Biomedical Applications of CNT-Based Fibers
Abstract
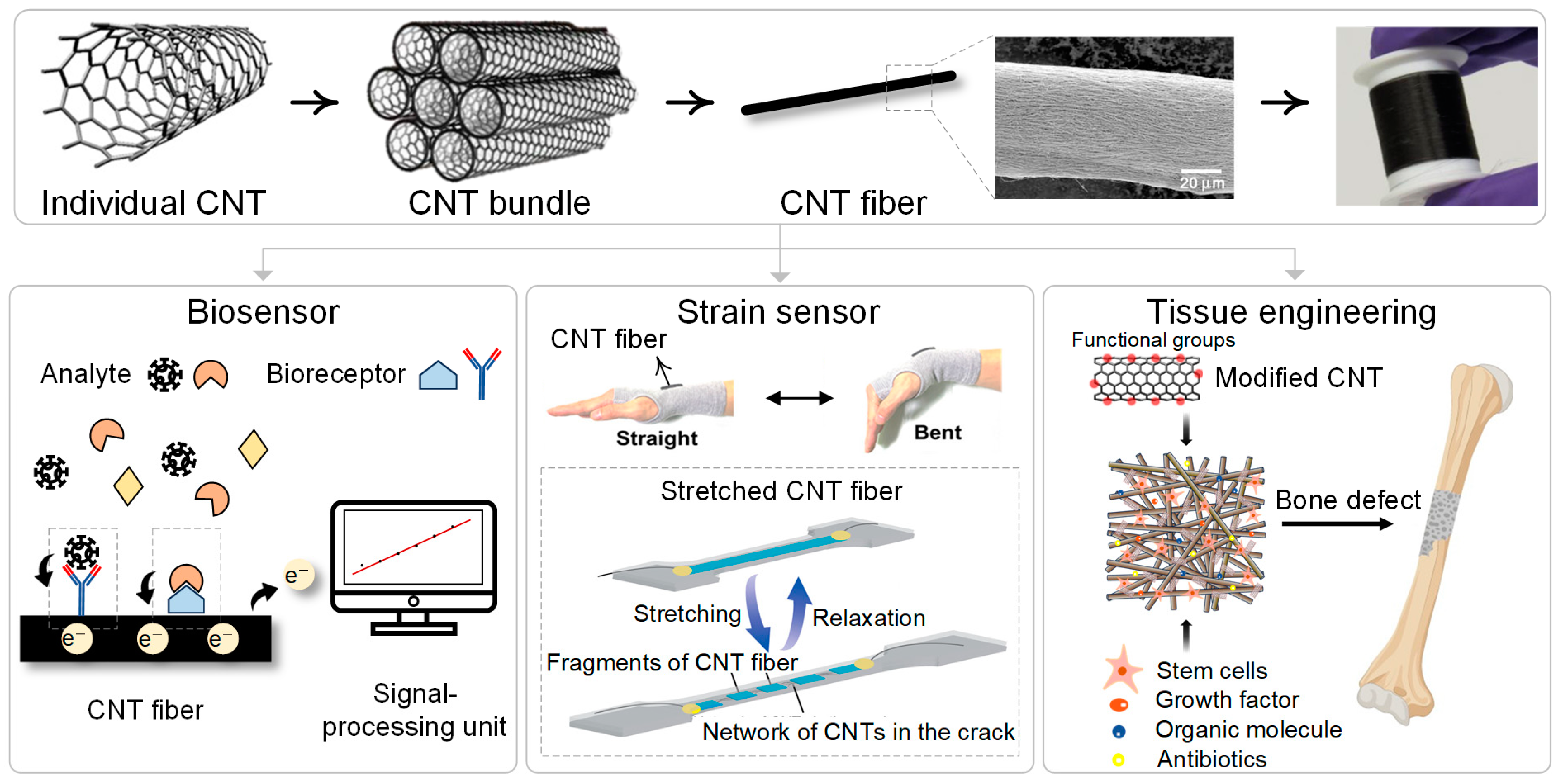
:1. Introduction
2. Carbon Nanotube Fibers
3. CNT Fibers-Based Biosensors
3.1. Basic Principles of Biosensors
3.2. CNT Fiber-Based Biosensors
3.3. CNTF-Based Biosensors for Detecting Various Analytes
3.3.1. Glucose
3.3.2. Dopamine
3.3.3. Ascorbic Acid
3.3.4. Oxygen and pH
3.3.5. Malaria Biomarker (PfHRP2)
3.3.6. Catechol
4. Flexible Strain Sensors
4.1. CNTs/Polymer Composite Fiber Strain Sensors
4.2. CNTF-Based Strain Sensors

| Year | Structural | Stretchability [%] | Gauge Factor | Linear Region | Durability | Ref |
|---|---|---|---|---|---|---|
| 2013 | Twisted CNTF | 25 | 0.1 (at 25% strain) | 1000 (at 25% strain) | [179] | |
| 2013 | Overtwisted CNTF | 800 | 0.1 (at 500% strain) | 400 (at 500% strain) | [180] | |
| 2015 | CNTF embedded in Ecoflex | 900 | 0.5 (under 440% strain) | 10,000 (at 300% strain) | [178] | |
| 54 (within 440–900% strain) | ||||||
| [0.56 (under 200% strain), | ||||||
| 47 (within 200–440% strain)] * | ||||||
| 2016 | CNTF embedded in PDMS | 15 | 100,000 (at 15% strain) | 5000 (at 12% strain) | [175] | |
| 2018 | PVA coating on CNTF | 14 | 2.3 (at 12% strain) | 20 (at 5% strain) | [182] | |
| 2018 | CNTF wrapped by TPE | 250 | 425 (within 20–100% strain) | 20–100% | 3250 (within 20–100% strain) | [178] |
| 2018 | CNTF wrapped by silicone | 330 | 18,181 (at 330% strain) | 10,000 (at 100% strain) | [184] | |
| [1378 (at 330 strain)] * | ||||||
| 2021 | Epoxy coating on CNTF | 11 | 8.65 (at 2% strain) | Up to 2% | 20 (at 2% strain) | [181] |
5. CNT-Incorporated Nanofibers for Tissue Engineering
5.1. CNT Nanofibers for Tissue Engineering Scaffolds
5.2. CNT-Nanofiber-Based Tissue Engineering
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon Nanotubes for Lithium Ion Batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon Nanotubes: A Novel Material for Multifaceted Applications in Human Healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Tang, Q.; Zeng, S.; Yang, Q.; Yang, X.; Tong, X.; Zhu, G.; Lei, L.; Li, S. Emerging Biomaterials for Tumor Immunotherapy. Biomater. Res. 2023, 27, 47. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Q.; Yang, Y.; Li, Y.; Lin, W. Recent Trends in Therapeutic Application of Engineered Blood Purification Materials for Kidney Disease. Biomater. Res. 2022, 26, 5. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Zhou, X.; Tan, W.; Luo, H.; Lei, S.; Yang, Y. GelMA-Based Bioactive Hydrogel Scaffolds with Multiple Bone Defect Repair Functions: Therapeutic Strategies and Recent Advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef]
- Rajendran, A.K.; Sankar, D.; Amirthalingam, S.; Kim, H.D.; Rangasamy, J.; Hwang, N.S. Trends in Mechanobiology Guided Tissue Engineering and Tools to Study Cell-Substrate Interactions: A Brief Review. Biomater. Res. 2023, 27, 55. [Google Scholar] [CrossRef]
- Li, M.; Xia, W.; Khoong, Y.M.; Huang, L.; Huang, X.; Liang, H.; Zhao, Y.; Mao, J.; Yu, H.; Zan, T. Smart and Versatile Biomaterials for Cutaneous Wound Healing. Biomater. Res. 2023, 27, 87. [Google Scholar] [CrossRef]
- Meskher, H.; Mustansar, H.C.; Thakur, A.K.; Sathyamurthy, R.; Lynch, I.; Singh, P.; Han, T.K.; Saidur, R. Recent Trends in Carbon Nanotube (CNT)-Based Biosensors for the Fast and Sensitive Detection of Human Viruses: A Critical Review. Nanoscale Adv. 2022, 5, 992–1010. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.J.; Oralkan, O.; et al. Carbon Nanotubes as Photoacoustic Molecular Imaging Agents in Living Mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.K.; Jain, A.K.; Lodhi, N.; Raj, R.; Dubey, V.; Mishra, D.; Nahar, M.; Jain, N.K. Challenges in the Use of Carbon Nanotubes for Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 169–206. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Ter Waarbeek, R.F.; De Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef]
- Koziol, K.; Vilatela, J.; Moisala, A.; Motta, M.; Cunniff, P.; Sennett, M.; Windle, A. High-Performance Carbon Nanotube Fiber. Science 2007, 318, 1892–1895. [Google Scholar] [CrossRef]
- Tsentalovich, D.E.; Headrick, R.J.; Mirri, F.; Hao, J.; Behabtu, N.; Young, C.C.; Pasquali, M. Influence of Carbon Nanotube Characteristics on Macroscopic Fiber Properties. ACS Appl. Mater. Interfaces 2017, 9, 36189–36198. [Google Scholar] [CrossRef]
- Lu, W.; Zu, M.; Byun, J.H.; Kim, B.S.; Chou, T.W. State of the Art of Carbon Nanotube Fibers: Opportunities and Challenges. Adv. Mater. 2012, 24, 1805–1833. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One Hundred Fold Increase in Current Carrying Capacity in a Carbon Nanotube-Copper Composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.W.; Cheon, J.Y.; Kim, J.H.; Jung, Y.; Lee, K.; Park, J.S.; Park, J.Y.; Song, K.S.; Lee, S.B.; Kim, T.; et al. All-in-One Flexible Supercapacitor with Ultrastable Performance under Extreme Load. Sci. Adv. 2022, 8, eabl8631. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.G.; Hong, S.; Madrona, C.; Oh, Y.; Park, M.; Komatsu, N.; Taylor, L.W.; Chung, B.; Kim, J.; et al. Ultrahigh Strength, Modulus, and Conductivity of Graphitic Fibers by Macromolecular Coalescence. Sci. Adv. 2022, 8, eabn0939. [Google Scholar] [CrossRef] [PubMed]
- Vilatela, J.J.; Windle, A.H. Yarn-like Carbon Nanotube Fibers. Adv. Mater. 2010, 22, 4959–4963. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.M.; Jung, Y.; Park, J.; Lee, H.S.; Kim, Y.K.; Park, C.R.; Jeong, H.S.; Kim, S.M. Direct Spinning and Densification Method for High-Performance Carbon Nanotube Fibers. Nat. Commun. 2019, 10, 2962. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Zhang, X.; Zhou, B.; Wen, S.; Zhou, Y.; Chen, S.; Jiang, L.; Jerrams, S.; Zhou, F. Biodegradable Polyurethane Fiber-Based Strain Sensor with a Broad Sensing Range and High Sensitivity for Human Motion Monitoring. ACS Sustain. Chem. Eng. 2022, 10, 8788–8798. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, H.; Xu, X.; Han, F.; Lubineau, G. Ultrasensitive, Stretchable Strain Sensors Based on Fragmented Carbon Nanotube Papers. ACS Appl. Mater. Interfaces 2017, 9, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, Q.; Fan, S. Nanotechnology: Spinning Continuous Carbon Nanotube Yarns. Nature 2002, 419, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, K.; Feng, C.; Liu, P.; Zhang, L.; Kong, J.; Zhang, T.; Li, Q.; Fan, S. Spinning and Processing Continuous Yarns from 4-Inch Wafer Scale Super-Aligned Carbon Nanotube Arrays. Adv. Mater. 2006, 18, 1505–1510. [Google Scholar] [CrossRef]
- Lee, J.; Oh, E.; Kim, H.J.; Cho, S.; Kim, T.; Lee, S.; Park, J.; Kim, H.J.; Lee, K.H. The Reason for an Upper Limit to the Height of Spinnable Carbon Nanotube Forests. J. Mater. Sci. 2013, 48, 6897–6904. [Google Scholar] [CrossRef]
- Vigolo, B.; Penicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Poulin, P. Macroscopic Fibers and Ribbons of Oriented Carbon Nanotubes. Science 2000, 290, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Ericson, L.M.; Davis, V.A.; Saini, R.K.; Kittrell, C.; Pasquali, M.; Billups, W.E.; Adams, W.W.; Hauge, R.H.; Smalley, R.E. Dissolution of Pristine Single Walled Carbon Nanotubes in Superacids by Direct Protonation. J. Phys. Chem. B 2004, 108, 8794–8798. [Google Scholar] [CrossRef]
- Ericson, L.M.; Fan, H.; Peng, H.; Davis, V.A.; Zhou, W.; Sulpizio, J.; Wang, Y.; Booker, R.; Vavro, J.; Guthy, C.; et al. Macroscopic, Neat, Single-Walled Carbon Nanotube Fibers. Science 2004, 305, 1447–1451. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.M.; Kim, Y.K.; Jeong, H.S.; Kim, S.M. Significantly Increased Solubility of Carbon Nanotubes in Superacid by Oxidation and Their Assembly into High-Performance Fibers. Small 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.; Park, J.H.; Lee, D.M.; Lee, A.; Moon, S.Y.; Lee, S.Y.; Jeong, H.S.; Kim, S.M. Deep-Injection Floating-Catalyst Chemical Vapor Deposition to Continuously Synthesize Carbon Nanotubes with High Aspect Ratio and High Crystallinity. Carbon 2021, 173, 901–909. [Google Scholar] [CrossRef]
- A Device That Uses Specific Biochemical Reactions Mediated by Isolated Enzymes, Immunosystems, Tissues, Organelles or Whole Cells to Detect Chemical Compounds Usually by Electrical, Thermal or Optical Signals. PAC 2014, 143, 2014. [CrossRef]
- Situ, C.; Mooney, M.H.; Elliott, C.T.; Buijs, J. Advances in Surface Plasmon Resonance Biosensor Technology towards High-Throughput, Food-Safety Analysis. TrAC-Trends Anal. Chem. 2010, 29, 1305–1315. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic Biosensors in Medicine—A Review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Rogers, K.R. Recent Advances in Biosensor Techniques for Environmental Monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Sandifer, J.R.; Voycheck, J.J. A Review of Biosensor and Industrial Applications of PH-ISFETs and an Evaluation of Honeywell’s “DuraFET”. Mikrochim. Acta 1999, 131, 91–98. [Google Scholar] [CrossRef]
- Kisaalita, W.S. Biosensor Standards Requirements. Biosens. Bioelectron. 1992, 7, 613–620. [Google Scholar] [CrossRef]
- Coulet, P.R.; Blum, L.J. Biosensor Principles and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sethi, R.S. Transducer Aspects of Biosensors. Biosens. Bioelectron. 1994, 9, 243–264. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Cullum, B. Biosensors and Biochips: Advances in Biological and Medical Diagnostics. Fresenius. J. Anal. Chem. 2000, 366, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Sadik, K. A Roadmap of Biomedical Engineers and Milestones; IntechOpen: London, UK, 2012. [Google Scholar]
- Bitew, Z.; Amare, M. Electrochemical Determination of Ascorbic Acid in Pharmaceutical Tablets Using Carbon Paste Electrode. Org. Med. Chem. 2019, 8, 555749. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2000; ISBN 0471282723. [Google Scholar]
- Baur, J.E.; Kristensen, E.W.; May, L.J.; Wiedemann, D.J.; Wightman, R.M. Fast-Scan Voltammetry of Biogenic Amines. Anal. Chem. 1988, 60, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Ewing, A.G.; Dayton, M.A.; Wightman, R.M. Pulse Voltammetry with Microvoltammetric Electrodes. Anal. Chem. 1981, 53, 1842–1847. [Google Scholar] [CrossRef]
- Kissinger, P.; Heineman, W.R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed.; Revised and Expanded—CRC Press Book; CRC Press: Boca Raton, FL, USA, 1996; ISBN 0824794451. [Google Scholar]
- Huffman, M.L.; Venton, B.J. Carbon-Fiber Microelectrodes for in Vivo Applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Venton, B.J.; Troyer, K.P.; Wightman, R.M. Response Times of Carbon Fiber Microelectrodes to Dynamic Changes in Catecholamine Concentration. Anal. Chem. 2002, 74, 539–546. [Google Scholar] [CrossRef]
- Huffman, M.L.; Venton, B.J. Electrochemical Properties of Different Carbon-Fiber Microelectrodes Using Fast-Scan Cyclic Voltammetry. Electroanalysis 2008, 20, 2422–2428. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Ivanov, I.N.; Nguyen, M.D.; Zestos, A.G.; Venton, B.J. High Temporal Resolution Measurements of Dopamine with Carbon Nanotube Yarn Microelectrodes. Anal. Chem. 2014, 86, 5721–5727. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Wei, H.; Yao, L.; Sun, L.; Yu, P.; Su, B.; Mao, L. In Vivo Monitoring of Oxygen in Rat Brain by Carbon Fiber Microelectrode Modified with Antifouling Nanoporous Membrane. Anal. Chem. 2019, 91, 3645–3651. [Google Scholar] [CrossRef]
- Peltola, E.; Sainio, S.; Holt, K.B.; Palomäki, T.; Koskinen, J.; Laurila, T. Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes. Anal. Chem. 2018, 90, 1408–1416. [Google Scholar] [CrossRef]
- Vitale, F.; Summerson, S.R.; Aazhang, B.; Kemere, C.; Pasquali, M. Neural Stimulation and Recording with Bidirectional, Soft Carbon Nanotube Fiber Microelectrodes. ACS Nano 2015, 9, 4465–4474. [Google Scholar] [CrossRef]
- Gooding, J.J. Nanostructuring Electrodes with Carbon Nanotubes: A Review on Electrochemistry and Applications for Sensing. Electrochim. Acta 2005, 50, 3049–3060. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Cai, C.; Chen, J. Direct Electron Transfer of Glucose Oxidase Promoted by Carbon Nanotubes. Anal. Biochem. 2004, 332, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Dong, Z.; Shanov, V.N.; Doepke, A.; Heineman, W.R.; Halsall, H.B.; Bhattacharya, A.; Wong, D.K.Y.; Schulz, M.J. Fabrication and Characterization of Carbon Nanotube Array Electrodes with Gold Nanoparticle Tips. Sens. Actuators B Chem. 2008, 133, 208–212. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Advances in Carbon Nanotube Based Electrochemical Sensors for Bioanalytical Applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yantasee, W.; Wang, J. Carbon Nanotubes (CNTs) for the Development of Electrochemical Biosensors. Front. Biosci. 2005, 10, 492–505. [Google Scholar] [CrossRef]
- Nugent, J.M.; Santhanam, K.S.V.; Rubio, A.; Ajayan, P.M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Lett. 2001, 1, 87–91. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Vickrey, T.L.; Venton, B.J. Functional Groups Modulate the Sensitivity and Electron Transfer Kinetics of Neurochemicals at Carbon Nanotube Modified Microelectrodes. Analyst 2011, 136, 3557–3565. [Google Scholar] [CrossRef]
- Xiao, N.; Venton, B.J. Rapid, Sensitive Detection of Neurotransmitters at Microelectrodes Modified with Self-Assembled SWCNT Forests. Anal. Chem. 2012, 84, 7816–7822. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Müller, L.; Riediker, M.; Wick, P.; Mohr, M.; Gehr, P.; Rothen-Rutishauser, B. Oxidative Stress and Inflammation Response after Nanoparticle Exposure: Differences between Human Lung Cell Monocultures and an Advanced Three-Dimensional Model of the Human Epithelial Airways. J. R. Soc. Interface 2010, 7, S27. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Mu, Q.; Zhang, B.; Yan, B. Exploring the Immunotoxicity of Carbon Nanotubes. Nanoscale Res. Lett. 2008, 3, 271–277. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Falck, G.C.M.; Suhonen, S.; Vippola, M.; Vanhala, E.; Catalán, J.; Savolainen, K.; Norppa, H. Genotoxicity of Nanomaterials: DNA Damage and Micronuclei Induced by Carbon Nanotubes and Graphite Nanofibres in Human Bronchial Epithelial Cells in Vitro. Toxicol. Lett. 2009, 186, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Song, W.; Burugapalli, K.; Moussy, F.; Li, Y.L.; Zhong, X.H. Nano-Yarn Carbon Nanotube Fiber Based Enzymatic Glucose Biosensor. Nanotechnology 2010, 21, 165501. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Mao, F.; Heller, A. Characteristics of a Miniature Compartment-Less Glucose-O2 Biofuel Cell and Its Operation in a Living Plant. J. Am. Chem. Soc. 2003, 125, 6588–6594. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.S.; Orecchioni, M.; Vitale, F.; Coco, J.A.; Duret, G.; Antonucci, S.; Pamulapati, S.S.; Taylor, L.W.; Dewey, O.S.; Di Sante, M.; et al. Biocompatibility Studies of Macroscopic Fibers Made from Carbon Nanotubes: Implications for Carbon Nanotube Macrostructures in Biomedical Applications. Carbon 2021, 173, 462–476. [Google Scholar] [CrossRef]
- Zestos, A.G.; Jacobs, C.B.; Trikantzopoulos, E.; Ross, A.E.; Venton, B.J. Polyethylenimine Carbon Nanotube Fiber Electrodes for Enhanced Detection of Neurotransmitters. Anal. Chem. 2014, 86, 8568–8575. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deo, R.P.; Poulin, P.; Mangey, M. Carbon Nanotube Fiber Microelectrodes. J. Am. Chem. Soc. 2003, 125, 14706–14707. [Google Scholar] [CrossRef] [PubMed]
- Viry, L.; Derré, A.; Garrigue, P.; Sojic, N.; Poulin, P.; Kuhn, A. Optimized Carbon Nanotube Fiber Microelectrodes as Potential Analytical Tools. Anal. Bioanal. Chem. 2007, 389, 499–505. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized Helical Fibre Bundles of Carbon Nanotubes as Electrochemical Sensors for Long-Term in Vivo Monitoring of Multiple Disease Biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Moussy, F.; Li, Y.; Milne, W.I. Design of Carbon Nanotube Fiber Microelectrode for Glucose Biosensing. J. Chem. Technol. Biotechnol. 2012, 87, 256–262. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, D.; Cho, S.P.; Choi, J.H.; Sung, S.J.; Hong, S.; Yu, W.R. Semiconducting Carbon Nanotube Fibers for Electrochemical Biosensor Platforms. Mater. Des. 2020, 192, 108740. [Google Scholar] [CrossRef]
- Manesh, K.M.; Kim, H.T.; Santhosh, P.; Gopalan, A.I.; Lee, K.P. A Novel Glucose Biosensor Based on Immobilization of Glucose Oxidase into Multiwall Carbon Nanotubes-Polyelectrolyte-Loaded Electrospun Nanofibrous Membrane. Biosens. Bioelectron. 2008, 23, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.E.; Mattoso, L.H.C.; Medeiros, E.S.; Zucolotto, V. Poly(Lactic Acid)/Carbon Nanotube Fibers as Novel Platforms for Glucose Biosensors. Biosensors 2012, 2, 70–82. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.; Bai, Y.; Wang, X.; Zhou, X.; Zhang, Y. Macroscopic Carbon Nanotube Fiber Film Based Glucose Biosensor. Int. J. Electrochem. Sci. 2013, 8, 10100–10111. [Google Scholar] [CrossRef]
- Muqaddas, S.; Javed, M.; Nadeem, S.; Asghar, M.A.; Haider, A.; Ahmad, M.; Ashraf, A.R.; Nazir, A.; Iqbal, M.; Alwadai, N.; et al. Carbon Nanotube Fiber-Based Flexible Microelectrode for Electrochemical Glucose Sensors. ACS Omega 2023, 8, 2272–2280. [Google Scholar] [CrossRef]
- Ali, A.; Muqaddas, S.; Aldosari, H.; Rashid, S.; Hafiz, A.; Saeed, M.U.; Ahmad, A.; Ahmad, M. Novel Structured Carbon Nanotubes Fiber Based Microelectrodes for Efficient Electrochemical Water Splitting and Glucose Sensing. Carbon 2024, 218, 118709. [Google Scholar] [CrossRef]
- Viry, L.; Derré, A.; Poulin, P.; Kuhn, A. Discrimination of Dopamine and Ascorbic Acid Using Carbon Nanotube Fiber Microelectrodes. Phys. Chem. Chem. Phys. 2010, 12, 9993–9995. [Google Scholar] [CrossRef] [PubMed]
- Harreither, W.; Trouillon, R.; Poulin, P.; Neri, W.; Ewing, A.G.; Safina, G. Carbon Nanotube Fiber Microelectrodes Show a Higher Resistance to Dopamine Fouling. Anal. Chem. 2013, 85, 7447–7453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.; Wang, J.; Li, H.; Chen, C.; Feng, J.; Guo, Y.; Yu, H.; Sun, X.; Peng, H. Flexible Dopamine-Sensing Fiber Based on Potentiometric Method for Long-Term Detection in Vivo. Sci. China Chem. 2021, 64, 1763–1769. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Wang, X.; Zhu, Y.; Sombers, L.A. Carbon Nanotube Yarn Electrodes for Enhanced Detection of Neurotransmitter Dynamics in Live Brain Tissue. ACS Nano 2013, 7, 7864–7873. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Trikantzopoulos, E.; Jacobs, C.B.; Venton, B.J. Evaluation of Carbon Nanotube Fiber Microelectrodes for Neurotransmitter Detection: Correlation of Electrochemical Performance and Surface Properties. Anal. Chim. Acta 2017, 965, 1–8. [Google Scholar] [CrossRef]
- Yang, C.; Trikantzopoulos, E.; Nguyen, M.D.; Jacobs, C.B.; Wang, Y.; Mahjouri-Samani, M.; Ivanov, I.N.; Venton, B.J. Laser Treated Carbon Nanotube Yarn Microelectrodes for Rapid and Sensitive Detection of Dopamine in Vivo. ACS Sens. 2016, 1, 508–515. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Sun, X.; Wei, G.F.; Tian, Y.; Liu, Z.P.; Huang, R.; Yu, Y.; Peng, H. Engineering Carbon Nanotube Fiber for Real-Time Quantification of Ascorbic Acid Levels in a Live Rat Model of Alzheimer’s Disease. Anal. Chem. 2017, 89, 1831–1837. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Yang, T.; Wang, Z.; Hasebe, Y.; Lv, T.; Zhang, Z. A Novel Flexible Electrochemical Ascorbic Acid Sensor Constructed by Ferrocene Methanol Doped Multi-Walled Carbon Nanotube Yarn. Electroanalysis 2021, 33, 2445–2451. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, F.; Liu, W.; Zhu, T.; Zhang, J.Z.H.; Chen, C.; Dai, Z.; Peng, H.; Huang, J.L.; Hu, Q.; et al. An Electrochemical Biosensor with Dual Signal Outputs: Toward Simultaneous Quantification of PH and O2 in the Brain upon Ischemia and in a Tumor during Cancer Starvation Therapy. Angew. Chem.-Int. Ed. 2017, 56, 10471–10475. [Google Scholar] [CrossRef]
- Paul, B.; Panigrahi, A.K.; Singh, V.; Singh, S.G. A Multi-Walled Carbon Nanotube-Zinc Oxide Nanofiber Based Flexible Chemiresistive Biosensor for Malaria Biomarker Detection. Analyst 2017, 142, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Bourourou, M.; Holzinger, M.; Bossard, F.; Hugenell, F.; Maaref, A.; Cosnier, S. Chemically Reduced Electrospun Polyacrilonitrile-Carbon Nanotube Nanofibers Hydrogels as Electrode Material for Bioelectrochemical Applications. Carbon 2015, 87, 233–238. [Google Scholar] [CrossRef]
- Parker, L. Value of Screening for Neuroblastoma? Lancet 1995, 346, 1419. [Google Scholar] [CrossRef]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef]
- Haque, A.M.J.; Nandhakumar, P.; Yang, H. Specific and Rapid Glucose Detection Using NAD-Dependent Glucose Dehydrogenase, Diaphorase, and Osmium Complex. Electroanalysis 2019, 31, 876–882. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Katakis, I.; Domínguez, E. Catalytic Electrooxidation of NADH for Dehydrogenase Amperometric Biosensors. Mikrochim. Acta 1997, 126, 11–32. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thomas, D.F.; Chen, A. Based on Nanoporous PtPb Networks Glucose Sensor Based on Three-Dimensional PtPb Net- One-Step Hydrothermal Method. The Surface Morphology. Anal. Chem. 2008, 80, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.S.; Gifford, R. Biosensors for Real-Time in Vivo Measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef] [PubMed]
- Bockrath, M.; Cobden, D.H.; McEuen, P.L.; Chopra, N.G.; Zettl, A.; Thess, A.; Smalley, R.E. Single-Electron Transport in Ropes of Carbon Nanotubes. Science 1997, 275, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution Blow Spinning: A New Method to Produce Micro-and Nanofibers from Polymer Solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Owen, M.J.; Murray, R.M. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol. Psychiatry 2017, 81, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Dopamine and Depression: A Review of Recent Evidence. I. Empirical Studies. Brain Res. Rev. 1983, 6, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The Substantia Nigra of the Human Brain: II. Patterns of Loss of Dopamine-Containing Neurons in Parkinson’s Disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Britto, P.J.; Santhanam, K.S.V.; Ajayan, P.M. Carbon Nanotube Electrode for Oxidation of Dopamine. Bioelectrochem. Bioenerg. 1996, 41, 121–125. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, R.; Chai, Y.; Li, X. Investigation of the Electrochemical and Electrocatalytic Behavior of Positively Charged Gold Nanoparticle and L-Cysteine Film on an Au Electrode. Anal. Chim. Acta 2007, 596, 99–105. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G. Polyeugenol-Modified Platinum Electrode for Selective Detection of Dopamine in the Presence of Ascorbic Acid. Anal. Chem. 1999, 71, 1055–1061. [Google Scholar] [CrossRef]
- Duvall, S.H.; McCreery, R.L. Self-Catalysis by Catechols and Quinones during Heterogeneous Electron Transfer at Carbon Electrodes. J. Am. Chem. Soc. 2000, 122, 6759–6764. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Zhan, D.; Liu, H.; Zhao, Q.; Kou, Y.; Shao, Y.; Li, M.; Zhuang, Q.; Zhu, Z. Selective Detection of Dopamine in the Presence of Ascorbic Acid and Uric Acid by a Carbon Nanotubes-Ionic Liquid Gel Modified Electrode. Talanta 2005, 66, 51–57. [Google Scholar] [CrossRef]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Dalton, A.B.; Collins, S.; Razal, J.; Munoz, E.; Ebron, V.H.; Kim, B.G.; Coleman, J.N.; Ferraris, J.P.; Baughman, R.H. Continuous Carbon Nanotube Composite Fibers: Properties, Potential Applications, and Problems. J. Mater. Chem. 2004, 14, 1–3. [Google Scholar] [CrossRef]
- Muñoz, E.; Suh, D.S.; Collins, S.; Selvidge, M.; Dalton, A.B.; Kim, B.G.; Razal, J.M.; Ussery, G.; Rinzler, A.G.; Martínez, M.T.; et al. Highly Conducting Carbon Nanotube/Polyethyleneimine Composite Fibers. Adv. Mater. 2005, 17, 1064–1067. [Google Scholar] [CrossRef]
- Roberts, J.G.; Moody, B.P.; McCarty, G.S.; Sombers, L.A. Specific Oxygen-Containing Functional Groups on the Carbon Surface Underlie an Enhanced Sensitivity to Dopamine at Electrochemically Pretreated Carbon Fiber Microelectrodes. Langmuir 2010, 26, 9116–9122. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.C.; Bode, A.M. Biology of Free Radical Scavengers: An Evaluation of Ascorbate. FASEB J. 1993, 7, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological Significance of Ascorbic Acid (Vitamin C) in Human Health—A Review. Pak. J. Nutr. 2003, 3, 5–13. [Google Scholar] [CrossRef]
- Crespi, F. Concomitant in vivo electrophysiological and voltammetric analysis indicate that ascorbic acid is a biochemical index of early ischaemia. Neurosci. Lett. 1996, 215, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, H.; Hamamoto, M.; Ueda, M.; Nito, C.; Yamaguchi, H.; Katayama, Y. The Effect of Ascorbic Acid on the Pharmacokinetics of Levodopa in Elderly Patients with Parkinson Disease. Clin. Neuropharmacol. 2004, 27, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Acuña, A.I.; Esparza, M.; Kramm, C.; Beltrán, F.A.; Parra, A.V.; Cepeda, C.; Toro, C.A.; Vidal, R.L.; Hetz, C.; Concha, I.I.; et al. A Failure in Energy Metabolism and Antioxidant Uptake Precede Symptoms of Huntington’s Disease in Mice. Nat. Commun. 2013, 4, 2917. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical Methods for Ascorbic Acid Determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Ndubuizu, O.; LaManna, J.C. Brain Tissue Oxygen Concentration Measurements. Antioxid. Redox Signal. 2007, 9, 1207–1219. [Google Scholar] [CrossRef]
- Jesberger, J.A.; Richardson, J.S. Oxygen Free Radicals and Brain Dysfunction. Int. J. Neurosci. 1991, 57, 1–17. [Google Scholar] [CrossRef]
- Ziemann, A.E.; Schnizler, M.K.; Albert, G.W.; Severson, M.A.; Howard, M.A.; Welsh, M.J.; Wemmie, J.A. Seizure Termination by Acidosis Depends on ASIC1a. Nat. Neurosci. 2008, 11, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ding, C.; Zhou, J.; Tian, Y. 2D Ratiometric Fluorescent PH Sensor for Tracking of Cells Proliferation and Metabolism. Biosens. Bioelectron. 2015, 70, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Lassen, N.A. Brain Extracellular Ph: The Main Factor Controlling Cerebral Blood Flow. Scand. J. Clin. Lab. Investig. 1968, 22, 247–251. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- WHO World Malaria Report 2020; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789241564106.
- Newton, P.; White, N. Malaria: New Developments in Treatment and Prevention. Annu. Rev. Med. 1999, 50, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Rao, V.K.; Agarwal, G.S.; Rai, G.P.; Gopalan, N.; Prakash, S.; Sharma, S.K.; Vijayaraghavan, R. Highly Sensitive Amperometric Immunosensor for Detection of Plasmodium Falciparum Histidine-Rich Protein 2 in Serum of Humans with Malaria: Comparison with a Commercial Kit. J. Clin. Microbiol. 2008, 46, 3759–3765. [Google Scholar] [CrossRef]
- Nazari, M.; Kashanian, S.; Moradipour, P.; Maleki, N. A Novel Fabrication of Sensor Using ZnO-Al2O3 Ceramic Nanofibers to Simultaneously Detect Catechol and Hydroquinone. J. Electroanal. Chem. 2018, 812, 122–131. [Google Scholar] [CrossRef]
- Erogul, S.; Bas, S.Z.; Ozmen, M.; Yildiz, S. A New Electrochemical Sensor Based on Fe3O4 Functionalized Graphene Oxide-Gold Nanoparticle Composite Film for Simultaneous Determination of Catechol and Hydroquinone. Electrochim. Acta 2015, 186, 302–313. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Pitanga, B.P.S.; Grangeiro, M.S.; Lima, R.M.F.; Costa, M.F.D.; Costa, S.L.; Clarêncio, J.; El-Bacha, R.S. Catechol Cytotoxicity in Vitro: Induction of Glioblastoma Cell Death by Apoptosis. Hum. Exp. Toxicol. 2010, 29, 199–212. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, L.; Yu, S.; Wang, C. An In-ZnO Nanosheet-Modified Carbon Nanotube-Polyimide Film Sensor for Catechol Detection. J. Mater. Chem. A 2014, 2, 6656–6662. [Google Scholar] [CrossRef]
- Ruzgas, T.; Gorton, L.; Emnéus, J.; Marko-Varga, G. Kinetic Models of Horseradish Peroxidase Action on a Graphite Electrode. J. Electroanal. Chem. 1995, 391, 41–49. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Skorobogatko, O.V.; Vartanov, S.S.; Varfolomeyev, S. La Cease. Appl. Biochem. Biotechnol. 1994, 49, 257–280. [Google Scholar] [CrossRef]
- Ortega, F.; Domínguez, E.; Jönsson-Pettersson, G.; Gorton, L. Amperometric Biosensor for the Determination of Phenolic Compounds Using a Tyrosinase Graphite Electrode in a Flow Injection System. J. Biotechnol. 1993, 31, 289–300. [Google Scholar] [CrossRef]
- Mu, S. Catechol Sensor Using Poly(Aniline-Co-o-Aminophenol) as an Electron Transfer Mediator. Biosens. Bioelectron. 2006, 21, 1237–1243. [Google Scholar] [CrossRef]
- Ruzgas, T.; Emnéus, J.; Gorton, L.; Marko-Varga, G. The Development of a Peroxidase Biosensor for Monitoring Phenol and Related Aromatic Compounds. Anal. Chim. Acta 1995, 311, 245–253. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, J.; Yang, D.; Park, B.C.; Ryu, S.; Park, I. A Stretchable Strain Sensor Based on a Metal Nanoparticle Thin Film for Human Motion Detection. Nanoscale 2014, 6, 11932–11939. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Yang, Y. Graphene–Polymer Nanocomposite-Based Redox-Induced Electricity for Flexible Self-Powered Strain Sensors. Adv. Energy Mater. 2018, 8, 1800961. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Yu, W.; Wang, K.; Wei, J.; Wu, D.; Cao, A.; Li, Z.; Cheng, Y.; Zheng, Q.; et al. Stretchable and Highly Sensitive Graphene-on-Polymer Strain Sensors. Sci. Rep. 2012, 2, 870. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, S.; Tebyetekerwa, M.; Li, Y.; Pionteck, J.; Sun, B.; Qin, Z.; Zhu, M. Highly Sensitive and Stretchable Piezoresistive Strain Sensor Based on Conductive Poly(Styrene-Butadiene-Styrene)/Few Layer Graphene Composite Fiber. Compos. Part A Appl. Sci. Manuf. 2018, 105, 291–299. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Wu, Z.; Luo, J.; Li, B.; Huang, X.; Xue, H.; Gao, J. Super-Hydrophobic, Durable and Cost-Effective Carbon Black/Rubber Composites for High Performance Strain Sensors. Compos. Part B Eng. 2019, 176, 107358. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.; Chen, P. Flexible Strain Sensor Based on Carbon Black/Silver Nanoparticles Composite for Humanmotion Detection. Materials 2018, 11, 1836. [Google Scholar] [CrossRef]
- Qu, M.; Qin, Y.; Sun, Y.; Xu, H.; Schubert, D.W.; Zheng, K.; Xu, W.; Nilsson, F. Biocompatible, Flexible Strain Sensor Fabricated with Polydopamine-Coated Nanocomposites of Nitrile Rubber and Carbon Black. ACS Appl. Mater. Interfaces 2020, 12, 42140–42152. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire-Elastomer Nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, L.; Zhong, J.; Ding, T.; Liu, Y.; Cai, Z.; Rong, Y.; Han, H.; Zhou, J.; Wang, Z.L. High-Strain Sensors Based on ZnO Nanowire/Polystyrene Hybridized Flexible Films. Adv. Mater. 2011, 23, 5440–5444. [Google Scholar] [CrossRef]
- Tombler, T.W.; Zhou, C.; Alexseyev, L.; Kong, J.; Dai, H.; Liu, L.; Jayanthi, C.S.; Tang, M.; Wu, S.Y. Reversible Electromechanical Characteristics of Carbon Nanotubes under Local-Probe Manipulation. Nature 2000, 405, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Alamusi; Hu, N.; Fukunaga, H.; Atobe, S.; Liu, Y.; Li, J. Piezoresistive Strain Sensors Made from Carbon Nanotubes Based Polymer Nanocomposites. Sensors 2011, 11, 10691–10723. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Wang, R.; Sun, L.; Zhu, X.; Ge, W.; Li, H.; Li, Z.; Zhang, H.; Huang, Y.; Li, Z.; Zhang, Y.F.; et al. Carbon Nanotube-Based Strain Sensors: Structures, Fabrication, and Applications. Adv. Mater. Technol. 2022, 8, 2200855. [Google Scholar] [CrossRef]
- Kanoun, O.; Müller, C.; Benchirouf, A.; Sanli, A.; Dinh, T.N.; Al-Hamry, A.; Bu, L.; Gerlach, C.; Bouhamed, A. Flexible Carbon Nanotube Films for High Performance Strain Sensors. Sensors 2014, 14, 10042–10071. [Google Scholar] [CrossRef]
- Qi, H.; Schulz, B.; Vad, T.; Liu, J.; Mäder, E.; Seide, G.; Gries, T. Novel Carbon Nanotube/Cellulose Composite Fibers As Multifunctional Materials. ACS Appl. Mater. Interfaces 2015, 7, 22404–22412. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Quijano, J.R.; Pötschke, P.; Brünig, H.; Heinrich, G. Strain Sensing, Electrical and Mechanical Properties of Polycarbonate/Multiwall Carbon Nanotube Monofilament Fibers Fabricated by Melt Spinning. Polymer 2016, 82, 181–189. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Xiang, H.; Zhu, L.; Tebyetekerwa, M.; Zhu, M. Superior Piezoresistive Strain Sensing Behaviors of Carbon Nanotubes in One-Dimensional Polymer Fiber Structure. Carbon 2018, 140, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Yue, X.; Yu, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. A Highly Stretchable Carbon Nanotubes/Thermoplastic Polyurethane Fiber-Shaped Strain Sensor with Porous Structure for Human Motion Monitoring. Compos. Sci. Technol. 2018, 168, 126–132. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Zhou, G.; Jung, B.M.; Kim, T.H.; Park, B.J.; Byun, J.H.; Chou, T.W. High Conductive Free-Written Thermoplastic Polyurethane Composite Fibers Utilized as Weight-Strain Sensors. Compos. Sci. Technol. 2020, 189, 108011. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, C.; Meng, T.; Mojtaba, M.; Teng, Y.; Feng, Q.; Li, D. Multifunctional Wet-Spun Filaments through Robust Nanocellulose Networks Wrapping to Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 42808–42817. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.N.; Lee, G.S.; Kim, B.; Dinh Xuan, H.; Kim, D.; Yoo, S.I.; Yoon, J. Microfluidic Preparation of Highly Stretchable Natural Rubber Microfiber Containing CNT/PEDOT:PSS Hybrid for Fabric-Sewable Wearable Strain Sensor. Compos. Sci. Technol. 2021, 210, 108811. [Google Scholar] [CrossRef]
- Li, L.; Du, Z.; Sun, B.; Li, W.; Jiang, L.; Zhou, Y.; Ma, J.; Chen, S.; Zhou, F.L. Fabrication of Electrically Conductive Poly(Styrene-b-Ethylene-Ran-Butylene-b-Styrene)/Multi-Walled Carbon Nanotubes Composite Fiber and Its Application in Ultra-Stretchable Strain Sensor. Eur. Polym. J. 2022, 169, 111121. [Google Scholar] [CrossRef]
- He, Z.; Byun, J.H.; Zhou, G.; Park, B.J.; Kim, T.H.; Lee, S.B.; Yi, J.W.; Um, M.K.; Chou, T.W. Effect of MWCNT Content on the Mechanical and Strain-Sensing Performance of Thermoplastic Polyurethane Composite Fibers. Carbon 2019, 146, 701–708. [Google Scholar] [CrossRef]
- He, Z.; Zhou, G.; Byun, J.H.; Lee, S.K.; Um, M.K.; Park, B.; Kim, T.; Lee, S.B.; Chou, T.W. Highly Stretchable Multi-Walled Carbon Nanotube/Thermoplastic Polyurethane Composite Fibers for Ultrasensitive, Wearable Strain Sensors. Nanoscale 2019, 11, 5884–5890. [Google Scholar] [CrossRef]
- Guo, T.; Wan, Z.; Li, D.; Song, J.; Rojas, O.J.; Jin, Y. Intermolecular Self-Assembly of Dopamine-Conjugated Carboxymethylcellulose and Carbon Nanotubes toward Supertough Filaments and Multifunctional Wearables. Chem. Eng. J. 2021, 416, 128981. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Sun, B.; Zhou, Y.; Ma, J.; Chen, S.; Jiang, L.; Zhou, F.-L. Styrene-Ethylene-Butadiene-Styrene Copolymer/Carbon Nanotubes Composite Fiber Based Strain Sensor with Wide Sensing Range and High Linearity for Human Motion Detection. J. Ind. Text. 2022, 52, 152808372211219. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Real-Time in Situ Sensing of Damage Evolution in Advanced Fiber Composites Using Carbon Nanotube Networks. Nanotechnology 2008, 19, 215713. [Google Scholar] [CrossRef]
- Hu, N.; Karube, Y.; Yan, C.; Masuda, Z.; Fukunaga, H. Tunneling Effect in a Polymer/Carbon Nanotube Nanocomposite Strain Sensor. Acta Mater. 2008, 56, 2929–2936. [Google Scholar] [CrossRef]
- Zhang, R.; Baxendale, M.; Peijs, T. Universal Resistivity-Strain Dependence of Carbon Nanotube/Polymer Composites. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 76, 2–6. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Hodak, M.; Lee, R.S. Carbon Nanotubes, Buckyballs, Ropes, and a Universal Graphitic Potential. Phys. Rev. B-Condens. Matter Mater. Phys. 2000, 62, 13104–13110. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Yu, H.; Lubineau, G. Deformable and Wearable Carbon Nanotube Microwire-Based Sensors for Ultrasensitive Monitoring of Strain, Pressure and Torsion. Nanoscale 2017, 9, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Bradford, P.D.; Zhou, Q.; Jia, Q.; Yuan, F.G.; Zhu, Y. Carbon Nanotube Yarn Strain Sensors. Nanotechnology 2010, 21, 305502. [Google Scholar] [CrossRef] [PubMed]
- Abu Obaid, A.; Heider, D.; Gillespie, J.W. Investigation of Electro-Mechanical Behavior of Carbon Nanotube Yarns during Tensile Loading. Carbon 2015, 93, 731–741. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.G. Extremely Elastic Wearable Carbon Nanotube Fiber Strain Sensor for Monitoring of Human Motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, Y.; He, X.; Zhang, L.; Li, Z.; Li, P.; Shi, E.; Wu, S.; Cao, A. Elastic Carbon Nanotube Straight Yarns Embedded with Helical Loops. Nanoscale 2013, 5, 2403–2410. [Google Scholar] [CrossRef]
- Li, Y.; Shang, Y.; He, X.; Peng, Q.; Du, S.; Shi, E.; Wu, S.; Li, Z.; Li, P.; Cao, A. Overtwisted, Resolvable Carbon Nanotube Yarn Entanglement as Strain Sensors and Rotational Actuators. ACS Nano 2013, 7, 8128–8135. [Google Scholar] [CrossRef]
- Shang, Y.; Hua, C.; Xu, W.; Hu, X.; Wang, Y.; Zhou, Y.; Zhang, Y.; Li, X.; Cao, A. Meter-Long Spiral Carbon Nanotube Fibers Show Ultrauniformity and Flexibility. Nano Lett. 2016, 16, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, F.; Liu, W.; Gao, Y.; Zhang, K.; Zhang, X.; Qiu, Y. Flexible Strain Sensor Based on Aerogel-Spun Carbon Nanotube Yarn with a Core-Sheath Structure. Compos. Part A Appl. Sci. Manuf. 2018, 108, 107–113. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Xin, Y.; Lubineau, G. Coaxial Thermoplastic Elastomer-Wrapped Carbon Nanotube Fibers for Deformable and Wearable Strain Sensors. Adv. Funct. Mater. 2018, 28, 1705591. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Wang, F.; Bian, C.; Chen, Y.; Wang, Y.; Li, B. Highly Stretchable Core-Sheath Fibers via Wet-Spinning for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 6624–6635. [Google Scholar] [CrossRef]
- Ma, H.; Gao, Y.; Liu, W.; Farha, F.I.; Zhang, K.; Guo, L.; Xu, F. Light-Weight Strain Sensor Based on Carbon Nanotube/Epoxy Composite Yarn. J. Mater. Sci. 2021, 56, 13156–13164. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Shi, S.; Wang, F.; Li, B. Coaxial Carbon Nanotube/Polymer Fibers as Wearable Piezoresistive Sensors. Sens. Actuators A Phys. 2018, 284, 85–95. [Google Scholar] [CrossRef]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone Cell Proliferation on Carbon Nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef]
- Song, L.; Wang, K.; Li, Y.; Yang, Y. Nanotopography Promoted Neuronal Differentiation of Human Induced Pluripotent Stem Cells. Colloids Surf. B Biointerfaces 2016, 148, 49–58. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, H.S. Therapeutic Application of Electrospun Nanofibrous Meshes. Nanomedicine 2014, 9, 517–533. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, T.H.; Mandakhbayar, N.; Singh, R.K.; Jang, J.H.; Lee, J.H.; Kim, H.W. Coating Biopolymer Nanofibers with Carbon Nanotubes Accelerates Tissue Healing and Bone Regeneration through Orchestrated Cell- and Tissue-Regulatory Responses. Acta Biomater. 2020, 108, 97–110. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Using Wet Electrospun PCL/Gelatin/CNT Yarns to Fabricate Textile-Based Scaffolds for Vascular Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 2627–2637. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Tsai, S.W.; Huang, C.C.; Rau, L.R.; Hsu, F.Y. Fabrication of Aligned Carbon Nanotube/Polycaprolactone/Gelatin Nanofibrous Matrices for Schwann Cell Immobilization. J. Nanomater. 2014, 2014, 498131. [Google Scholar] [CrossRef]
- Por Hajrezaei, S.; Haghbin Nazarpak, M.; Hojjati Emami, S.; Shahryari, E. Biocompatible and Electroconductive Nanocomposite Scaffolds with Improved Piezoelectric Response for Bone Tissue Engineering. Int. J. Polym. Sci. 2022, 2022, 4521937. [Google Scholar] [CrossRef]
- Karbasi, S.; Alizadeh, Z.M. Effects of Multi-Wall Carbon Nanotubes on Structural and Mechanical Properties of Poly(3-Hydroxybutyrate)/Chitosan Electrospun Scaffolds for Cartilage Tissue Engineering. Bull. Mater. Sci. 2017, 40, 1247–1253. [Google Scholar] [CrossRef]
- Zadehnajar, P.; Akbari, B.; Karbasi, S.; Mirmusavi, M.H. Preparation and Characterization of Poly ε-Caprolactone-Gelatin/Multi-Walled Carbon Nanotubes Electrospun Scaffolds for Cartilage Tissue Engineering Applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 326–337. [Google Scholar] [CrossRef]
- Su, W.T.; Shih, Y.A. Nanofiber Containing Carbon Nanotubes Enhanced PC12 Cell Proliferation and Neuritogenesis by Electrical Stimulation. Biomed. Mater. Eng. 2015, 26, S189–S195. [Google Scholar] [CrossRef] [PubMed]
- Lewitus, D.Y.; Landers, J.; Branch, J.R.; Smith, K.L.; Callegari, G.; Kohn, J.; Neimark, A.V. Biohybrid Carbon Nanotube/Agarose Fibers for Neural Tissue Engineering. Adv. Funct. Mater. 2011, 21, 2624–2632. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, N.; Derakhshan, M.A.; Mansoori, K.; Ghanbari, H. Improvement of Sciatic Nerve Regeneration by Multichannel Nanofibrous Membrane-Embedded Electro-Conductive Conduits Functionalized with Laminin. J. Mater. Sci. Mater. Med. 2022, 33, 50. [Google Scholar] [CrossRef] [PubMed]
- Yardimci, A.I.; Aypek, H.; Ozturk, O.; Yilmaz, S.; Ozcivici, E.; Mese, G.; Selamet, Y. CNT Incorporated Polyacrilonitrile/Polypyrrole Nanofibers as Keratinocytes Scaffold. J. Biomim. Biomater. Biomed. Eng. 2019, 41, 69–81. [Google Scholar] [CrossRef]
- Shokraei, N.; Asadpour, S.; Shokraei, S.; Nasrollahzadeh Sabet, M.; Faridi-Majidi, R.; Ghanbari, H. Development of Electrically Conductive Hybrid Nanofibers Based on CNT-Polyurethane Nanocomposite for Cardiac Tissue Engineering. Microsc. Res. Tech. 2019, 82, 1316–1325. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT Nanofibers as Electrically Conductive Scaffolds for Cardiovascular Tissue Engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- Razal, J.M.; Gilmore, K.J.; Wallace, G.G. Carbon Nanotube Biofiber Formation in a Polymer-Free Coagulation Bath. Adv. Funct. Mater. 2008, 18, 61–66. [Google Scholar] [CrossRef]
- Zheng, T.; Xu, N.; Kan, Q.; Li, H.; Lu, C.; Zhang, P.; Li, X.; Zhang, D.; Wang, X. Wet-Spinning Assembly of Continuous, Highly Stable Hyaluronic/Multiwalled Carbon Nanotube Hybrid Microfibers. Polymers 2019, 11, 867. [Google Scholar] [CrossRef]





| Analyte | Fiber Type | Spinning Method | Sensitivity | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Glucose | CNT | Wet spinning | [76] | ||
| CNT | Forest spinning | 5.6 nA μM−1 | 50 μM | [77] | |
| CNT | Direct spinning | 7.2 nA mM−1 | 2 mM | [71] | |
| CNT | Direct spinning | 2.7 nA mM−1 | 25 μM | [78] | |
| CNT | Wet spinning | 0.5 μM | [79] | ||
| PMMA/CNT | Electrospinning | 3.7048 nA mM−1 | 1 μM | [80] | |
| PLA/CNT | Solution blow spinning | 358 nA mM−1 | 0.08 mM | [81] | |
| CNT | Direct spinning | 4.867 nA mM−1 | [82] | ||
| CNT | Wet spinning | 3.025 mA (cm2 mM)−1 | 1.4 μM | [83] | |
| CNT | Wet spinning | 813 mA (cm2 mM)−1 | 0.59 μM | [84] | |
| Dopamine | CNT | Wet spinning | [85] | ||
| CNT | Wet spinning | [86] | |||
| CNT | Forest spinning | 0.28 mV nM−1 | 5 nM | [87] | |
| CNT | Forest spinning | 13.4 ± 1.7 nM | [88] | ||
| CNT | Forest spinning | 10 ± 0.8 nM | [54] | ||
| PEI/CNT | Wet spinning | 5 nM | [74] | ||
| PEI/CNT, CNT | Wet spinning, Forest spinning | [89] | |||
| CNT | Forest spinning | 4.6 ± 0.9 nM | [90] | ||
| Ascorbic acid | CNT | Forest spinning | [91] | ||
| CNT | 1.32 μM | [92] | |||
| Oxygen, pH | CNT | Forest spinning | [93] | ||
| Malaria biomarker(PfHRP2) | ZnO/CNT | Electrospinning | 8.29 kΩg−1 mL | 0.97 fg mL−1 | [94] |
| Catechol | PAN/CNT | Electrospinning | 118 mA M−1 | 0.9 μM | [95] |
| Year | Materials | Spinning Method | Stretchability [%] | Gauge Factor | Linear Region | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| 2015 | MWCNTs/cellulose | Wet | 14.3 | 18 (at 14.3% strain) | [159] | ||
| 2016 | MWCNTs/PC | Melt | 9 | 16 (at 5% strain) | [160] | ||
| 2018 | MWCNTs/SBS | Wet | 260 | 20,000 (at 14.3% strain) | Up to 1% | [161] | |
| 175 (at 50% strain) * | |||||||
| 2018 | MWCNTs/TPU | Wet | 320 | 22 (under 160% strain) | 0–160% | 9700 | [162] |
| 97 (within 160–320% strain) | 160–320% | (at 100% strain) | |||||
| 2019 | MWCNTs/TPU | Wet | 117 | 71 (at 35% strain) | [167] | ||
| 5200 (at 35% strain) * | |||||||
| 2019 | MWCNTs/TPU | Wet | 100 | 27 (at 100% strain) | [168] | ||
| 2800 (at 100% strain) * | |||||||
| 2020 | MWCNTs/Ag NW/TPU | Wet | 254 | 49 (at 254% strain) | [163] | ||
| 2021 | MWCNTs/PEDOT:PSS/ natural rubber | Wet | 1000 | 2 (at 100% strain) 3.8 (at 1000% strain) * | Up to 1000% | 2000 (at 200% strain) | [165] |
| 2022 | MWCNTs/SEBS | Wet | 506 | 58.2 (under 275% strain) | 0–275% | 2500 | [166] |
| 197.9 (within 275–506% strain) | 275–506% | (at 20% strain) | |||||
| 2022 | MWCNTs/biodegradable PU | Wet | 250 | 100 (at 200% strain) | 3000 | [23] | |
| 15 (at 100% strain), | (at 50% strain) | ||||||
| 2468 (at 250% strain) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.H.; Kwon, M.; Shin, S.; Lee, J.; Kim, K.S. Biomedical Applications of CNT-Based Fibers. Biosensors 2024, 14, 137. https://doi.org/10.3390/bios14030137
Jeong YH, Kwon M, Shin S, Lee J, Kim KS. Biomedical Applications of CNT-Based Fibers. Biosensors. 2024; 14(3):137. https://doi.org/10.3390/bios14030137
Chicago/Turabian StyleJeong, Yun Ho, Mina Kwon, Sangsoo Shin, Jaegeun Lee, and Ki Su Kim. 2024. "Biomedical Applications of CNT-Based Fibers" Biosensors 14, no. 3: 137. https://doi.org/10.3390/bios14030137
APA StyleJeong, Y. H., Kwon, M., Shin, S., Lee, J., & Kim, K. S. (2024). Biomedical Applications of CNT-Based Fibers. Biosensors, 14(3), 137. https://doi.org/10.3390/bios14030137







