Extremozyme-Based Biosensors for Environmental Pollution Monitoring: Recent Developments
Abstract
:1. Introduction
2. Extremozymes: Varieties and Significance
2.1. Cold-Active Enzymes
2.2. Thermostable Enzymes
2.3. Salt-Adapted Enzymes
2.4. Alkalistable Enzymes
2.5. Acidostable Enzymes
2.6. Extremozymes with Prospecting Applications in Environmental Monitoring
3. Characteristics of Extremozyme-Based Biosensors for Environmental Monitoring
3.1. Overview of Enzyme-Based Biosensors for Environmental Monitoring
3.2. Performances of Extremozyme-Based Biosensors and Bioassays
3.3. Immobilization Methods Used with Extremozymes
3.4. Selectivity of Extremozyme-Based Biosensors
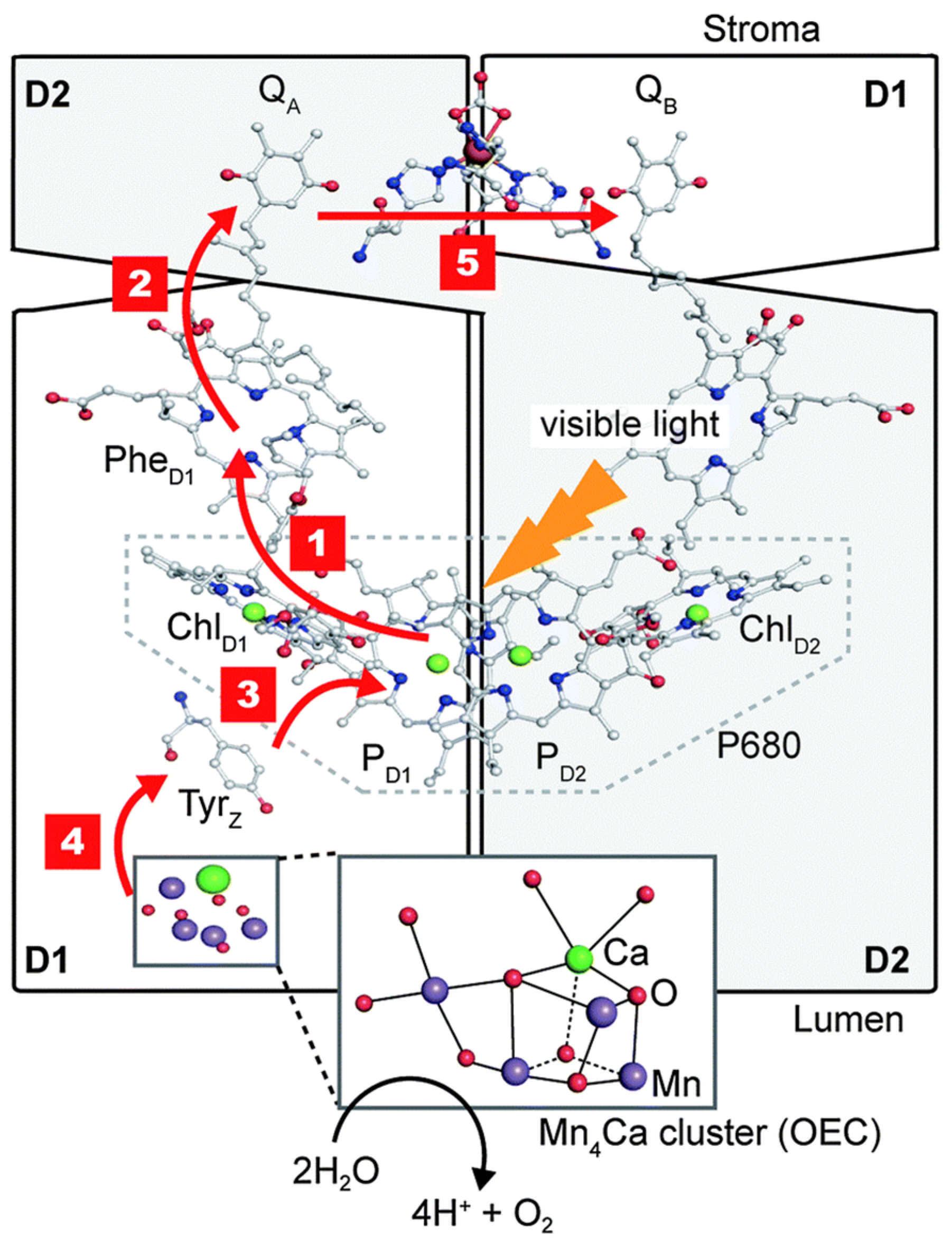
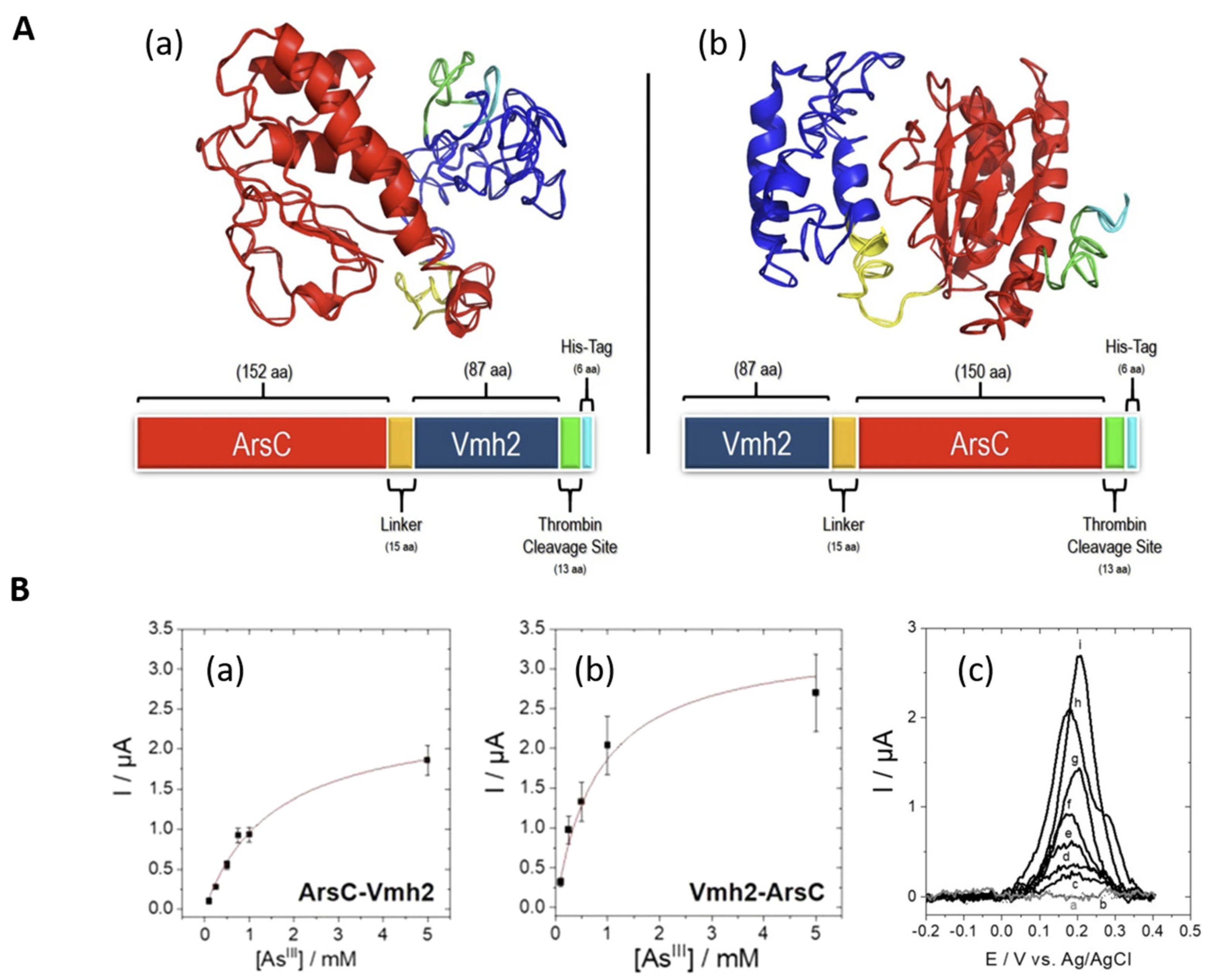
4. Design of Extremozyme-Based Biosensors: Case Study of Biosensors for Photosynthesis Inhibitors
4.1. Photosynthesis Inhibitors
4.2. Configuration of the Sensing Layer
- Physical procedures [167] by the
- (1)
- adsorption on filter paper, alumina, glass microfiber, or DEAE cellulose;
- (2)
- inclusion in polysaccharide (agar, agarose, carrageenan, alginate), protein (gelatin) or synthetic gels (polyacrylamide, polyurethane, poly(vinyl alcohol) and poly(vinylalcohol) functionalized with styrylpyridium groups, vinyl and photocrosslinkable resin);
- (3)
- adsorption on electrodes modified with conductive redox polymeric films;
- (4)
- layer-by-layer deposition of coatings with alternating positive and negative charge;
- (1)
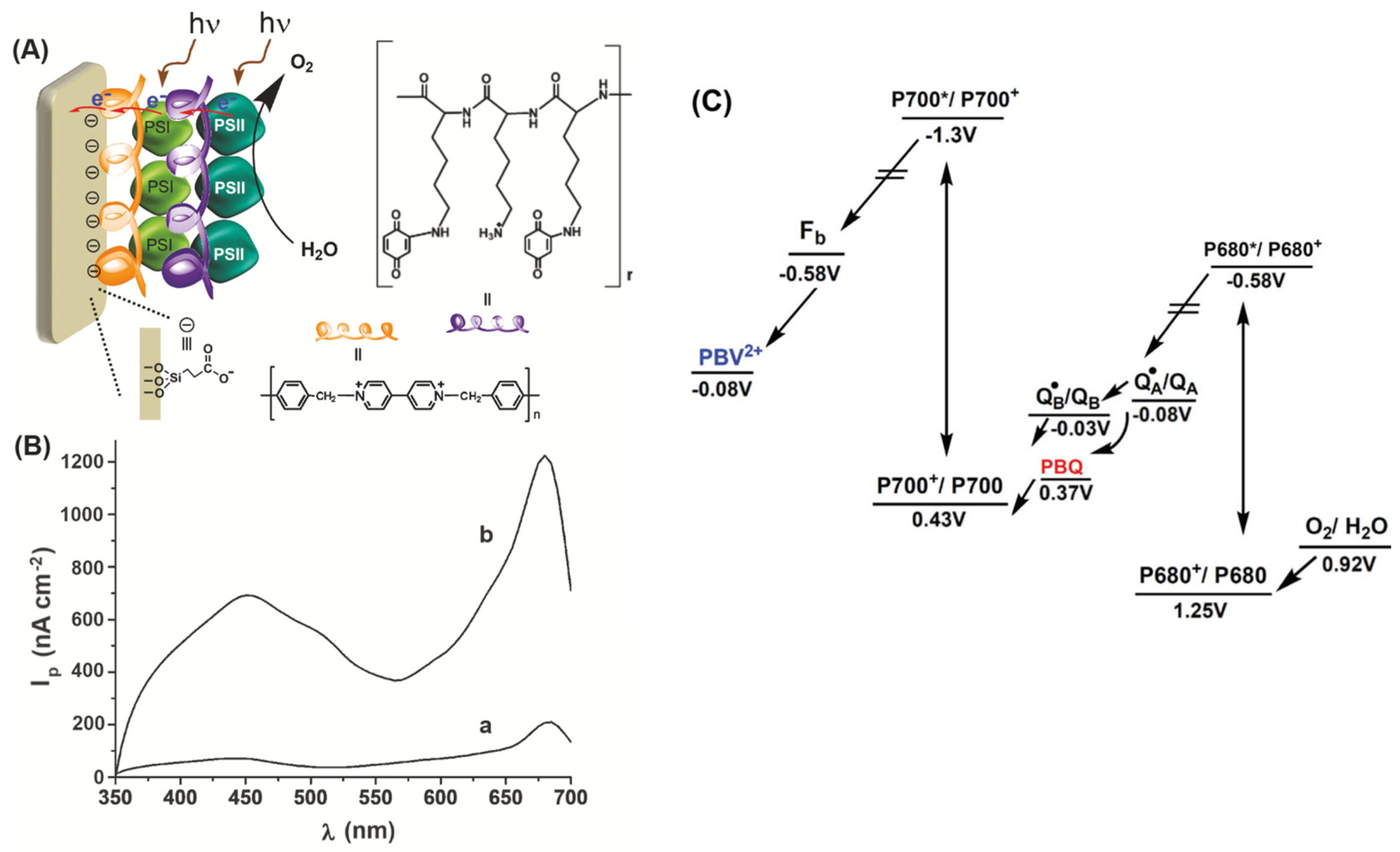
- the non-oriented covalent attachment of photosynthetic materials to sensing surfaces: amino groups from the lysine residues in the photosynthetic reaction centers (RC) were bound via bifunctional reagents such as glutaraldehyde to other amino groups in the other RC or in proteins such as bovine serum albumin, collagen, or gelatin, which protect the biological material against denaturation [152]. A special case is represented by the “wiring” of photosystems I and II to surfaces by cross-linking them to redox polymers. The polymer serves as both an immobilization matrix and as a facilitator of the electron transfer from the photosystem to the electrode surface.
- (2)
- the covalent, oriented immobilization of RC was achieved by chemisorption using the thiol group in the cysteine residue in the RC [168]. Alternatively, phosphonic acid linkers with carboxylic end groups were used to attract the PS by electrostatic interactions and the covalent bonds were formed via carbodiimide chemistry between the carboxylic groups of the linker and the amine groups in the RC.
- (3)
- non-covalent, oriented attachment of photosynthetic RC with engineered poly(His) tag to electrodes modified with a complex of nickel and nitrilotriacetic acid (Ni-NTA) via metal histidine affinity [169].
| Biological Preparation | Immobilization Matrix | Applied Potential | Current Density (μA cm−2) | Reference |
|---|---|---|---|---|
| Thylakoid membranes from pea plants (Pisum sativum L.) | Adsorbed on carbon paper | 1 V vs. Ag/AgCl | 14 | [174] |
| Thylakoid membranes from spinach | Thin film of thylakoids, osmium redox polymer, and ITO NP on a porous graphite electrode | 0.4 V vs. Ag/AgCl | 500 | [173] |
| PSII and PSI from Mastigocladus laminosus | ITO | (a) PBV2+/PSI/PBV2+/PSI: −0.05 V vs. Ag/AgCl (b) PBV2+/PSII/PBV2+/PSII; 0 V vs. Ag/AgCl (c) PBV2+/PSI/PBQ/PSII, OCV | (a) 2.2 (b) 0.5 (c) 1.2 | [175] |
| PSI extracted from baby spinach | PSI multilayer deposited | 0.1 V vs. Ag/AgCl | 7.9 | [176] |
| on electrode surface | ||||
| PSI from Mastigocladus laminosus | Bis aniline-crosslinked Pt NPs/PSI composite-modified surface | 0.3 V vs. Ag/AgCl | 4 | [177] |
| PSII from Thermosynechococcus elongatus | PSII immobilized in Os2+/3+ complex containing hydrogel-modified electrode | 0.3 V vs. Ag/AgCl | 45 | [172] |
| PSII from Thermosynechococcus vulcanus | His-tag/PSII-modified Au electrode | 0.3 V vs. Ag/AgCl | 2.4 | [178] |
| PSII from Thermosynechococcus elongatus | Mesoporous ITO electrode | 0.5 V vs. NHE | 1.6 ± 0.3 2.2 ± 0.5 (45 °C) 12 ± 1 (with NQS) 22 ± 2 (with DCBQ) | [179] |
| PSII from Thermosynechococcus elongatus | IO-ITO-Os-based polymer–PSII IO-ITO-PSII | 0.5 V vs. SHE | 381 ± 31 33 ± 5 μA | [171] |
| PSII from Thermosynechococcus elongatus | MgAl-[FeCN)6]/PSII CoAl–[FeCN)6]/PSII | +0.5 V vs. NHE | 0.07 ± 0.02 2.3 ± 0.2 | [180] |
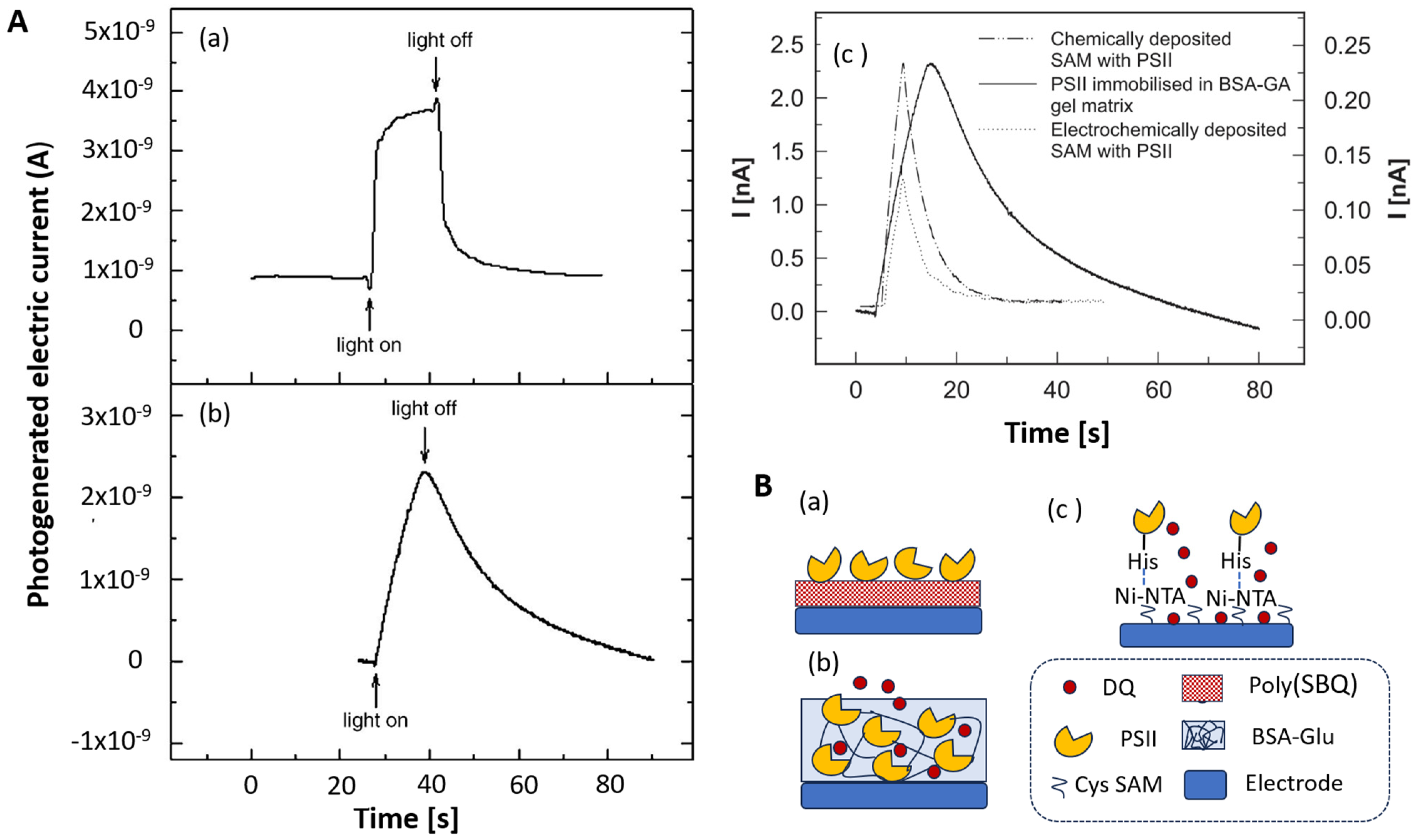
4.2.1. Stability versus High Current Density and Short Response Time
4.2.2. Interfering Contributions to the Output Signal
5. Applications of Extremozyme-Based Biosensors
5.1. Detection of Heavy Metals
5.2. Detection of Pesticides
5.2.1. Detection of Organophosphorus Pesticides
5.2.2. Detection of Dithiocarbamate Fungicides
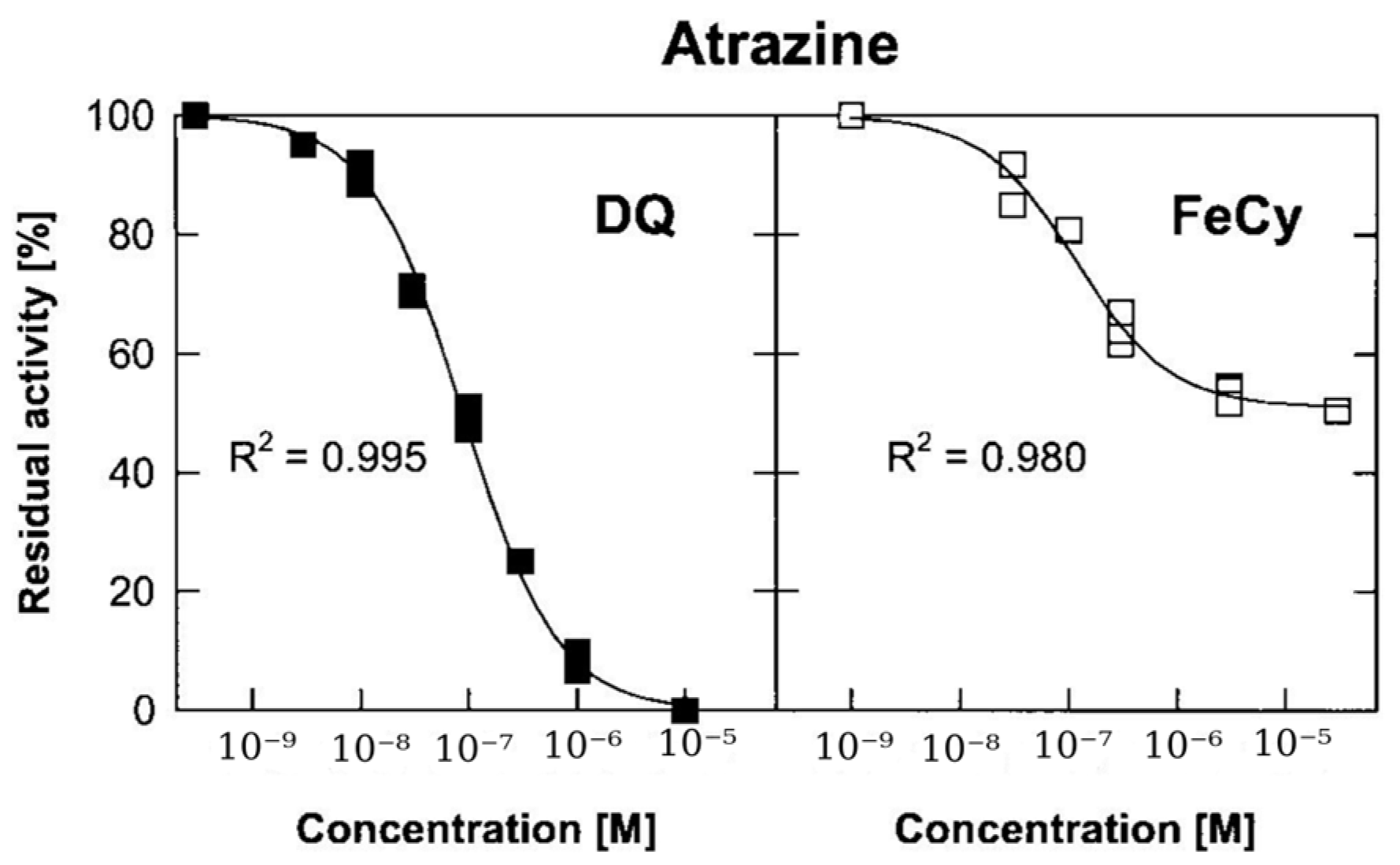
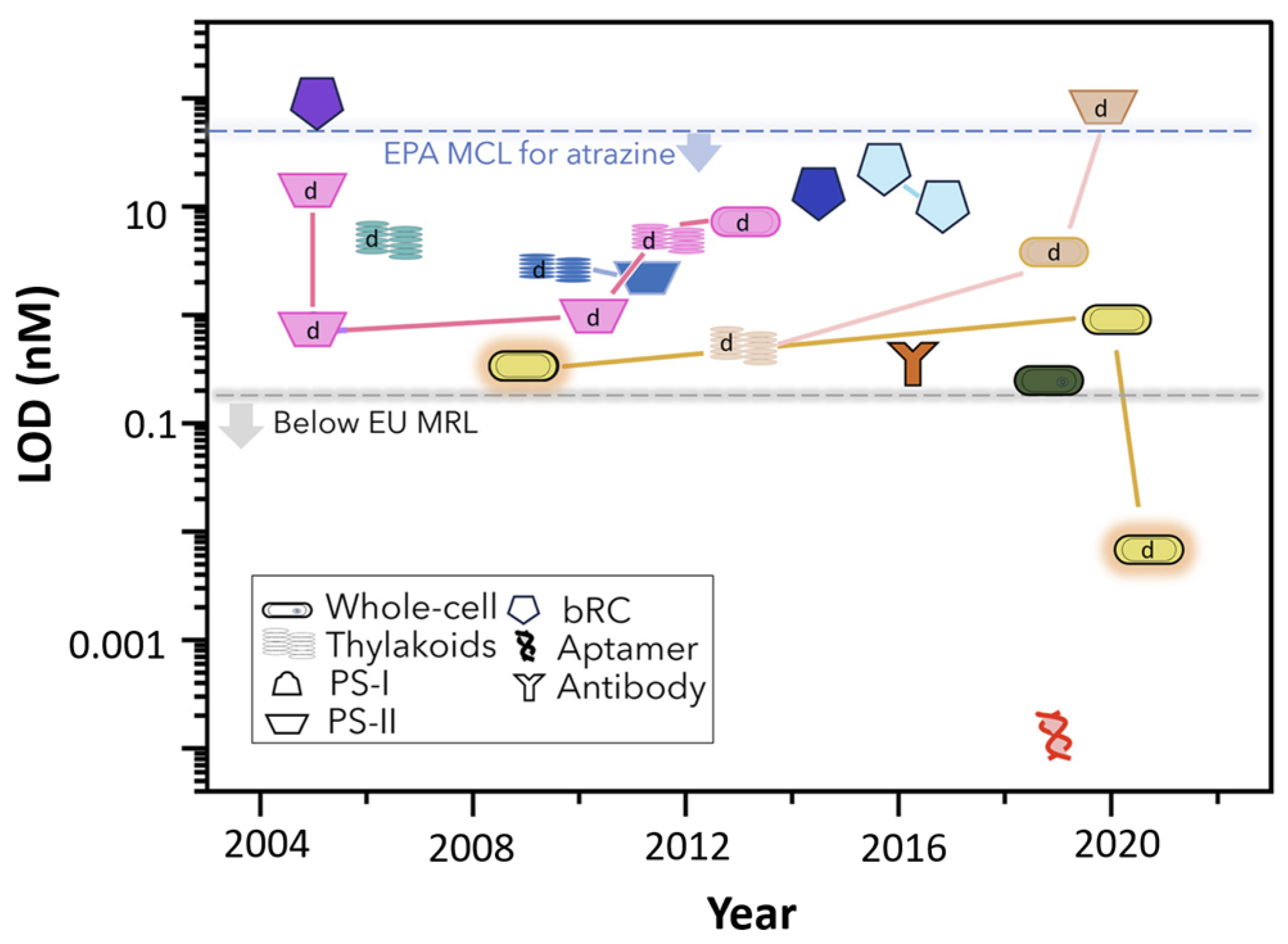
5.2.3. Detection of Photosynthesis Inhibiting Herbicides
5.3. Detection of Phenols
6. Challenges and Perspectives of Extremozyme-Based Biosensors
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallo, G.; Puopolo, R.; Carbonaro, M.; Maresca, E.; Fiorentino, G. Extremophiles, a Nifty Tool to Face Environmental Pollution: From Exploitation of Metabolism to Genome Engineering. Int. J. Environ. Res. Public Health 2021, 18, 5228. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, C.; Nguyen, M.K.; Hussain, A.; Bui, X.T.; Ngo, H.H. A review of biosensor for environmental monitoring: Principle, application, and corresponding achievement of sustainable development goals. Bioengineered 2023, 14, 58–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zeng, Z.; Zeng, G.; Xiao, R.; Wang, Y.; Hu, Y.; Tang, L.; Feng, C. Sensors for the environmental pollutant detection: Are we already there? Coord. Chem. Rev. 2021, 431, 213681. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezginturk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Gavrilas, S.; Ursachi, C.S.; Perta-Crisan, S.; Munteanu, F.D. Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors 2022, 22, 1513. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Bhardwaj, A. Biosensor technology for pesticides—A review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.D.; Marty, J.L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Staiano, M.; Pennacchio, A.; Varriale, A.; Capo, A.; Majoli, A.; Capacchione, C.; D’Auria, S. Enzymes as Sensors. Methods Enzymol. 2017, 589, 115–131. [Google Scholar] [CrossRef]
- Soy, S.; Sharma, S.R.; Nigam, V.K. Bio-fabrication of thermozyme-based nano-biosensors: Their components and present scenario. J. Mater. Sci. Mater. Electron. 2022, 33, 5523–5533. [Google Scholar] [CrossRef]
- Espina, G.; Atalah, J.; Blamey, J.M. Extremophilic Oxidoreductases for the Industry: Five Successful Examples with Promising Projections. Front. Bioeng. Biotechnol. 2021, 9, 710035. [Google Scholar] [CrossRef]
- Dopson, M.; Ni, G.; Sleutels, T.H. Possibilities for extremophilic microorganisms in microbial electrochemical systems. FEMS Microbiol. Rev. 2016, 40, 164–181. [Google Scholar] [CrossRef]
- Dumorne, K.; Cordova, D.C.; Astorga-Elo, M.; Renganathan, P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef]
- Espina, G.; Muñoz-Ibacache, S.A.; Cáceres-Moreno, P.; Amenabar, M.J.; Blamey, J.M. From the Discovery of Extremozymes to an Enzymatic Product: Roadmap Based on Their Applications. Front. Bioeng. Biotechnol. 2021, 9, 752281. [Google Scholar] [CrossRef]
- Miteva, V. Bacteria in Snow and Glacier Ice. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.-C., Gerday, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 31–50. [Google Scholar]
- Yadav, A.N.; Yadav, N.; Sachan, S.G.; Saxena, A.K. Biodiversity of psychrotrophic microbes and their biotechnological applications. J. Appl. Biol. Biotechnol. 2019, 7, 99–108. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Ma, J.; Zhou, Y.; Wei, Q.; Tian, C.; Fang, Y.; Zhong, R.; Chen, G.; Zhang, S. Advances in cold-adapted enzymes derived from microorganisms. Front. Microbiol. 2023, 14, 1152847. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Annapure, U.S.; Pratisha, N. Chapter 14—Psychrozymes: A novel and promising resource for industrial applications. In Microbial Extremozymes; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 185–195. [Google Scholar]
- Watanabe, K.; Kitamura, K.; Suzuki, Y. Analysis of the critical sites for protein thermostabilization by proline substitution in oligo-1,6-glucosidase from Bacillus coagulans ATCC 7050 and the evolutionary consideration of proline residues. Appl. Environ. Microbiol. 1996, 62, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Wang, Y.F.; Hou, Y.H.; Shi, Y.L.; Han, H.; Miao, M.; Wu, Y.Y.; Liu, Y.P.; Yue, X.N.; Li, Y.J. Cloning, expression and biochemical characterization of recombinant superoxide dismutase from Antarctic psychrophilic bacterium Pseudoalteromonas sp. ANT506. J. Basic. Microbiol. 2016, 56, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Liu, W.; Chen, C.C.; Ko, T.P.; He, M.; Xu, Z.; Liu, M.; Luo, H.; Guo, R.T.; et al. Structural insight into potential cold adaptation mechanism through a psychrophilic glycoside hydrolase family 10 endo-β-1,4-xylanase. J. Struct. Biol. 2016, 193, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Necula-Petrareanu, G.; Lavin, P.; Paun, V.I.; Gheorghita, G.R.; Vasilescu, A.; Purcarea, C. Highly Stable, Cold-Active Aldehyde Dehydrogenase from the Marine Antarctic Flavobacterium sp. PL002. Fermentation 2022, 8, 7. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Kazuoka, T.; Yoshida, M.; Yamanaka, K.; Oikawa, T.; Soda, K. Thermostable aldehyde dehydrogenase from psychrophile, Cytophaga sp. KUC-1: Enzymological characteristics and functional properties. Biochem. Biophys. Res. Commun. 2002, 298, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Kim, S.M.; Choi, J.I. Purification, Characterization, and Cloning of a Cold-Adapted Protease from Antarctic Janthinobacterium lividum. J. Microbiol. Biotechnol. 2018, 28, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Vieille, C.; Burdette, D.S.; Zeikus, J.G. Thermozymes. Biotechnol. Annu. Rev. 1996, 2, 1–83. [Google Scholar] [CrossRef] [PubMed]
- Stetter, K.O. History of discovery of the first hyperthermophiles. Extremophiles 2006, 10, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cen, Z.; Zhao, J. The survival mechanisms of thermophiles at high temperatures: An angle of omics. Physiology (Bethesda) 2015, 30, 97–106. [Google Scholar] [CrossRef]
- Razvi, A.; Scholtz, J.M. Lessons in stability from thermophilic proteins. Protein Sci. 2006, 15, 1569–1578. [Google Scholar] [CrossRef]
- Kumar, S.; Tsai, C.J.; Nussinov, R. Factors enhancing protein thermostability. Protein Eng. 2000, 13, 179–191. [Google Scholar] [CrossRef]
- Bruins, M.E.; Janssen, A.E.M.; Boom, R.M. Thermozymes and their applications. Appl. Biochem. Biotechnol. 2001, 90, 155–186. [Google Scholar] [CrossRef]
- Ward, D.E.; Shockley, K.R.; Chang, L.S.; Levy, R.D.; Michel, J.K.; Conners, S.B.; Kelly, R.M. Proteolysis in hyperthermophilic microorganisms. Archaea 2002, 1, 63–74. [Google Scholar] [CrossRef]
- Ruginescu, R.; Gomoiu, I.; Popescu, O.; Cojoc, R.; Neagu, S.; Lucaci, I.; Batrinescu-Moteau, C.; Enache, M. Bioprospecting for Novel Halophilic and Halotolerant Sources of Hydrolytic Enzymes in Brackish, Saline and Hypersaline Lakes of Romania. Microorganisms 2020, 8, 1903. [Google Scholar] [CrossRef]
- Oren, A. Properties of Halophilic Proteins. In Halophilic Microorganisms and Their Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 233–278. [Google Scholar]
- Tadeo, X.; Lopez-Mendez, B.; Trigueros, T.; Lain, A.; Castano, D.; Millet, O. Structural basis for the aminoacid composition of proteins from halophilic archea. PLoS Biol. 2009, 7, e1000257. [Google Scholar] [CrossRef]
- Siglioccolo, A.; Paiardini, A.; Piscitelli, M.; Pascarella, S. Structural adaptation of extreme halophilic proteins through decrease of conserved hydrophobic contact surface. BMC Struct. Biol. 2011, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Gunde-Cimerman, N.; Plemenitas, A.; Oren, A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Amoozegar, M.A.; Safarpour, A.; Noghabi, K.A.; Bakhtiary, T.; Ventosa, A. Halophiles and Their Vast Potential in Biofuel Production. Front. Microbiol. 2019, 10, 1895. [Google Scholar] [CrossRef]
- Horikoshi, K. Alkaliphiles: Some applications of their products for biotechnology. Microbiol. Mol. Biol. Rev. 1999, 63, 735–750. [Google Scholar] [CrossRef]
- Dubnovitsky, A.P.; Kapetaniou, E.G.; Papageorgiou, A.C. Enzyme adaptation to alkaline pH: Atomic resolution (1.08 A) structure of phosphoserine aminotransferase from Bacillus alcalophilus. Protein Sci. 2005, 14, 97–110. [Google Scholar] [CrossRef]
- Sarethy, I.P.; Saxena, Y.; Kapoor, A.; Sharma, M.; Sharma, S.K.; Gupta, V.; Gupta, S. Alkaliphilic bacteria: Applications in industrial biotechnology. J. Ind. Microbiol. Biotechnol. 2011, 38, 769–790. [Google Scholar] [CrossRef]
- Preiss, L.; Hicks, D.B.; Suzuki, S.; Meier, T.; Krulwich, T.A. Alkaliphilic Bacteria with Impact on Industrial Applications, Concepts of Early Life Forms, and Bioenergetics of ATP Synthesis. Front. Bioeng. Biotechnol. 2015, 3, 75. [Google Scholar] [CrossRef]
- Johnson, D.B. Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol. Ecol. 1998, 27, 307–317. [Google Scholar] [CrossRef]
- Reed, C.J.; Lewis, H.; Trejo, E.; Winston, V.; Evilia, C. Protein adaptations in archaeal extremophiles. Archaea 2013, 2013, 373275. [Google Scholar] [CrossRef] [PubMed]
- Schwermann, B.; Pfau, K.; Liliensiek, B.; Schleyer, M.; Fischer, T.; Bakker, E.P. Purification, Properties and Structural Aspects of a Thermoacidophilic α-Amylase from Alicyclobacillus Acidocaldarius Atcc 27009. Eur. J. Biochem. 1994, 226, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kawarabayasi, Y.; Satyanarayana, T. Acidophilic bacteria and archaea: Acid stable biocatalysts and their potential applications. Extremophiles 2012, 16, 1–19. [Google Scholar] [CrossRef]
- Athens Research & Technology, Atlanta, Georgia, USA. Glutamate Dehydrogenase, Recombinant Microbial (Swissaustral USA). Available online: https://www.athensresearch.com/products/enzymes-and-inhibitors/ (accessed on 7 December 2023).
- Kerafast, B. USA. Recombinant Microbial Glutamate Dehydrogenase (GDH) Datasheet. 2023. Available online: https://www.kerafast.com/ (accessed on 7 December 2023).
- Swissaustral USA. Catalase. Available online: http://www.swissaustral.com (accessed on 7 December 2023).
- Wang, Z.; Cai, Y.; Liao, X.; Zhang, F.; Zhang, D.; Li, Z. Production and characterization of a novel laccase with cold adaptation and high thermal stability from an isolated fungus. Appl. Biochem. Biotech. 2010, 162, 280–294. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, M.; Zhang, M.; Wang, C.; Liu, Y.; Fan, X.; Li, H. Characterization of a novel, cold-adapted, and thermostable laccase-like enzyme with high tolerance for organic solvents and salt and potent dye decolorization ability, derived from a marine metagenomic library. Front. Microbiol. 2018, 9, 426783. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K. A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 2005, 9, 415–425. [Google Scholar] [CrossRef]
- Brander, S.; Mikkelsen, J.D.; Kepp, K.P. TtMCO: A highly thermostable laccase-like multicopper oxidase from the thermophilic Thermobaculum terrenum. J. Mol. Catal. B Enzym. 2015, 112, 59–65. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Song, W.-Y.; Kim, T.-Y.; Hur, H.-G. A novel laccase from thermoalkaliphilic bacterium Caldalkalibacillus thermarum strain TA2.A1 able to catalyze dimerization of a lignin model compound. Appl. Microbiol. Biotechnol. 2018, 102, 4075–4086. [Google Scholar] [CrossRef]
- Uthandi, S.; Saad, B.; Humbard Matthew, A.; Maupin-Furlow Julie, A. LccA, an Archaeal Laccase Secreted as a Highly Stable Glycoprotein into the Extracellular Medium by Haloferax volcanii. Appl. Environ. Microb. 2010, 76, 733–743. [Google Scholar] [CrossRef]
- Sulistyaningdyah, W.T.; Ogawa, J.; Tanaka, H.; Maeda, C.; Shimizu, S. Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G-4 in comparison with bilirubin oxidase. FEMS Microbiol. Lett. 2004, 230, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Tetsch, L.; Bend, J.; Hölker, U. Molecular and enzymatic characterisation of extra- and intracellular laccases from the acidophilic ascomycete Hortaea acidophila. Antonie van Leeuwenhoek 2006, 90, 183–194. [Google Scholar] [CrossRef]
- Tan, S.-I.; Ng, I.S.; Yu, Y.-J. Heterologous expression of an acidophilic multicopper oxidase in Escherichia coli and its applications in biorecovery of gold. Bioresour. Bioprocess. 2017, 4, 20. [Google Scholar] [CrossRef]
- Kim, H.; Yeon, Y.J.; Choi, Y.R.; Song, W.; Pack, S.P.; Choi, Y.S. A cold-adapted tyrosinase with an abnormally high monophenolase/diphenolase activity ratio originating from the marine archaeon Candidatus Nitrosopumilus koreensis. Biotechnol. Lett. 2016, 38, 1535–1542. [Google Scholar] [CrossRef]
- Kong, K.-H.; Hong, M.-P.; Choi, S.-S.; Kim, Y.-T.; Cho, S.-H. Purification and characterization of a highly stable tyrosinase from Thermomicrobium roseum. Biotechnol. Appl. Biochem. 2000, 31, 113–118. [Google Scholar] [CrossRef]
- Suzuki, S.; Hirahara, T.; Horinouchi, S.; Beppu, T. Purification and Properties of Thermostable Tryptophanase from an Obligately Symbiotic Thermophile, Symbiobactevium thermophilum. Agric. Biol. Chem. 1991, 55, 3059–3066. [Google Scholar] [CrossRef]
- Harir, M.; Bellahcene, M.; Baratto, M.C.; Pollini, S.; Rossolini, G.M.; Trabalzini, L.; Fatarella, E.; Pogni, R. Isolation and characterization of a novel tyrosinase produced by Sahara soil actinobacteria and immobilization on nylon nanofiber membranes. J. Biotechnol. 2018, 265, 54–64. [Google Scholar] [CrossRef]
- Ishida, Y.; Tsuruta, H.; Tsuneta, S.T.; Uno, T.; Watanabe, K.; Aizono, Y. Characteristics of Psychrophilic Alkaline Phosphatase. Biosci. Biotechnol. Biochem. 1998, 62, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Rina, M.; Pozidis, C.; Mavromatis, K.; Tzanodaskalaki, M.; Kokkinidis, M.; Bouriotis, V. Alkaline phosphatase from the Antarctic strain TAB5. Eur. J. Biochem. 2000, 267, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Gregory Zeikus, J. Purification and characterization of alkaline phosphatase from Thermotoga neapolitana. Enzym. Microb. Technol. 1997, 21, 335–340. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Yang, F. Expression and Characterization of Recombinant Thermostable Alkaline Phosphatase from a Novel Thermophilic Bacterium Thermus thermophilus XM. Acta Biochim. Biophys. Sin. 2007, 39, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Yamashita, S.; Tokunaga, M. Characterization of Halophilic Alkaline Phosphatase from Halomonas sp. 593, a Moderately Halophilic Bacterium. Biosci. Biotechnol. Biochem. 2005, 69, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Hecht, K.; Eisenberg, H.; Mevarech, M. Extracellular Ca2(+)-dependent inducible alkaline phosphatase from extremely halophilic archaebacterium Haloarcula marismortui. J. Bacteriol. 1990, 172, 7065–7070. [Google Scholar] [CrossRef]
- Paun, V.I.; Banciu, R.M.; Lavin, P.; Vasilescu, A.; Fanjul-Bolado, P.; Purcarea, C. Antarctic aldehyde dehydrogenase from Flavobacterium PL002 as a potent catalyst for acetaldehyde determination in wine. Sci. Rep. 2022, 12, 17301. [Google Scholar] [CrossRef]
- Rosli, N.E.; Ali, M.S.; Kamarudin, N.H.; Masomian, M.; Latip, W.; Saadon, S.; Rahman, R.N. Structure Prediction and Characterization of Thermostable Aldehyde Dehydrogenase from Newly Isolated Anoxybacillus geothermalis Strain D9. Microorganisms 2022, 10, 1444. [Google Scholar] [CrossRef]
- Kim, H.-j.; Joo, W.-A.; Cho, C.-W.; Kim, C.-W. Halophile Aldehyde Dehydrogenase from Halobacterium salinarum. J. Proteome Res. 2006, 5, 192–195. [Google Scholar] [CrossRef]
- Cao, Y.; Liao, L.; Xu, X.-W.; Oren, A.; Wu, M. Aldehyde dehydrogenase of the haloalkaliphilic archaeon Natronomonas pharaonis and its function in ethanol metabolism. Extremophiles 2008, 12, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Miyanaga, A.; Kanaya, S.; Morikawa, M. Gene cloning and characterization of an aldehyde dehydrogenase from long-chain alkane-degrading Geobacillusthermoleovorans B23. Extremophiles 2010, 14, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Inoue, Y. Highly Purified Thermo-Stable Oxygen-Evolving Photosystem II Core Complex from the Thermophilic Cyanobacterium Synechococcus elongatus Having His-Tagged CP43. Plant Cell Physiol. 1999, 40, 1219–1231. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors 2021, 11, 12. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Zhang, N.; He, Y.; Zhang, D.; Liu, B.; Fang, Y. Recent advances in enzyme inhibition based-electrochemical biosensors for pharmaceutical and environmental analysis. Talanta 2023, 253, 124092. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Liu, Z.; Chu, Z.; Jin, W. Nanomaterial-based electrochemical enzymatic biosensors for recognizing phenolic compounds in aqueous effluents. Environ. Res. 2022, 214, 113858. [Google Scholar] [CrossRef]
- González-González, R.B.; Flores-Contreras, E.A.; González-González, E.; Torres Castillo, N.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Biosensor Constructs for the Monitoring of Persistent Emerging Pollutants in Environmental Matrices. Ind. Eng. Chem. Res. 2023, 62, 4503–4520. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Rangasamy, G.; Nisha Pauline, J.M.; Ramaraju, P.; Mohanasundaram, S.; Nguyen Vo, D.-V. Biosensor for heavy metals detection in wastewater: A review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Le, W.; Jie, Z.; Ruiqin, F.; Bilal, M.; Tang, L. Recent advances on engineered enzyme-conjugated biosensing modalities and devices for halogenated compounds. TrAC Trends Anal. Chem. 2021, 134, 116145. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS ES&T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Coronado-Apodaca, K.G.; González-Meza, G.M.; Aguayo-Acosta, A.; Araújo, R.G.; Gonzalez-Gonzalez, R.B.; Oyervides-Muñoz, M.A.; Martínez-Ruiz, M.; Melchor-Martínez, E.M.; Barceló, D.; Parra-Saldívar, R.; et al. Immobilized Enzyme-based Novel Biosensing System for Recognition of Toxic Elements in the Aqueous Environment. Top. Catal. 2023, 66, 606–624. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Laccase and Tyrosinase Biosensors Used in the Determination of Hydroxycinnamic Acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Rodríguez, A.M.; Parra-Arroyo, L.; González-González, R.B.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Laccase-assisted biosensing constructs—Robust modalities to detect and remove environmental contaminants. Case Stud. Chem. Environ. Eng. 2022, 5, 100180. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Khoo, K.S.; Soto-Moscoso, M. Enzyme Immobilized Nanomaterials: An Electrochemical Bio-Sensing and Biocatalytic Degradation Properties toward Organic Pollutants. Top. Catal. 2023, 66, 691–706. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Fan, Y.-F.; Guo, Z.-B.; Ge, G.-B. Enzyme-Based Biosensors and Their Applications. Biosensors 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Sosa-Hernández, J.E.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Enzyme (Single and Multiple) and Nanozyme Biosensors: Recent Developments and Their Novel Applications in the Water-Food-Health Nexus. Biosensors 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, E.G.; Vlachakis, D.; Papageorgiou, A.C.; Ataya, F.S.; Labrou, N.E. Structure-based design and application of an engineered glutathione transferase for the development of an optical biosensor for pesticides determination. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sok, V.; Fragoso, A. Amperometric biosensor for glyphosate based on the inhibition of tyrosinase conjugated to carbon nano-onions in a chitosan matrix on a screen-printed electrode. Microchim. Acta 2019, 186, 569. [Google Scholar] [CrossRef] [PubMed]
- Dabhade, A.; Jayaraman, S.; Paramasivan, B. Development of glucose oxidase-chitosan immobilized paper biosensor using screen-printed electrode for amperometric detection of Cr(VI) in water. 3 Biotech 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. A novel amperometric enzyme inhibition biosensor based on xanthine oxidase immobilised onto glassy carbon electrodes for bisphenol A determination. Talanta 2018, 184, 388–393. [Google Scholar] [CrossRef]
- Mohd Razib, M.S.; Latip, W.; Abdul Rashid, J.I.; Knight, V.F.; Wan Yunus, W.M.Z.; Ong, K.K.; Mohd Kasim, N.A.; Mohd Noor, S.A. An Enzyme-Based Biosensor for the Detection of Organophosphate Compounds Using Mutant Phosphotriesterase Immobilized onto Reduced Graphene Oxide. J. Chem. 2021, 2021, 2231089. [Google Scholar] [CrossRef]
- Liu, L.; Chen, C.; Chen, C.; Kang, X.; Zhang, H.; Tao, Y.; Xie, Q.; Yao, S. Poly(noradrenalin) based bi-enzyme biosensor for ultrasensitive multi-analyte determination. Talanta 2019, 194, 343–349. [Google Scholar] [CrossRef]
- Dabhade, A.; Jayaraman, S.; Paramasivan, B. Colorimetric paper bioassay by horseradish peroxidase for the detection of catechol and resorcinol in aqueous samples. Prep. Biochem. Biotech. 2020, 50, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Dhaka, S.; Paul, A.K.; Dayananda, S.; Deep, A. Organophosphate hydrolase conjugated UiO-66-NH2 MOF based highly sensitive optical detection of methyl parathion. Environ. Res. 2019, 174, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hondred, J.A.; Breger, J.C.; Alves, N.J.; Trammell, S.A.; Walper, S.A.; Medintz, I.L.; Claussen, J.C. Printed Graphene Electrochemical Biosensors Fabricated by Inkjet Maskless Lithography for Rapid and Sensitive Detection of Organophosphates. ACS Appl. Mater. Interf. 2018, 10, 11125–11134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, M.; Zhang, X.; Cao, J.; She, Y.; Cao, Z.; Wang, J.; Abd El-Aty, A.M. Acetylcholinesterase Immobilized on Magnetic Mesoporous Silica Nanoparticles Coupled with Fluorescence Analysis for Rapid Detection of Carbamate Pesticides. ACS Appl. Nano Mater. 2022, 5, 1327–1338. [Google Scholar] [CrossRef]
- da Silva, W.; Ghica, M.E.; Brett, C.M.A. Choline oxidase inhibition biosensor based on poly(brilliant cresyl blue)—Deep eutectic solvent/carbon nanotube modified electrode for dichlorvos organophosphorus pesticide. Sens. Actuators B Chem. 2019, 298, 126862. [Google Scholar] [CrossRef]
- Rigo, A.A.; Cezaro, A.M.D.; Muenchen, D.K.; Martinazzo, J.; Manzoli, A.; Steffens, J.; Steffens, C. Heavy metals detection in river water with cantilever nanobiosensor. J. Environ. Sci. Health B 2020, 55, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Noori, R.; Perwez, M.; Mazumder, J.A.; Sardar, M. Development of low-cost paper-based biosensor of polyphenol oxidase for detection of phenolic contaminants in water and clinical samples. Environ. Sci. Pollut. Res. 2020, 27, 30081–30092. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, Z.; Wang, M.; Chen, J.; Zhang, X. Biosensor based on polyaniline-polyacrylonitrile-graphene hybrid assemblies for the determination of phenolic compounds in water samples. J. Hazard. Mater. 2019, 378, 120714. [Google Scholar] [CrossRef]
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Lorenzo, E.; Morais, S. Laccase bioconjugate and multi-walled carbon nanotubes-based biosensor for bisphenol A analysis. Bioelectrochemistry 2022, 144, 108033. [Google Scholar] [CrossRef]
- Evli, S.; Uygun, D.A. Amperometric biosensor based on immobilized laccase onto Cys-Ag@Fe3O4 magnetic nanoparticles for selective catechol detection. J. Appl. Electrochem. 2023, 53, 1687–1700. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Tempesta, E.; Cartoni, A.; Plescia, P.; Bolognesi, P.; Chiarinelli, J.; Calandra, P.; Cicco, N.; Verrastro, M.F.; Centonze, D.; et al. Fabrication of a New, Low-Cost, and Environment-Friendly Laccase-Based Biosensor by Electrospray Immobilization with Unprecedented Reuse and Storage Performances. ACS Sustain. Chem. Eng. 2022, 10, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Butmee, P.; Tumcharern, G.; Songsiriritthigul, C.; Durand, M.J.; Thouand, G.; Kerr, M.; Kalcher, K.; Samphao, A. Enzymatic electrochemical biosensor for glyphosate detection based on acid phosphatase inhibition. Anal. Bioanal. Chem. 2021, 413, 5859–5869. [Google Scholar] [CrossRef]
- Iyer, R.; Pavlov, V.; Katakis, I.; Bachas, L.G. Amperometric Sensing at High Temperature with a “Wired” Thermostable Glucose-6-phosphate Dehydrogenase from Aquifex aeolicus. Anall Chem. 2003, 75, 3898–3901. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Bachas, L. Biosensor for Asparagine Using a Thermostable Recombinant Asparaginase from Archaeoglobus fulgidus. Anal. Chem. 2002, 74, 3336–3341. [Google Scholar] [CrossRef]
- Ozkan, M.; Erhan, E.; Terzi, O.; Tan, İ.; Ozöner, S.K.J.T. Thermostable amperometric lactate biosensor with Clostridium thermocellum L-LDH for the measurement of blood lactate. Talanta 2009, 79, 1412–1417. [Google Scholar] [CrossRef]
- Wei, Z.; Knaus, T.; Liu, Y.; Zhai, Z.; Gargano, A.F.G.; Rothenberg, G.; Yan, N.; Mutti, F.G. A high-performance electrochemical biosensor using an engineered urate oxidase. Chem. Commun. 2023, 59, 8071–8074. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, N.; Annamalai, J.; Kim, P. Extremozymes and Extremoproteins in Biosensor Applications. In Encyclopedia of Marine Biotechnology; Kim, S.-K., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1711–1736. [Google Scholar] [CrossRef]
- Purcarea, C.; Necula-Petrareanu, G.; Vasilescu, A. Chapter 7—Extremophile-assisted nanomaterial production and nanomaterial-based biosensing. In Functional Nanostructured Interfaces for Environmental and Biomedical Applications; Dinca, V., Suchea, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–180. [Google Scholar]
- Koblizek, M.; Masojidek, J.; Komenda, J.; Kucera, T.; Pilloton, R.; Mattoo, A.K.; Giardi, M.T. A sensitive photosystem II-based biosensor for detection of a class of herbicides. Biotechnol. Bioeng. 1998, 60, 664–669. [Google Scholar] [CrossRef]
- Koblížek, M.; Malý, J.; Masojídek, J.; Komenda, J.; Kučera, T.; Giardi, M.T.; Mattoo, A.K.; Pilloton, R. A biosensor for the detection of triazine and phenylurea herbicides designed using Photosystem II coupled to a screen-printed electrode. Biotechnol. Bioeng. 2002, 78, 110–116. [Google Scholar] [CrossRef]
- Maly, J.; Di Meo, C.; De Francesco, M.; Masci, A.; Masojidek, J.; Sugiura, M.; Volpe, A.; Pilloton, R. Reversible immobilization of engineered molecules by Ni-NTA chelators. Bioelectrochemistry 2004, 63, 271–275. [Google Scholar] [CrossRef]
- Malý, J.; Klem, K.; Lukavská, A.; Masojídek, J. Degradation and Movement in Soil of the Herbicide Isoproturon Analyzed by a Photosystem II–Based Biosensor. J. Environ. Qual. 2005, 34, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Masojídek, J.; Souček, P.; Máchová, J.; Frolík, J.; Klem, K.; Malý, J. Detection of photosynthetic herbicides: Algal growth inhibition test vs. electrochemical photosystem II biosensor. Ecotox Environ. Safe 2011, 74, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Maly, J.; Masojidek, J.; Masci, A.; Ilie, M.; Cianci, E.; Foglietti, V.; Vastarella, W.; Pilloton, R. Direct mediatorless electron transport between the monolayer of photosystem II and poly(mercapto-p-benzoquinone) modified gold electrode—New design of biosensor for herbicide detection. Biosens. Bioelectron. 2005, 21, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, F.; Hartmann, V.; Nowaczyk, M.M.; Ruff, A.; Schuhmann, W.; Conzuelo, F. Reassessing the rationale behind herbicide biosensors: The case of a photosystem II/redox polymer-based bioelectrode. Bioelectrochemistry 2020, 136, 107597. [Google Scholar] [CrossRef] [PubMed]
- Noguer, T.; Gradinaru, A.; Ciucu, A.; Marty, J.-L. A New Disposable Biosensor for the Accurate and Sensitive Detection of Ethylenebis (Dithiocarbamate) Fungicides. Anal. Lett. 1999, 32, 1723–1738. [Google Scholar] [CrossRef]
- Febbraio, F.; Merone, L.; Cetrangolo, G.P.; Rossi, M.; Nucci, R.; Manco, G. Thermostable Esterase 2 from Alicyclobacillus acidocaldarius as Biosensor for the Detection of Organophosphate Pesticides. Anal. Chem. 2011, 83, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Cetrangolo, G.P.; Rusko, J.; Gori, C.; Carullo, P.; Manco, G.; Chino, M.; Febbraio, F. Highly Sensitive Detection of Chemically Modified Thio-Organophosphates by an Enzymatic Biosensing Device: An Automated Robotic Approach. Sensors 2020, 20, 1365. [Google Scholar] [CrossRef]
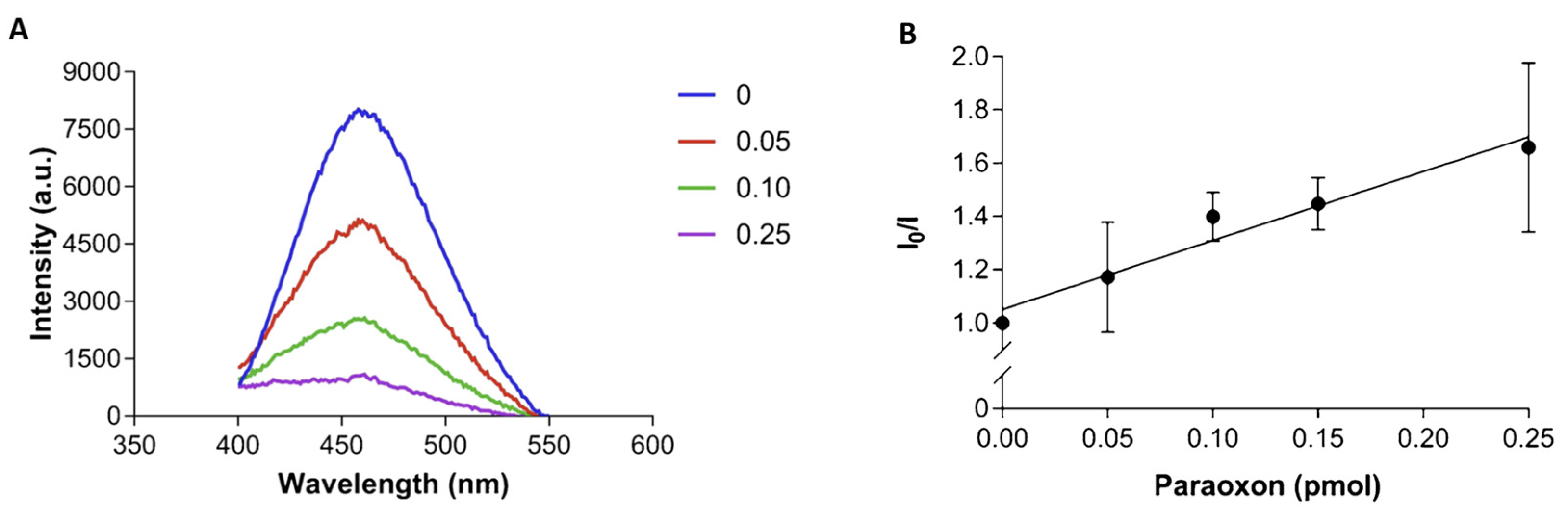
- Rodrigues, A.C.M.; Barbieri, M.V.; Chino, M.; Manco, G.; Febbraio, F. A FRET Approach to Detect Paraoxon among Organophosphate Pesticides Using a Fluorescent Biosensor. Sensors 2022, 22, 561. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Barbieri, M.V.; Febbraio, F. Monitoring of pesticide amount in fruit and vegetables by a fluorescence-based sensor. EFSA J. 2022, 20, e200419. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Barbieri, M.V.; Chino, M.; Manco, G.; Febbraio, F. A 3D printable adapter for solid-state fluorescence measurements: The case of an immobilized enzymatic bioreceptor for organophosphate pesticides detection. Anal. Bioanal. Chem. 2022, 414, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Bachas-Daunert, P.G.; Sellers, Z.P.; Wei, Y. Detection of halogenated organic compounds using immobilized thermophilic dehalogenase. Anal. Bioanal. Chem. 2009, 395, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.; Reiss, M.; Hartmeier, W. Amperometric phenol biosensor based on a thermostable phenol hydroxylase1This paper was presented at the fifth World Congress on Biosensors, Berlin, Germany, 3–5 June 1998.1. Biosens. Bioelectron. 1998, 13, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Politi, J.; Spadavecchia, J.; Fiorentino, G.; Antonucci, I.; De Stefano, L. Arsenate reductase from Thermus thermophilus conjugated to polyethylene glycol-stabilized gold nanospheres allow trace sensing and speciation of arsenic ions. J. R. Soc. Interface 2016, 13, 20160629. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, R.; Sorrentino, I.; Gallo, G.; Piscitelli, A.; Giardina, P.; Le Goff, A.; Fiorentino, G. Self-assembling thermostable chimeras as new platform for arsenic biosensing. Sci. Rep. 2021, 11, 2991. [Google Scholar] [CrossRef]
- Puopolo, R.; Gallo, G.; Limauro, D.; Contursi, P.; Fiorentino, G. A New Strategy for As(V) Biosensing Based on the Inhibition of the Phosphatase Activity of the Arsenate Reductase from Thermus thermophilus. Int. J. Mol. Sci. 2022, 23, 2942. [Google Scholar] [CrossRef]
- Compagnone, D.; McNeil, C.J.; Athey, D.; Di Ilio, C.; Guilbault, G.G. An amperometric NADH biosensor based on NADH oxidase from Thermus aquaticus. Enzym. Microb. Technol. 1995, 17, 472–476. [Google Scholar] [CrossRef]
- Antiochia, R.; Cass, A.E.G.; Palleschi, G. Purification and sensor applications of an oxygen insensitive, thermophilic diaphorase. Anal. Chim. Acta 1997, 345, 17–28. [Google Scholar] [CrossRef]
- Jeffries, C.; Pasco, N.; Baronian, K.; Gorton, L. Evaluation of a thermophile enzyme for a carbon paste amperometric biosensor: L-glutamate dehydrogenase. Biosens. Bioelectron. 1997, 12, 225–232. [Google Scholar] [CrossRef]
- Pasco, N.; Jeffries, C.; Davies, Q.; Downard, A.J.; Roddick-Lanzilotta, A.D.; Gorton, L. Characterisation of a thermophilic L-glutamate dehydrogenase biosensor for amperometric determination of L-glutamate by flow injection analysis. Biosens. Bioelectron. 1999, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Suzuki, H.; Ishimaru, Y.; Toyama, S.; Ikariyama, Y.; Iida, T. Thermophilic glucokinase-based sensors for the detection of various saccharides and glycosides. Sens. Actuators B Chem. 2005, 108, 727–732. [Google Scholar] [CrossRef]
- Tani, Y.; Tanaka, K.; Yabutani, T.; Mishima, Y.; Sakuraba, H.; Ohshima, T.; Motonaka, J. Development of a d-amino acids electrochemical sensor based on immobilization of thermostable d-Proline dehydrogenase within agar gel membrane. Anal. Chim. Acta 2008, 619, 215–220. [Google Scholar] [CrossRef]
- Iwasa, H.; Hiratsuka, A.; Yokoyama, K.; Uzawa, H.; Orihara, K.; Muguruma, H. Thermophilic Talaromyces emersonii Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase Bioanode for Biosensor and Biofuel Cell Applications. ACS Omega 2017, 2, 1660–1665. [Google Scholar] [CrossRef]
- Liu, D.; Li, W.; Jiang, X.; Bai, S.; Liu, J.; Liu, X.; Shi, Y.; Kuai, Z.; Kong, W.; Gao, R.; et al. Using near-infrared enhanced thermozyme and scFv dual-conjugated Au nanorods for detection and targeted photothermal treatment of Alzheimer’s disease. Theranostics 2019, 9, 2268–2281. [Google Scholar] [CrossRef]
- Titoiu, A.M.; Necula-Petrareanu, G.; Visinescu, D.; Dinca, V.; Bonciu, A.; Mihailescu, C.N.; Purcarea, C.; Boukherroub, R.; Szunerits, S.; Vasilescu, A. Flow injection enzymatic biosensor for aldehydes based on a Meldola Blue-Ni complex electrochemical mediator. Microchim. Acta 2020, 187, 550. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wohlschlager, L.; Csarman, F.; Ruff, A.; Schuhmann, W.; Scheiblbrandner, S.; Ludwig, R. Real-Time Measurement of Cellobiose and Glucose Formation during Enzymatic Biomass Hydrolysis. Anal. Chem. 2021, 93, 7732–7738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, M.; Zhang, J.; Jiang, Y. Synergistic catalysis and detection of hydrogen peroxide based on a 3D-dimensional molybdenum disulfide interspersed carbon nanotubes nanonetwork immobilized chloroperoxidase biosensor. Bioelectrochemistry 2023, 154, 108507. [Google Scholar] [CrossRef]
- Cai, P.; Li, G.; Li, J.; Jia, Y.; Zhang, Z.; Li, J. Photosystem II Based Multilayers. In Supramolecular Chemistry of Biomimetic Systems; Li, J., Ed.; Springer: Singapore, 2017; pp. 109–133. [Google Scholar]
- Wang, F.; Liu, X.; Willner, I. Integration of Photoswitchable Proteins, Photosynthetic Reaction Centers and Semiconductor/Biomolecule Hybrids with Electrode Supports for Optobioelectronic Applications. Adv. Mater. 2013, 25, 349–377. [Google Scholar] [CrossRef]
- Kato, M.; Zhang, J.Z.; Paul, N.; Reisner, E. Protein film photoelectrochemistry of the water oxidation enzyme photosystem II. Chem. Soc. Rev. 2014, 43, 6485–6497. [Google Scholar] [CrossRef]
- Yehezkeli, O.; Tel-Vered, R.; Michaeli, D.; Willner, I.; Nechushtai, R. Photosynthetic reaction center-functionalized electrodes for photo-bioelectrochemical cells. Photosynth. Res. 2014, 120, 71–85. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Enzyme Immobilization. Molecules 2023, 28, 1373. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Cowan, D.A.; Wood, A.N.P. Hyperstabilization of a thermophilic esterase by multipoint covalent attachment. Enzym. Microb. Technol. 1995, 17, 366–372. [Google Scholar] [CrossRef]
- Fernandez–Lafuente, R.; Guisan, J.M.; Ali, S.; Cowan, D. Immobilization of functionally unstable catechol-2,3-dioxygenase greatly improves operational stability. Enzym. Microb. Technol. 2000, 26, 568–573. [Google Scholar] [CrossRef]
- Yan, X.; Tang, J.; Ma, S.; Tanner, D.; Ludwig, R.; Ulstrup, J.; Xiao, X. Engineering bio-interfaces for the direct electron transfer of Myriococcum thermophilum cellobiose dehydrogenase: Towards a mediator-less biosupercapacitor/biofuel cell hybrid. Biosens. Bioelectron. 2022, 210, 114337. [Google Scholar] [CrossRef]
- Satomura, T.; Horinaga, K.; Tanaka, S.; Takamura, E.; Sakamoto, H.; Sakuraba, H.; Ohshima, T.; Suye, S.-I. Construction of a novel bioanode for amino acid powered fuel cells through an artificial enzyme cascade pathway. Biotechnol. Lett. 2019, 41, 605–611. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.; Rios, N.S.; Rodrigues, R.C.; Tardioli, P.W.; Berenguer-Murcia, Á.; Rocha-Martin, J.; Fernández-Lafuente, R. Immobilization of enzymes on nanomaterials: Necessity, opportunities, and drawbacks. In Bionanocatalysis: From Design to Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 419–450. [Google Scholar]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Bucur, B.; Purcarea, C.; Andreescu, S.; Vasilescu, A. Addressing the Selectivity of Enzyme Biosensors: Solutions and Perspectives. Sensors 2021, 21, 3038. [Google Scholar] [CrossRef]
- Bengtson Nash, S.M.; Goddard, J.; Müller, J.F. Phytotoxicity of surface waters of the Thames and Brisbane River Estuaries: A combined chemical analysis and bioassay approach for the comparison of two systems. Biosens. Bioelectron. 2006, 21, 2086–2093. [Google Scholar] [CrossRef]
- Febbraio, F.; D’Andrea, S.E.; Mandrich, L.; Merone, L.; Rossi, M.; Nucci, R.; Manco, G. Irreversible inhibition of the thermophilic esterase EST2 from Alicyclobacillus acidocaldarius. Extremophiles 2008, 12, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Trebst, A. Inhibitors in the functional dissection of the photosynthetic electron transport system. Photosynth. Res. 2007, 92, 217–224. [Google Scholar] [CrossRef]
- Battaglino, B.; Grinzato, A.; Pagliano, C. Binding Properties of Photosynthetic Herbicides with the QB Site of the D1 Protein in Plant Photosystem II: A Combined Functional and Molecular Docking Study. Plants 2021, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Zhao, X.; Zazubovich, V. Detection of explosive compounds using Photosystem II-based biosensor. J. Electroanal. Chem. 2011, 657, 84–90. [Google Scholar] [CrossRef]
- Rouillon, R.; Piletsky, S.A.; Piletska, E.V.; Euzet, P.; Carpentier, R. Comparison of the Immobilization Techniques for Photosystem II. In Biotechnological Applications of Photosynthetic Proteins: Biochips, Biosensors and Biodevices; Giardi, M.T., Piletska, E.V., Eds.; Springer: Boston, MA, USA, 2006; pp. 73–83. [Google Scholar]
- Katz, E. Application of bifunctional reagents for immobilization of proteins on a carbon electrode surface: Oriented immobilization of photosynthetic reaction centers. J. Electroanal. Chem. 1994, 365, 157–164. [Google Scholar] [CrossRef]
- Trammell, S.A.; Wang, L.; Zullo, J.M.; Shashidhar, R.; Lebedev, N. Orientated binding of photosynthetic reaction centers on gold using Ni NTA self-assembled monolayers. Biosens. Bioelectron. 2004, 19, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Reisner, E. Advancing photosystem II photoelectrochemistry for semi-artificial photosynthesis. Nat. Rev. Chem. 2020, 4, 6–21. [Google Scholar] [CrossRef]
- Sokol, K.P.; Mersch, D.; Hartmann, V.; Zhang, J.Z.; Nowaczyk, M.M.; Rögner, M.; Ruff, A.; Schuhmann, W.; Plumeré, N.; Reisner, E. Rational wiring of photosystem II to hierarchical indium tin oxide electrodes using redox polymers. Energy Environ. Sci. 2016, 9, 3698–3709. [Google Scholar] [CrossRef]
- Badura, A.; Guschin, D.; Esper, B.; Kothe, T.; Neugebauer, S.; Schuhmann, W.; Rögner, M. Photo-Induced Electron Transfer between Photosystem 2 via Cross-linked Redox Hydrogels. Electroanalysis 2008, 20, 1043–1047. [Google Scholar] [CrossRef]
- Lee, J.; Shin, H.; Kang, C.; Kim, S. Solar Energy Conversion through Thylakoid Membranes Wired by Osmium Redox Polymer and Indium Tin Oxide Nanoparticles. ChemSusChem 2021, 14, 2216–2225. [Google Scholar] [CrossRef]
- Lettieri, S.; Battaglino, B.; Sacco, A.; Saracco, G.; Pagliano, C. A green and easy-to-assemble electrochemical biosensor based on thylakoid membranes for photosynthetic herbicides detection. Biosens. Bioelectron. 2022, 198, 113838. [Google Scholar] [CrossRef] [PubMed]
- Yehezkeli, O.; Tel-Vered, R.; Michaeli, D.; Nechushtai, R.; Willner, I. Photosystem I (PSI)/Photosystem II (PSII)-Based Photo-Bioelectrochemical Cells Revealing Directional Generation of Photocurrents. Small 2013, 9, 2970–2978. [Google Scholar] [CrossRef]
- Ciesielski, P.N.; Faulkner, C.J.; Irwin, M.T.; Gregory, J.M.; Tolk, N.H.; Cliffel, D.E.; Jennings, G.K. Enhanced Photocurrent Production by Photosystem I Multilayer Assemblies. Adv. Funct. Mater. 2010, 20, 4048–4054. [Google Scholar] [CrossRef]
- Yehezkeli, O.; Wilner, O.I.; Tel-Vered, R.; Roizman-Sade, D.; Nechushtai, R.; Willner, I. Generation of Photocurrents by Bis-aniline-Cross-Linked Pt Nanoparticle/Photosystem I Composites on Electrodes. J. Phys. Chem. B 2010, 114, 14383–14388. [Google Scholar] [CrossRef]
- Terasaki, N.; Iwai, M.; Yamamoto, N.; Hiraga, T.; Yamada, S.; Inoue, Y. Photocurrent generation properties of Histag-photosystem II immobilized on nanostructured gold electrode. Thin Solid Films 2008, 516, 2553–2557. [Google Scholar] [CrossRef]
- Kato, M.; Cardona, T.; Rutherford, A.W.; Reisner, E. Photoelectrochemical Water Oxidation with Photosystem II Integrated in a Mesoporous Indium–Tin Oxide Electrode. J. Am. Chem. Soc. 2012, 134, 8332–8335. [Google Scholar] [CrossRef]
- Kato, M.; Sato, H.; Yagi, I.; Sugiura, M. Bio-inorganic hybrid photoanodes of photosystem II and ferricyanide-intercalated layered double hydroxide for visible-light-driven water oxidation. Electrochim. Acta 2018, 264, 386–392. [Google Scholar] [CrossRef]
- Weliwatte, N.S.; Grattieri, M.; Minteer, S.D. Rational design of artificial redox-mediating systems toward upgrading photobioelectrocatalysis. Photochem. Photobiol. Sci. 2021, 20, 1333–1356. [Google Scholar] [CrossRef]
- Kanso, H.; Pankratova, G.; Bollella, P.; Leech, D.; Hernandez, D.; Gorton, L. Sunlight photocurrent generation from thylakoid membranes on gold nanoparticle modified screen-printed electrodes. J. Electroanal. Chem. 2018, 816, 259–264. [Google Scholar] [CrossRef]
- Maly, J.; Krejci, J.; Ilie, M.; Jakubka, L.; Masojídek, J.; Pilloton, R.; Sameh, K.; Steffan, P.; Stryhal, Z.; Sugiura, M. Monolayers of photosystem II on gold electrodes with enhanced sensor response—Effect of porosity and protein layer arrangement. Anal. Bioanal. Chem. 2005, 381, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Kothe, T.; Schuhmann, W.; Rögner, M. Wiring photosynthetic enzymes to electrodes. Energy Environ. Sci. 2011, 4, 3263–3274. [Google Scholar] [CrossRef]
- Kato, M.; Cardona, T.; Rutherford, A.W.; Reisner, E. Covalent Immobilization of Oriented Photosystem II on a Nanostructured Electrode for Solar Water Oxidation. J. Am. Chem. Soc. 2013, 135, 10610–10613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Hartmann, V.; Ruff, A.; Nowaczyk, M.M.; Rögner, M.; Schuhmann, W.; Conzuelo, F. Unravelling electron transfer processes at photosystem 2 embedded in an Os-complex modified redox polymer. Electrochim. Acta 2018, 290, 451–456. [Google Scholar] [CrossRef]
- Brinkert, K.; Le Formal, F.; Li, X.; Durrant, J.; Rutherford, A.W.; Fantuzzi, A. Photocurrents from photosystem II in a metal oxide hybrid system: Electron transfer pathways. Biochim. Biophys. Acta (BBA)—Bioenerg. 2016, 1857, 1497–1505. [Google Scholar] [CrossRef]
- Ulas, G.; Brudvig, G.W. Redirecting Electron Transfer in Photosystem II from Water to Redox-Active Metal Complexes. J. Am. Chem. Soc. 2011, 133, 13260–13263. [Google Scholar] [CrossRef]
- da Costa, F.P.; Cipolatti, E.P.; Furigo Júnior, A.; Oliveira Henriques, R. Nanoflowers: A New Approach of Enzyme Immobilization. Chem. Rec. 2022, 22, e20210029322. [Google Scholar] [CrossRef]
- Tel-Vered, R.; Willner, I. Photo-bioelectrochemical Cells for Energy Conversion, Sensing, and Optoelectronic Applications. ChemElectroChem 2014, 1, 1778–1797. [Google Scholar] [CrossRef]
- Gacitua, M.; Urrejola, C.; Carrasco, J.; Vicuña, R.; Srain, B.M.; Pantoja-Gutiérrez, S.; Leech, D.; Antiochia, R.; Tasca, F. Use of a Thermophile Desiccation-Tolerant Cyanobacterial Culture and Os Redox Polymer for the Preparation of Photocurrent Producing Anodes. Front. Bioeng. Biotechnol. 2020, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yuan, H.; Liu, B.; Peng, J.; Xu, L.; Yang, D. Review of the distribution and detection methods of heavy metals in the environment. Anal. Methods 2020, 12, 5747–5766. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency, Drinking Water Regulations. Available online: https://www.epa.gov/dwreginfo/drinking-water-regulations (accessed on 30 December 2023).
- Mukherjee, S.; Bhattacharyya, S.; Ghosh, K.; Pal, S.; Halder, A.; Naseri, M.; Mohammadniaei, M.; Sarkar, S.; Ghosh, A.; Sun, Y.; et al. Sensory development for heavy metal detection: A review on translation from conventional analysis to field-portable sensor. Trends Food Sci. Technol. 2021, 109, 674–689. [Google Scholar] [CrossRef]
- Sarkar, A.; Sarkar, K.D.; Amrutha, V.; Dutta, K. Chapter 15—An overview of enzyme-based biosensors for environmental monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Kaur Brar, S., Hegde, K., Pachapur, V.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–329. [Google Scholar]
- Ashrafi, A.M.; Sýs, M.; Sedláčková, E.; Shaaban Farag, A.; Adam, V.; Přibyl, J.; Richtera, L. Application of the Enzymatic Electrochemical Biosensors for Monitoring Non-Competitive Inhibition of Enzyme Activity by Heavy Metals. Sensors 2019, 19, 2939. [Google Scholar] [CrossRef]
- Hui, Y.; Huang, Z.; Alahi, M.E.E.; Nag, A.; Feng, S.; Mukhopadhyay, S.C. Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosensors 2022, 12, 551. [Google Scholar] [CrossRef]
- Tabibi, Z.; Massah, J.; Asefpour Vakilian, K. A biosensor for the sensitive and specific measurement of arsenite using gold nanoparticles. Measurement 2022, 187, 110281. [Google Scholar] [CrossRef]
- Sanllorente-Méndez, S.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Immobilization of Acetylcholinesterase on Screen-Printed Electrodes. Application to the Determination of Arsenic(III). Sensors 2010, 10, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Sanllorente-Méndez, S.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Development of acid phosphatase based amperometric biosensors for the inhibitive determination of As(V). Talanta 2012, 93, 301–306. [Google Scholar] [CrossRef]
- Statista. Global Pesticide Agricultural Use 2021, by Leading Country. 2023. Available online: https://www.statista.com/statistics/1263077/global-pesticide-agricultural-use/ (accessed on 5 January 2024).
- Statista. Global Number of Banned Highly Hazardous Pesticides (HHPs) in 2022, by Select Country. 2023. Available online: https://www.statista.com/statistics/1382175/number-of-banned-highly-hazardous-pesticides-worldwide-by-select-country/ (accessed on 5 January 2024).
- Clercq, G.D. (Reuters). European Parliament Scraps Pesticides Bill in Latest Setback for Green Legislation. 2023. Available online: https://www.reuters.com/world/europe/european-parliament-scraps-pesticides-bill-latest-setback-green-legislation-2023-11-22/ (accessed on 5 January 2024).
- Food and Agriculature Organisation of the United Nations; World Health Organisation; Codex Alimentarius International Food Standards. Codex Pesticides Residues in Food Online Database. 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/en/ (accessed on 5 January 2024).
- European Commission, EU Pesticides Database. 2023. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 5 January 2024).
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC-Trend Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef]
- Rafaqat, S.; Raqba; Ali, N.; Hussain, A. Validating role of different enzymes (laccases and catalases) based voltammetric biosensors in detection of pesticide and dye. Mater. Chem. Phys. 2022, 290, 126545. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Li, M.; Wang, M.; Liu, G.; Ping, J. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. TrAC-Trend Anal. Chem. 2023, 165, 117152. [Google Scholar] [CrossRef]
- Kumaran, A.; Vashishth, R.; Singh, S.; Surendran, U.; James, A.; Chellam, P.V. Biosensors for detection of organophosphate pesticides: Current technologies and future directives. Microchem. J. 2022, 178, 107420. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, X.; Zhou, G.; Ge, L.; Li, F. Acetylcholinesterase-catalyzed silver deposition for ultrasensitive electrochemical biosensing of organophosphorus pesticides. Analyst 2020, 145, 2339–2344. [Google Scholar] [CrossRef]
- Pundir, C.S.; Malik, A.; Preety. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Obare, S.O.; Wei, J. Molecularly imprinted polymers as chemosensors for organophosphate pesticide detection and environmental applications. TrAC Trends Anal. Chem. 2023, 167, 117231. [Google Scholar] [CrossRef]
- Ding, R.; Li, Z.; Xiong, Y.; Wu, W.; Yang, Q.; Ho, X. Electrochemical (bio)sensors for the detection of organophosphorus pesticides based on nanomaterial-modified electrodes: A review. Crit. Rev. Anal. Chem. 2023, 53, 1766–1791. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, Y.; Li, X.; Lan, L.; Jiang, C.; Ping, J. Metallic transition metal dichalcogenide nanosheets as an effective and biocompatible transducer for electrochemical detection of pesticide. Anal. Chem. 2018, 90, 11658–11664. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Yola, M.L.; Atar, N.; Orooji, Y.; Karimi, F.; Kumar, P.S.; Rouhi, J.; Baghayeri, M. A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J. Coll. Interf. Sci. 2021, 592, 174–185. [Google Scholar] [CrossRef]
- Bala, R.; Kumar, M.; Bansal, K.; Sharma, R.K.; Wangoo, N. Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens. Bioelectron. 2016, 85, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef] [PubMed]
- Reportlinker. Fungicides Market Report—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2017–2025. Available online: https://www.prnewswire.com/news-releases/fungicides-market-mancozeb-chlorothalonil-metalaxyl-strobilurin-and-others-for-cereals--grains-oilseeds--pulses-fruits--vegetables-and-other-crops---global-industry-analysis-size-share-growth-trends-and-forecast-20-300243678.html (accessed on 26 November 2023).
- Oliveira, T.M.B.F.; Fátima Barroso, M.; Morais, S.; Araújo, M.; Freire, C.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Laccase–Prussian blue film–graphene doped carbon paste modified electrode for carbamate pesticides quantification. Biosens. Bioelectron. 2013, 47, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.B.F.; Barroso, M.F.; Morais, S.; Araújo, M.; Freire, C.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Sensitive bi-enzymatic biosensor based on polyphenoloxidases–gold nanoparticles–chitosan hybrid film–graphene doped carbon paste electrode for carbamates detection. Bioelectrochemistry 2014, 98, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Noguer, T.; Balasoiu, A.-M.; Avramescu, A.; Marty, J.-L. Development of a disposable biosensor for the detection of metam-sodium and its metabolite MITC. Anal. Lett. 2001, 34, 513–528. [Google Scholar] [CrossRef]
- Noguer, T.; Marty, J.-L. High sensitive bienzymic sensor for the detection of dithiocarbamate fungicides. Anal. Chim. Acta 1997, 347, 63–70. [Google Scholar] [CrossRef]
- Ibáñez, D.; Izquierdo-Bote, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Development of a New Screen-Printed Transducer for the Electrochemical Detection of Thiram. Chemosensors 2021, 9, 303. [Google Scholar] [CrossRef]
- Shortall, K.; Arshi, S.; Bendl, S.; Xiao, X.; Belochapkine, S.; Demurtas, D.; Soulimane, T.; Magner, E. Coupled immobilized bi-enzymatic flow reactor employing cofactor regeneration of NAD+ using a thermophilic aldehyde dehydrogenase and lactate dehydrogenase. Green. Chem. 2023, 25, 4553–4564. [Google Scholar] [CrossRef]
- Vasilescu, A.; Titoiu, A.M.; Purcarea, C.; Necula-Petrareanu, G. Method for Determining the Fungicide Thiram Based on Enzymatic Inhibition and Electrochemical Sensor (In Romanian: Metoda de Determinare a Fungicidului tiram bazată pe Inhibiție Enzimatică șl Senzor Electrochimic). Patent Application (Romania) OSIM no A00587/13.08.2018. 2018. Available online: http://pub.osim.ro/publication-server/pdf-document?PN=RO133890%20RO%20133890&iDocId=12891&iepatch=.pdf (accessed on 5 January 2024).
- Xiao, L.; Feng, S.; Hua, M.Z.; Lu, X. Rapid determination of thiram on apple using a flexible bacterial cellulose-based SERS substrate. Talanta 2023, 254, 124128. [Google Scholar] [CrossRef]
- Antonacci, A.; Zappi, D.; Giardi, M.T.; Scognamiglio, V. Photosynthesis-based biosensors for environmental analysis of herbicides. Case Stud. Chem. Environ. Eng. 2021, 4, 100157. [Google Scholar] [CrossRef]
- Modak, N.; Friebe, V.M. Amperometric biosensors: Harnessing photosynthetic reaction centers for herbicide detection. Curr. Opin. Electrochem. 2023, 42, 101414. [Google Scholar] [CrossRef]
- Antonacci, A.; Attaallah, R.; Arduini, F.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A dual electro-optical biosensor based on Chlamydomonas reinhardtii immobilised on paper-based nanomodified screen-printed electrodes for herbicide monitoring. J. Nanobiotechnol. 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, Y.; Zhang, Z.; Du, B.; Lu, G.; Liu, M. Immobilization-free photoelectrochemical aptasensor for atrazine based on bifunctional graphene signal amplification and a controllable sulfhydryl-assembled BiOBr/AgNP microinterface. Anal. Chem. 2023, 95, 15736–15744. [Google Scholar] [CrossRef]
- Boron, I.; Juárez, A.; Battaglini, F. Portable Microalgal Biosensor for Herbicide Monitoring. ChemElectroChem 2020, 7, 1623–1630. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, E.V.R.; Fraceto, L.F.; Palleschi, G. An eco-designed paper-based algal biosensor for nanoformulated herbicide optical detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; Pezzotti, G.; Turemis, M.; Sanchís, J.; Farré, M.; Denaro, R.; Giacobbe, M.G.; Crisafi, F.; Giardi, M.T. Fast pesticide pre-screening in marine environment using a green microalgae-based optical bioassay. Mar. Pollut. Bull. 2018, 129, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Theriot, C.M.; Du, X.; Tove, S.R.; Grunden, A.M. Improving the catalytic activity of hyperthermophilic Pyrococcus prolidases for detoxification of organophosphorus nerve agents over a broad range of temperatures. Appl. Microbiol. Biotechnol. 2010, 87, 1715–1726. [Google Scholar] [CrossRef]
- Hassan, H.A.; Dawah, S.E.; El-Sheekh, M.M. Monitoring the degradation capability of novel haloalkaliphilic tributyltin chloride (TBTCl) resistant bacteria from butyltin-polluted site. Rev. Argent. Microbiol. 2019, 51, 39–46. [Google Scholar] [CrossRef]
- Gu, C.; Su, X.; Liu, B.; Zheng, C.; Wang, S.; Tian, Y.; Ma, J.; Wu, J. Recent progress in advanced materials for electrochemical determination of phenolic contaminants. Microchem. J. 2023, 195, 109513. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Calandra, P.; Antonacci, A.; Scognamiglio, V. The convergence of forefront technologies in the design of laccase-based biosensors—An update. TrAC-Trend Anal. Chem. 2019, 119, 115615. [Google Scholar] [CrossRef]
- Niu, K.; Gao, J.; Wu, L.; Lu, X.; Chen, J. Nitrogen-Doped Graphdiyne as a Robust Electrochemical Biosensing Platform for Ultrasensitive Detection of Environmental Pollutants. Anal. Chem. 2021, 93, 8656–8662. [Google Scholar] [CrossRef]
- Verrastro, M.; Cicco, N.; Crispo, F.; Morone, A.; Dinescu, M.; Dumitru, M.; Favati, F.; Centonze, D. Amperometric biosensor based on Laccase immobilized onto a screen-printed electrode by Matrix Assisted Pulsed Laser Evaporation. Talanta 2016, 154, 438–445. [Google Scholar] [CrossRef]
- Panwar, V.; Lzaod, S.; Dutta, T. Thermostable Bacterial Laccase: Catalytic Properties and Its Application in Biotransformation of Emerging Pollutants. ACS Omega 2023, 8, 34710–34719. [Google Scholar] [CrossRef]
- Kavetskyy, T.; Smutok, O.; Demkiv, O.; Kukhazh, Y.; Stasyuk, N.; Leonenko, E.; Kiv, A.; Kobayashi, Y.; Kinomura, A.; Šauša, O.; et al. Improvement of laccase biosensor characteristics using sulfur-doped TiO2 nanoparticles. Bioelectrochemistry 2022, 147, 108215. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, D.; Geng, X.; Li, Z.; Gao, R. Real-time regulation of catalysis by remote-controlled enzyme-conjugated gold nanorod composites for aldol reaction-based applications. Catal. Sci. Technol. 2019, 9, 2221–2230. [Google Scholar] [CrossRef]
- da Silva, R.T.P.; Ribeiro de Barros, H.; Sandrini, D.M.F.; Córdoba de Torresi, S.I. Stimuli-Responsive Regulation of Biocatalysis through Metallic Nanoparticle Interaction. Bioconjugate Chem. 2022, 33, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Freitas, A.I.; Domingues, L.; Aguiar, T.Q. Tag-mediated single-step purification and immobilization of recombinant proteins toward protein-engineered advanced materials. J. Adv. Res. 2022, 36, 249–264. [Google Scholar] [CrossRef]
- George, S.P.; Ahmad, A.; Rao, M.B. A novel thermostable xylanase from Thermomonospora sp.: Influence of additives on thermostability. Bioresour. Technol. 2001, 78, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotechnol. Appl. Biochem. 2004, 39, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Reyes-De-Corcuera, J.I.; Olstad, H.E.; García-Torres, R. Stability and Stabilization of Enzyme Biosensors: The Key to Successful Application and Commercialization. Ann. Rev. Food Sci. Technol. 2018, 9, 293–322. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants—A Review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef]
- Tschmelak, J.; Proll, G.; Riedt, J.; Kaiser, J.; Kraemmer, P.; Bárzaga, L.; Wilkinson, J.S.; Hua, P.; Patrick Hole, J.; Nudd, R.; et al. Biosensors for unattended, cost-effective and continuous monitoring of environmental pollution: Automated Water Analyser Computer Supported System (AWACSS) and River Analyser (RIANA). Int. J. Environ. Anal. Chem. 2005, 85, 837–852. [Google Scholar] [CrossRef]
- Hough, D.W.; Danson, M.J. Extremozymes. Curr. Opin. Chem. Biol. 1999, 3, 39–46. [Google Scholar] [CrossRef]
- Economou, A.; Karapetis, S.; Nikoleli, G.-P.; Nikolelis, D.; Bratakou, S.; Varzakas, T. Enzyme-based Sensors. In Advances in Food Diagnostics, 2nd ed.; Toldrá, F., Nollet, L.M.L., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2017; pp. 231–250. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed Evolution: Methodologies and Applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef]
- Andon, J.S.; Lee, B.; Wang, T. Enzyme directed evolution using genetically encodable biosensors. Org. Biomol. Chem. 2022, 20, 5891–5906. [Google Scholar] [CrossRef]
- Takano, K.; Okamoto, T.; Okada, J.; Tanaka, S.-I.; Angkawidjaja, C.; Koga, Y.; Kanaya, S. Stabilization by Fusion to the C-terminus of Hyperthermophile Sulfolobus tokodaii RNase HI: A Possibility of Protein Stabilization Tag. PLoS ONE 2011, 6, e16226. [Google Scholar] [CrossRef]
- Rusling, J.F.; Forster, R.J. Biosensors Designed for Clinical Applications. Biomedicines 2021, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Pezzotti, G.; Pezzotti, I.; Cano, J.; Buonasera, K.; Giannini, D.; Giardi, M.T. Biosensors for effective environmental and agrifood protection and commercialization: From research to market. Microchim. Acta 2010, 170, 215–225. [Google Scholar] [CrossRef]
- Antonacci, A.; Scognamiglio, V. Biotechnological Advances in the Design of Algae-Based Biosensors. Trends Biotechnol. 2020, 38, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Cheng, K.-C.; Liao, V.H.-C. A novel approach for rapidly and cost-effectively assessing toxicity of toxic metals in acidic water using an acidophilic iron-oxidizing biosensor. Chemosphere 2017, 186, 446–452. [Google Scholar] [CrossRef]
- Gaffney, E.M.; Simoska, O.; Minteer, S.D. The Use of Electroactive Halophilic Bacteria for Improvements and Advancements in Environmental High Saline Biosensing. Biosensors 2021, 11, 48. [Google Scholar] [CrossRef]
- Pansook, S.; Incharoensakdi, A.; Phunpruch, S. Effects of the Photosystem II Inhibitors CCCP and DCMU on Hydrogen Production by the Unicellular Halotolerant Cyanobacterium Aphanothece halophytica. Sci. World J. 2019, 2019, 1030236. [Google Scholar] [CrossRef]
- Larionova, M.D.; Markova, S.V.; Vysotski, E.S. The novel extremely psychrophilic luciferase from Metridia longa: Properties of a high-purity protein produced in insect cells. Biochem. Biophys. Res. Commun. 2017, 483, 772–778. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Kalyabina, V.P.; Kopylova, K.V.; Torgashina, I.G.; Kratasyuk, V.A. Design of bioluminescent biosensors for assessing contamination of complex matrices. Talanta 2021, 233, 122509. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Pesticide Sales in Europe. Available online: https://ec.europa.eu/eurostat/databrowser/view/aei_fm_salpest09/default/table?lang=en (accessed on 5 January 2024).

| Enzyme | Source | Extreme Features | Potential Uses of Biosensor | Reference |
|---|---|---|---|---|
| Laccase | Pycnoporus sp. | Active at 0–100 °C (optimally 70 °C), at pH 4–10; half-life > 85 h at 60 °C | Detection of carbamate pesticides, dyes, and phenolic compounds in cold environments, such as marine areas | [50] |
| Marine metagenome | Active at 0–70 °C (optimally 60 °C), at pH 4–8 (optimally pH 7); resistance to organic solvents and >1 M NaCl; stable > 2 h at 70 °C | [51] | ||
| Thermus thermophilus | Optimal activity at 92 °C, at pH 5; half-life > 14 h at 80 °C | Detection of carbamate pesticides, dyes, and phenolic compounds in thermal basins | [52] | |
| Thermobacullum terrenum | Active at 70–90 °C, at pH 4.5; half-life > 2 days at 70 °C | [53] | ||
| Caldalkalibacillus thermarum | Optimal activity at 70 °C, at pH 8; active at >1 M NaCl; resistance to various organic solvents, surfactants, and halides; half-life 12 h at 90 °C | [54] | ||
| Haloferax volcanii | Active at 21–60 °C (optimally 45 °C), at pH 6–8.4; active and stable at >1.4 M NaCl; resistance to various organic solvents; half-life 31 h at 50 °C | Detection of carbamate pesticides, dyes, and phenolic compounds in hypersaline lakes, marine areas or wastewaters | [55] | |
| Myrothecium verrucaria | Active and stable at pH 8–11.5 (optimally pH 9); optimal activity at 70 °C; stable > 1 h at 50 °C | Detection of carbamate pesticides, dyes and phenolic compounds in wastewater treatment plants (alkaline) and mining areas (acidic) | [56] | |
| Hortaea acidophila | Active and stable at pH 2–7; optimal activity on ABTS * at pH 2 | [57] | ||
| Proteus hauseri | Optimal activity at pH 2.2, at 50–65 °C (optimally at 55 °C) | [58] | ||
| Tyrosinase | Candidatus Nitrosopumilus koreensis | Active at 0–60 °C (optimally 20 °C), at pH 5–8 (optimally 6); retained 50% of maximal activity at 0 °C | Detection of pesticides, hormones, and phenolic compounds in cold environments, such as marine areas | [59] |
| Thermomicrobium roseum | Optimal activity at 70 °C and pH 9.5; retained 70% activity at pH 8.5–10; stable at <70 °C | Detection of pesticides, hormones, and phenolic compounds in thermal basins and hot industrial effluents | [60] | |
| Symbiobacterium thermophilum | Active at 50–80 °C (optimally 80 °C) and pH 6–9 (optimally pH 7); stable at pH 6–11 and at <80 °C | [61] | ||
| Streptomyces cyaneofuscatus | Active at 50–70 °C (optimally 55 °C) and pH 5–10 (optimally pH 6.5–7.5); stable at 40 °C and pH 5–10 | [62] | ||
| Alkaline phosphatase | Shewanella sp. | Active and stable at 0–80 °C (optimally 40 °C), at pH 6–11 (optimally pH 9.8) | Detection of heavy metals, pesticides and inorganic salts in marine areas, thermal basins, saline wastewater, etc. | [63] |
| Antarctic strain TAB5 | Active at 0–25 °C (optimally 25 °C), at pH 8.5 | [64] | ||
| Thermotoga neapolitana | Active at 20–90 °C (optimally 70 °C), at pH 7.5–11 (optimally 9.9); the half-life was 4 h at 90 °C | [65] | ||
| Thermus thermophilus | Active at 40–95 °C (optimally 75–80 °C), at pH 8–12.5 (optimally pH 12); retained >50% activity after 6 h at 80 °C | [66] | ||
| Halomonas sp. | Active at <2 M NaCl, at 37–50 °C and pH 6–11 (optimally pH 10.5) | [67] | ||
| Haloarcula marismortui | Active and stable up to 3 M NaCl and KCl, at pH 7.5–10 (optimally pH 8.5) | [68] | ||
| Aldehyde dehydrogenase | Flavobacterium sp. | Active and stable at 10–40 °C (optimally 35 °C), at pH 7.5; resistance to various organic solvents and salts | Detection of dithiocarbamate fungicides in marine areas, thermal basins, saline wastewater, etc. | [69] |
| Anoxybacillus geothermalis | Active and stable at 30–80 °C (optimally 60 °C), at pH 6–9 (optimally pH 8); tolerance to various organic solvents | [70] | ||
| Halobacterium salinarum | Active at 0–3 M NaCl (optimally 1 M), at pH 7.2, and at room temperature | [71] | ||
| Natronomonas pharaonis | Optimal activity at 60 °C, at pH 8, and at 0.25 M NaCl | [72] | ||
| Geobacillus thermoleovorans | Optimal activity at pH 10, at 50–55 °C | [73] | ||
| Photosystem II | Synechococcus elongatus | Stabile for >21 days at 20 °C | Detection of herbicides, heavy metals, and endocrine-disrupting chemicals in marine areas and other environments | [74] |
| Enzyme(s) | Analyte | Method | Analytical Characteristics | Sample | Reference |
|---|---|---|---|---|---|
| Glutathione transferase mutant Phe117Ile entrapped in sol-gel | α-endosulfan | Spectrophotometry | LR: 6.25 × 10−7–3 × 10−5 M | Spiked mineral and drinking water | [91] |
| Butyrylcholinesterase, alkaline phosphatase, and tyrosinase; origami paper device; carbon black modified screen-printed electrodes on office paper | Paraoxon, 2,4-dichlorophenoxyacetic acid (2,4-D), atrazine | CA | LOD: 2 ppb (paraoxon) 50 ppb (2,4-D) 10 ppb (atrazine) LR: 2–20 ppb (paraoxon) 100–600 ppb (2,4-D) 10–100 ppb (atrazine) | Spiked river water | [92] |
| Tyrosinase conjugated with carbon nano onions and immobilized in a chitosan matrix | Glyphosate | AMP | LOD: 6.5 × 10−9 M LR: 1.5 × 10−8–1.0 × 10−5 M | Water and soil samples taken from irrigation of a rice field | [93] |
| Glucose oxidase entrapped in chitosan on filter paper combined with SPCE | Cr(VI) | AMP | LOD: 0.05 ppm LR: 0.05–1 ppm | Water | [94] |
| Xanthine oxidase/cross-linking with glutaraldehyde/GC electrode | Bisphenol | AMP | Ki app: 8.15 × 10−9 M LOD: 1 × 10−9 M LR: 1 × 10−9 M–4 × 10−8 M | Mineral and river water | [95] |
| Mutant phosphotriesterase YT-PTE covalently immobilized on rGO, then drop-casted on SPCE | Organophosphates | DPV | LOD: 1.1 × 10−7 M | Spiked lake water, drain water, and soil run-off water | [96] |
| HRP and glucose oxidase co-immobilized in poly(noradrenaline) on a Pt electrode | Cr (VI) Cr (III) Glucose H2O2 | AMP | LOD: 2 × 10−10 M (Cr(VI)) LOD: 1 × 10−8 M (Cr(III)) 8 × 10−8 M (glucose) 1 × 10−5 M (H2O2) | Water samples | [97] |
| Horseradish peroxidase entrapped in chitosan on filter paper | Catechol Resorcinol | Image analysis | LOD: 0.45 mM (catechol) LOD: 0.09 mM (resorcinol) | Water samples | [98] |
| Hexahistidine-tagged organophosphorus hydrolase immobilized on Zr-MOF (UiO-66-NH2) | Methyl parathion | Fluorescence | LOD: 10 ppb (3.4 ×·10−8 M) LR: 10–106 ppb | Spiked tomato and orange | [99] |
| Phosphotriesterase cross-linked with glutaraldehyde on graphene electrode with Pt NP | Paraoxon | AMP | LOD: 3 × 10−9 M LR:1 × 10−7 M–1 × 10−6 M | Tap water, river water, soil slurry | [100] |
| Acetylcholinesterase covalently immobilized on magnetic mesoporous silica nanoparticles | Carbofuran Methomyl Isoprocarb Carbaryl | FL | LOD: 1 × 10−8 M LOD: 22 × 10−8 M LOD: 26 × 10−8 M LOD: 43 × 10−8 M | Spiked Chinese cabbage and cucumber | [101] |
| Choline oxidase immobilized in poly(brilliant cresyl blue)—on MWCNT/GCE | Dichlorvos | AMP | LOD: 1.55 × 10−9 M LR: 2.5 × 10−9 M–60 × 10−9 M | Spiked orange juice | [102] |
| Alkaline phosphatase covalently immobilized on SAM of 16-mercaptoundecanoic acid | Pb, Ni, Cd, Zn, Co, and Al | Nanocantilever | LOD: 0.32 ± 0.06 ppb (Pb) 0.87 ± 0.03 ppb (Ni) 0.33 ± 0.01 ppb (Cd) 0.48 ± 0.01 ppb (Zn) 0.42 ± 0.02 ppb (Co) 0.39 ± 0.01 ppb (Al) | River water | [103] |
| Polyphenol oxidase on filter paper | Catechol, phenol, p-cresol, 4-methyl catechol | Colorimetry/Image analysis | LOD: 5 × 10−7 M | River water Urine | [104] |
| Polyphenol oxidase entrapment in polyaniline-polyacrylonitrile-graphene hybrid and cross-linking with glutaraldehyde/Pt electrode | p-Cresol | AMP | LOD: 2.65 × 10−7 M (p-cresol) KMapp: 2.53 × 10−6 M | Spiked river and sea water | [105] |
| Laccase mixed with BMIMBF4-and chitosan/MWCNT electrode | Bisphenol A | AMP | LOD: 8.4 ± 0.3 × 10−9 M LR: 0.5–12 × 10−6 M | River water | [106] |
| Laccase immobilized on L-cysteine functionalized-silver-coated magnetic Fe3O4 nanoparticles/SPE | Catechol | AMP | LOD: 6 × 10−8 M LR: 1 × 10−7–1 × 10−4 M | Lake and tap water | [107] |
| Laccase immobilized by electrospray deposition on SPCE | Catechol | AMP | LOD: 1.7 × 10−6 M LR: 2 × 10−6–1 × 10−4 M | Lake water | [108] |
| Acid phosphatase cross-linked with GA on a GO-AgNP/SPCE | Glyphosate | AMP | LOD: 15 ppb LR1: 50–500 ppb LR2: 500 ppb–22 ppm | Spiked water and soil | [109] |
| Compound | Extremozyme | Technique | Analytical Characteristics | Reference |
|---|---|---|---|---|
| Diuron Atrazine Simazine Ioxynil Bromoxynil Dinoseb | PSII from Synechococcus elongatus immobilized on the surface of a Clark oxygen electrode by using a dialysis membrane | AMP | Diuron: LOD: 5 × 10−10 M I50: 8 × 10−8 M Atrazine: LOD: 2 × 10−9 M I50: 3 × 10−7 M Simazine: 1 × 10−8 M I50: 8 × 10−7 M Ioxynil: LOD: 9 × 10−9 M I50: 4 × 10−7 M Bromoxynil: LOD: 2 × 10−7 M I50: 1 × 10−6 M Dinoseb: LOD: 6 × 10−8 M I50: 8 × 10−7 M | [117] |
| Diuron Simazine Atrazine | PSII from Synechococcus elongatus cross-linked in a BSA matrix (BSA-GLU) or entrapped in a gel of agarose, alginate or gelatin; screen-printed graphite electrode | AMP | Immobilized in BSA-GLU: LOD: 1 × 10−9 M (diuron) LOD: 4 × 10−9 M (simazine) LOD: 2 × 10−9 M (atrazine) | [118] |
| Atrazine | His-tagged PSII from thermophilic Synechococcus elongatus immobilized on Ni-NTA/cysteine SAM without or with OCT or by cross-linking in BSA-GLU; Au screen-printed electrode | AMP | I50: 2 × 10−8 M (CYS–NTA–PSII) I50: 5 × 10−10 M (CYS + OCT) I50: 9 × 10−8 M (BSA–GA–PSII) | [119] |
| Isoproturon | PSII from Synechococcus elongatus cross-linked in BSA-GLU/Pt electrode | AMP | LOD: 9.1 × 10−8 M ED50: 2 × 10−6 M | [120] |
| Atrazine Isoproturon Diuron | PSII from thermophilic Synechococcus elongatus f. thermalis, strain KOVROV 1972/8, cross-linked into BSA-GLU-glycerol/Pt screen-printed electrode | AMP | LOD: 1.6 × 10−9 M LOD: 9.9 × 10−9 M LOD: 1 × 10−9 M | [121] |
| Diuron | PSII from Synechococcus bigranulatus strain Kovrov 1972/8 adsorbed on poly(SBQ)-Au electrode | AMP | LOD: 7 × 10−10 M I50: 9 × 10−9 M | [122] |
| Dinoterb, bromoxynil, 2,4-dinitrophenol | PSII from Thermosynechococcus elongatus immobilized by cross-linking in an Os-based redox polymer | AMP | Enhanced stability of the biosensor with herbicide co-immobilized with PSII, no inhibition | [123] |
| Ethylenebis(Dithiocarbamate) Fungicides Zineb, nabam | Aldehyde dehydrogenase from yeast co-immobilized with NADH oxidase from Thermus thermophilus on a Pt-sputtered screen-printed electrode | AMP | LOD: 8 ppb nabam LR: 10–80 ppb (nabam and zineb) | [124] |
| Organophosphorus pesticides | Esterase-2 (EST2) from Alicyclobacillus acidocaldarius immobilized on nitrocellulose membrane | COL/Image analysis | Detection of paraoxon in spiked fruit juices Detection range: up to 9 × 10−7 M paraoxon | [125] |
| Organophosphorus pesticides | EST2 in solution | FL | LOD: 205.5 ± 6.98 × 10−15 M (10% inhibition for paraoxon) | [126] |
| Organophosphorus pesticides | IAEDANS-labeled EST2-S35C (mutant of Esterase-2 from Alicyclobacillus acidocaldarius with the serine 35 replaced by a cysteine residue) developed to bind an extrinsic fluorescent probe | FRET | LOD: 2 × 10−9 M paraoxon | [127] |
| Organophosphorus pesticides | Recombinant EST2 from Alicyclobacillus acidocaldarius immobilized on a polyvinylidene difluoride (PVDF) membrane | FRET | LOD: 9 × 10−8 M LR: up to 3 × 10−5 M | [128] |
| Organophosphorus pesticides | IAEDANS-labeled EST2-S35C immobilized by physical adsorption on a polyvinylidene difluoride membrane | FL | Paraoxon: LOD: 9 × 10−8 M LOQ:3.1 × 10−7 M | [129] |
| Halogenated organic compounds | L-HADST (dehalogenase) from Sulfolobus tokodai covalently linked to N-hydroxysuccinimidyl Sepharose | POT | KMimm = 4.9 × 10−3 M KMfree = 4.9 × 10−3 M | [130] |
| Phenol | Phenol hydroxylase (EC 1.14.13.7) Bacillus stearothermophilus immobilized by sol-gel at the surface of a Clark type oxygen electrode | AMP | LR = 2.5 × 10−6–4 × 10−4 M | [131] |
| As(III) and As(V) | Arsenate reductase from Thermus thermophilus bound to PEG-stabilized Au NPs | UV-VIS y | LOD: 10 ± 3 × 10−12 M for As (III) and LOD = 7.7 ± 0.3 × 10−12 M for As(V) | [132] |
| As(III) | Thermostable chimeras Vmh2-ArsC and ArsC-Vmh2 immobilized on a Au electrode | SWV | AsrC-Vmh2: KAsIII = 650 (±100) L·mol−1 buffer. Vmh2-ArsC KAsIII = 1200 (±300)L·mol−1 at 60 °C in 50 mM Tris–HCl pH 7.5 | [133] |
| As (V) | Arsenate reductase from Thermus thermophilus | COL | LOD: 0.28 ± 0.02 × 10−6 M | [134] |
| NADH | NADH oxidase from Thermus aquaticus immobilized on a Immobilon AV membrane | AMP | Measurement of lactate dehydrogenase activity in serum LOD: 2 × 10−7 M LR: 5 × 10−7–2 × 10−5 M | [135] |
| NADH | Diaphorase from Bacillus stearothermophilus immobilized on a Immobilon AV membrane at the surface of a Pt electrode; covered by a polycarbonate 0.33 μm membrane | AMP | Bienzymatic sensors (diaphorase and NAD-dependent dehydrogenase) for alcohol, lactate and β-hidroxybutyrate LOD (NADH): 5 × 10−8 M LR(NADH): 1 × 10−7–1 × 10−3 M | [136] |
| Glutamate | Glutamate dehydrogenase from the hyperthermophilic sulfur-reducing archaeon Pyrococcus woesei, immobilized in carbon paste with a polyethyleneimine- Toluidine Blue O redox polymer and lactitol/DEAE dextran | AMP | KMapp = 2.11–2.26 × 10−3 M at pH 7 and 45 °C KMapp = 1.17–1.32 × 10−2 M at pH 8.9 and 35 °C | [137] |
| Glutamate | Glutamate dehydrogenase from thermophilic archaebacterial isolate AN1 immobilized in carbon paste wax with toluidine blue O–acrylamide redox polymer and [Ru(NH3)6]Cl3 | AMP(FIA) | LOD: 3 × 10−4 M LR: up to 4 × 10−2 M | [138] |
| Asparagine | Asparaginase from Archaeoglobus fulgidus retained on an ammonia ISE by a dialysis membrane | POT | LOD (L-asparagine): 6 × 10−5 M KM (L-asparagine) = 8 × 10−5 M at 37 °C and pH 9.2 | [111] |
| Glucose-6-phosphate | Glucose-6-phosphate dehydrogenase from Aquifex aeolicus cross linked in a redox polymer of osmium (1,10-phenanthroline-5,6-dione)2-poly(4-vinylpyridine on graphite electrodes) | AMP | LOD: 2 × 10−4 M LR: 6 × 10−4–2 × 10−2 M KMimmob = 2.9 × 10−3 M KMfree = 0.18 × 10−3 M) | [110] |
| Glucose-6-phosphate | Glucose-6-phosphate dehydrogenase from B. stearothermophilus cross-linked on a Pt black electrode; covered by cross-linked BSA film | AMP | LR: 1–50 × 10−3 M | [139] |
| Salicilin, esculin, cellobiose, lactose | Glucokinase and hydrolase from Bacillus stearothermophilus and β-D-glucosidase from Caldocellum saccharolyticum immobilized on a ISFET by cross-linking in BSA film | ISFET | At pH 7.0 and 40 °C. DR: 0.3–30 × 10−3 M salicilin Output (10 × 10−3 M solutions): Salicilin: 7.6 mV Esculin: 5.4 mV Cellobiose: 1.6 mV Lactose: <0.1 mV | [139] |
| D-proline | D-proline dehydrogenase from the hyperthermophilic archaeon Pyrobaculum islandicum, immobilized in agar on a GCE | AMP | Detection of D-amino acids in urine LR: 5 × 10−4–5 × 10−3 M D-proline KMapp = 7.9 × 10−3 M | [140] |
| L-lactate | L-lactate dehydrogenase from Clostridium thermocellum immobilized on Au electrode in a polyglutaraldehyde-polypyrrole film | AMP | Detection in blood LOD: 1.55 × 10−4 M Dynamic range: 4 × 10−2–1.6 × 10−1 M | [112] |
| Glucose | Recombinant Flavin Adenine Dinucleotide-dependent glucose dehydrogenase from Talaromyces emersonii immobilized on a SWCNT/PPF/Au electrode and covered by PPF | AMP | Detection of glucose in diabetic patients LOD: 5 × 10−5 M LR: 5 × 10−6–2.6 × 10−2 M | [141] |
| Aβ1-40 fibrils | Recombinant acylpeptide hydrolase from Sulfolobus tokodaii; complex with a single chain variable fragment (scFv 12B4), and gold nanorods | COL | Monitoring amyloid aggregation Theranostics | [142] |
| Benzaldehyde | Recombinant aldehyde dehydrogenase “F-ALDH” from Flavobacterium PL002 immobilized by cross-linking with GA in a BSA matrix on a CNF SPE | AMP (FIA) | Detection of benzaldehyde in benzoic acid raw material LOD:1 × 10−5 M LR: 3 × 10−5–3 × 10−4 M Kmapp = 3.86 × 10−4 M Kmfree = 1.45 × 10−4 M | [143] |
| Acetaldehyde | Recombinant aldehyde dehydrogenase “ALDS2” from Flavobacterium PL002; CNT SPE | AMP | Detection of acetaldehyde in wines LR: 1.25 × 10−4–2.5 × 10−3 M | [69] |
| Uric acid | Engineered urate oxidase from Bacilllus sp. TB-90 covalently immobilized on GCE modified with AuNPs and thioglycolic acid | AMP | LOD: 9.16 × 10−9 M LR: 5 × 10−8–1 × 10−3 M | [113] |
| Cellobiose | Cellobiose dehydrogenase from Phanerochaete chrysosporium cross-linked in a Os-based redox polymer with PEDGDE, on a GCE | AMPy | LOD: 2.55 × 10−6 M LR: up to 1 × 10−4 M Used together with a glucose biosensor to monitor the hydrolysis of cellulose and milled corncob | [144] |
| H2O2 | Extremophilic fungus Caldariomyces fumango covalently immobilized on 3D-CNT@MoS2/ILEM/GCE | EC | LOD: 0.097 × 10−6 M LR: 0.2–997 × 10−6 M Detection of H2O2 in human urine | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcarea, C.; Ruginescu, R.; Banciu, R.M.; Vasilescu, A. Extremozyme-Based Biosensors for Environmental Pollution Monitoring: Recent Developments. Biosensors 2024, 14, 143. https://doi.org/10.3390/bios14030143
Purcarea C, Ruginescu R, Banciu RM, Vasilescu A. Extremozyme-Based Biosensors for Environmental Pollution Monitoring: Recent Developments. Biosensors. 2024; 14(3):143. https://doi.org/10.3390/bios14030143
Chicago/Turabian StylePurcarea, Cristina, Robert Ruginescu, Roberta Maria Banciu, and Alina Vasilescu. 2024. "Extremozyme-Based Biosensors for Environmental Pollution Monitoring: Recent Developments" Biosensors 14, no. 3: 143. https://doi.org/10.3390/bios14030143
APA StylePurcarea, C., Ruginescu, R., Banciu, R. M., & Vasilescu, A. (2024). Extremozyme-Based Biosensors for Environmental Pollution Monitoring: Recent Developments. Biosensors, 14(3), 143. https://doi.org/10.3390/bios14030143







