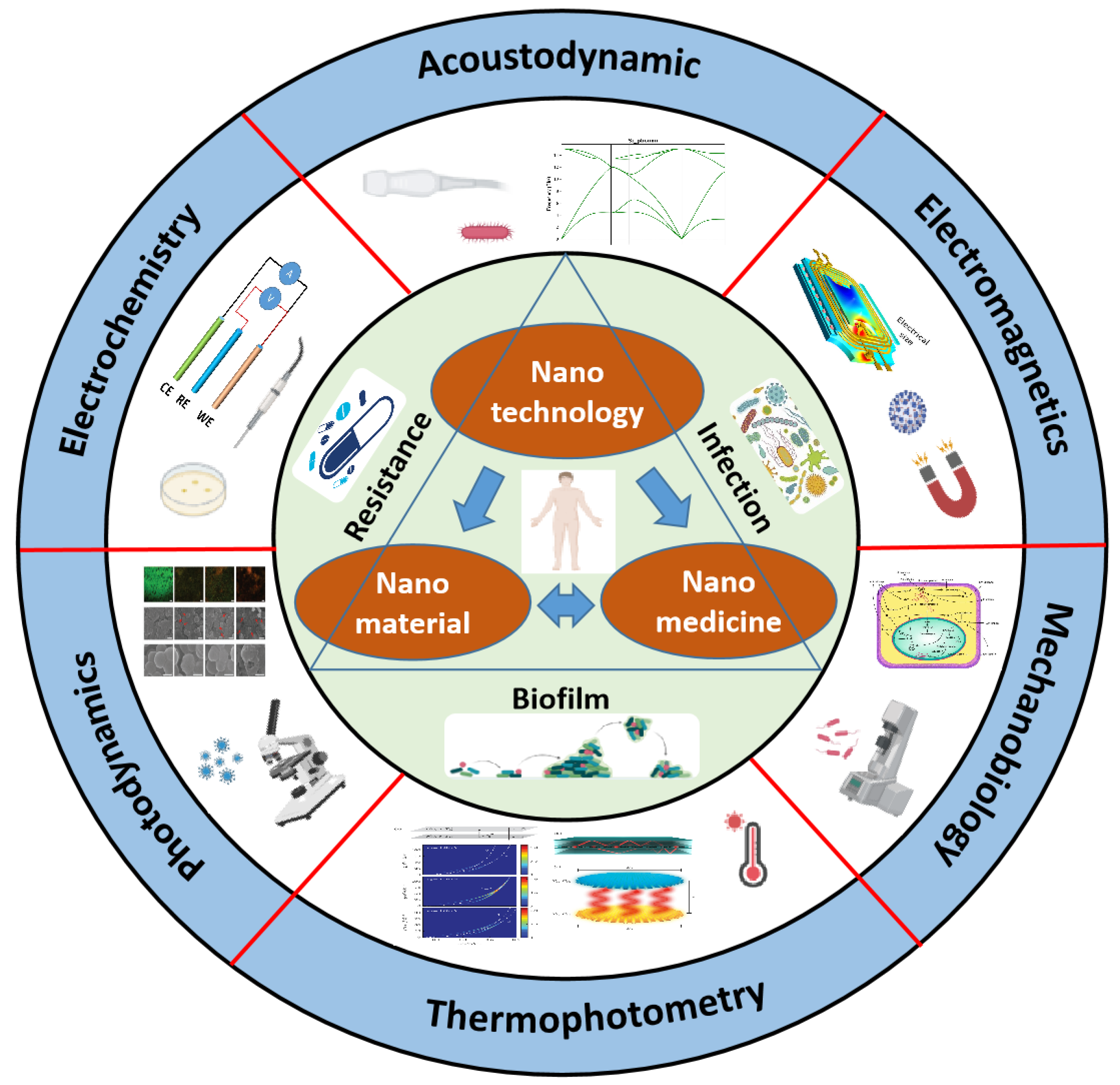
Advances in Engineered Nano-Biosensors for Bacteria Diagnosis and Multidrug Resistance Inhibition
Abstract
1. Introduction
1.1. Traditional Techniques
1.2. Nano-Biosensor Techniques
2. Bacterial-Related Infection
2.1. Bacterial Infection
2.2. Bacterial Resistance
2.3. Bacterial Biofilm
3. Engineered Nano-Biosensors for Precise Bacterial Diagnosis and Treatment
3.1. Photodynamics-Based Nano-Biosensors
3.1.1. Phototherapeutic Drug Nanomaterials
3.1.2. Photodynamic Combined Treatment
3.1.3. Nano-Biosensors upon NIR Light Irradiation
3.1.4. Nanocage-Based Biosensor for Targeted Phototherapy
3.2. Electrochemistry-Based Nano-Biosensors
3.2.1. Custom-Designed Electrochemical Cell
3.2.2. Three-Dimensional Electrode Scaffold
3.2.3. Nanopore Electrical Evaluation
3.2.4. Microfluidic Impedance Biosensors
3.3. Acoustic-Dynamics-Based Nano-Biosensors
3.3.1. Ultrasound-Switchable Nanozyme System
3.3.2. Low-Frequency Ultrasonic Sterilization
3.3.3. Ultrasound-Activated Chemokinetic Therapy
3.3.4. Antibacterial Sonodynamic Nanocapturer
3.4. Electromagnetism-Based Nano-Biosensors
3.4.1. Magneto-Controlled Micromotor
3.4.2. Magnetic Cantilevers
3.4.3. Magnetotactic Bacteria
3.4.4. Magnetic Liquid Metal Nanoparticles
3.5. Photothermal Nano-Biosensors
3.5.1. Photothermal Ablation
3.5.2. Hybrid Coating
3.5.3. Bacterial Affinity Photothermal Carbon Dots
3.5.4. Intelligent Hybrid Hydrogels
3.6. Mechanobiology-Based Nano-Biosensors
3.6.1. Extracellular Matrix Stiffness Regulation
3.6.2. Three-Dimensional Extracellular Matrix Rigidities
3.7. In Situ Bio-Assembly Nano-Biosensors for Bacteria-Related Disease Theranostics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deusenbery, C.; Wang, Y.; Shukla, A. Recent Innovations in Bacterial Infection Detection and Treatment. ACS Infect. Dis. 2021, 7, 695–720. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Jia, Q.; Huang, H.; Zhang, J.; Li, P.; Dong, X.; Huang, W. Emerging Photothermal-Derived Multimodal Synergistic Therapy in Combating Bacterial Infections. Chem. Soc. Rev. 2021, 50, 8762–8789. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial Biofilm Formation: A Need to Act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Wächter, J.; Vestweber, P.K.; Jung, N.; Windbergs, M. Imitating the Microenvironment of Native Biofilms Using Nanofibrous Scaffolds to Emulate Chronic Wound Infections. J. Mater. Chem. B 2023, 11, 3212–3225. [Google Scholar] [CrossRef]
- Lazcka, O.; Campo, F.J.D.; Muñoz, F.X. Pathogen Detection: A Perspective of Traditional Methods and Biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and Promise of Bacterial Quorum Sensing Research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019, 119, 700–726. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Munir, M.U.; Ahmad, M.M. Nanomaterials Aiming to Tackle Antibiotic-Resistant Bacteria. Pharmaceutics 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, H.; Liu, X.; Wang, X. Tunable Nanomaterials of Intracellular Crystallization for In Situ Biolabeling and Biomedical Imaging. Chem. Biomed. Imaging 2023, 1, 767–784. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Wang, Y.; Xiao, J.; Liu, X.; Jiang, H.; Wang, X. Intracellular Liquid-Liquid Phase Separation Induces Tunable Anisotropic Nanocrystal Growth for Multidimensional Analysis. Adv. Funct. Mater. 2023, 33, 2302136. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology Based Drug Delivery System: Current Strategies and Emerging Therapeutic Potential for Medical Science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Krishnan, Y.; Seeman, N.C. Introduction: Nucleic Acid Nanotechnology. Chem. Rev. 2019, 119, 6271–6272. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s Plenty of Room at the Bottom. Resonance 2011, 16, 890–905. [Google Scholar] [CrossRef]
- Al-Shuja’a, O.; Obeid, A.; El-Shekeil, Y.; Hashim, M.; Al-Washali, Z. New Strategy for Chemically Attachment of Imine Group on Multi-Walled Carbon Nanotubes Surfaces: Synthesis, Characterization and Study of DC Electrical Conductivity. J. Mater. Sci. Chem. Eng. 2017, 5, 11. [Google Scholar] [CrossRef][Green Version]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the Science and Technology of Carbon Nanotubes and Their Composites: A Review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Wang, H.; Agarwal, P.; Jiang, B.; Stewart, S.; Liu, X.; Liang, Y.; Hancioglu, B.; Webb, A.; Fisher, J.P.; Liu, Z.; et al. Bioinspired One Cell Culture Isolates Highly Tumorigenic and Metastatic Cancer Stem Cells Capable of Multilineage Differentiation. Adv. Sci. 2020, 7, 2000259. [Google Scholar] [CrossRef]
- Du, G.; Moulin, E.; Jouault, N.; Buhler, E.; Giuseppone, N. Muscle-like Supramolecular Polymers: Integrated Motion from Thousands of Molecular Machines. Angew. Chem. Int. Ed. 2012, 51, 12504–12508. [Google Scholar] [CrossRef]
- Campbell, E.K.; Holz, M.; Gerlich, D.; Maier, J.P. Laboratory Confirmation of C60+ as the Carrier of Two Diffuse Interstellar Bands. Nature 2015, 523, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Cai, X.; Tu, M.W.-Y.; Zhang, X.; Huang, B.; Wilson, N.P.; Seyler, K.L.; Zhu, L.; Taniguchi, T.; Watanabe, K.; et al. Giant Tunneling Magnetoresistance in Spin-Filter van Der Waals Heterostructures. Science 2018, 360, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Frochot Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Mo, L.; Chen, C.; Zhong, H.; Guo, Z.; Liu, Z. Progress in the Development and Application of Transitional Technology of Surface-Enhanced Raman Spectroscopy. Colloid Interface Sci. Commun. 2021, 43, 100443. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Mo, L.; Sun, Y.; Zhang, J.; Liu, X.; Liu, Z. Quantitative Label-free Optical Technique to Analyze the Ultrastructure Changes and Spatiotemporal Relationship of Enamel Induced by Msx2 Deletion. J. Biophotonics 2021, 14, e202100165. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Wang, Y.; Jiang, H.; Wang, X. Advances and Challenges in Metallic Nanomaterial Synthesis and Antibacterial Applications. J. Mater. Chem. B 2020, 8, 4764–4777. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Xiu, W.; Wan, L.; Yang, K.; Li, X.; Yuwen, L.; Dong, H.; Mou, Y.; Yang, D.; Wang, L. Potentiating Hypoxic Microenvironment for Antibiotic Activation by Photodynamic Therapy to Combat Bacterial Biofilm Infections. Nat. Commun. 2022, 13, 3875. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Y.; Ye, W.; Tian, L.; Niu, S.; Ming, W.; Zhao, J.; Ren, L. Near-Infrared Light Triggered Photodynamic and Nitric Oxide Synergistic Antibacterial Nanocomposite Membrane. Chem. Eng. J. 2021, 417, 128049. [Google Scholar] [CrossRef]
- Qin, Z.; Zheng, Y.; Du, T.; Wang, Y.; Gao, H.; Quan, J.; Zhang, Y.; Du, Y.; Yin, L.; Wang, X.; et al. Cysteamine: A Key to Trigger Aggregation-Induced NIR-II Photothermal Effect and Silver Release Booming of Gold-Silver Nanocages for Synergetic Treatment of Multidrug-Resistant Bacteria Infection. Chem. Eng. J. 2021, 414, 128779. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv. Mater. 2018, 30, 1801808. [Google Scholar] [CrossRef] [PubMed]
- Mehrjou, B.; Wu, Y.; Liu, P.; Wang, G.; Chu, P.K. Design and Properties of Antimicrobial Biomaterials Surfaces. Adv. Healthc. Mater. 2023, 12, 2202073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.W.; McPherson, A.; Milne, K.; Kroeger, D.R.; Hamilton, P.T.; Miranda, A.; Funnell, T.; Little, N.; De Souza, C.P.E.; Laan, S.; et al. Interfaces of Malignant and Immunologic Clonal Dynamics in Ovarian Cancer. Cell 2018, 173, 1755–1769. [Google Scholar] [CrossRef] [PubMed]
- Horejs, C.-M. Bubbly for Bacteria. Nat. Rev. Mater. 2018, 3, 457. [Google Scholar] [CrossRef]
- Tibbits, G.; Mohamed, A.; Call, D.R.; Beyenal, H. Rapid Differentiation of Antibiotic-Susceptible and -Resistant Bacteria through Mediated Extracellular Electron Transfer. Biosens. Bioelectron. 2022, 197, 113754. [Google Scholar] [CrossRef]
- Fang, X.; Kalathil, S.; Divitini, G.; Wang, Q.; Reisner, E. A Three-Dimensional Hybrid Electrode with Electroactive Microbes for Efficient Electrogenesis and Chemical Synthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 5074–5080. [Google Scholar] [CrossRef]
- Escosura-Muñiz, A.D.L.; Ivanova, K.; Tzanov, T. Electrical Evaluation of Bacterial Virulence Factors Using Nanopores. ACS Appl. Mater. Interfaces 2019, 11, 13140–13146. [Google Scholar] [CrossRef]
- Yao, L.; Wang, L.; Huang, F.; Cai, G.; Xi, X.; Lin, J. A Microfluidic Impedance Biosensor Based on Immunomagnetic Separation and Urease Catalysis for Continuous-Flow Detection of E. coli O157:H7. Sens. Actuators B Chem. 2018, 259, 1013–1021. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, B.; Cheng, J.; Tang, J.; Song, B.; Wang, H.; He, Y. Bacteria Eat Nanoprobes for Aggregation-Enhanced Imaging and Killing Diverse Microorganisms. Nat. Commun. 2022, 13, 1255. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/Nanoparticle-Augmented Sonodynamic Therapy (SDT): Breaking the Depth Shallow of Photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, L.; Zheng, L.; Zhou, Q.; Liu, K.; Mao, Y.; Song, S. Sonodynamic Therapy-Derived Multimodal Synergistic Cancer Therapy. Cancer Lett. 2021, 497, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Pang, X.; Cheng, Y.; Ming, J.; Xiang, S.; Zhang, C.; Lv, P.; Chu, C.; Chen, X.; Liu, G.; et al. Ultrasound-Switchable Nanozyme Augments Sonodynamic Therapy against Multidrug-Resistant Bacterial Infection. ACS Nano 2020, 14, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Dai, Z. Design and Challenges of Sonodynamic Therapy System for Cancer Theranostics: From Equipment to Sensitizers. Adv. Sci. 2021, 8, 2002178. [Google Scholar] [CrossRef]
- Ouyang, J.; Tang, Z.; Farokhzad, N.; Kong, N.; Kim, N.Y.; Feng, C.; Blake, S.; Xiao, Y.; Liu, C.; Xie, T.; et al. Ultrasound Mediated Therapy: Recent Progress and Challenges in Nanoscience. Nano Today 2020, 35, 100949. [Google Scholar] [CrossRef]
- Mitragotri, S.; Kost, J. Low-Frequency Sonophoresis. Adv. Drug Deliv. Rev. 2004, 56, 589–601. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Weng, C.-X.; Cai, Y. Low-Frequency Ultrasound Enhances Antimicrobial Activity of Colistin–Vancomycin Combination against Pan-Resistant Biofilm of Acinetobacter baumannii. Ultrasound Med. Biol. 2016, 42, 1968–1975. [Google Scholar] [CrossRef]
- Song, M.; Cheng, Y.; Tian, Y.; Chu, C.; Zhang, C.; Lu, Z.; Chen, X.; Pang, X.; Liu, G. Sonoactivated Chemodynamic Therapy: A Robust ROS Generation Nanotheranostic Eradicates Multidrug-Resistant Bacterial Infection. Adv. Funct. Mater. 2020, 30, 2003587. [Google Scholar] [CrossRef]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial Biofilm Modulation by Ultrasound: Current Concepts and Controversies. Ultrason. Sonochem. 2014, 21, 15–22. [Google Scholar] [CrossRef]
- Pang, X.; Liu, X.; Cheng, Y.; Zhang, C.; Ren, E.; Liu, C.; Zhang, Y.; Zhu, J.; Chen, X.; Liu, G. Sono-Immunotherapeutic Nanocapturer to Combat Multidrug-Resistant Bacterial Infections. Adv. Mater. 2019, 31, 1902530. [Google Scholar] [CrossRef]
- Wu, M.-C.; Deokar, A.R.; Liao, J.-H.; Shih, P.-Y.; Ling, Y.-C. Graphene-Based Photothermal Agent for Rapid and Effective Killing of Bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.S.; Rodriguez, J.A.; Galán-Vidal, C.A.; Cepeda, A.; Miranda, J.M. Magnetic Solid Phase Extraction Applied to Food Analysis. J. Chem. 2015, 2015, 919414. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Xu, L.; Zhang, Y.; Chen, Y.; Chen, H.; Pei, R. Selection and Characterization of Thioflavin T Aptamers for the Development of Light-up Probes. Anal. Methods 2016, 8, 8461–8465. [Google Scholar] [CrossRef]
- Ji, H.; Hu, H.; Tang, Q.; Kang, X.; Liu, X.; Zhao, L.; Jing, R.; Wu, M.; Li, G.; Zhou, X.; et al. Precisely Controlled and Deeply Penetrated Micro-Nano Hybrid Multifunctional Motors with Enhanced Antibacterial Activity against Refractory Biofilm Infections. J. Hazard. Mater. 2022, 436, 129210. [Google Scholar] [CrossRef] [PubMed]
- Leulmi Pichot, S.; Joisten, H.; Grant, A.J.; Dieny, B.; Cowburn, R.P. Magneto-Mechanically Actuated Microstructures to Efficiently Prevent Bacterial Biofilm Formation. Sci. Rep. 2020, 10, 15470. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.P.; Maratea, D.; Wolfe, R.S. Isolation and Pure Culture of a Freshwater Magnetic Spirillum in Chemically Defined Medium. J. Bacteriol. 1979, 140, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, C.-F.; Yi, Y.; Chen, L.-J.; Wu, L.-F.; Song, T. Construction of a Microrobot System Using Magnetotactic Bacteria for the Separation of Staphylococcus Aureus. Biomed. Microdevices 2014, 16, 761–770. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Wang, P.; Wu, L.-F.; Song, T. Magnetically-Induced Elimination of Staphylococcus Aureus by Magnetotactic Bacteria under a Swing Magnetic Field. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 363–370. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Yi, Y.; Chen, C.; Wu, L.-F.; Song, T. Killing of Staphylococcus Aureus via Magnetic Hyperthermia Mediated by Magnetotactic Bacteria. Appl. Environ. Microbiol. 2016, 82, 2219–2226. [Google Scholar] [CrossRef]
- Ye, P.; Li, F.; Zou, J.; Luo, Y.; Wang, S.; Lu, G.; Zhang, F.; Chen, C.; Long, J.; Jia, R.; et al. In Situ Generation of Gold Nanoparticles on Bacteria-Derived Magnetosomes for Imaging-Guided Starving/Chemodynamic/Photothermal Synergistic Therapy against Cancer. Adv. Funct. Mater. 2022, 32, 2110063. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Chen, H.; Mo, L.; Guo, Z.; Ye, B.; Liu, Z. 2D-PROTACs with Augmented Protein Degradation for Super-Resolution Photothermal Optical Coherence Tomography Guided Momentary Multimodal Therapy. Chem. Eng. J. 2022, 446, 137039. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-Based Photothermal Therapy and Its Potentials in Antibacterial Treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zheng, H.; Wang, W.; Wu, Q.; Zhang, Q.; Guo, J.; Pu, B.; Shi, X.; Li, J.; Chen, X.; et al. Rapid Eradication of Antibiotic-Resistant Bacteria and Biofilms by MXene and near-Infrared Light through Photothermal Ablation. Sci. China Mater. 2021, 64, 748–758. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Sun, Y.; Zhang, Y.; Ding, X.; Zhao, N.; Yu, B.; Zhao, H.; Duan, S.; Xu, F.-J. Well-Defined Gold Nanorod/Polymer Hybrid Coating with Inherent Antifouling and Photothermal Bactericidal Properties for Treating an Infected Hernia. ACS Nano 2020, 14, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mo, L.; Chen, H.; Chen, C.; Wu, J.; Tang, Z.; Guo, Z.; Hu, C.; Liu, Z. Carbon Dots with Intrinsic Bioactivities for Photothermal Optical Coherence Tomography, Tumor-Specific Therapy and Postoperative Wound Management. Adv. Healthc. Mater. 2022, 11, 2101448. [Google Scholar] [CrossRef] [PubMed]
- Qie, X.; Zan, M.; Gui, P.; Chen, H.; Wang, J.; Lin, K.; Mei, Q.; Ge, M.; Zhang, Z.; Tang, Y.; et al. Design, Synthesis, and Application of Carbon Dots with Synergistic Antibacterial Activity. Front. Bioeng. Biotechnol. 2022, 10, 894100. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Liu, X.; Mo, L.; Chen, C.; Guo, Z.; Liu, Z. Dual-Responsive Ultrathin 1T-Phase Niobium Telluride Nanosheet-Based Delivery Systems for Enhanced Chemo-Photothermal Therapy. J. Mater. Chem. B 2021, 9, 8109–8120. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Guo, L.; Wang, Y.; Feng, L. Intelligent Hybrid Hydrogels for Rapid In Situ Detection and Photothermal Therapy of Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 39685–39694. [Google Scholar] [CrossRef]
- Grote, A.; Earl, A.M. Within-Host Evolution of Bacterial Pathogens during Persistent Infection of Humans. Curr. Opin. Microbiol. 2022, 70, 102197. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Liu, X.; Zhong, H.; Mo, L.; Chen, C.; Guo, Z.; Ye, B. Facile Synthesis of Tannic Acid Modified NbTe2 Nanosheets for Effective Photothermal Ablation of Bacterial Pathogens. Colloid Interface Sci. Commun. 2021, 41, 100383. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, K.; Duan, X.; Wang, P.; Han, Y.; Peng, W.; Huang, J. Extracellular Matrix Stiffness Modulates Host-Bacteria Interactions and Antibiotic Therapy of Bacterial Internalization. Biomaterials 2021, 277, 121098. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, N.; Xu, H.; Yuan, Z.; Xiu, J.; Mao, S.; Liu, X.; Huang, J. Extracellular Matrix Rigidities Regulate the Tricarboxylic Acid Cycle and Antibiotic Resistance of Three-Dimensionally Confined Bacterial Microcolonies. Adv. Sci. 2023, 10, 2206153. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Cai, W.; Feng, H.; Du, T.; Liu, W.; Jiang, H.; Pasquarelli, A.; Weizmann, Y.; Wang, X. In Situ Self-Assembling Au-DNA Complexes for Targeted Cancer Bioimaging and Inhibition. Proc. Natl. Acad. Sci. USA 2020, 117, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Singh, P.; Li, H.-B.; Song, Q.-Q.; Singh, R.K. Microbial Biofilms: Development, Structure, and Their Social Assemblage for Beneficial Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–138. ISBN 978-0-444-64279-0. [Google Scholar]
- Zheng, Y.; Liu, W.; Chen, Y.; Li, C.; Jiang, H.; Wang, X. Conjugating Gold Nanoclusters and Antimicrobial Peptides: From Aggregation-Induced Emission to Antibacterial Synergy. J. Colloid Interface Sci. 2019, 546, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zeng, J.; Liu, W.; Chen, Y.; Jiang, H.; Weizmann, Y.; Wang, X. Formation of Bio-Responsive Nanocomposites for Targeted Bacterial Bioimaging and Disinfection. Chem. Eng. J. 2021, 426, 130726. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, Z.; Wang, Y.; Qin, Z.; Ma, Y.; Jiang, H.; Weizmann, Y.; Wang, X. Intelligent Bio-Assembly Imaging-Guided Platform for Real-Time Bacteria Sterilizing and Infectious Therapy. Nano Res. 2022, 15, 4164–4174. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Q.; Huang, K.; Yin, L.; Jiang, H.; Liu, X.; Wang, X. Ultrafast Cancer Cells Imaging for Liquid Biopsy via Dynamic Self-Assembling Fluorescent Nanoclusters. Biosensors 2023, 13, 602. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; Jiang, H.; Liu, X.; Yin, L.; Wang, X. Advances in Engineered Nano-Biosensors for Bacteria Diagnosis and Multidrug Resistance Inhibition. Biosensors 2024, 14, 59. https://doi.org/10.3390/bios14020059
Xia Q, Jiang H, Liu X, Yin L, Wang X. Advances in Engineered Nano-Biosensors for Bacteria Diagnosis and Multidrug Resistance Inhibition. Biosensors. 2024; 14(2):59. https://doi.org/10.3390/bios14020059
Chicago/Turabian StyleXia, Qingxiu, Hui Jiang, Xiaohui Liu, Lihong Yin, and Xuemei Wang. 2024. "Advances in Engineered Nano-Biosensors for Bacteria Diagnosis and Multidrug Resistance Inhibition" Biosensors 14, no. 2: 59. https://doi.org/10.3390/bios14020059
APA StyleXia, Q., Jiang, H., Liu, X., Yin, L., & Wang, X. (2024). Advances in Engineered Nano-Biosensors for Bacteria Diagnosis and Multidrug Resistance Inhibition. Biosensors, 14(2), 59. https://doi.org/10.3390/bios14020059






