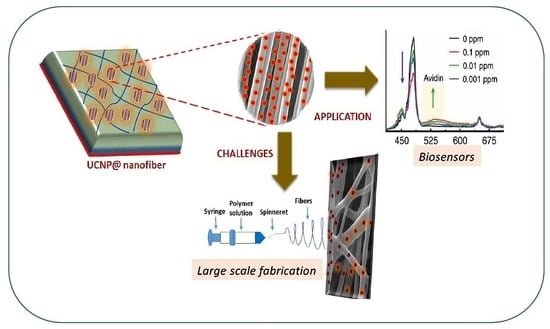
Miniaturized Biosensors Based on Lanthanide-Doped Upconversion Polymeric Nanofibers
Abstract
1. Introduction
1.1. Impact of Morphology of UCNP@nanofibers on Luminescence Efficiency
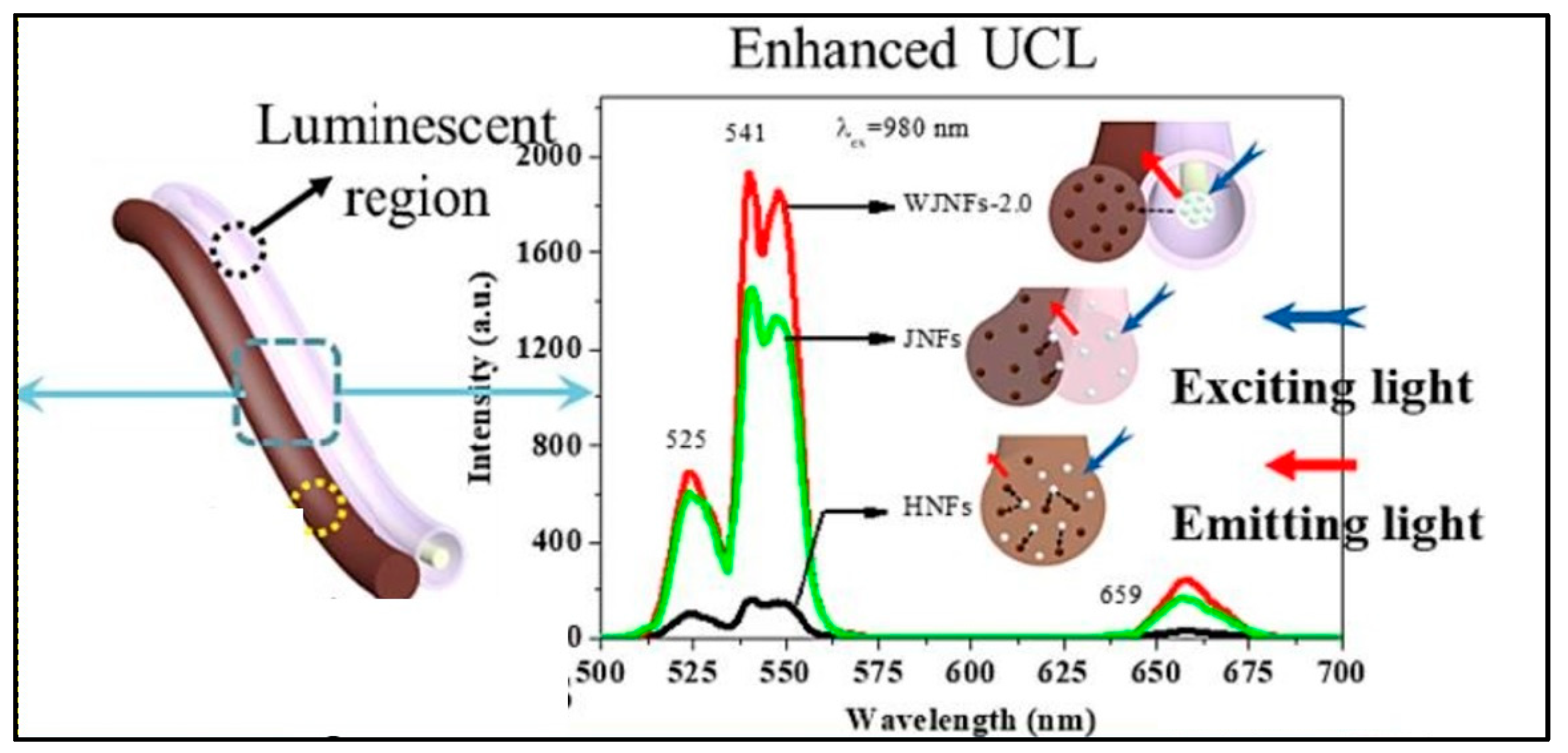
1.2. Impact of Design of UCNP@nanofibers on Luminescence Efficiency
2. Trends in Embedding UCNP’s in Nanofiber
2.1. Recent Techniques Used for Embedding UCNPs into Nanofiber
2.2. Advantages of Entrapment of Upconversion Nanoparticles inside Nanofibers
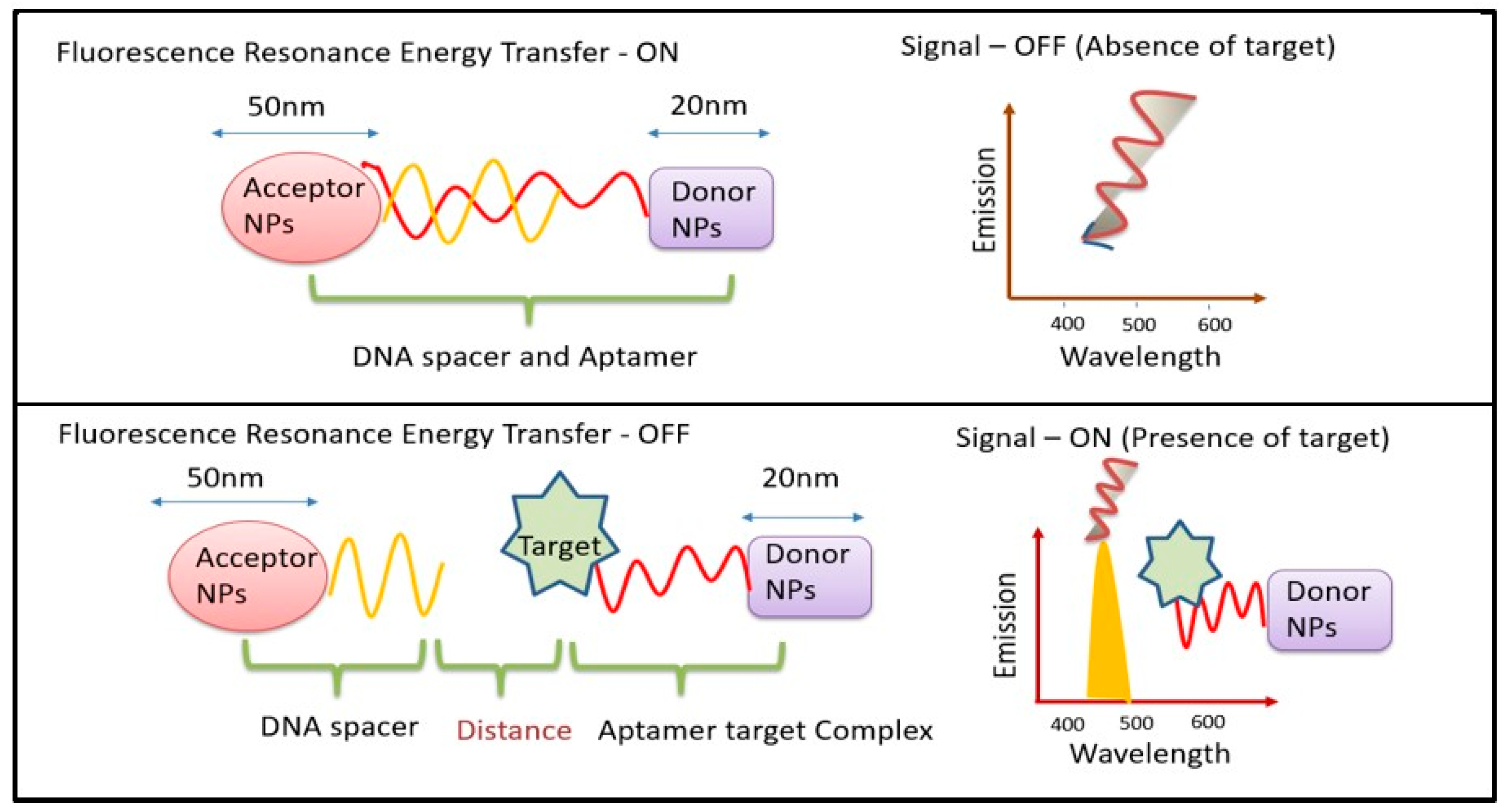
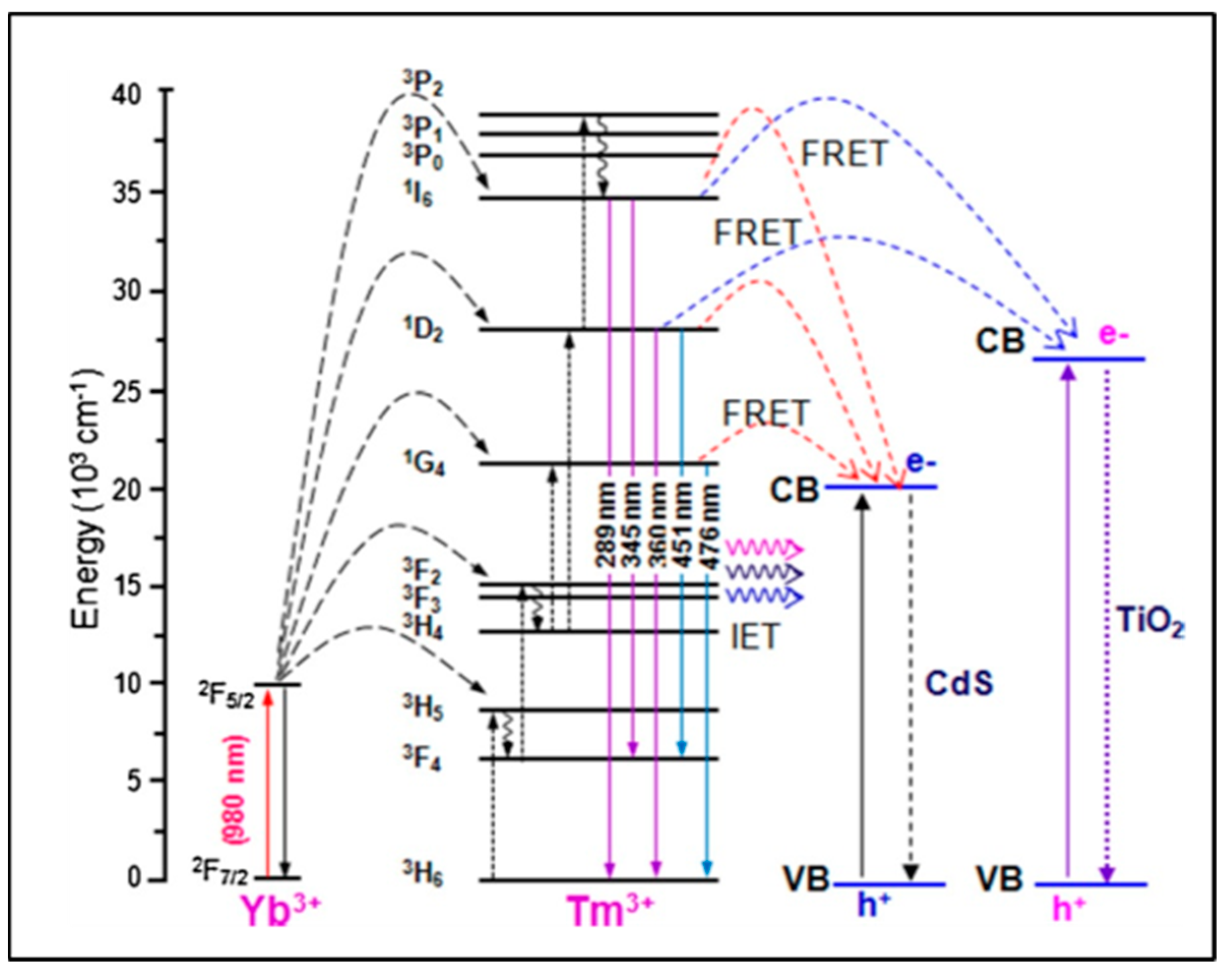
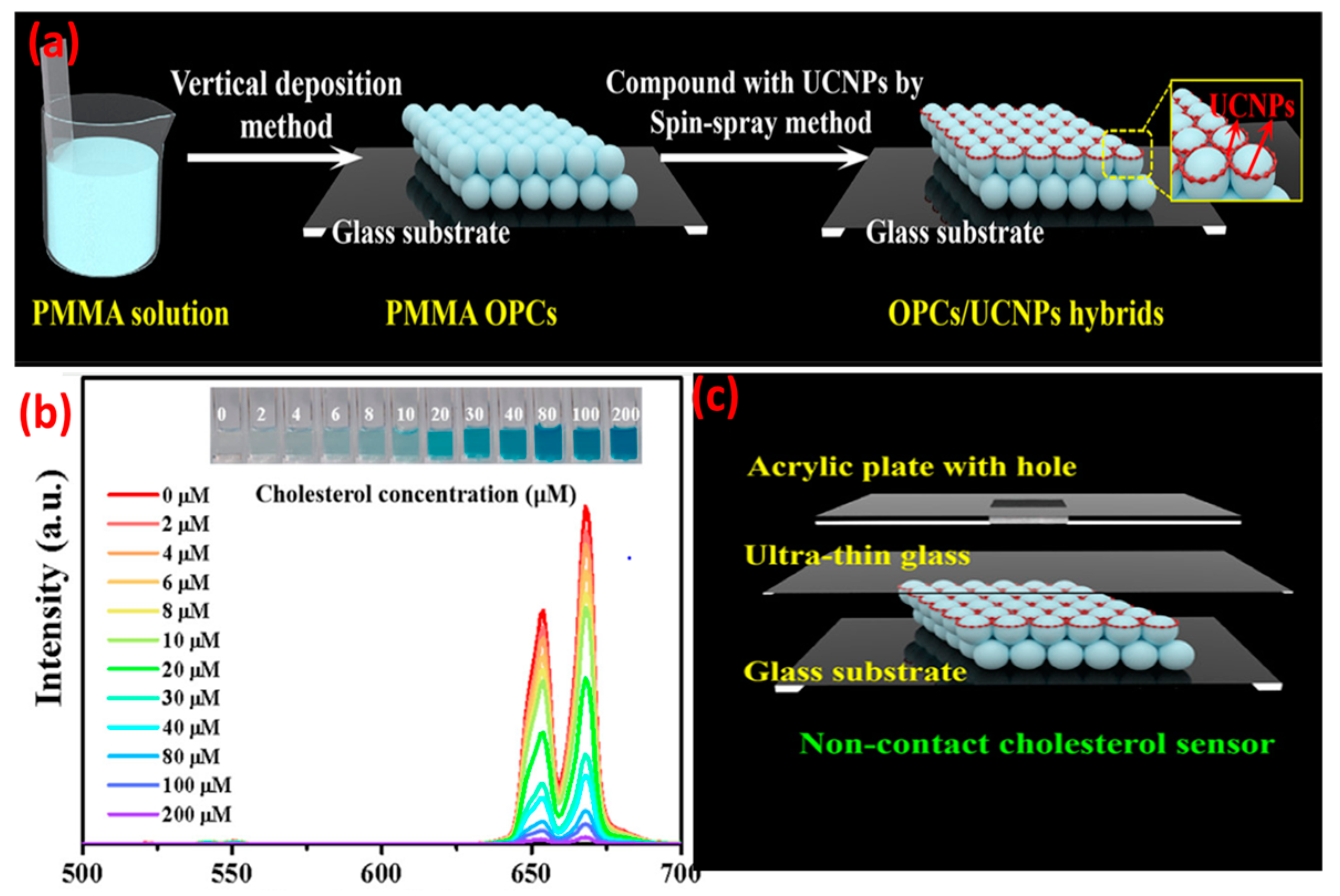
2.3. UCNP@nanofibers as Sensing Platform for Bio(analytical) Assays
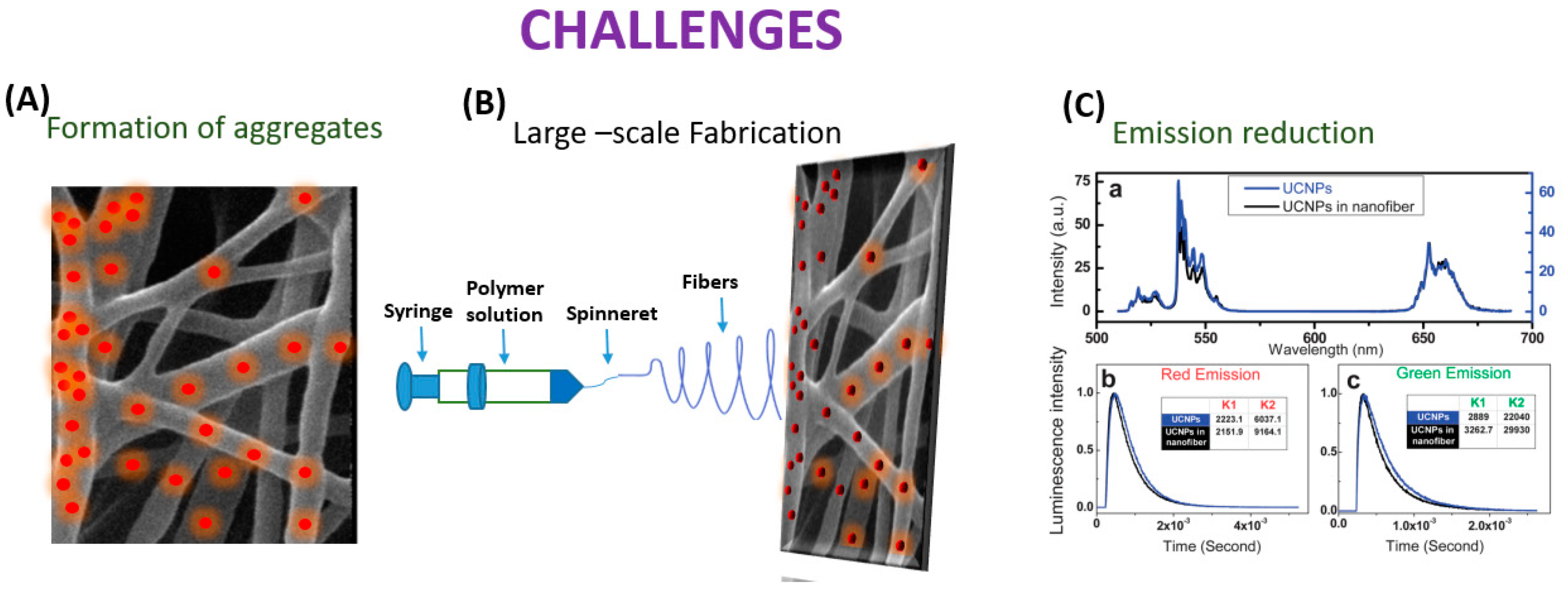
3. Challenges in Embedding UCNP’s in Nanofiber
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khandurina, J.; Guttman, A. Bioanalysis in microfluidic devices. J. Chromatogr. 2002, 2, 159–183. [Google Scholar] [CrossRef]
- Li, X.; Soler, M.; Belushkin, A.; Yesilkoÿ, F.; Altug, H. Optofluidic nanoplasmonic biosensor for label-free live cell analysis in real time. In Plasmonics in Biology and Medicine XV; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; p. 105090. [Google Scholar]
- Rezaei, Z.; Mahmoudifard, M. Pivotal role of electrospun nanofibers in microfluidic diagnostic systems—A review. J. Mater. Chem. B 2019, 30, 4602–4619. [Google Scholar] [CrossRef]
- Yang, D.; Ma, P.; Hou, Z.; Cheng, Z.; Li, C.; Lin, J. Current advances in lanthanide ion (Ln3+)-based upconversion nano-materials for drug delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef]
- Qin, H.; Wu, D.; Sathian, J.; Xie, X.; Ryan, M.; Xie, F. Tuning the upconversion photoluminescence lifetimes of NaYF4:Yb3+, Er3+ through lanthanide Gd3+ doping. Sci. Rep. 2018, 8, 12683. [Google Scholar] [CrossRef]
- Yin, W.; Tian, G.; Ren, W.; Yan, L.; Jin, S.; Gu, Z.; Zhou, L.; Lia, J.; Zhao, Y. Design of multifunctional alkali ion doped CaF2 upconversion nanoparticles for simultaneous bioimaging and therapy. Dalton Trans. 2014, 43, 3861–3870. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Huang, L.; Yang, Y.; Zhao, Y.; El-Banna, G.; Han, G. Nanoscale “fluorescent stone”: Luminescent calcium fluoride nanoparticles as theranostic platforms. Theranostics 2016, 13, 2380–2393. [Google Scholar] [CrossRef] [PubMed]
- Garfield, D.J.; Borys, N.J.; Hamed, S.M.; Torquato, N.A.; Tajon, C.A.; Tian, B.; Shevitski, B.; Barnard, E.S.; Suh, Y.D.; Aloni, S.; et al. Enrichment of molecular antenna triplets amplifies upconverting nanoparticle emission. Nat. Photonics 2018, 12, 402–407. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 1, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. Advances in the theoretical understanding of photon upconversion in rare-earth activated nanophosphors. Chem. Soc. Rev. 2015, 6, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Luu, Q.A.N.; Zhao, Y.; Fong, A.; May, P.S.; Jiang, C. Upconversion polymeric nanofibers containing lantha-nide-doped nanoparticles via electrospinning. Nanoscale 2012, 4, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organo- phosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef]
- Singh, R.; Dumlupinar, G.; Andersson-Engels, S.; Melgar, S. Emerging applications of upconverting nanoparticles in in-testinal infection and colorectal cancer. Int. J. Nanomed. 2019, 14, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, F. Emerging frontiers of upconversion nanoparticles. Trends Chem. 2020, 5, 427–439. [Google Scholar] [CrossRef]
- Schroter, A.; Hirsch, T. Control of Luminescence and Interfacial Properties as Perspective for Upconversion Nanoparticles. Small 2023, 2306042. [Google Scholar] [CrossRef]
- Dubey, N.; Chandra, S. Upconversion nanoparticles: Recent strategies and mechanism based applications. J. Rare Earths 2022, 9, 1343–1359. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fibre fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Watkins, Z.; Taylor, J.; D’Souza, S.; Britton, J.; Nyokong, T.J. Fluorescence behaviour and singlet oxygen production of aluminium phthalocyanine in the presence of upconversion nanoparticles. J. Fluoresc. 2015, 25, 1417–1429. [Google Scholar] [CrossRef]
- Simsek, M.; Hoecherl, K.; Schlosser, M.; Baeumner, A.J.; Wongkaew, N. Printable 3D carbon nanofiber networks with embedded metal nanocatalysts. ACS App. Mater. Interfaces 2020, 12, 39533–39540. [Google Scholar] [CrossRef]
- Presley, K.; Hwang, J.; Cheong, S.; Tilley, R.; Collins, J.; Viapiano, M.; Lannutti, J. Nanoscale upconversion for oxygen sensing. Mater. Sci. Eng. C 2017, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, T.; Wang, G.; Gu, T.; Xie, C.; Huang, J.; Li, X.; Best, S.; Han, G. Production of a fluorescence resonance energy transfer(FRET) biosensor membrane for microRNA detection. J. Mater. Chem. B 2017, 65, 87. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qin, G.; Zhao, D.; Zheng, K.; Qin, W. Synthesis and upconversion properties of Ln3+ doped YOF nanofibers. J. Fluo. Chem. 2012, 140, 38–42. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.-L.; Peng, H.-Y.; Cong, H.-P.; Qian, H.-S. Near-infrared photocatalytic upconversion nanoparticles/TiO2 nanofibers assembled in large scale by electrospinning. Part. Part. Syst. Charact. 2016, 33, 248–253. [Google Scholar] [CrossRef]
- Santos, M.C.D.; Algar, W.R.; Medintz, I.L.; Hildebrandt, N. Quantum dots for Förster Resonance Energy Transfer (FRET). TrAC Trends Anal. Chem. 2020, 125, 115819. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.-L.; Wang, W.-N.; Cong, H.-P.; Qian, H.-S. Titanium dioxide/upconversion nanoparticles/cadmium sulfide nanofibers enable enhanced full-spectrum absorption for superior solar light driven photocatalysis. ChemSusChem 2016, 9, 1449–1454. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Habault, D.; Branda, N.R.; Zhao, Y. Near infrared light triggered release of biomacromolecules from hydrogels loaded with upconversion nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. [Google Scholar] [CrossRef]
- Arppe, R.; Hyppänen, I.; Perälä, N.; Peltomaa, R.; Kaiser, M.; Würth, C.; Christ, C.; Resch-Genger, U.; Schäferlinga, M.; Soukkaa, T. Quenching of the upconversion luminescence of NaYF4:Yb3+,Er3+ and NaYF4:Yb3+,Tm3+ nanophosphors by water: The role of the sensitizer Yb3+ in non-radiative relaxation. Nanoscale 2015, 7, 11746–11757. [Google Scholar] [CrossRef]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of ligand-free colloidally stable water dispersible brightly luminescent lanthanide-doped upconverting nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef]
- Dai, M.; Jin, S.; Nugen, S.R. Water-soluble electrospun nanofibers as a method for on-chip reagent storage. Biosensors 2012, 2, 388–395. [Google Scholar] [CrossRef]
- Ostrowski, A.D.; Chan, E.M.; Gargas, D.J.; Katz, E.M.; Han, G.; Schuck, P.J.; Milliron, D.J.; Cohen, B.E. Controlled Synthesis and Single-Particle Imaging of Bright, Sub-10 nm Lanthanide-Doped Upconverting Nanocrystals. ACS Nano 2012, 3, 2686–2692. [Google Scholar] [CrossRef]
- Chan, E.M.; Xu, C.X.; Mao, A.W.; Han, G.; Owen, J.S.; Cohen, B.E.; Milliron, D.J. Reproducible, High-Throughput Synthesis of Colloidal Nanocrystals for Optimization in Multidimensional Parameter Space. Nano Lett. 2010, 10, 1874–1885. [Google Scholar] [CrossRef]
- Dong, G.; Xiao, X.; Chi, Y.; Qian, B.; Liu, X.; Ma, Z.; Wu, E.; Zeng, H.; Chen, D.; Qiu, J. Size-dependent polarized pho-toluminescence from Y 3 Al 5 O 12: Eu3+ single crystalline nanofiber prepared by electrospinning. J. Mater. Chem. 2010, 8, 1587–1593. [Google Scholar] [CrossRef]
- Zhang, W.; Hongrui, J.; Ye, H.; Dai, T.; Yin, X.; He, J.; Chen, R.; Wang, Y.; Pang, X. Facile fabrication of transparent and upconversion photoluminescent nanofiber mats with tunable optical properties. ACS Omega. 2018, 3, 8220–8225. [Google Scholar] [CrossRef]
- Dimitriev, O.P. Effect of confinement on photophysical properties of P3HT chains in PMMA matrix. Nanoscale Res. Lett. 2017, 12, 510. [Google Scholar] [CrossRef]
- Zou, P.; Hong, X.; Ding, Y.; Zhang, Z.; Chu, X.; Shaymurat, T.; Shao, C.; Liu, Y. Up-Conversion luminescence of NaYF4:Yb3+/Er3+ nanoparticles embedded into PVP nanotubes with controllable diameters. J. Phys. Chem. C 2012, 116, 5787–5791. [Google Scholar] [CrossRef]
- Lahtinen, S.; Liisberg, M.K.; Raiko, K.; Krause, S.; Soukka, T.; Vosch, T. Thulium- and erbium-doped nanoparticles with poly(acrylic acid) coating for upconversion cross-correlation spectroscopy-based sandwich immunoassays in plasma. ACS Appl. Nano Mater. 2021, 4, 432–440. [Google Scholar] [CrossRef]
- Oakland, C.; Andrews, M.; Burgess, L.; Jones, A.; Hay, S.; Harvey, P.; Natrajan, L. Expanding the scope of biomolecule monitoring with ratiometric signaling from rare-earth upconverting phosphors. Eur. J. Inorg. Chem. 2017, 44, 5176–5185. [Google Scholar] [CrossRef]
- Harvey, P.; Oakland, C.; Driscoll, M.D.; Hay, S.; Natrajan, L.S. Ratiometric detection of enzyme turnover and flavin reduction using rare-earth upconverting phosphors. Dalton Trans. 2014, 43, 5265–5268. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, L.; Wegner, K.D.; Hildebrandt, N.; Soukka, T. Upconverting nanoparticle to quantum dot FRET for homoge-neous double-nano biosensors. RSC Adv. 2015, 5, 13270–13277. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, S.; Lu, Y.; Jiang, B.; Liu, X.; Li, X.; Zhao, X.; Yan, X.; Wang, C.; Jia, X.; et al. Photonic crystal effects on upconversion enhancement of LiErF4:0.5%Tm3+@LiYF4 for noncontact cholesterol detection. ACS Appl. Mater. Interfaces 2022, 14, 428–438. [Google Scholar] [CrossRef]
- Elnabawy, E.; Sun, D.; Shearer, N.; Shyha, I. Electro-Blown Spinning: New Insight into the Effect of Electric Field and Airflow Hybridized Forces on the Production Yield and Characteristics of Nanofiber Membranes. J. Sci. Adv. Mater. Dev. 2023, 8, 100552. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Fang, Y.; Zheng, F.; Li, J.; He, J. Fabrication of highly oriented nanoporous fibers via airflow bub-ble-spinning. Appl. Surf. Sci. 2017, 421, 61–67. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Shen, X.; Li, H.; Takai, O.; Saito, N. Plasma-induced synthesis of CuO nanofibers and ZnO nanoflowers in water. Plasma Chem. Plasma Process. 2014, 34, 1129–1139. [Google Scholar] [CrossRef]
- Suzuki, A.; Mikuni, T.; Hasegawa, T. Nylon 66 nanofibers prepared by CO2 laser supersonic drawing. J. Appl. Polym. Sci. 2014, 6, 40015. [Google Scholar] [CrossRef]
- Wu, C.; Su, B.; Xin, N.; Tang, J.; Xiao, J.; Luo, H.; Wei, D.; Luo, F.; Sun, J.; Fan, H. An upconversion nanoparticle-integrated fibrillar scaffold combined with a NIR-optogenetic strategy to regulate neural cell performance. J. Mater. Chem. B 2023, 11, 430–440. [Google Scholar] [CrossRef]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M.A. Comprehensive review of the covalent immobi-lization of biomolecules onto electrospun nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Shen, W.; Ao, F. Application of blocking and immobilization of electrospun fiber in the biomedical field. RSC Adv. 2020, 10, 37246–37265. [Google Scholar] [CrossRef]
- Wohnhaas, C.; Friedemann, K.; Busko, D.; Landfester, K.; Baluschev, S.; Crespy, D.; Turshatov, A. All organic nanofibers as ultralight versatile support for triplet− triplet annihilation upconversion. ACS Macro. Lett. 2013, 2, 446–450. [Google Scholar] [CrossRef]
- Buchner, M.; Ngoensawat, U.; Schenck, M.; Fenzl, C.; Wongkaew, N.; Matlock-Colangelo, L.; Hirsch, T.; Duerkop, A.; Baeummer, A.J. Embedded nanolamps in electrospun nanofibers enabling online monitoring and ratiometric measurements. J. Mater. Chem. C 2017, 5, 9712–9720. [Google Scholar] [CrossRef]
- Haghju, S.; Bari, M.R.; Khaled-Abad, M.A. Affecting parameters on fabrication of β-D-galactosidase immobilized chi-tosan/poly (vinyl alcohol) electrospun nanofibers. Carbohydr. Polym. 2018, 200, 137–143. [Google Scholar] [CrossRef]
- Gupta, S.K.; Hernandez, C.; Zuniga, J.P.; Lozano, K.; Mao, Y. Luminescent PVDF nanocomposite films and fibers encapsulated with La2Hf2O7:Eu3+ nanoparticles. SN Appl. Sci. 2020, 2, 616. [Google Scholar] [CrossRef]
- Hou, Z.; Li, C.; Ma, P.; Li, G.; Cheng, Z.; Peng, C.; Yang, D.; Yang, P.; Lin, J. Electrospinning Preparation and Drug-Delivery Properties of an Up-conversion Luminescent Porous NaYF4:Yb3+, Er3+@Silica Fiber Nanocomposite. Adv. Funct. Mater. 2011, 12, 2356–2365. [Google Scholar] [CrossRef]
- Song, H.; Yu, L.; Lu, S.; Liu, Z.; Yang, L.; Wang, T. Improved photoluminescent properties in one-dimensional LaPO4: Eu3+ nanowires. Optics. Lett. 2005, 5, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Heer, S.; Kömpe, K.; Güdel, H.U.; Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Hou, Z.; Li, X.; Li, C.; Dai, Y.; Ma, P.; Zhang, X.; Kang, X.; Cheng, Z.; Lin, J. Electrospun upconversion composite fibers as dual drugs delivery system with individual release properties. Langmuir 2013, 29, 9473–9482. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Idris, N.M.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, L.-N.; Wang, Y.; Cheng, S.; Wang, W.-N.; Zhang, C.-L.; Xu, F.; Qian, H.-S. Hydrothermal-assisted crystallization for the synthesis of upconversion nanoparticles/CdS/TiO2 composite nanofibers by electrospinning. CrystEngComm 2016, 18, 6013–6018. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, P.; Chen, B.; Sun, J.; Cheng, L.; Li, X.; Zhang, J.; Xu, S. Electrospinning preparation and upconversion lumi-nescence of Y2Ti2O7:Tm/Yb nanofibers. Appl. Phys. A Mater. Sci. Process. 2020, 126, 690. [Google Scholar] [CrossRef]
- González-Béjar, M.; Pérez-Prieto, J. Upconversion luminescent nanoparticles in physical sensing and in monitoring physical processes in biological samples. Methods Appl. Fluoresc. 2015, 3, 042002. [Google Scholar] [CrossRef]
- Wiesholler, L.M.; Genslein, C.; Schroter, A.; Hirsch, T. Plasmonic enhancement of NIR to UV upconversion by a nanoen-gineered interface consisting of NaYF4:Yb, Tm nanoparticles and a gold nanotriangle array for optical detection of vitamin B12 in serum. Analy. Chem. 2018, 90, 14247–14254. [Google Scholar] [CrossRef]
- Peng, J.; Xu, W.; Teoh, C.L.; Han, S.; Kim, B.; Samanta, A.; Jun, C.; Wang, L.; Yuan, L.; Liu, X.; et al. High-efficiency in vitro and in vivo detection of Zn2+ by dye-assembled upconversion nanoparticles. J. Am. Chem. Soc. 2015, 137, 2336–2342. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, Q. Recent advances on functionalized upconversion nanoparticles for detection of small molecules and ions in biosystems. Adv. Sci. 2018, 5, 1700609. [Google Scholar] [CrossRef]
- Fu, J.; Qiao, H.; Li, D.; Luo, L.; Chen, K.; Wei, Q. Laccase biosensor based on electrospun copper/carbon composite nanofibers for catechol detection. Sensors 2014, 14, 3543–3556. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, M.A.; Agrawal, V.V.; Malhotra, B.D.; Sharma, A. Highly sensitive biofunctionalized mesoporous electrospun TiO2 nanofiber based interface for biosensing. ACS App. Mater. Inter. 2014, 6, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawrońska, E.; Biadasz, A.; Jesionowski, T. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Y.; Yang, X.; Li, Y.; Wang, C. Electrospun nanofibrous membranes containing epoxy groups and hydrophilic polyethylene oxide chain for highly active and stable covalent immobilization of lipase. J. Chem. Eng. 2018, 336, 456–464. [Google Scholar] [CrossRef]
- Uzun, S.D.; Kayaci, F.; Uyar, T.; Timur, S.; Toppare, L. Bioactive surface design based on functional composite electrospun nanofibers for biomolecule immobilization and biosensor applications. ACS Appl. Mater. Inter. 2014, 6, 5235–5243. [Google Scholar] [CrossRef]
- Fazel, R.; Torab, S.-F.; Naseri-Nosar, P.; Ghasempur, S.; Ranaei-Siadat, S.-O.; Khajeh, K. Electrospun polyvinyl alcohol/bovine serum albumin biocomposite membranes for horseradish peroxidase immobilization. Enzym. Microb. Technol. 2016, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Niu, J.; Liu, J.; Yin, L.; Xu, J. In situ encapsulation of laccase in microfibers by emulsion electrospinning: Preparation, characterization, and application. Bioresour. Technol. 2010, 101, 8942–8947. [Google Scholar] [CrossRef]
- Li, C.; Zuo, J.; Zhang, L.; Chang, Y.; Zhang, Y.; Tu, L.; Liu, X.; Xue, B.; Li, Q.; Zhao, H.; et al. Accurate quan-titative sensing of intracellular pH based on self-ratiometric upconversion luminescent nanoprobe. Sci. Rep. 2016, 6, 38617. [Google Scholar] [CrossRef]
- Wilhelm, S.; Barrio, M.; Heiland, J.; Himmelstoß, S.F.; Galban, J.; Wolfbeis, O.S.; Hirsch, T. Spectrally matched upconverting luminescent nanoparticles for monitoring enzymatic reactions. ACS Appl. Mater. Inter. 2014, 6, 15427–15433. [Google Scholar] [CrossRef]
- Long, Q.; Fang, A.; Wen, Y.; Li, H.; Zhang, Y.; Yao, S. Rapid and highly-sensitive uric acid sensing based on enzymatic ca-talysis-induced upconversion inner filter effect. Biosens. Bioelectron. 2016, 86, 109–114. [Google Scholar] [CrossRef]
- Ni, J.; Shan, C.; Li, B.; Zhang, L.; Ma, H.; Luo, Y.; Song, H. Assembling of functional cyclodextrin-decorated upconversion luminescence nanoplatform for cysteine-sensing. Chem. Commun. 2015, 51, 14054–14056. [Google Scholar] [CrossRef]
- Sheng, Y.; Qi, H.; Li, N.; Xie, Y.; Shao, H.; Hu, Y.; Li, D.; Ma, Q.; Liu, G.; Dong, X. Wire-in-tube nanofiber as one side to construct specific-shaped Janus nanofiber with improved upconversion luminescence and tunable magnetism. J. Colloid. Interface Sci. 2024, 655, 58–69. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Yuan, J.; Zhang, Z.; Zhang, X.; Dong, S.; Hao, J. Formation and degradation tracking of a composite hydrogel based on UCNPs@PDA. Macromolecules 2020, 53, 2430–2440. [Google Scholar] [CrossRef]
- Yao, J.; Ji, P.; Wang, B.; Wang, H.; Chen, S. Color-tunable luminescent macrofibers based on CdTe QDs-loaded bacterial cellulose nanofibers for pH and glucose sensing. Sens. Actuators B Chem. 2018, 254, 110–119. [Google Scholar] [CrossRef]
- Antoniadou, M.; Pilch-Wrobel, A.; Riziotis, C.; Tanasă, E.; Krasia-Christoforou, T. Fluorescent electrospun PMMA micro-fiber mats with embedded NaYF4:Yb/Er upconverting nanoparticles. Methods Appl. Fluoresc. 2019, 7, 034002. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-C.; Zhang, Z.-Y.; Shan, Z.-Q.; Feng, Z.-Q.; Li, J.-S.; Song, C.-L.; Bao, Y.-N.; Qi, X.-H.; Bong, B. A flexible and superhy-drophobic upconversion-luminescence membrane as an ultrasensitive fluorescence sensor for single droplet detection. Light. Sci. Appl. 2016, 5, 16136. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Song, H.; Yu, H.; Zhang, H.; Qin, R.; Bai, X.; Pan, G.; Lu, S.; Wang, F.; Fan, L.; et al. Upconversion properties of Ln3+ doped NaYF4/polymer composite fibers prepared by electrospinning. J. Phys. Chem. C 2008, 112, 1435–1440. [Google Scholar] [CrossRef]
- Ge, W.; Xu, M.; Shi, J.; Zhu, J.; Li, Y. Highly temperature-sensitive and blue upconversion luminescence properties of Bi2Ti2O7: Tm3+/Yb3+ nanofibers by electrospinning. J. Chem. Eng. 2020, 391, 123546. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Kong, X.; Pun, E.Y.B.; Lin, H. Excitation Mechanism Rearrangement in Yb3+-Introduced La2O2S: Er3+/Polyacrylonitrile Photon-Upconverted Nanofibers for Optical Temperature Sensors. ACS Appl. Nano Mater. 2023, 14, 13570–13581. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.X.; Sun, K.W.; Ling, X.C.; Sun, J.W.; Chen, D.H. Upconversion red light emission and luminescence thermometry of Gd2O3:Er3+@Gd2O3:Yb 3+ core-shell nanofibers synthesized via electrospinning. Chalcogenide Lett. 2023, 7, 1841–4834. [Google Scholar]
- Deng, Z.; Wu, H.; Mu, H.; Jiang, L.; Xi, W.; Xu, X.; Zheng, W. Preparation and properties of electrospun NaYF4: Yb3+, Er3+-PLGA-gelatin nanofibers. J. Appl. Polym. Sci. 2022, 26, 52422. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, J.C.; Yan, J.S.; Zhu, H.; Liu, J.J.; Li, R.; Ramakrishna, S.; Long, Y.Z. Ultrasensitive and reusable upconversion-luminescence nanofibrous indicator paper for in-situ dual detection of single drop-let. J. Chem. Eng. 2020, 382, 122779. [Google Scholar] [CrossRef]
- Han, W.; Wang, Y.; Su, J.; Xin, X.; Guo, Y.; Long, Y.Z.; Ramakrishna, S. Fabrication of nanofibrous sensors by electrospinning. Sci. China Technol. Sci. 2019, 62, 886–894. [Google Scholar] [CrossRef]
- Toncelli, A. RE-based inorganic-crystal nanofibers produced by electrospinning for photonic applications. Materials 2021, 10, 2679. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Mananquil, T.; Scenna, R.; Dennis, E.S.; Castillo-Rodriguez, J.; Koivisto, B.D. Light-Driven Energy and Charge Transfer Processes between Additives within Electrospun Nanofibres. Molecules 2023, 12, 4857. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-based nanofiber–nanoparticle hybrids and their medical applications. Polymers 2022, 2, 351. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, T.; Golmohammadi, H.; Yousefi, H.; Hosseinifard, M.; Kostiv, U.; Horak, D.; Merkoci, A. Chitin nanofiber paper toward optical (bio) sensing applications. ACS Appl. Mater. Int. 2020, 13, 15538–15552. [Google Scholar] [CrossRef]
- Kumar, B.; Malhotra, K.; Fuku, R.; Houten, J.V.; Qu, G.Y.; Piunno, P.A.E.; Krull, U.J. Recent trends in the developments of analytical probes based on lanthanide-doped upconversion nanoparticles. TrAC Trends Anal. Chem. 2021, 139, 116256. [Google Scholar] [CrossRef]
- Tsai, E.S.; Himmelstoß, S.F.; Wiesholler, L.M.; Hirsch, T.; Hall, E.A. Upconversion nanoparticles for sensing pH. Analyst 2019, 18, 5547–5557. [Google Scholar] [CrossRef]
- Shen, J.; Chen, G.; Ohulchanskyy, T.Y.; Kesseli, S.J.; Buchholz, S.; Li, Z.; Prasad, P.N.; Han, G. Tunable near infrared to ul-traviolet upconversion luminescence enhancement in (α-NaYF4:Yb, Tm)/CaF2 core/shell nanoparticles for in situ real-time recorded biocompatible photoactivation. Small 2013, 9, 3213–3217. [Google Scholar] [CrossRef] [PubMed]


| Sr. No | Polymer | UCNPs | Applications | References |
|---|---|---|---|---|
| 1 | Polysulfone | LiYF4:Yb,Tm | Oxygen sensor | [20] |
| 2 | Biomolecules | Phosphor doped with Yb and Tm | Detection of presence of flavoprotein | [37] |
| 3 | PMMA | LiErF4:0.5%Tm@LiYF4 | Detection of oxidase and cholestrol | [40] |
| 4 | PVP | NaYF4:Yb/Er and 10%Gd@NaYF4 | Sensing the photoreaction | [49] |
| 5 | CdS/PVP/TBT | Β NaYF4:Yb/Tm@NaYF4 | Degradation of rhodium B dyes | [57] |
| 6 | PAA | NaYF4:Yb/Tm@NaYF4 | Monitoring of Zn | [61] |
| 7 | PDA | NaGdF4:Yb/Er@NaGdF4@PDA | Hydrogel degradation | [75] |
| 8 | PS | NaYF4:Yb/Tm and NaYF4:Yb/Er | Fluorescence sensor for detecting avidin | [78] |
| 9 | PAN | Gd2O3:Er3+@Gd2O3:Yb3+ | Temperature sensing | [81] |
| 10 | PLGA | NaYF4:Yb3+, Er3 | In vivo bioimaging | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, N.; Chandra, S. Miniaturized Biosensors Based on Lanthanide-Doped Upconversion Polymeric Nanofibers. Biosensors 2024, 14, 116. https://doi.org/10.3390/bios14030116
Dubey N, Chandra S. Miniaturized Biosensors Based on Lanthanide-Doped Upconversion Polymeric Nanofibers. Biosensors. 2024; 14(3):116. https://doi.org/10.3390/bios14030116
Chicago/Turabian StyleDubey, Neha, and Sudeshna Chandra. 2024. "Miniaturized Biosensors Based on Lanthanide-Doped Upconversion Polymeric Nanofibers" Biosensors 14, no. 3: 116. https://doi.org/10.3390/bios14030116
APA StyleDubey, N., & Chandra, S. (2024). Miniaturized Biosensors Based on Lanthanide-Doped Upconversion Polymeric Nanofibers. Biosensors, 14(3), 116. https://doi.org/10.3390/bios14030116