Detection of Parasites in the Field: The Ever-Innovating CRISPR/Cas12a
Abstract
:1. Introduction
2. Application of Nucleic Acid Amplification Tests in Parasite Detection
3. CRISPR/Cas12a for POCT
3.1. Discovery of CRISPR
3.2. CRISPR/Cas12a System
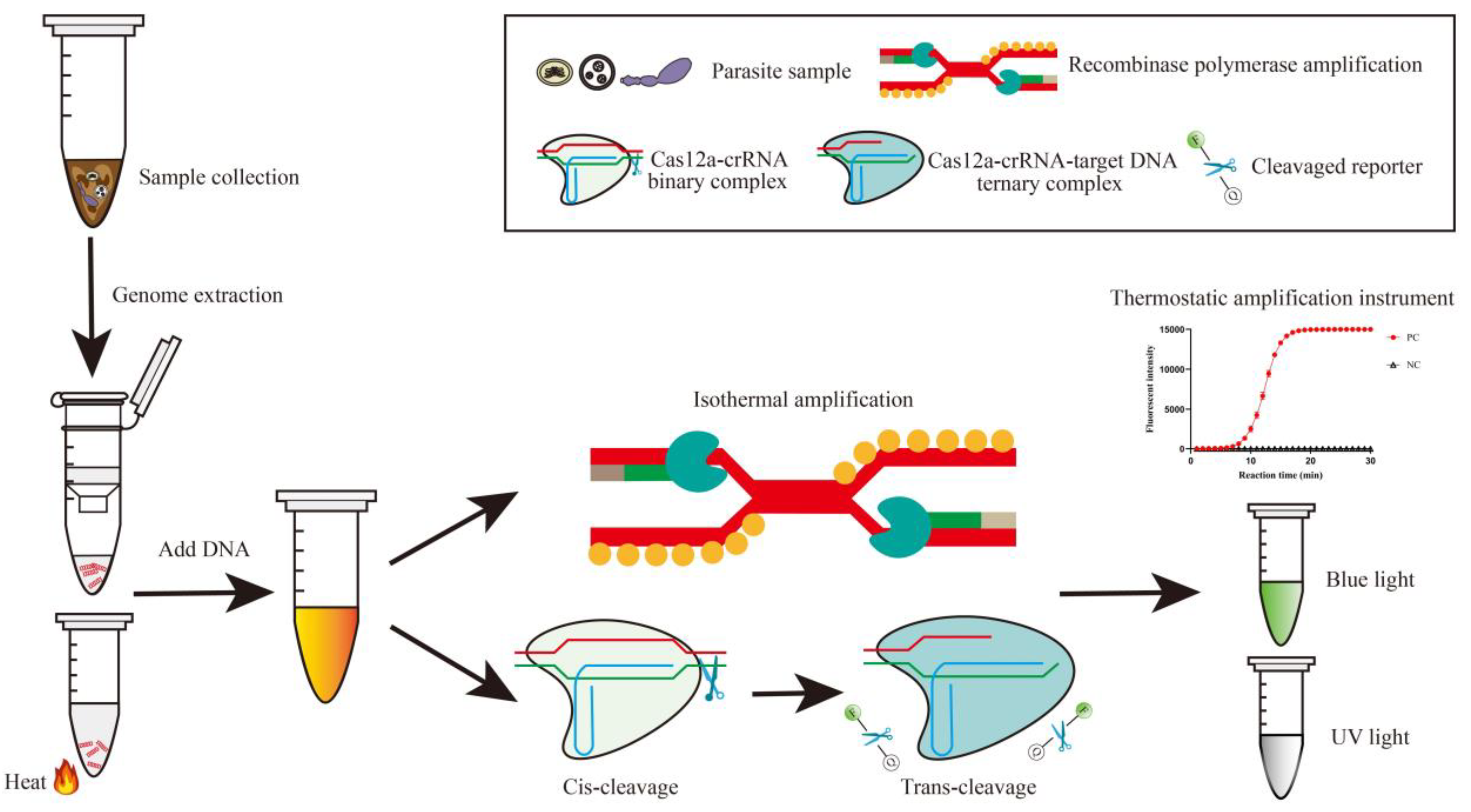
3.3. CRISPR/Cas12a for Rapid One-Site Detection
3.4. Application of CRISPR/Cas12a for Parasite Detection
4. Optimization of the CRISPR/Cas12a One-Pot Detection Assay
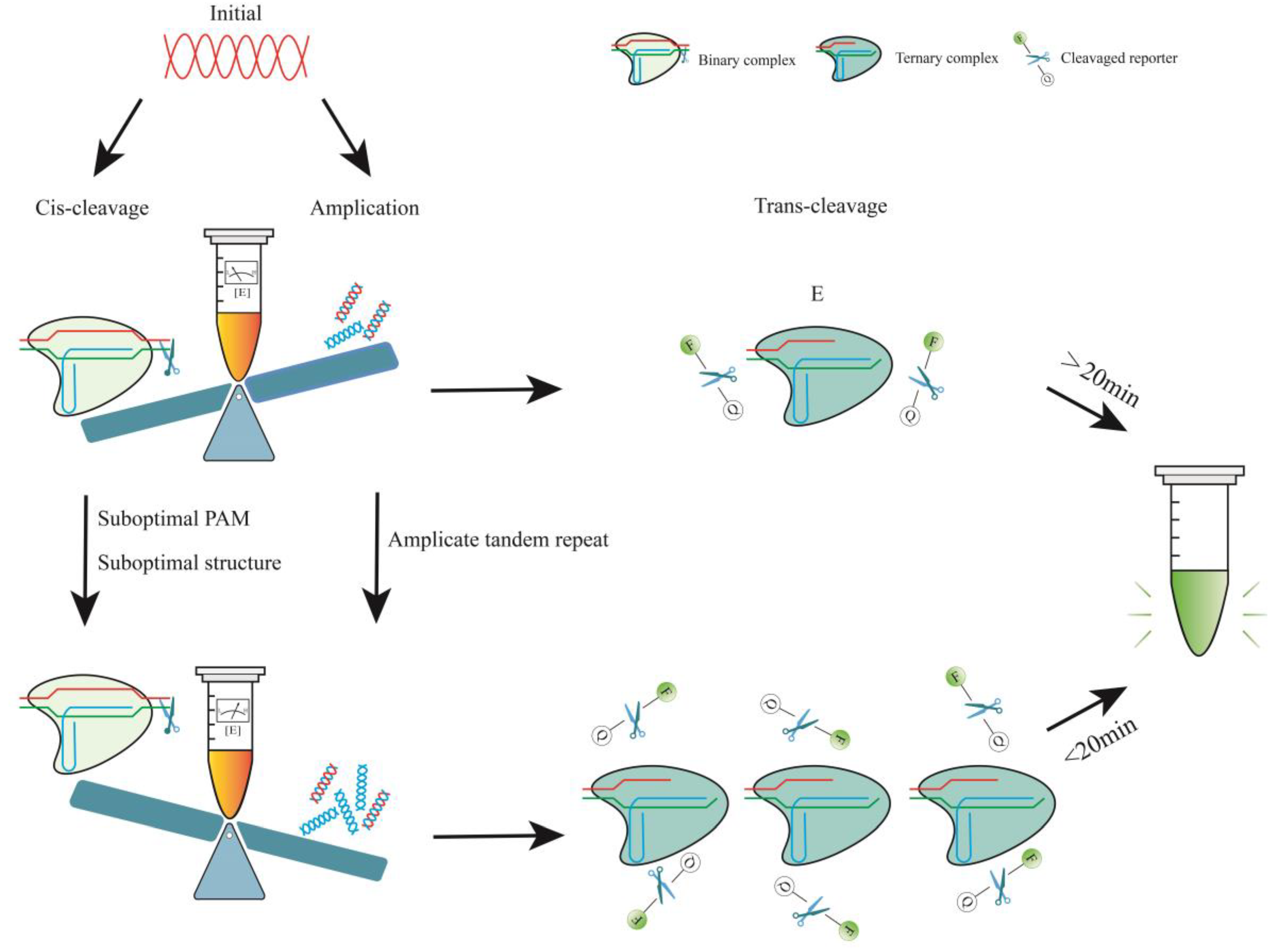
4.1. One-Pot One-Step Reaction
4.1.1. Determinants of Cas12a Enzyme Kinetics
4.1.2. Reduced crRNA Efficiency by PAM
4.1.3. Reduced crRNA Efficiency by Structure
4.2. One-Pot Reactions with Two Steps
4.2.1. Light-Activated crRNA to Initiate Cleavage
4.2.2. Physical Separation of the Two Processes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Nordling, L. Malaria’s modelling problem. Nature 2023, 618, S34–S35. [Google Scholar] [CrossRef] [PubMed]
- WHO. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. Available online: https://www.who.int/publications-detail-redirect/9789241564090 (accessed on 11 February 2023).
- The, L. Neglected tropical diseases: Ending the neglect of populations. Lancet 2022, 399, 411. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fevre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 6 December 2023).
- Meinel, T.R.; Gottstein, B.; Geib, V.; Keel, M.J.; Biral, R.; Mohaupt, M.; Brügger, J. Vertebral alveolar echinococcosis—A case report, systematic analysis, and review of the literature. Lancet Infect. Dis. 2018, 18, e87–e98. [Google Scholar] [CrossRef]
- Wong, S.S.; Fung, K.S.; Chau, S.; Poon, R.W.; Wong, S.C.; Yuen, K.Y. Molecular diagnosis in clinical parasitology: When and why? Exp. Biol. Med. 2014, 239, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, S.; Cantacessi, C.; Arsic-Arsenijevic, V.; Otranto, D.; Tasic-Otasevic, S. Rapid diagnosis of parasitic diseases: Current scenario and future needs. Clin. Microbiol. Infect. 2019, 25, 290–309. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Fan, C.; Chen, H.-Y. Biosensing: CRISPR-powered diagnostics. Nat. Biomed. Eng. 2017, 1, 0091. [Google Scholar] [CrossRef]
- Yue, H.; Huang, M.; Tian, T.; Xiong, E.; Zhou, X. Advances in Clustered, Regularly Interspaced Short Palindromic Repeats (CRISPR)-Based Diagnostic Assays Assisted by Micro/Nanotechnologies. ACS Nano 2021, 15, 7848–7859. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Y.; Liu, G.; Gooding, J.J. CRISPR Mediated Biosensing Toward Understanding Cellular Biology and Point-of-Care Diagnosis. Angew. Chem. Int. Ed. Engl. 2020, 59, 20754–20766. [Google Scholar] [CrossRef]
- Chertow, D.S. Next-generation diagnostics with CRISPR. Science 2018, 360, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, R.; Li, J. CRISPR-Cas-based techniques for pathogen detection: Retrospect, recent advances, and future perspectives. J. Adv. Res. 2023, 50, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mallon, J.; Poddar, A.; Wang, Y.; Tippana, R.; Yang, O.; Bailey, S.; Ha, T. Real-time observation of DNA target interrogation and product release by the RNA-guided endonuclease CRISPR Cpf1 (Cas12a). Proc. Natl. Acad. Sci. USA 2018, 115, 5444–5449. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Li, C.; Hao, Y.; Tang, Y.; Yuan, Y.; Chai, L.; Fan, T.; Yu, J.; Ma, X.; et al. CRISPR-Cas12a-Empowered Electrochemical Biosensor for Rapid and Ultrasensitive Detection of SARS-CoV-2 Delta Variant. Nano-Micro Lett. 2022, 14, 159. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Dong, K.; Shu, W.; Zhang, J.; Zhang, J.; Zhao, R.; Wei, S.; Feng, D.; Xiao, X.; et al. A universal and specific RNA biosensor via DNA circuit-mediated PAM-independent CRISPR/Cas12a and PolyA-rolling circle amplification. Biosens. Bioelectron. 2023, 226, 115139. [Google Scholar] [CrossRef]
- van Dongen, J.E.; Berendsen, J.T.W.; Steenbergen, R.D.M.; Wolthuis, R.M.F.; Eijkel, J.C.T.; Segerink, L.I. Point-of-care CRISPR/Cas nucleic acid detection: Recent advances, challenges and opportunities. Biosens. Bioelectron. 2020, 166, 112445. [Google Scholar] [CrossRef]
- Chen, S.J.; Rai, C.I.; Wang, S.C.; Chen, Y.C. Point-of-Care Testing for Infectious Diseases Based on Class 2 CRISPR/Cas Technology. Diagnostics 2023, 13, 2255. [Google Scholar] [CrossRef]
- Lu, S.; Tong, X.; Han, Y.; Zhang, K.; Zhang, Y.; Chen, Q.; Duan, J.; Lei, X.; Huang, M.; Qiu, Y.; et al. Fast and sensitive detection of SARS-CoV-2 RNA using suboptimal protospacer adjacent motifs for Cas12a. Nat. Biomed. Eng. 2022, 6, 286–297. [Google Scholar] [CrossRef]
- Lee, R.A.; Puig, H.; Nguyen, P.Q.; Angenent-Mari, N.M.; Donghia, N.M.; McGee, J.P.; Dvorin, J.D.; Klapperich, C.M.; Pollock, N.R.; Collins, J.J. Ultrasensitive CRISPR-based diagnostic for field-applicable detection of Plasmodium species in symptomatic and asymptomatic malaria. Proc. Natl. Acad. Sci. USA 2020, 117, 25722–25731. [Google Scholar] [CrossRef]
- Lei, R.; Li, L.; Wu, P.; Fei, X.; Zhang, Y.; Wang, J.; Zhang, D.; Zhang, Q.; Yang, N.; Wang, X. RPA/CRISPR/Cas12a-Based On-Site and Rapid Nucleic Acid Detection of Toxoplasma gondii in the Environment. ACS Synth. Biol. 2022, 11, 1772–1781. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, M.; Yang, S.; Xing, C.; Li, Q.; Zhu, Y.; Ji, Y.; Du, Y. CRISPR/Cas12a combined with RPA for detection of T. gondii in mouse whole blood. Parasites Vectors 2023, 16, 256. [Google Scholar] [CrossRef]
- Li, Y.; Deng, F.; Hall, T.; Vesey, G.; Goldys, E.M. CRISPR/Cas12a-powered immunosensor suitable for ultra-sensitive whole Cryptosporidium oocyst detection from water samples using a plate reader. Water Res. 2021, 203, 117553. [Google Scholar] [CrossRef] [PubMed]
- Cherkaoui, D.; Mesquita, S.G.; Huang, D.; Lugli, E.B.; Webster, B.L.; McKendry, R.A. CRISPR-assisted test for Schistosoma haematobium. Sci. Rep. 2023, 13, 4990. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Chiodini, P.L. Laboratory investigations and diagnosis of tropical diseases in travelers. Infect. Dis. Clin. N. Am. 2012, 26, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Kettelhut, M.M.; Chiodini, P.L.; Edwards, H.; Moody, A. External quality assessment schemes raise standards: Evidence from the UKNEQAS parasitology subschemes. J. Clin. Pathol. 2003, 56, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.E. Laboratory diagnosis of infections due to blood and tissue parasites. Clin. Infect. Dis. 2009, 49, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, D.; Sakkas, H.; Mohammed, B.; Vartholomatos, G.; Malamos, K.; Sreekantam, S.; Kanavaros, P.; Kalogeropoulos, C. Ocular toxoplasmosis: A review of the current diagnostic and therapeutic approaches. Int. Ophthalmol. 2022, 42, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.; Denniston, A.K.; Murray, P.I. False Negative Toxoplasma Serology in an Immunocompromised Patient with PCR Positive Ocular Toxoplasmosis. Ocul. Immunol. Inflamm. 2018, 26, 1200–1202. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.G.; Han, D.H.; Lee, J.S.; Lee, S.J.; Park, J.K. Pushbutton-activated microfluidic dropenser for droplet digital PCR. Biosens. Bioelectron. 2021, 181, 113159. [Google Scholar] [CrossRef] [PubMed]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. A Bimon. Publ. Int. Union. Biochem. Mol. Biol. 2021, 49, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, V.; Filion, M. New Developments in Quantitative Real-time Polymerase Chain Reaction Technology. Curr. Issues Mol. Biol. 2014, 16, 1–6. [Google Scholar] [PubMed]
- Kobets, T.; Badalova, J.; Grekov, I.; Havelkova, H.; Svobodova, M.; Lipoldova, M. Leishmania parasite detection and quantification using PCR-ELISA. Nat. Protoc. 2010, 5, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yan, W.; Lv, C.; Bai, R.; Wang, T.; Wei, Z.; Zhang, M. Molecular Detection and Genotyping of Toxoplasma gondii and Neospora caninum in Slaughtered Goats in Central China. Foodborne Pathog. Dis. 2020, 17, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Waitumbi, J.N.; Gerlach, J.; Afonina, I.; Anyona, S.B.; Koros, J.N.; Siangla, J.; Ankoudinova, I.; Singhal, M.; Watts, K.; Polhemus, M.E.; et al. Malaria prevalence defined by microscopy, antigen detection, DNA amplification and total nucleic acid amplification in a malaria-endemic region during the peak malaria transmission season. Trop. Med. Int. Health 2011, 16, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.; Li, Z.; Poole, C.B.; Carlow, C.K. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends Parasitol. 2015, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Piubelli, C.; Perandin, F.; Bisoffi, Z. Digital PCR: A new technology for diagnosis of parasitic infections. Clin. Microbiol. Infect. 2019, 25, 1510–1516. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef]
- Lalremruata, A.; Nguyen, T.T.; McCall, M.B.B.; Mombo-Ngoma, G.; Agnandji, S.T.; Adegnika, A.A.; Lell, B.; Ramharter, M.; Hoffman, S.L.; Kremsner, P.G.; et al. Recombinase Polymerase Amplification and Lateral Flow Assay for Ultrasensitive Detection of Low-Density Plasmodium falciparum Infection from Controlled Human Malaria Infection Studies and Naturally Acquired Infections. J. Clin. Microbiol. 2020, 58, 10-1128. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Faisal, K.; Chowdhury, R.; Nath, R.; Ghosh, P.; Ghosh, D.; Hossain, F.; Abd El Wahed, A.; Mondal, D. Evaluation of molecular assays to detect Leishmania donovani in Phlebotomus argentipes fed on post-kala-azar dermal leishmaniasis patients. Parasit. Vectors 2021, 14, 465. [Google Scholar] [CrossRef]
- Xu, J.; Rong, R.; Zhang, H.Q.; Shi, C.J.; Zhu, X.Q.; Xia, C.M. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int. J. Parasitol. 2010, 40, 327–331. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, W.; Zhang, X.Z.; Yao, X.R.; Huang, W.; Jia, H.; Liu, X.L.; Hou, S.H.; Wang, X.J. Development of a real-time recombinase-aided amplification (RT-RAA) molecular diagnosis assay for sensitive and rapid detection of Toxoplasma gondii. Vet. Parasitol. 2021, 298, 109489. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, G.; Li, P.; Jin, Q.; Lu, Q.; Zhang, G. Development and application of a recombinase-aided amplification and lateral flow assay for rapid detection of pseudorabies virus from clinical crude samples. Int. J. Biol. Macromol. 2023, 224, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2020, 60, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Zhou, X.M.; Liu, L. Improved Strategies for CRISPR-Cas12-based Nucleic Acids Detection. J. Anal. Test. 2022, 6, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.A.; von Sternberg, R. Why repetitive DNA is essential to genome function. Biol. Rev. Camb. Philos. Soc. 2005, 80, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, W.; Zhang, L.; Zhang, Z.; Li, J.; Lu, G.; Zhu, Y.; Wang, Y.; Huang, Y.; Liu, J.; et al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat. Genet. 2013, 45, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Prediger, E.; Huecas, M.E.; Nogueira, N.; Lizardi, P.M. Minichromosomal repetitive DNA in Trypanosoma cruzi: Its use in a high-sensitivity parasite detection assay. Proc. Natl. Acad. Sci. USA 1984, 81, 3356–3360. [Google Scholar] [CrossRef]
- Homan, W.L.; Vercammen, M.; De Braekeleer, J.; Verschueren, H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000, 30, 69–75. [Google Scholar] [CrossRef]
- Demas, A.; Oberstaller, J.; DeBarry, J.; Lucchi, N.W.; Srinivasamoorthy, G.; Sumari, D.; Kabanywanyi, A.M.; Villegas, L.; Escalante, A.A.; Kachur, S.P.; et al. Applied genomics: Data mining reveals species-specific malaria diagnostic targets more sensitive than 18S rRNA. J. Clin. Microbiol. 2011, 49, 2411–2418. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D.P.; Gupta, R.; Savargaonkar, D.; Singh, O.P.; Nanda, N.; Bhatt, R.M.; Valecha, N. Comparison of three PCR-based assays for the non-invasive diagnosis of malaria: Detection of Plasmodium parasites in blood and saliva. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014, 33, 1631–1639. [Google Scholar] [CrossRef]
- Azam, M.; Upmanyu, K.; Gupta, R.; Sruthy, K.S.; Matlani, M.; Savargaonkar, D.; Singh, R. Development of Two-Tube Loop-Mediated Isothermal Amplification Assay for Differential Diagnosis of Plasmodium falciparum and Plasmodium vivax and Its Comparison with Loopamp™ Malaria. Diagnostics 2021, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Rosenzvit, M.C.; Canova, S.G.; Kamenetzky, L.; Ledesma, B.A.; Guarnera, E.A. Echinococcus granulosus: Cloning and characterization of a tandemly repeated DNA element. Exp. Parasitol. 1997, 87, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, I.; Hamburger, J.; Raoul, F.; Craig, P.S.; Campos-Ponce, M.; Branzburg, A.; Hafez, S.K.A. Copro-Diagnosis of Echinococcus granulosus Infection in Dogs by Amplification of a Newly Identified Repeated DNA Sequence. Am. J. Trop. Med. Hyg. 2003, 69, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.; Vallejo, V.; Mossie, K.G.; Ortiz, D.; Agabian, N.; Flisser, A. Isolation and characterization of species-specific DNA probes from Taenia solium and Taenia saginata and their use in an egg detection assay. J. Clin. Microbiol. 1995, 33, 1283–1288. [Google Scholar] [CrossRef]
- González, L.M.; Montero, E.; Harrison, L.J.; Parkhouse, R.M.; Garate, T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J. Clin. Microbiol. 2000, 38, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, J.; Turetski, T.; Kapeller, I.; Deresiewicz, R. Highly repeated short DNA sequences in the genome of Schistosoma mansoni recognized by a species-specific probe. Mol. Biochem. Parasitol. 1991, 44, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for Diagnosis of Schistosoma mansoni Infection in Faecal Samples. J. Parasitol. Res. 2018, 2018, 1267826. [Google Scholar] [CrossRef]
- Hamburger, J.; Xu, Y.X.; Ramzy, R.M.; Jourdane, J.; Ruppel, A. Development and laboratory evaluation of a polymerase chain reaction for monitoring Schistosoma mansoni infestation of water. Am. J. Trop. Med. Hyg. 1998, 59, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, J.; He, N.; Abbasi, I.; Ramzy, R.M.; Jourdane, J.; Ruppel, A. Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: A potential tool for monitoring schistosome-infested water. Am. J. Trop. Med. Hyg. 2001, 65, 907–911. [Google Scholar] [CrossRef]
- Hertel, J.; Hamburger, J.; Haberl, B.; Haas, W. Detection of bird schistosomes in lakes by PCR and filter-hybridization. Exp. Parasitol. 2002, 101, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lodh, N.; Caro, R.; Sofer, S.; Scott, A.; Krolewiecki, A.; Shiff, C. Diagnosis of Strongyloides stercoralis: Detection of parasite-derived DNA in urine. Acta Trop. 2016, 163, 9–13. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, L.A.; DeSimone, S.M.; Williams, S.A. Cloning and comparison of repeated DNA sequences from the human filarial parasite Brugia malayi and the animal parasite Brugia pahangi. Proc. Natl. Acad. Sci. USA 1986, 83, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.; Sartono, E.; Wahyuni, S.; Yazdanbakhsh, M.; Maizels, R.M.; Klarmann-Schulz, U.; Pfarr, K.; Hoerauf, A. Real-time PCR detection of the HhaI tandem DNA repeat in pre- and post-patent Brugia malayi Infections: A study in Indonesian transmigrants. Parasit. Vectors 2014, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; McCarthy, J.; Bierwert, L.; Lizotte-Waniewski, M.; Chanteau, S.; Nutman, T.B.; Ottesen, E.A.; Williams, S.A. A polymerase chain reaction assay for detection of the parasite Wuchereria bancrofti in human blood samples. Am. J. Trop. Med. Hyg. 1996, 54, 357–363. [Google Scholar] [CrossRef]
- Rao, R.U.; Atkinson, L.J.; Ramzy, R.M.; Helmy, H.; Farid, H.A.; Bockarie, M.J.; Susapu, M.; Laney, S.J.; Williams, S.A.; Weil, G.J. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am. J. Trop. Med. Hyg. 2006, 74, 826–832. [Google Scholar] [CrossRef]
- Saul, A.; Yeganeh, F.; Howard, R.J. Cloning and characterization of a novel multicopy, repetitive sequence of Plasmodium falciparum, REP51. Immunol. Cell Biol. 1992, 70 Pt 5, 357–359. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Raimon, S.; Abd-Elfarag, G.; Carter, J.Y.; Sebit, W.; Suliman, A.; Siewe Fodjo, J.N.; De Witte, P.; Logora, M.Y.; Colebunders, R.; et al. Onchocerca volvulus is not detected in the cerebrospinal fluid of persons with onchocerciasis-associated epilepsy. Int. J. Infect. Dis. 2020, 91, 119–123. [Google Scholar] [CrossRef]
- Macfarlane, C.L.; Quek, S.; Pionnier, N.; Turner, J.D.; Wanji, S.; Wagstaff, S.C.; Taylor, M.J. The insufficiency of circulating miRNA and DNA as diagnostic tools or as biomarkers of treatment efficacy for Onchocerca volvulus. Sci. Rep. 2020, 10, 6672. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Fessler, M.K.; Bloomfield, R.A.; Sandke, W.D.; Malekshahi, C.R.; Keroack, C.D.; Duignan, P.J.; Torquato, S.D.; Williams, S.A. A novel quantitative real-time PCR diagnostic assay for fecal and nasal swab detection of an otariid lungworm, Parafilaroides decorus. Int. J. Parasitol. Parasites Wildl. 2020, 12, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Embden, J.D.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef]
- Mohanraju, P.; Makarova, K.S.; Zetsche, B.; Zhang, F.; Koonin, E.V.; van der Oost, J. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science 2016, 353, aad5147. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- East-Seletsky, A.; O’Connell, M.R.; Burstein, D.; Knott, G.J.; Doudna, J.A. RNA Targeting by Functionally Orthogonal Type VI-A CRISPR-Cas Enzymes. Mol. Cell 2017, 66, 373–383.e3. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.B.; Harrington, L.B.; Da Costa, M.; Tian, X.R.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- East-Seletsky, A.; O’Connell, M.R.; Knight, S.C.; Burstein, D.; Cate, J.H.; Tjian, R.; Doudna, J.A. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 2016, 538, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, C.; An, C.; Zheng, X.; Wen, S.; Chen, W.; Liu, X.; Lv, Z.; Yang, P.; Xu, W.; et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol. Cancer 2021, 20, 126. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.C.; Jinek, M. Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol. Cell 2019, 73, 589–600.e584. [Google Scholar] [CrossRef]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V.; et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.G.; Pitts, R.J. PrimedSherlock: A tool for rapid design of highly specific CRISPR-Cas12 crRNAs. BMC Bioinform. 2022, 23, 428. [Google Scholar] [CrossRef]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat. Biotechnol. 2017, 35, 31–34. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q.; et al. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.I.; Makhawi, A.M. SHERLOCK and DETECTR: CRISPR-Cas Systems as Potential Rapid Diagnostic Tools for Emerging Infectious Diseases. J. Clin. Microbiol. 2021, 59, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Kostyusheva, A.; Brezgin, S.; Babin, Y.; Vasilyeva, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. CRISPR-Cas systems for diagnosing infectious diseases. Methods 2022, 203, 431–446. [Google Scholar] [CrossRef]
- Li, H.; Cui, X.; Sun, L.; Deng, X.; Liu, S.; Zou, X.; Li, B.; Wang, C.; Wang, Y.; Liu, Y.; et al. High concentration of Cas12a effector tolerates more mismatches on ssDNA. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21153. [Google Scholar] [CrossRef]
- Li, S.Y.; Cheng, Q.X.; Liu, J.K.; Nie, X.Q.; Zhao, G.P.; Wang, J. CRISPR-Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res. 2018, 28, 491–493. [Google Scholar] [CrossRef]
- Strohkendl, I.; Saifuddin, F.A.; Rybarski, J.R.; Finkelstein, I.J.; Russell, R. Kinetic Basis for DNA Target Specificity of CRISPR-Cas12a. Mol. Cell 2018, 71, 816–824.e3. [Google Scholar] [CrossRef]
- Nalefski, E.A.; Patel, N.; Leung, P.J.Y.; Islam, Z.; Kooistra, R.M.; Parikh, I.; Marion, E.; Knott, G.J.; Doudna, J.A.; Le Ny, A.M.; et al. Kinetic analysis of Cas12a and Cas13a RNA-Guided nucleases for development of improved CRISPR-Based diagnostics. iScience 2021, 24, 102996. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Somoza, R.A.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the Trans-Cleavage Activity of CRISPR-Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor. Angew. Chem. Int. Ed. Engl. 2019, 58, 17399–17405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mansour, H.; Watson, C.J.F.; Tang, Y.; MacNeil, A.J.; Li, F. Amplified detection of nucleic acids and proteins using an isothermal proximity CRISPR Cas12a assay. Chem. Sci. 2021, 12, 2133–2137. [Google Scholar] [CrossRef]
- Liang, M.; Li, Z.; Wang, W.; Liu, J.; Liu, L.; Zhu, G.; Karthik, L.; Wang, M.; Wang, K.F.; Wang, Z.; et al. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat. Commun. 2019, 10, 3672. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Xie, S.; Tian, R.; Wang, S.; Lu, Q.; Gao, D.; Lei, C.; Zhu, H.; Nie, Z. A CRISPR-Cas autocatalysis-driven feedback amplification network for supersensitive DNA diagnostics. Sci. Adv. 2021, 7, eabc7802. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, J.; Yang, Z.; Mou, Q.; Ma, Y.; Xiong, Y.; Lu, Y. Functional DNA Regulated CRISPR-Cas12a Sensors for Point-of-Care Diagnostics of Non-Nucleic-Acid Targets. J. Am. Chem. Soc. 2020, 142, 207–213. [Google Scholar] [CrossRef]
- Wang, S.Y.; Du, Y.C.; Wang, D.X.; Ma, J.Y.; Tang, A.N.; Kong, D.M. Signal amplification and output of CRISPR/Cas-based biosensing systems: A review. Anal. Chim. Acta 2021, 1185, 338882. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zeng, H.; Liu, X.; Jiang, W.; Wang, Y.; Ouyang, W.; Tang, X. RPA-Cas12a-FS: A frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem. 2021, 334, 127608. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, K.; Wang, Y.; Li, D.; Cui, Z.; Huang, J.; Zhang, S.; Li, X.; Zhang, L. CRISPR/Cas12a-based on-site diagnostics of Cryptosporidium parvum IId-subtype-family from human and cattle fecal samples. Parasit. Vectors 2021, 14, 208. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, D.; Zhou, X. M-CDC: Magnetic pull-down-assisted colorimetric method based on the CRISPR/Cas12a system. Methods 2022, 203, 259–267. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z.; et al. opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosens. Bioelectron. 2021, 172, 112766. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, M.; Liu, Y.; Ma, P.; Dang, L.; Meng, Q.; Wan, W.; Ma, X.; Liu, J.; Yang, G.; et al. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci. Bull. 2020, 65, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.E.; Lee, P.W.; Trick, A.Y.; Park, J.S.; Chen, L.; Shah, K.; Mostafa, H.; Carroll, K.C.; Hsieh, K.; Wang, T.H. Point-of-care CRISPR-Cas-assisted SARS-CoV-2 detection in an automated and portable droplet magnetofluidic device. Biosens. Bioelectron. 2021, 190, 113390. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020, 11, 4711. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Hu, J.; Huang, C.; Feng, W.; Liu, Y.; Kumblathan, T.; Tao, J.; Xu, J.; Le, X.C.; Zhang, H. CRISPR techniques and potential for the detection and discrimination of SARS-CoV-2 variants of concern. Trends Anal. Chem. 2023, 161, 117000. [Google Scholar] [CrossRef]
- Tian, T.; Shu, B.; Jiang, Y.; Ye, M.; Liu, L.; Guo, Z.; Han, Z.; Wang, Z.; Zhou, X. An Ultralocalized Cas13a Assay Enables Universal and Nucleic Acid Amplification-Free Single-Molecule RNA Diagnostics. ACS Nano 2021, 15, 1167–1178. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Sfeir, M.M.; Liu, C. Sensitive quantitative detection of SARS-CoV-2 in clinical samples using digital warm-start CRISPR assay. Biosens. Bioelectron. 2021, 184, 113218. [Google Scholar] [CrossRef]
- Moon, J.; Liu, C. Asymmetric CRISPR enabling cascade signal amplification for nucleic acid detection by competitive crRNA. Nat. Commun. 2023, 14, 7504. [Google Scholar] [CrossRef]
- Lee, I.; Kwon, S.J.; Sorci, M.; Heeger, P.S.; Dordick, J.S. Highly Sensitive Immuno-CRISPR Assay for CXCL9 Detection. Anal. Chem. 2021, 93, 16528–16534. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Song, T.; Gao, L.; Yin, S.; Ma, M.; Tan, Y.; Wu, L.; Yang, Y.; Wang, Y.; Lin, T.; et al. A CRISPR-based ultrasensitive assay detects attomolar concentrations of SARS-CoV-2 antibodies in clinical samples. Nat. Commun. 2022, 13, 4667. [Google Scholar] [CrossRef]
- Cheng, M.; Xiong, E.; Tian, T.; Zhu, D.; Ju, H.Q.; Zhou, X. A CRISPR-driven colorimetric code platform for highly accurate telomerase activity assay. Biosens. Bioelectron. 2021, 172, 112749. [Google Scholar] [CrossRef]
- Tian, T.; Zhou, X. CRISPR-Based Biosensing Strategies: Technical Development and Application Prospects. Annu. Rev. Anal. Chem. 2023, 16, 311–332. [Google Scholar] [CrossRef]
- Sam, I.K.; Chen, Y.Y.; Ma, J.; Li, S.Y.; Ying, R.Y.; Li, L.X.; Ji, P.; Wang, S.J.; Xu, J.; Bao, Y.J.; et al. TB-QUICK: CRISPR-Cas12b-assisted rapid and sensitive detection of Mycobacterium tuberculosis. J. Infect. 2021, 83, 54–60. [Google Scholar] [CrossRef]
- Kachwala, M.J.; Smith, C.W.; Nandu, N.; Yigit, M.V. Reprogrammable Gel Electrophoresis Detection Assay Using CRISPR-Cas12a and Hybridization Chain Reaction. Anal. Chem. 2021, 93, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Qiu, Z.; Jiang, Y.; Zhu, D.; Zhou, X. Exploiting the orthogonal CRISPR-Cas12a/Cas13a trans-cleavage for dual-gene virus detection using a handheld device. Biosens. Bioelectron. 2022, 196, 113701. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing-xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Liu, G. CRISPR/Cas Multiplexed Biosensing: A Challenge or an Insurmountable Obstacle? Trends Biotechnol. 2019, 37, 792–795. [Google Scholar] [CrossRef]
- Shao, N.; Han, X.; Song, Y.; Zhang, P.; Qin, L. CRISPR-Cas12a Coupled with Platinum Nanoreporter for Visual Quantification of SNVs on a Volumetric Bar-Chart Chip. Anal. Chem. 2019, 91, 12384–12391. [Google Scholar] [CrossRef]
- Zhao, R.; Tang, Y.; Song, D.; Liu, M.; Li, B. CRISPR/Cas12a-Responsive Hydrogels for Conjugation-Free and Universal Indicator Release in Colorimetric Detection. Anal. Chem. 2023, 95, 18522–18529. [Google Scholar] [CrossRef] [PubMed]
- Kosack, C.S.; Page, A.L.; Klatser, P.R. A guide to aid the selection of diagnostic tests. Bull. World Health Organ. 2017, 95, 639–645. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yu, D.; Bao, M.; Korensky, G.; Chen, J.; Shin, M.; Kim, J.; Park, M.; Qin, P.; Du, K. High-throughput and all-solution phase African Swine Fever Virus (ASFV) detection using CRISPR-Cas12a and fluorescence based point-of-care system. Biosens. Bioelectron. 2020, 154, 112068. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, J.; Liu, Y.; Cheng, W.; Huang, H.; Liang, X.; Huang, W.; Lin, L.; Zheng, Y.; Chen, W.; et al. Rapid and Ultrasensitive Detection of Plasmodium spp. Parasites via the RPA-CRISPR/Cas12a Platform. ACS Infect. Dis. 2023, 9, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Lin, H.; Li, H.; Zhou, Y.; Liu, J.; Zhong, G.; Wu, L.; Jiang, W.; Du, H.; Yang, J.; et al. Cas12a-Based On-Site and Rapid Nucleic Acid Detection of African Swine Fever. Front. Microbiol. 2019, 10, 2830. [Google Scholar] [CrossRef] [PubMed]
- Mukama, O.; Yuan, T.; He, Z.; Li, Z.; Habimana, J.d.D.; Hussain, M.; Li, W.; Yi, Z.; Liang, Q.; Zeng, L. A high fidelity CRISPR/Cas12a based lateral flow biosensor for the detection of HPV16 and HPV18. Sens. Actuators B Chem. 2020, 316, 128119. [Google Scholar] [CrossRef]
- Tsou, J.H.; Leng, Q.; Jiang, F. A CRISPR Test for Detection of Circulating Nuclei Acids. Transl. Oncol. 2019, 12, 1566–1573. [Google Scholar] [CrossRef]
- Yuan, C.; Tian, T.; Sun, J.; Hu, M.; Wang, X.; Xiong, E.; Cheng, M.; Bao, Y.; Lin, W.; Jiang, J.; et al. Universal and Naked-Eye Gene Detection Platform Based on the Clustered Regularly Interspaced Short Palindromic Repeats/Cas12a/13a System. Anal. Chem. 2020, 92, 4029–4037. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, Y.; Que, H.; Yang, T.; Cheng, X.; Ding, S.; Zhang, X.; Cheng, W. CRISPR/Cas12a-Mediated Interfacial Cleaving of Hairpin DNA Reporter for Electrochemical Nucleic Acid Sensing. ACS Sens. 2020, 5, 557–562. [Google Scholar] [CrossRef]
- English, M.A.; Soenksen, L.R.; Gayet, R.V.; de Puig, H.; Angenent-Mari, N.M.; Mao, A.S.; Nguyen, P.Q.; Collins, J.J. Programmable CRISPR-responsive smart materials. Science 2019, 365, 780–785. [Google Scholar] [CrossRef]
- WHO. Malaria Rapid Diagnostic Test Performance: Summary Results of WHO Product Testing of Malaria RDTs: Round 8 (2016–2018). Available online: https://www.who.int/publications/i/item/9789241514965 (accessed on 23 April 2023).
- Ma, Q.N.; Wang, M.; Zheng, L.B.; Lin, Z.Q.; Ehsan, M.; Xiao, X.X.; Zhu, X.Q. RAA-Cas12a-Tg: A Nucleic Acid Detection System for Toxoplasma gondii Based on CRISPR-Cas12a Combined with Recombinase-Aided Amplification (RAA). Microorganisms 2021, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Galvani, A.T.; Christ, A.P.G.; Padula, J.A.; Barbosa, M.R.F.; de Araújo, R.S.; Sato, M.I.Z.; Razzolini, M.T.P. Real-time PCR detection of Toxoplasma gondii in surface water samples in São Paulo, Brazil. Parasitol. Res. 2019, 118, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Kanitchinda, S.; Srisala, J.; Suebsing, R.; Prachumwat, A.; Chaijarasphong, T. CRISPR-Cas fluorescent cleavage assay coupled with recombinase polymerase amplification for sensitive and specific detection of Enterocytozoon hepatopenaei. Biotechnol. Rep. 2020, 27, e00485. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, L.; Li, J.; Li, X.; Li, S.; Wang, X.; Zhang, N.; Yu, Y.; Zhang, X.; Zhao, Z.; et al. Rapid, sensitive, and visual detection of Clonorchis sinensis with an RPA-CRISPR/Cas12a-based dual readout portable platform. Int. J. Biol. Macromol. 2023, 249, 125967. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Peng, D.; Jiang, C.; Zhao, W.; Li, G.; Huang, W.; Kong, L.; Gao, H.; Zheng, J.; Peng, H. Rapid and Visual Detection of Heterodera schachtii Using Recombinase Polymerase Amplification Combined with Cas12a-Mediated Technology. Int. J. Mol. Sci. 2021, 22, 2577. [Google Scholar] [CrossRef] [PubMed]
- Fleming, K.A.; Horton, S.; Wilson, M.L.; Atun, R.; DeStigter, K.; Flanigan, J.; Sayed, S.; Adam, P.; Aguilar, B.; Andronikou, S.; et al. The Lancet Commission on diagnostics: Transforming access to diagnostics. Lancet 2021, 398, 1997–2050. [Google Scholar] [CrossRef] [PubMed]
- Huyke, D.A.; Ramachandran, A.; Bashkirov, V.I.; Kotseroglou, E.K.; Kotseroglou, T.; Santiago, J.G. Enzyme Kinetics and Detector Sensitivity Determine Limits of Detection of Amplification-Free CRISPR-Cas12 and CRISPR-Cas13 Diagnostics. Anal. Chem. 2022, 94, 9826–9834. [Google Scholar] [CrossRef]
- Ramachandran, A.; Santiago, J.G. CRISPR Enzyme Kinetics for Molecular Diagnostics. Anal. Chem. 2021, 93, 7456–7464. [Google Scholar] [CrossRef]
- Garneau, J.E.; Dupuis, M.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef]
- Dong, D.; Ren, K.; Qiu, X.; Zheng, J.; Guo, M.; Guan, X.; Liu, H.; Li, N.; Zhang, B.; Yang, D.; et al. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 2016, 532, 522–526. [Google Scholar] [CrossRef]
- Gao, P.; Yang, H.; Rajashankar, K.R.; Huang, Z.; Patel, D.J. Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 2016, 26, 901–913. [Google Scholar] [CrossRef]
- Yamano, T.; Zetsche, B.; Ishitani, R.; Zhang, F.; Nishimasu, H.; Nureki, O. Structural Basis for the Canonical and Non-canonical PAM Recognition by CRISPR-Cpf1. Mol. Cell 2017, 67, 633–645.e633. [Google Scholar] [CrossRef]
- Kim, H.K.; Song, M.; Lee, J.; Menon, A.V.; Jung, S.; Kang, Y.M.; Choi, J.W.; Woo, E.; Koh, H.C.; Nam, J.W.; et al. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat. Methods 2017, 14, 153–159. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, K.; Han, Y.; Li, T.; Duan, M.; Ji, R.; Wang, X.; Zhou, X.; Zhang, Y.; Yin, H. Fast and sensitive CRISPR detection by minimized interference of target amplification. Nat. Chem. Biol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Corsi, G.I.; Qu, K.; Alkan, F.; Pan, X.; Luo, Y.; Gorodkin, J. CRISPR/Cas9 gRNA activity depends on free energy changes and on the target PAM context. Nat. Commun. 2022, 13, 3006. [Google Scholar] [CrossRef]
- Chen, Q.; Chuai, G.; Zhang, H.; Tang, J.; Duan, L.; Guan, H.; Li, W.; Li, W.; Wen, J.; Zuo, E.; et al. Genome-wide CRISPR off-target prediction and optimization using RNA-DNA interaction fingerprints. Nat. Commun. 2023, 14, 7521. [Google Scholar] [CrossRef]
- Li, Q.; Song, Z.L.; Zhang, Y.; Zhu, L.; Yang, Q.; Liu, X.; Sun, X.; Chen, X.; Kong, R.; Fan, G.C.; et al. Synergistic Incorporation of Two ssDNA Activators Enhances the Trans-Cleavage of CRISPR/Cas12a. Anal. Chem. 2023, 95, 8879–8888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qian, X.; Zheng, M.; Deng, K.; Li, C. Enhancement and inactivation effect of CRISPR/Cas12a via extending hairpin activators for detection of transcription factors. Mikrochim. Acta 2023, 191, 43. [Google Scholar] [CrossRef] [PubMed]
- Kocak, D.D.; Josephs, E.A.; Bhandarkar, V.; Adkar, S.S.; Kwon, J.B.; Gersbach, C.A. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat. Biotechnol. 2019, 37, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mei, Y.; Zhao, X.; Jiang, X. Reagents-Loaded, Automated Assay that Integrates Recombinase-Aided Amplification and Cas12a Nucleic Acid Detection for a Point-of-Care Test. Anal. Chem. 2020, 92, 14846–14852. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Zhang, J.; Lee, J.-H.; Jiao, J.; Cheng, D.; Liu, L.; Kim, H.-W.; Tao, Y.; Li, M. Spatiotemporal control of CRISPR/Cas9 gene editing. Signal Transduct. Target. Ther. 2021, 6, 238. [Google Scholar] [CrossRef]
- Zhou, W.; Brown, W.; Bardhan, A.; Delaney, M.; Ilk, A.S.; Rauen, R.R.; Kahn, S.I.; Tsang, M.; Deiters, A. Spatiotemporal Control of CRISPR/Cas9 Function in Cells and Zebrafish using Light-Activated Guide RNA. Angew. Chem. Int. Ed. 2020, 59, 8998–9003. [Google Scholar] [CrossRef]
- Jain, P.K.; Ramanan, V.; Schepers, A.G.; Dalvie, N.S.; Panda, A.; Fleming, H.E.; Bhatia, S.N. Development of Light-Activated CRISPR Using Guide RNAs with Photocleavable Protectors. Angew. Chem. Int. Ed. Engl. 2016, 55, 12440–12444. [Google Scholar] [CrossRef]
- Hu, M.; Qiu, Z.; Bi, Z.; Tian, T.; Jiang, Y.; Zhou, X. Photocontrolled crRNA activation enables robust CRISPR-Cas12a diagnostics. Proc. Natl. Acad. Sci. USA 2022, 119, e2202034119. [Google Scholar] [CrossRef]
- Hu, M.; Liu, R.; Qiu, Z.; Cao, F.; Tian, T.; Lu, Y.; Jiang, Y.; Zhou, X. Light-Start CRISPR-Cas12a Reaction with Caged crRNA Enables Rapid and Sensitive Nucleic Acid Detection. Angew. Chem. Int. Ed. 2023, 62, e202300663. [Google Scholar] [CrossRef]
- Pang, B.; Xu, J.; Liu, Y.; Peng, H.; Feng, W.; Cao, Y.; Wu, J.; Xiao, H.; Pabbaraju, K.; Tipples, G.; et al. Isothermal Amplification and Ambient Visualization in a Single Tube for the Detection of SARS-CoV-2 Using Loop-Mediated Amplification and CRISPR Technology. Anal. Chem. 2020, 92, 16204–16212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, Y.; Chen, Y.; Yang, Z.; Wu, H.; Zhou, Z.; Li, J.; Ping, J.; He, L.; Shen, H.; et al. Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection. Biosens. Bioelectron. 2020, 169, 112642. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Liu, Y.; Yang, M.; Zheng, J.; Liu, C.; Ye, W.; Song, S.; Bai, T.; Song, C.; Wang, M.; et al. The engineered CRISPR-Mb2Cas12a variant enables sensitive and fast nucleic acid-based pathogens diagnostics in the field. Plant Biotechnol. J. 2023, 21, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, B.; Yang, L.; Zhao, C.; Wang, Y.; Tang, Y.; Yang, G.; Wang, P.; Gao, S. CRISPR/Cas12a combined with recombinase polymerase amplification for rapid and sensitive detection of Vibrio vulnificus in one tube. Acta Biochim. Biophys. Sin. 2023, 55, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Shebanova, R.; Nikitchina, N.; Shebanov, N.; Mekler, V.; Kuznedelov, K.; Ulashchik, E.; Vasilev, R.; Sharko, O.; Shmanai, V.; Tarassov, I.; et al. Efficient target cleavage by Type V Cas12a effectors programmed with split CRISPR RNA. Nucleic Acids Res. 2022, 50, 1162–1173. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Smith, B.M.; Jain, P.K. Enhancement of trans-cleavage activity of Cas12a with engineered crRNA enables amplified nucleic acid detection. Nat. Commun. 2020, 11, 4906. [Google Scholar] [CrossRef]
- Ooi, K.H.; Liu, M.M.; Tay, J.W.D.; Teo, S.Y.; Kaewsapsak, P.; Jin, S.; Lee, C.K.; Hou, J.; Maurer-Stroh, S.; Lin, W.; et al. An engineered CRISPR-Cas12a variant and DNA-RNA hybrid guides enable robust and rapid COVID-19 testing. Nat. Commun. 2021, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, W.; Ma, S.; Li, Z.; Yao, Y.; Fei, T. A chemical-enhanced system for CRISPR-Based nucleic acid detection. Biosens. Bioelectron. 2021, 192, 113493. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Zhao, G.; Wang, T.H. Applying biosensor development concepts to improve preamplification-free CRISPR/Cas12a-Dx. Analyst 2020, 145, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Meng, Q.; Sun, B.; Zhao, B.; Dang, L.; Zhong, M.; Liu, S.; Xu, H.; Mei, H.; Liu, J.; et al. MeCas12a, a Highly Sensitive and Specific System for COVID-19 Detection. Adv. Sci. 2020, 7, 2001300. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Shu, B.; Tian, T.; Xiong, E.; Huang, M.; Zhu, D.; Sun, J.; Liu, Q.; Wang, S.; Li, Y.; et al. Droplet Cas12a Assay Enables DNA Quantification from Unamplified Samples at the Single-Molecule Level. Nano Lett. 2021, 21, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, J.; Zhang, J.; Chen, Y.; Yin, L.; Jin, D.; Gu, D.; Zhao, H.; Xu, Y.; Wang, J. Definition of CRISPR Cas12a T rans-Cleavage Units to Facilitate CRISPR Diagnostics. Front. Microbiol. 2021, 12, 766464. [Google Scholar] [CrossRef]
- Rossetti, M.; Merlo, R.; Bagheri, N.; Moscone, D.; Valenti, A.; Saha, A.; Arantes, P.R.; Ippodrino, R.; Ricci, F.; Treglia, I.; et al. Enhancement of CRISPR/Cas12a trans-cleavage activity using hairpin DNA reporters. Nucleic Acids Res. 2022, 50, 8377–8391. [Google Scholar] [CrossRef]
- Li, T.; Hu, R.; Xia, J.; Xu, Z.; Chen, D.; Xi, J.; Liu, B.F.; Zhu, J.; Li, Y.; Yang, Y.; et al. G-triplex: A new type of CRISPR-Cas12a reporter enabling highly sensitive nucleic acid detection. Biosens. Bioelectron. 2021, 187, 113292. [Google Scholar] [CrossRef]
- Paul, R.; Ostermann, E.; Wei, Q. Advances in point-of-care nucleic acid extraction technologies for rapid diagnosis of human and plant diseases. Biosens. Bioelectron. 2020, 169, 112592. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Chen, F.; Bai, H.; Xiu, L.; Zhou, X.; Guo, X.; Hu, Q.; Yin, K. Amplification-free CRISPR/Cas detection technology: Challenges, strategies, and perspectives. Chem. Soc. Rev. 2023, 52, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.B.; Lalbakhsh, A.; Talla, J.; Peroutka, Z.; Hadjilooei, F.; Lalbakhsh, P.; Jamshidi, M.; Spada, L.; Mirmozafari, M.; Dehghani, M.; et al. Artificial Intelligence and COVID-19: Deep Learning Approaches for Diagnosis and Treatment. IEEE Access Pract. Innov. Open Solut. 2020, 8, 109581–109595. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Zhou, M.; Han, Y.; Zhang, M.; Gao, Z.; Liu, Z.; Chen, P.; Du, W.; Zhang, X.; et al. Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence. Nat. Commun. 2023, 14, 1341. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Mukherjee, S.; Saha, N.; Yarravarapu, N.; Lone, S.N.; Masoodi, T.; Chauhan, R.; Maacha, S.; Bagga, P.; et al. Integration of CRISPR/Cas9 with artificial intelligence for improved cancer therapeutics. J. Transl. Med. 2022, 20, 534. [Google Scholar] [CrossRef]
- Gonatopoulos-Pournatzis, T.; Aregger, M.; Brown, K.R.; Farhangmehr, S.; Braunschweig, U.; Ward, H.N.; Ha, K.C.H.; Weiss, A.; Billmann, M.; Durbic, T.; et al. Genetic interaction mapping and exon-resolution functional genomics with a hybrid Cas9-Cas12a platform. Nat. Biotechnol. 2020, 38, 638–648. [Google Scholar] [CrossRef]
- Baisya, D.; Ramesh, A.; Schwartz, C.; Lonardi, S.; Wheeldon, I. Genome-wide functional screens enable the prediction of high activity CRISPR-Cas9 and -Cas12a guides in Yarrowia lipolytica. Nat. Commun. 2022, 13, 922. [Google Scholar] [CrossRef] [PubMed]

| Discipline | Strength | Weakness |
|---|---|---|
| Morphology | Accuracy (gold standard) Can detect multiple species at the same time | Lower sensitivity Difficulty distinguishing parasite-like egg High demand for professional skills |
| Immunology | Robust specificity Robust sensitivity | High cost and time consuming False positives for cross-reactivity False negatives in immunocompromised patients Inability to differentiate between ongoing and past infections |
| Molecular biology | Robust specificity Robust sensitivity Robust repeatability | High cost Limitations related to sample preparation and equipment Logistics systems requiring fresh sample analysis (e.g., cryogenic) |
| Parasite | Repeat Sequence Name | Length (bp) | Quantity | GenBank Accession | Refs |
|---|---|---|---|---|---|
| Protozoa | |||||
| Trypanosoma cruzi | TCNRE | 195 | 12% of the total genome | K01772 | [53] |
| Toxoplasma gondii | / | 529 | 200–300 copies per genome | AF146527 | [54] |
| Plasmodium falciparum | Pfr364 | 716 | 41 copies per genome | / | [55,56] |
| Plasmodium vivax | Pvr47 | 333 | 14 copies per genome | / | [55,56,57] |
| Cestodes | |||||
| Echinococcus granulosus | EgG1 Hae III repeat | 269 | 6900 copies per haploid genome (1% of E. granulosus genomic DNA) | DQ157697 | [58,59] |
| Taenia solium | Tsol-9 | 158 | / | U45987 | [60] |
| Taenia saginata | HDP1 | 1272 | 0.4% of the T. saginata DNA | AJ133764 | [61] |
| Trematodes | |||||
| Schistosoma mansoni | Sml-7 (DraI) | 121 | 12% of the total genome | M61098 | [62,63,64] |
| Schistosoma haematobium | DraI | 121 | over 15% of the S. haematobium genome | DQ157698 | [65] |
| Trichobilharzia ocellata | ToSau3A | 396 | 10,000 copies per haploid genome (1.5% of the T. ocellata genome) | AF442689 | [66] |
| Nematodes | |||||
| Strongyloides stercoralis | / | 765 | / | AY028262 | [67] |
| Brugia malayi | HhaI repeat | 320 | several thousand copies per haploid genome (about 12% of the genome) | M12691 | [68,69] |
| Wuchereria bancrofti | SspI | 195 | 300 copies per haploid genome | L20344 | [70] |
| LDR | 1674 | / | AY297458 | [71] | |
| Onchocerca volvulus | O-150 | 149 | 4500 copies per haploid genome | J04659 | [72,73,74] |
| Parafilaroides decorus | Pd65 | 689 | / | MT053285 | [75] |
| Technology | Device Dependency | Specificity | Reaction Time (min) | Number of Primers | Quantification | Cost | Results View Method | POCT Potential |
|---|---|---|---|---|---|---|---|---|
| PCR | Moderate | Robust | 60–180 | 2 | No | High | Gel electrophoresis | Moderate |
| qPCR (RT-qPCR) | High | Robust | >60 | 2 | Yes | Extremely high | Fluorescent and computer system | LOW |
| dPCR | High | Robust | >60 | 2 | Yes | Extremely high | Fluorescent and computer system | LOW |
| LAMP | Low | Robust | <60 | 4–6 | No | Low | Gel electrophoresis Color Turbidity | High |
| RPA/RAA | Low | Moderate | 20–60 | 2 | No | Low | Gel electrophoresis Fluorescent Lateral flow | High |
| Cas12a | Low | Robust | 20–60 | 2 | No | Low | fluorescent Lateral flow | High |
| Species | Method | Time (min) | LOD | Sample | Refs |
|---|---|---|---|---|---|
| Plasmodium falciparum | Cas12a-RPA | 30 (+10) a | 0.36 parasites/μL | Serum/Whole blood/ Dried blood spot | [23,136] |
| Plasmodium vivax | Cas12a-RPA | 30 (+10) a | 1.2 parasites/μL | ||
| Toxoplasma gondii | Cas12a-RPA (two steps) | 30 + >15 | 1.5 copies/μL | Whole blood | [25] |
| Cas12a-RPA | 35 (+20) a | 99~115 copies/μL | Environmental samples (e.g., water and soil) | [24] | |
| Cas12a-RAA (two steps) | 20 + 50 | 1 fM | [144] | ||
| Schistosoma haematobium | Cas12a-RPA | 40 + (70) a | 2 eggs | Urine | [27] |
| Cryptosporidium parvum | Cas12a-RPA (two steps) | 30 + 60 (+20) a | 10 oocysts | Feces | [112] |
| Cas12a-RPA | 90 | 1 oocyst | Water | [26] | |
| Enterocytozoon hepatopenaei | Cas12a-RPA | 60 | 50 copies/μL | Tissue | [146] |
| Heterodera schachtii | Cas12a-RPA | 60 | 10−4 single cysts | Tissue | [148] |
| Clonorchis sinensis | Cas12a-RPA | <60 | 1 copy/μL | Feces/Tissue | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Dang, Z.; Tang, W.; Zhang, H.; Shao, J.; Jiang, R.; Zhang, X.; Huang, F. Detection of Parasites in the Field: The Ever-Innovating CRISPR/Cas12a. Biosensors 2024, 14, 145. https://doi.org/10.3390/bios14030145
Li X, Dang Z, Tang W, Zhang H, Shao J, Jiang R, Zhang X, Huang F. Detection of Parasites in the Field: The Ever-Innovating CRISPR/Cas12a. Biosensors. 2024; 14(3):145. https://doi.org/10.3390/bios14030145
Chicago/Turabian StyleLi, Xin, Zhisheng Dang, Wenqiang Tang, Haoji Zhang, Jianwei Shao, Rui Jiang, Xu Zhang, and Fuqiang Huang. 2024. "Detection of Parasites in the Field: The Ever-Innovating CRISPR/Cas12a" Biosensors 14, no. 3: 145. https://doi.org/10.3390/bios14030145
APA StyleLi, X., Dang, Z., Tang, W., Zhang, H., Shao, J., Jiang, R., Zhang, X., & Huang, F. (2024). Detection of Parasites in the Field: The Ever-Innovating CRISPR/Cas12a. Biosensors, 14(3), 145. https://doi.org/10.3390/bios14030145





