Effect of Protection Polymer Coatings on the Performance of an Amperometric Galactose Biosensor in Human Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
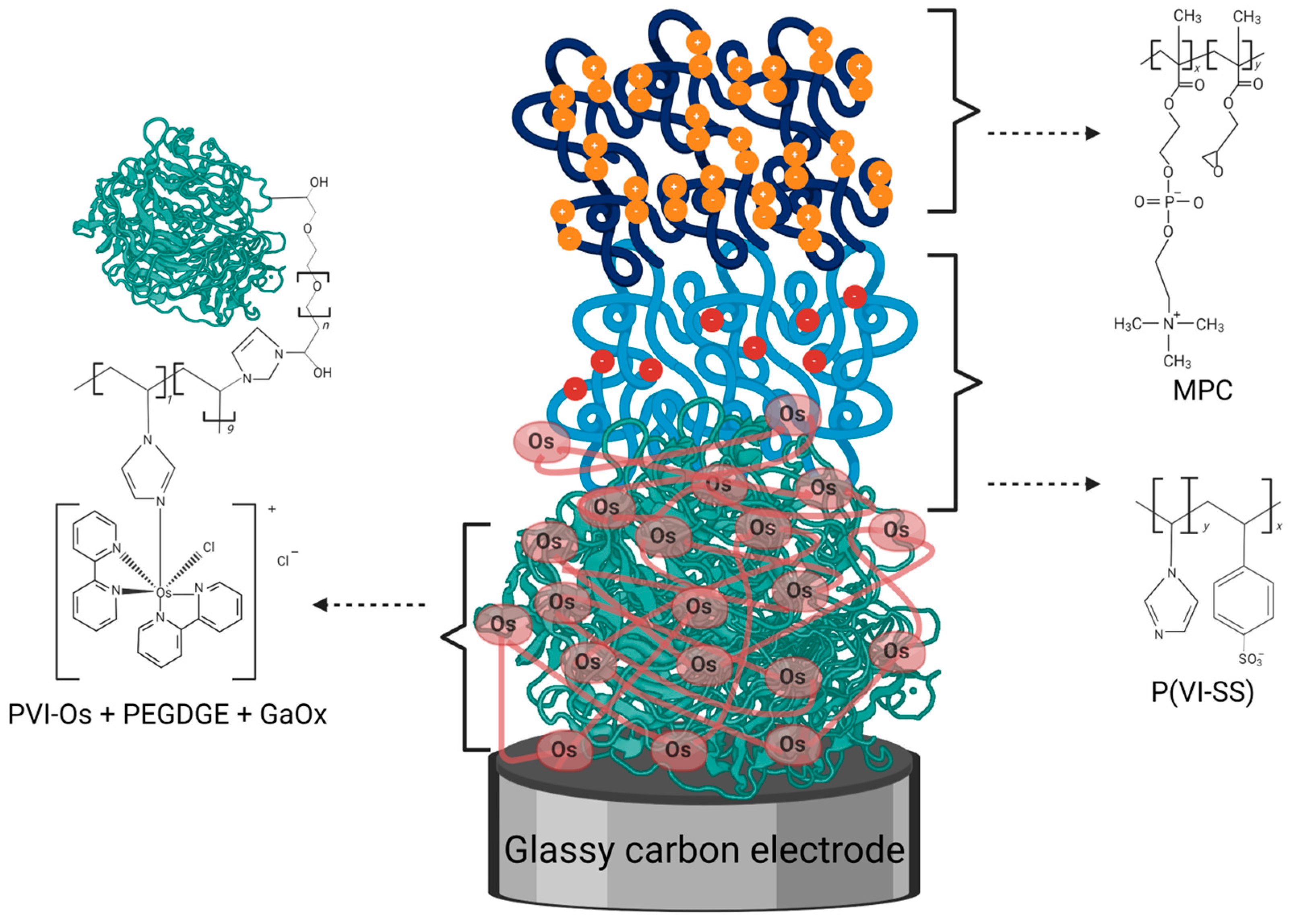
2.2. Electrode Modification
2.3. Electrochemical Measurements
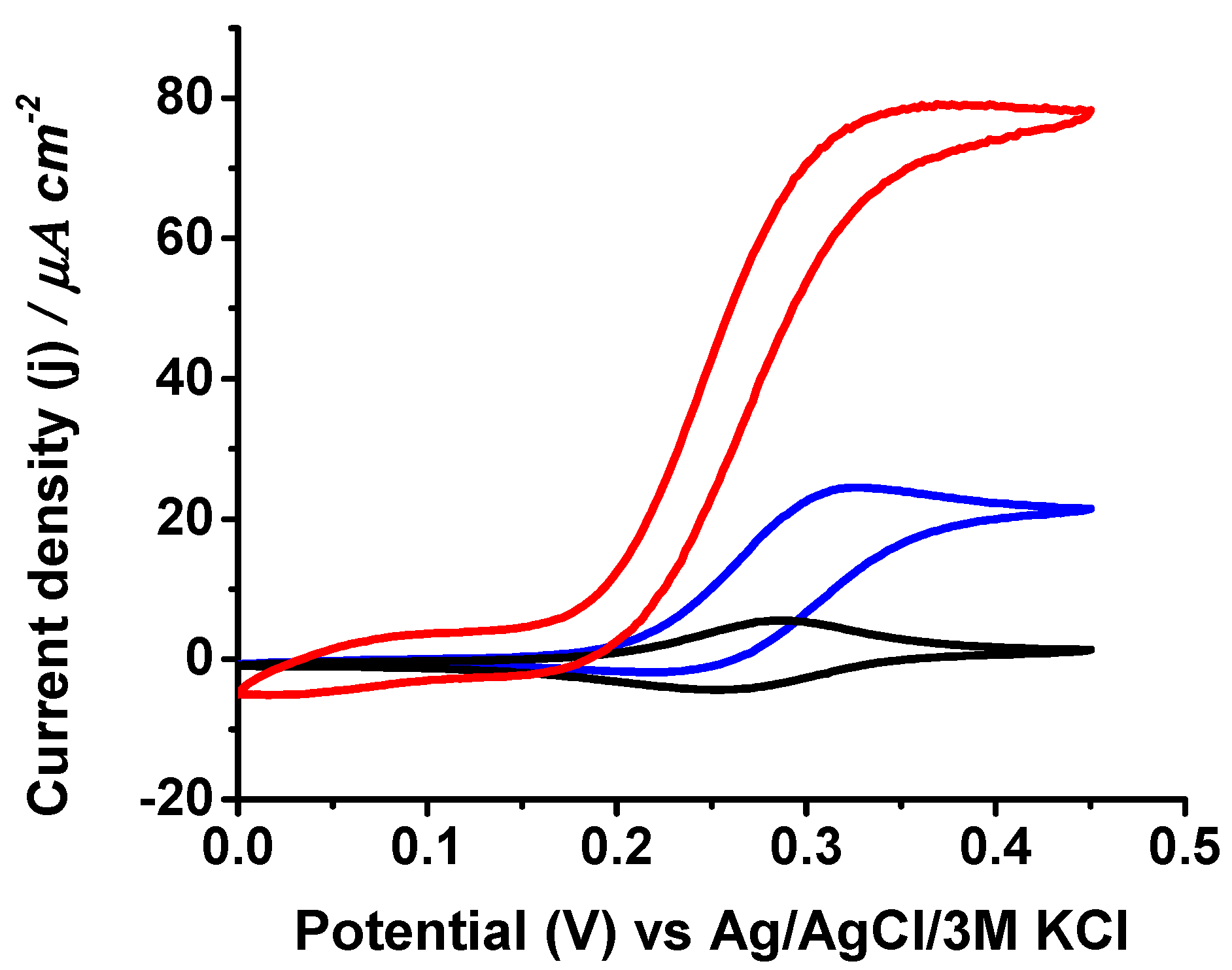
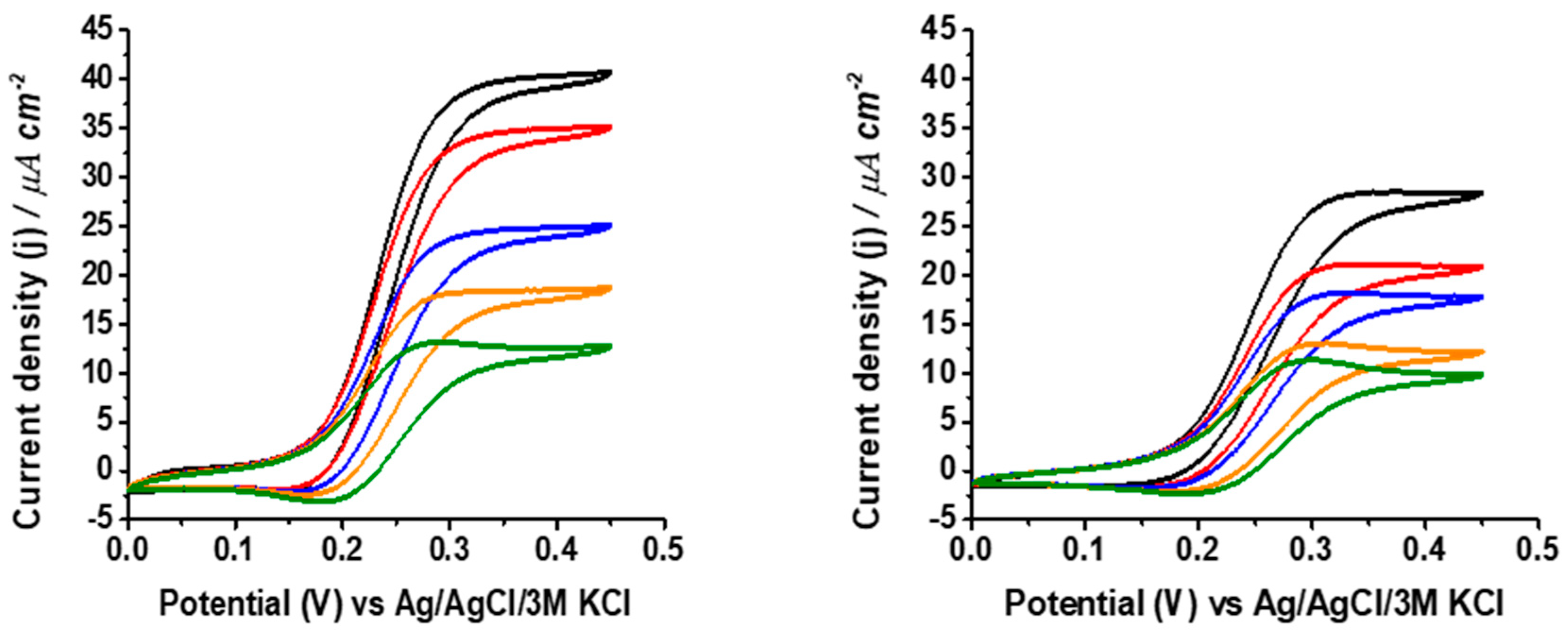
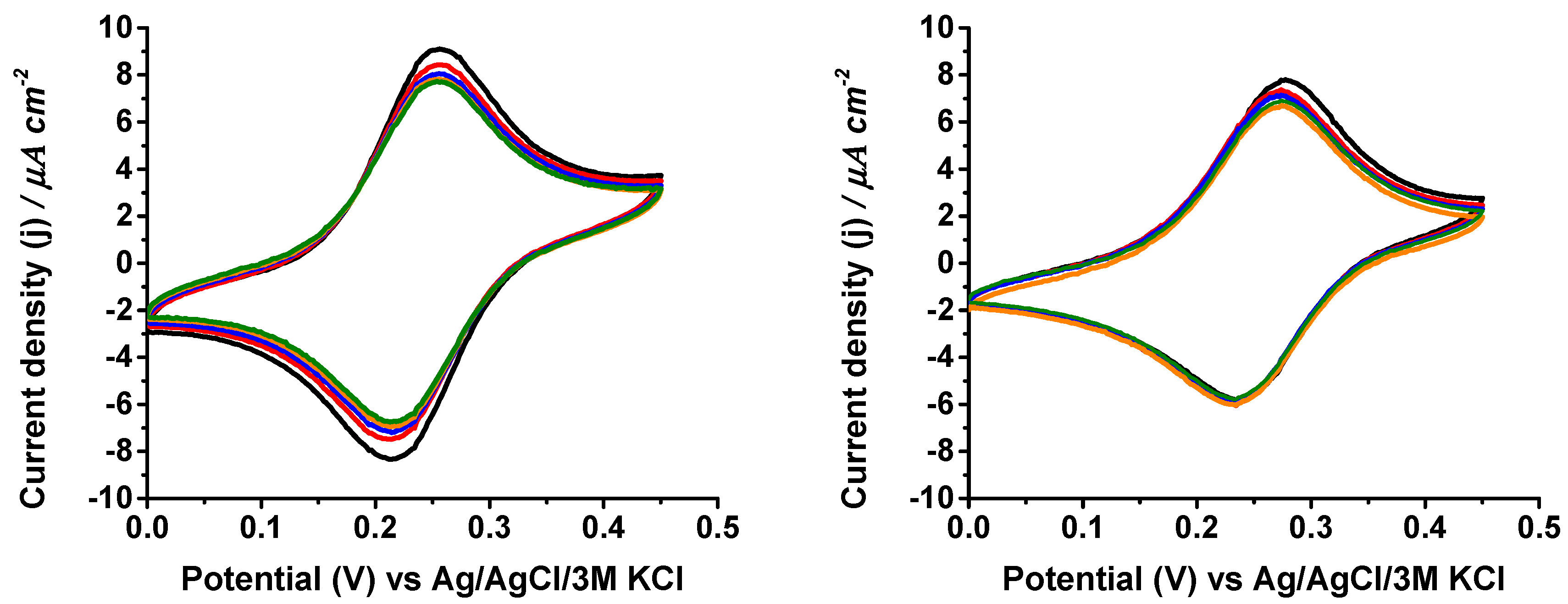
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiori, V.; et al. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.P.; Luo, X.J.; Liu, Y.Q.; Yan, C.L.; Li, H.X.; Lv, J.C.; Yang, L.; Cui, Y. A disposable printed amperometric biosensor for clinical evaluation of creatinine in renal function detection. Talanta 2022, 248, 123592. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Kotanen, C.N.; Moussy, F.G.; Carrara, S.; Guiseppi-Elie, A. Implantable enzyme amperometric biosensors. Biosens. Bioelectron. 2012, 35, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent progress in wearable biosensors: From healthcare monitoring to sports analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Baracu, A.M.; Dinu Gugoasa, L.A. Review—Recent advances in microfabrication, design and applications of amperometric sensors and biosensors. J. Electrochem. Soc. 2021, 168, 037503. [Google Scholar] [CrossRef]
- Capuani, S.; Malgir, G.; Chua, C.Y.X.; Grattoni, A. Advanced strategies to thwart foreign body response to implantable devices. Bioeng. Trans. Med. 2022, 7, e10300. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lee, H. Anti-biofouling strategies for long-term continuous use of implantable biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Falk, M.; Psotta, C.; Cirovic, S.; Shleev, S. Non-invasive electrochemical biosensors operating in human physiological fluids. Sensors 2020, 20, 6352. [Google Scholar] [CrossRef]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Common causes of glucose oxidase instability in in invo biosensing: A brief review. J. Diabetes Sci. Technol. 2013, 7, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Meehan, J.; Ward, C.; Langdon, S.P.; Kunkler, L.H.; Murray, A.; Argyle, D. Implantable biosensors and their contribution to the future of precision medicine. Vet. J. 2018, 239, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the foreign-body response to implanted biomaterials: Strategies and applications of new materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Z.; Morrin, A.; Luo, X. Low fouling strategies for electrochemical biosensors targeting disease biomarkers. Anal. Meth. 2019, 11, 702–711. [Google Scholar] [CrossRef]
- Tkac, J.; Whittaker, J.W.; Ruzgas, T. The use of single walled carbon nanotubes dispersed in a chitosan matrix for preparation of a galactose biosensor. Biosens. Bioelectron. 2007, 22, 1820–1824. [Google Scholar] [CrossRef]
- Jia, W.Z.; Wang, K.; Xia, X.H. Elimination of electrochemical interferences in glucose biosensors. Trends Anal. Chem. 2010, 29, 306–318. [Google Scholar] [CrossRef]
- Nederberg, F.; Watanabe, J.; Ishihara, K.; Hilborn, J.; Bowden, T. Biocompatible and biodegradable phosphorylcholine ionomers with reduced protein adsorption and cell adhesion. J. Biomater. Sci. 2006, 17, 605–614. [Google Scholar] [CrossRef]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.I.; Moluton, S.E.; Guijt, R.M.; Greene, G.W.; et al. Antifouling strategies for electrochemical biosensing: Mechanisms and performance toward point of care based diagnostic applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef]
- Rodriguez Emmenegger, C.; Brynda, E.; Riedel, T.; Sedlakova, Z.; Houska, M.; Bologna Alles, A. Interaction of blood plasma with antifouling surfaces. Langmuir 2009, 25, 6328–6333. [Google Scholar] [CrossRef]
- Bernhard, C.; Roeters, S.J.; Franz, J.; Weidner, T.; Bonn, M.; Gonella, G. Repelling and ordering: The influence of poly(ethylene glycol) on protein adsorption. Phys. Chem. Chem. Phys. 2017, 19, 28182–28188. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Doloff, J.C.; Yesilyurt, V.; Sadraei, A.; McGarrigle, J.J.; Omami, M.; Veiseh, O.; Farah, S.; Isa, D.; Ghani, S.; et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2018, 2, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chang, Y.; Ishihara, K. Reduced blood cell adhesion on polypropylene substrates through a simple surface zwitterionization. Langmuir 2017, 33, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, K.; Lielpetere, A.; Domingo-López, D.A.; Levey, R.E.; Duffy, G.P.; Schuhmann, W.; Leech, D. Tethering zwitterionic polymer coatings to mediated glucose biosensor enzyme electrodes can decrease sensor foreign body response yet retain sensitivity to glucose. Biosen. Bioelectron. 2023, 219, 114815. [Google Scholar] [CrossRef] [PubMed]
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry, molecular genetics, newborn screening and treatment. Biomolecules 2022, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- Delnoy, B.; Coelho, A.I.; Rubio-Gozalbo, M.E. Current and future treatments for classic galactosemia. J. Pers. Med. 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Timson, D.J. The molecular basis of galactosemia—Past, present and future. Gene 2016, 589, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tisa, I.B.; Achim, A.C.; Cozma-Petrut, A. The importance of neonatal prescreening for galactosemia. Nutrients 2023, 15, 10. [Google Scholar] [CrossRef]
- Timmers, I.; van den Hurk, J.; Di Salle, F.; Rubio-Gozalbo, M.E.; Jansma, B.M. Language production and working memory in classic galactosemia from a cognitive neuroscience perspective: Future research directions. J. Inherit. Metab. Dis. 2011, 34, 367–376. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Chen, Y.T. Disorders of carbohydrate metabolism. In Emery and Rimoin’s Principles and Practice of Medical Genetics, 6th ed.; Rimoin, D., Pyeritz, R., Korf, B., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1–36. [Google Scholar] [CrossRef][Green Version]
- Kanyong, P.; Krampa, F.D.; Aniweh, Y.; Awandare, G.A. Enzyme-based amperometric galactose biosensors: A review. Microchim. Acta 2017, 184, 3663–3671. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; De Lacey, A.L.; Pita, M. Electrochemical studies of galactose oxidase. Electrochem. Sci. Adv. 2022, 2, e2100171. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of an amperometric screen-printed galactose biosensor for serum analysis. Anal. Biochem. 2013, 435, 114–119. [Google Scholar] [CrossRef]
- Gwon, K.; Lee, S.; Nam, H.; Shin, J.H. Disposable strip-type biosensors for amperometric determination of galactose. J. Electrochem. Sci. Technol. 2020, 11, 310–317. [Google Scholar] [CrossRef]
- Yu, K.M.; Yang, P.; Huang, T.Y.; Shen, T.Y.S.; Lau, J.Y.N.; Hu, O.Y.P. A novel galactose electrochemical biosensor intended for point-of-care measurement of quantitative liver function using galactose single-point test. Anal. Bioanal. Chem. 2022, 414, 4067–4077. [Google Scholar] [CrossRef]
- Figueiredo, C.; García-Ortega, A.; Mandal, T.; Lielpetere, A.; Cervantes, F.; Demurtas, D.; Magner, E.; Plou, F.J.; Schuhmann, W.; Leech, D.; et al. An oxygen-insensitive amperometric galactose biosensor based on galactose oxidase co-immobilized with an Os-complex modified redox polymer. Electrochim. Acta 2023, 472, 143438. [Google Scholar] [CrossRef]
- Lielpetere, A.; Jayakumar, K.; Leech, D.; Schuhmann, W. Cross-linkable polymer-based multi-layers for protecting electrochemical glucose biosensors against uric acid, ascorbic acid, and biofouling interferences. ACS Sens. 2023, 8, 1756–1765. [Google Scholar] [CrossRef]
- Forster, R.J.; Vos, J.G. Synthesis, characterization, and properties of a series of osmium- and ruthenium-containing metallopolymers. Macromolecules 1990, 23, 4372–4377. [Google Scholar] [CrossRef]
- Kober, E.M.; Caspar, J.V.; Sullivan, B.P.; Meyer, T.J. Synthetic routes to new polypyridyl complexes of osmium (II). Inorg. Chem. 1988, 27, 4587–4598. [Google Scholar] [CrossRef]
- Reichhart, T.M.B.; Scheiblbrandner, S.; Sygmund, C.; Harreither, W.; Schenkenfelder, J.; Schulz, C.; Felice, A.K.G.; Gorton, L.; Ludwig, R. Interface engineering of cellobiose dehydrogenase improves interdomain electron transfer. Protein Sci. 2023, 32, e4702. [Google Scholar] [CrossRef]
- Zhao, F.; Brix, A.C.; Lielpetere, A.; Schuhmann, W.; Conzuelo, F. On the mediated electron transfer of immobilized galactose oxidase for biotechnological applications. Chem. Eur. J. 2022, 28, e202200868. [Google Scholar] [CrossRef] [PubMed]
- Gregg, B.A.; Heller, A. Redox polymer films containing enzymes. 2. Glucose oxidase containing enzyme electrodes. J. Phys. Chem. 1991, 95, 5976–5980. [Google Scholar] [CrossRef]
- Ohara, T.J.; Rajagopalan, R.; Heller, A. Glucose electrodes based on cross-linked [Os(bpy)2Cl]+/2+ complexed poly(1-vinylimidazole) films. Anal. Chem. 1993, 65, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, C.; Psotta, C.; Jayakumar, K.; Lielpetere, A.; Mandal, T.; Schuhmann, W.; Leech, D.; Falk, M.; Pita, M.; Shleev, S.; et al. Effect of Protection Polymer Coatings on the Performance of an Amperometric Galactose Biosensor in Human Plasma. Biosensors 2024, 14, 167. https://doi.org/10.3390/bios14040167
Figueiredo C, Psotta C, Jayakumar K, Lielpetere A, Mandal T, Schuhmann W, Leech D, Falk M, Pita M, Shleev S, et al. Effect of Protection Polymer Coatings on the Performance of an Amperometric Galactose Biosensor in Human Plasma. Biosensors. 2024; 14(4):167. https://doi.org/10.3390/bios14040167
Chicago/Turabian StyleFigueiredo, Carina, Carolin Psotta, Kavita Jayakumar, Anna Lielpetere, Tanushree Mandal, Wolfgang Schuhmann, Dónal Leech, Magnus Falk, Marcos Pita, Sergey Shleev, and et al. 2024. "Effect of Protection Polymer Coatings on the Performance of an Amperometric Galactose Biosensor in Human Plasma" Biosensors 14, no. 4: 167. https://doi.org/10.3390/bios14040167
APA StyleFigueiredo, C., Psotta, C., Jayakumar, K., Lielpetere, A., Mandal, T., Schuhmann, W., Leech, D., Falk, M., Pita, M., Shleev, S., & De Lacey, A. L. (2024). Effect of Protection Polymer Coatings on the Performance of an Amperometric Galactose Biosensor in Human Plasma. Biosensors, 14(4), 167. https://doi.org/10.3390/bios14040167






