Raman Flow Cytometry and Its Biomedical Applications
Abstract
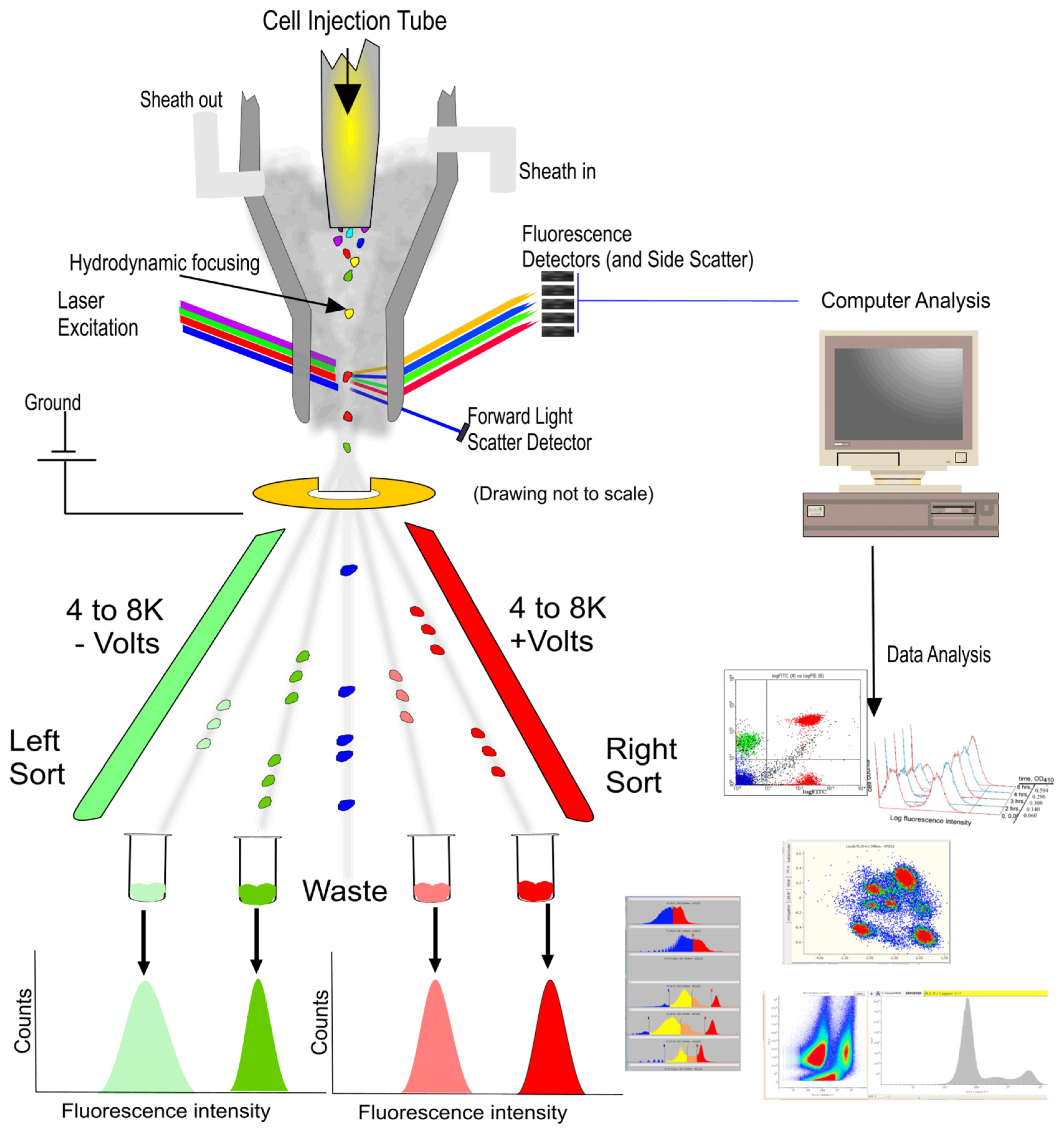
:1. Introduction
2. Basics of Raman Spectroscopy
3. Development and Advantages of RFC
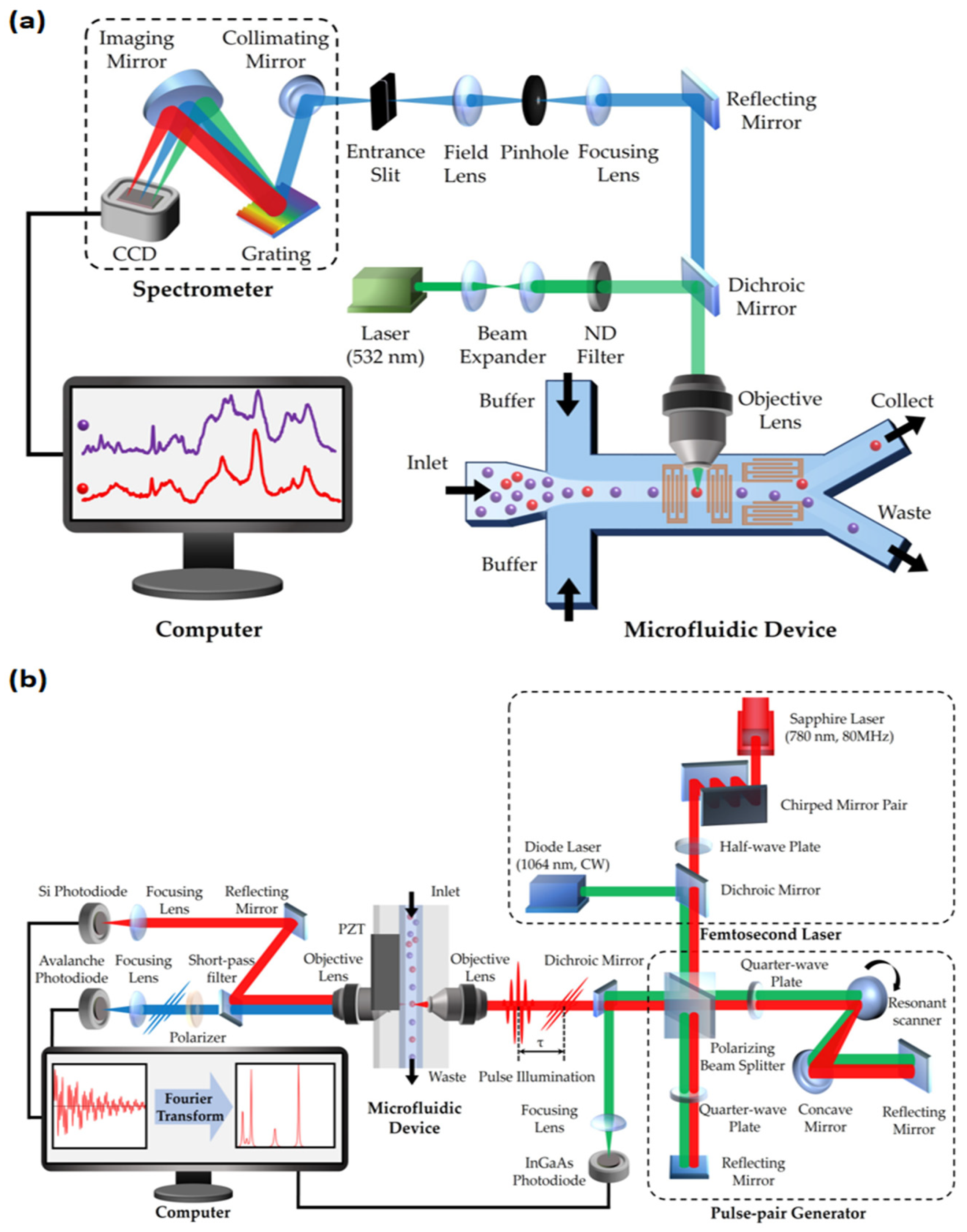
3.1. Development of RFC
3.2. Advantages of RFC
4. Biomedical Applications of RFC
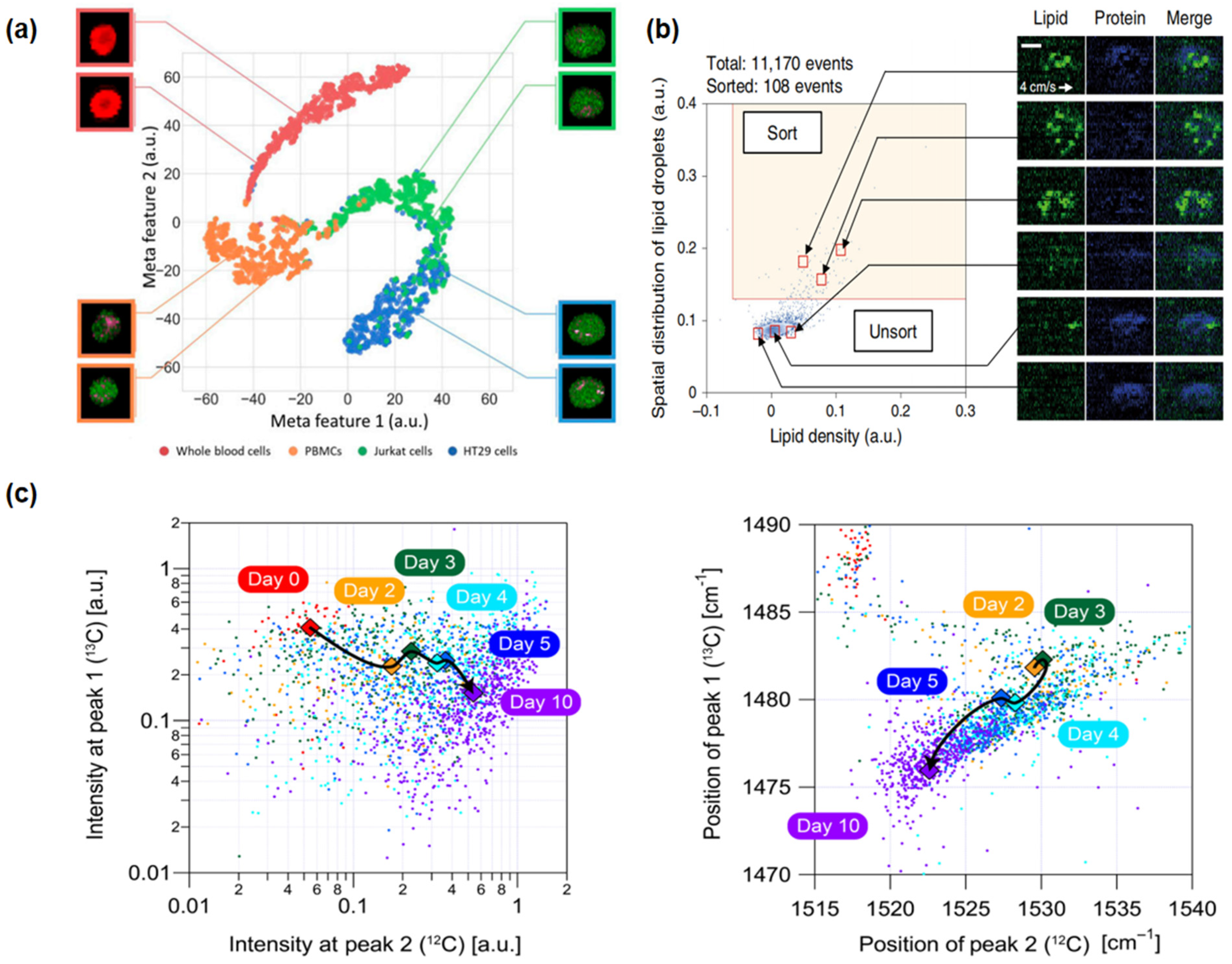
4.1. Cancer Cells and Cancer Detection
4.2. Stem Cells and Cellular Therapy
4.3. Microbial Cells and Subpopulation Differentiation
4.4. Drug Discovery and Sensitivity Assessment (Cellular Level)
5. Current Challenges and Future Directions
5.1. Sensitivity
5.2. Throughput
5.3. Instrumental Design and Data Processing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef] [PubMed]
- Herzenberg, L.A.; Parks, D.; Sahaf, B.; Perez, O.; Roederer, M.; Herzenberg, L.A. The History and Future of the Fluorescence Activated Cell Sorter and Flow Cytometry: A View from Stanford. Clin. Chem. 2002, 48, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.P.; Ostafe, R.; Iyengar, S.N.; Rajwa, B.; Fischer, R. Flow Cytometry: The Next Revolution. Cells 2023, 12, 1875. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.M.; Shah, P.; Nair, A. Flow Cytometry: Principles, Applications and Recent Advances. Bioanalysis 2021, 13, 181–198. [Google Scholar] [CrossRef] [PubMed]
- El-Hajjar, L.; Ali Ahmad, F.; Nasr, R. A Guide to Flow Cytometry: Components, Basic Principles, Experimental Design, and Cancer Research Applications. Curr. Protoc. 2023, 3, e721. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.A. Fluorochromes and Fluorescence. In Flow Cytometry: Principles and Applications; Macey, M.G., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 59–112. ISBN 978-1-59745-451-3. [Google Scholar]
- Austin Suthanthiraraj, P.P.; Graves, S.W. Fluidics. Curr. Protoc. Cytom. 2013, Chapter 1, 1.2.1–1.2.14. [Google Scholar] [CrossRef]
- Zorzi, F.; Bonfadini, S.; Aloisio, L.; Moschetta, M.; Storti, F.; Simoni, F.; Lanzani, G.; Criante, L. Optofluidic Flow Cytometer with In-Plane Spherical Mirror for Signal Enhancement. Sensors 2023, 23, 9191. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.F.; van den Engh, G. Flow Cytometry and Cell Sorting. In Cell Separation: Fundamentals, Analytical and Preparative Methods; Kumar, A., Galaev, I.Y., Mattiasson, B., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 19–39. ISBN 978-3-540-75263-9. [Google Scholar]
- Montante, S.; Brinkman, R.R. Flow Cytometry Data Analysis: Recent Tools and Algorithms. Int. J. Lab. Hematol. 2019, 41 (Suppl. 1), 56–62. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Reyer, M.A.; McLean, E.L.; Liu, W.; Fei, J. An Improved Method for Bacterial Immunofluorescence Staining to Eliminate Antibody Exclusion from the Fixed Nucleoid. Biochemistry 2019, 58, 4457–4465. [Google Scholar] [CrossRef]
- Fajrial, A.K.; He, Q.Q.; Wirusanti, N.I.; Slansky, J.E.; Ding, X. A Review of Emerging Physical Transfection Methods for CRISPR/Cas9-Mediated Gene Editing. Theranostics 2020, 10, 5532–5549. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Fixation and Permeabilization of Cells and Tissues. CSH Protoc. 2008, 2008, pdb.top36. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, F.; Li, M.; Wo, X.; Su, Y.-W.; Wang, W. Influence of Fixation and Permeabilization on the Mass Density of Single Cells: A Surface Plasmon Resonance Imaging Study. Front. Chem. 2019, 7, 588. [Google Scholar] [CrossRef]
- George, B. Regulations and Guidelines Governing Stem Cell Based Products: Clinical Considerations. Perspect. Clin. Res. 2011, 2, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Marks, P. The FDA’s Regulatory Framework for Chimeric Antigen Receptor-T Cell Therapies. Clin. Transl. Sci. 2019, 12, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M. Compensation in Flow Cytometry. Curr. Protoc. Cytom. 2002, Chapter 1, Unit 1.14. [Google Scholar] [CrossRef]
- Hunka, J.; Riley, J.T.; Debes, G.F. Approaches to Overcome Flow Cytometry Limitations in the Analysis of Cells from Veterinary Relevant Species. BMC Vet. Res. 2020, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Fedr, R.; Kahounová, Z.; Remšík, J.; Reiterová, M.; Kalina, T.; Souček, K. Variability of Fluorescence Intensity Distribution Measured by Flow Cytometry Is Influenced by Cell Size and Cell Cycle Progression. Sci. Rep. 2023, 13, 4889. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.A.; Brown, L.O.; Gaskill, D.F.; Naivar, M.; Graves, S.W.; Doorn, S.K.; Nolan, J.P. A Flow Cytometer for the Measurement of Raman Spectra. Cytom. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Gala de Pablo, J.; Lindley, M.; Hiramatsu, K.; Goda, K. High-Throughput Raman Flow Cytometry and Beyond. Acc. Chem. Res. 2021, 54, 2132–2143. [Google Scholar] [CrossRef]
- Song, Y.; Yin, H.; Huang, W.E. Raman Activated Cell Sorting. Curr. Opin. Chem. Biol. 2016, 33, 1–8. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Label-Free SERS Quantum Semiconductor Probe for Molecular-Level and in Vitro Cellular Detection: A Noble-Metal-Free Methodology. ACS Appl. Mater. Interfaces 2018, 10, 34886–34904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Fales, A.M.; Register, J.; Yuan, H.; Vo-Dinh, T. Direct Analysis of Traditional Chinese Medicines Using Surface-Enhanced Raman Scattering (SERS). Drug Test. Anal. 2014, 6, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Bumbrah, G.S.; Sharma, R.M. Raman Spectroscopy—Basic Principle, Instrumentation and Selected Applications for the Characterization of Drugs of Abuse. Egypt. J. Forensic Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- Jones, R.; Hooper, D.; Zhang, L.; Wolverson, D.; Valev, V. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Winterhalder, M.J.; Zumbusch, A. Beyond the Borders—Biomedical Applications of Non-Linear Raman Microscopy. Adv. Drug Deliv. Rev. 2015, 89, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.C.; Tare, R.S.; Oreffo, R.O.C.; Mahajan, S. Raman Spectroscopy and Coherent Anti-Stokes Raman Scattering Imaging: Prospective Tools for Monitoring Skeletal Cells and Skeletal Regeneration. J. R. Soc. Interface 2016, 13, 20160182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, Y.; Tan, S.P.H.; Bi, R.; Olivo, M. Molecular Fingerprint Detection Using Raman and Infrared Spectroscopy Technologies for Cancer Detection: A Progress Review. Biosensors 2023, 13, 557. [Google Scholar] [CrossRef]
- Shang, H.; Wang, H. Anharmonic Raman Spectra Simulation of Crystals from Deep Neural Networks. AIP Adv. 2021, 11, 035105. [Google Scholar] [CrossRef]
- Guo, G.; Guo, C.; Qie, X.; He, D.; Meng, S.; Su, L.; Liang, S.; Yin, S.; Yu, G.; Zhang, Z.; et al. Correlation Analysis between Raman Spectral Signature and Transcriptomic Features of Carbapenem-Resistant Klebsiella Pneumoniae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123699. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, S.; Lin, K.; Yi, X.; Sun, Y.; Xu, X.; He, N.; Zhang, Z.; Hu, H.; Qie, X.; et al. Leveraging Single-Cell Raman Spectroscopy and Single-Cell Sorting for the Detection and Identification of Yeast Infections. Anal. Chim. Acta 2023, 1239, 340658. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wu, G.; Liu, G. The Molecular Polarizability Derivatives and Their Implications as Interpreted from the Surface Enhanced Raman Intensities: A Case Study of Piperidine. J. Chem. Phys. 1987, 87, 7300–7306. [Google Scholar] [CrossRef]
- Guo, S.; Popp, J.; Bocklitz, T. Chemometric Analysis in Raman Spectroscopy from Experimental Design to Machine Learning–Based Modeling. Nat. Protoc. 2021, 16, 5426–5459. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.C.; Lednev, I.K. Raman Spectroscopy for Forensic Purposes: Recent Applications for Serology and Gunshot Residue Analysis. TrAC Trends Anal. Chem. 2018, 103, 215–222. [Google Scholar] [CrossRef]
- Understanding Raman Spectrometer Parameters. Available online: https://www.spectroscopyonline.com/view/understanding-raman-spectrometer-parameters (accessed on 4 March 2024).
- 7.3: Wavelength Selectors. Available online: https://chem.libretexts.org/Courses/Providence_College/CHM_331_Advanced_Analytical_Chemistry_1/07%3A_Components_of_Optical_Instruments_for_Molecular_Spectroscopy_in_the_UV_and_Visible/7.03%3A_Wavelength_Selectors (accessed on 16 December 2023).
- Microscopy and Raman Imaging: Open-System Raman Microscopy. Available online: https://www.laserfocusworld.com/test-measurement/spectroscopy/article/16551666/microscopy-and-raman-imaging-open-system-raman-microscopy (accessed on 16 December 2023).
- Photomultiplier Tubes Are at the Forefront of Low-Light Detection|Laser Focus World. Available online: https://www.laserfocusworld.com/detectors-imaging/article/16556324/photomultiplier-tubes-are-at-the-forefront-of-low-light-detection (accessed on 16 December 2023).
- Diffraction Grating Selection for Raman Spectroscopy | Spectroscopy Europe/World. Available online: https://www.spectroscopyeurope.com/applications/diffraction-grating-selection-raman-spectroscopy (accessed on 16 December 2023).
- Basic Principles of Raman Scattering and Spectroscopy. Available online: https://www.edmundoptics.com/knowledge-center/application-notes/lasers/basic-principles-of-raman-scattering-and-spectroscopy/ (accessed on 16 December 2023).
- Corp, J.G. Hamamatsu Selecting CCDs for Raman Spectroscopy. Available online: https://www.photonics.com/Articles/Selecting_CCDs_for_Raman_Spectroscopy/a45915 (accessed on 16 December 2023).
- Wahadoszamen, M.; Rahaman, A.; Hoque, N.M.R.; Talukder, A.I.; Abedin, K.M.; Haider, A.F.M.Y. Laser Raman Spectroscopy with Different Excitation Sources and Extension to Surface Enhanced Raman Spectroscopy. J. Spectrosc. 2015, 2015, e895317. [Google Scholar] [CrossRef]
- Qiu, Y.; Kuang, C.; Liu, X.; Tang, L. Single-Molecule Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 4889. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.-C.; Huang, T.-X.; Su, H.-S.; Zhong, J.-H.; Zeng, Z.-C.; Li, M.-H.; Ren, B. Tip-Enhanced Raman Spectroscopy for Surfaces and Interfaces. Chem. Soc. Rev. 2017, 46, 4020–4041. [Google Scholar] [CrossRef] [PubMed]
- Robert, B. Resonance Raman Spectroscopy. Photosynth. Res. 2009, 101, 147–155. [Google Scholar] [CrossRef]
- Deluca, M.; Hu, H.; Popov, M.N.; Spitaler, J.; Dieing, T. Advantages and Developments of Raman Spectroscopy for Electroceramics. Commun. Mater. 2023, 4, 78. [Google Scholar] [CrossRef]
- Tu, H.; Boppart, S.A. Coherent Anti-Stokes Raman Scattering Microscopy: Overcoming Technical Barriers for Clinical Translation. J. Biophotonics 2014, 7, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Manifold, B.; Fu, D. Quantitative Stimulated Raman Scattering Microscopy: Promises and Pitfalls. Annu. Rev. Anal. Chem. Palo Alto Calif 2022, 15, 269–289. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Front. 2022, 2022, 9764501. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Ideguchi, T.; Yonamine, Y.; Lee, S.; Luo, Y.; Hashimoto, K.; Ito, T.; Hase, M.; Park, J.-W.; Kasai, Y.; et al. High-Throughput Label-Free Molecular Fingerprinting Flow Cytometry. Sci. Adv. 2019, 5, eaau0241. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Yamada, K.; Lindley, M.; Suzuki, K.; Goda, K. Large-Scale Label-Free Single-Cell Analysis of Paramylon in Euglena Gracilis by High-Throughput Broadband Raman Flow Cytometry. Biomed. Opt. Express 2020, 11, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kobayashi, K.; Wakisaka, Y.; Deng, D.; Tanaka, S.; Huang, C.-J.; Lei, C.; Sun, C.-W.; Liu, H.; Fujiwaki, Y.; et al. Label-Free Chemical Imaging Flow Cytometry by High-Speed Multicolor Stimulated Raman Scattering. Proc. Natl. Acad. Sci. USA 2019, 116, 15842–15848. [Google Scholar] [CrossRef]
- McIlvenna, D.; Huang, W.E.; Davison, P.; Glidle, A.; Cooper, J.; Yin, H. Continuous Cell Sorting in a Flow Based on Single Cell Resonance Raman Spectra. Lab Chip 2016, 16, 1420–1429. [Google Scholar] [CrossRef]
- van Dam, P.A.; Watson, J.V.; Lowe, D.G.; Chard, T.; Shepherd, J.H. Comparative Evaluation of Fresh, Fixed, and Cryopreserved Solid Tumor Cells for Reliable Flow Cytometry of DNA and Tumor Associated Antigen. Cytometry 1992, 13, 722–729. [Google Scholar] [CrossRef]
- Nitta, N.; Iino, T.; Isozaki, A.; Yamagishi, M.; Kitahama, Y.; Sakuma, S.; Suzuki, Y.; Tezuka, H.; Oikawa, M.; Arai, F.; et al. Raman Image-Activated Cell Sorting. Nat. Commun. 2020, 11, 3452. [Google Scholar] [CrossRef]
- Aymanns, S.; Mauerer, S.; van Zandbergen, G.; Wolz, C.; Spellerberg, B. High-Level Fluorescence Labeling of Gram-Positive Pathogens. PLoS ONE 2011, 6, e19822. [Google Scholar] [CrossRef]
- Hussey, S.J.K.; Purves, J.; Allcock, N.; Fernandes, V.E.; Monks, P.S.; Ketley, J.M.; Andrew, P.W.; Morrissey, J.A. Air Pollution Alters Staphylococcus Aureus and Streptococcus Pneumoniae Biofilms, Antibiotic Tolerance and Colonisation. Environ. Microbiol. 2017, 19, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as Dynamic Regulators of Cell and Organismal Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, D.; Bates, M.; Jayaram, M.; Young, M.; He, J.; Raithel, A.L.; Hamann, T.W.; Zhang, W.; Borhan, B.; Lunt, R.R.; et al. Modulating Cellular Cytotoxicity and Phototoxicity of Fluorescent Organic Salts through Counterion Pairing. Sci. Rep. 2019, 9, 15288. [Google Scholar] [CrossRef]
- Marx, V. It’s Free Imaging—Label-Free, That Is. Nat. Methods 2019, 16, 1209–1212. [Google Scholar] [CrossRef]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170–182. [Google Scholar] [CrossRef]
- Huang, K.-C.; Li, J.; Zhang, C.; Tan, Y.; Cheng, J.-X. Multiplex Stimulated Raman Scattering Imaging Cytometry Reveals Lipid-Rich Protrusions in Cancer Cells under Stress Condition. iScience 2020, 23, 100953. [Google Scholar] [CrossRef]
- Alexander, C.M.; Puchalski, J.; Klos, K.S.; Badders, N.; Ailles, L.; Kim, C.F.; Dirks, P.; Smalley, M.J. Separating Stem Cells by Flow Cytometry: Reducing Variability for Solid Tissues. Cell Stem Cell 2009, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, C.M.; Mullaithilaga, N.; Yang, G.; Ip, S.Y.; Wang, C.; Walker, G.C. Surface-Enhanced Raman Scattering Dye-Labeled Au Nanoparticles for Triplexed Detection of Leukemia and Lymphoma Cells and SERS Flow Cytometry. Langmuir ACS J. Surf. Colloids 2013, 29, 1908–1919. [Google Scholar] [CrossRef]
- Pallaoro, A.; Hoonejani, M.R.; Braun, G.B.; Meinhart, C.D.; Moskovits, M. Rapid Identification by Surface-Enhanced Raman Spectroscopy of Cancer Cells at Low Concentrations Flowing in a Microfluidic Channel. ACS Nano 2015, 9, 4328–4336. [Google Scholar] [CrossRef]
- Wang, X.; Ren, L.; Diao, Z.; He, Y.; Zhang, J.; Liu, M.; Li, Y.; Sun, L.; Chen, R.; Ji, Y.; et al. Robust Spontaneous Raman Flow Cytometry for Single-Cell Metabolic Phenome Profiling via pDEP-DLD-RFC. Adv. Sci. Weinh. Baden Wurtt. Ger. 2023, 10, e2207497. [Google Scholar] [CrossRef]
- Rocha, R.A.; Fox, J.M.; Genever, P.G.; Hancock, Y. Biomolecular Phenotyping and Heterogeneity Assessment of Mesenchymal Stromal Cells Using Label-Free Raman Spectroscopy. Sci. Rep. 2021, 11, 4385. [Google Scholar] [CrossRef] [PubMed]
- Pascut, F.C.; Goh, H.T.; George, V.; Denning, C.; Notingher, I. Toward Label-Free Raman-Activated Cell Sorting of Cardiomyocytes Derived from Human Embryonic Stem Cells. J. Biomed. Opt. 2011, 16, 045002. [Google Scholar] [CrossRef]
- Camp, C.H.; Yegnanarayanan, S.; Eftekhar, A.A.; Adibi, A. Label-Free Flow Cytometry Using Multiplex Coherent Anti-Stokes Raman Scattering (MCARS) for the Analysis of Biological Specimens. Opt. Lett. 2011, 36, 2309–2311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, K.-C.; Rajwa, B.; Li, J.; Yang, S.; Lin, H.; Liao, C.; Eakins, G.; Kuang, S.; Patsekin, V.; et al. Stimulated Raman Scattering Flow Cytometry for Label-Free Single-Particle Analysis. Optica 2017, 4, 103–109. [Google Scholar] [CrossRef]
- Biris, A.S.; Galanzha, E.I.; Li, Z.; Mahmood, M.; Xu, Y.; Zharov, V.P. In Vivo Raman Flow Cytometry for Real-Time Detection of Carbon Nanotube Kinetics in Lymph, Blood, and Tissues. J. Biomed. Opt. 2009, 14, 021006. [Google Scholar] [CrossRef] [PubMed]
- Andor High Throughput Raman-Based Flow Cytometry. Available online: https://andor.oxinst.com/learning/view/article/spectral-flow-cytometry-raman-(sers)-flow-cytometry (accessed on 11 November 2023).
- Perrigue, P.M.; Murray, R.A.; Mielcarek, A.; Henschke, A.; Moya, S.E. Degradation of Drug Delivery Nanocarriers and Payload Release: A Review of Physical Methods for Tracing Nanocarrier Biological Fate. Pharmaceutics 2021, 13, 770. [Google Scholar] [CrossRef]
- Schröder, U.-C.; Ramoji, A.; Glaser, U.; Sachse, S.; Leiterer, C.; Csaki, A.; Hübner, U.; Fritzsche, W.; Pfister, W.; Bauer, M.; et al. Combined Dielectrophoresis–Raman Setup for the Classification of Pathogens Recovered from the Urinary Tract. Anal. Chem. 2013, 85, 10717–10724. [Google Scholar] [CrossRef]
- Schröder, U.-C.; Beleites, C.; Assmann, C.; Glaser, U.; Hübner, U.; Pfister, W.; Fritzsche, W.; Popp, J.; Neugebauer, U. Detection of Vancomycin Resistances in Enterococci within 3 ½ Hours. Sci. Rep. 2015, 5, 8217. [Google Scholar] [CrossRef]
- Song, Y.; Cui, L.; López, J.Á.S.; Xu, J.; Zhu, Y.-G.; Thompson, I.P.; Huang, W.E. Raman-Deuterium Isotope Probing for in-Situ Identification of Antimicrobial Resistant Bacteria in Thames River. Sci. Rep. 2017, 7, 16648. [Google Scholar] [CrossRef]
- Wang, J.; Lin, K.; Hu, H.; Qie, X.; Huang, W.E.; Cui, Z.; Gong, Y.; Song, Y. In Vitro Anticancer Drug Sensitivity Sensing through Single-Cell Raman Spectroscopy. Biosensors 2021, 11, 286. [Google Scholar] [CrossRef]
- Yi, X.; Song, Y.; Xu, X.; Peng, D.; Wang, J.; Qie, X.; Lin, K.; Yu, M.; Ge, M.; Wang, Y.; et al. Development of a Fast Raman-Assisted Antibiotic Susceptibility Test (FRAST) for the Antibiotic Resistance Analysis of Clinical Urine and Blood Samples. Anal. Chem. 2021, 93, 5098–5106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-X.; Xie, X.S. Vibrational Spectroscopic Imaging of Living Systems: An Emerging Platform for Biology and Medicine. Science 2015, 350, aaa8870. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.L.; Potma, E.O.; Xie, X.S. Coherent Anti-Stokes Raman Scattering Spectral Interferometry: Determination of the Real and Imaginary Components of Nonlinear Susceptibility Chi(3) for Vibrational Microscopy. Opt. Lett. 2004, 29, 2923–2925. [Google Scholar] [CrossRef] [PubMed]
- Potma, E.O.; Evans, C.L.; Xie, X.S. Heterodyne Coherent Anti-Stokes Raman Scattering (CARS) Imaging. Opt. Lett. 2006, 31, 241–243. [Google Scholar] [CrossRef]
- Littleton, B.; Kavanagh, T.; Festy, F.; Richards, D. Spectral Interferometric Implementation with Passive Polarization Optics of Coherent Anti-Stokes Raman Scattering. Phys. Rev. Lett. 2013, 111, 103902. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.R.; Park, J.H.; Kim, K.-S.; Lee, J.Y.; Kim, S. Multiplex CARS Imaging with Spectral Notch Shaped Laser Pulses Delivered by Optical Fibers. Opt. Express 2017, 25, 32178–32188. [Google Scholar] [CrossRef]
- Oron, D.; Dudovich, N.; Silberberg, Y. Femtosecond Phase-and-Polarization Control for Background-Free Coherent Anti-Stokes Raman Spectroscopy. Phys. Rev. Lett. 2003, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zeng, C.; Long, R.; Miao, Y.; Wei, L.; Xu, Q.; Min, W. Supermultiplexed Optical Imaging and Barcoding with Engineered Polyynes. Nat. Methods 2018, 15, 194–200. [Google Scholar] [CrossRef]
- Mikami, H.; Kawaguchi, M.; Huang, C.-J.; Matsumura, H.; Sugimura, T.; Huang, K.; Lei, C.; Ueno, S.; Miura, T.; Ito, T.; et al. Virtual-Freezing Fluorescence Imaging Flow Cytometry. Nat. Commun. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Okuno, M.; Hamaguchi, H. Multifocus Confocal Raman Microspectroscopy for Fast Multimode Vibrational Imaging of Living Cells. Opt. Lett. 2010, 35, 4096–4098. [Google Scholar] [CrossRef]
- Rane, A.S.; Rutkauskaite, J.; deMello, A.; Stavrakis, S. High-Throughput Multi-Parametric Imaging Flow Cytometry. Chem 2017, 3, 588–602. [Google Scholar] [CrossRef]
- Piyasena, M.E.; Austin Suthanthiraraj, P.P.; Applegate, R.W.; Goumas, A.M.; Woods, T.A.; López, G.P.; Graves, S.W. Multinode Acoustic Focusing for Parallel Flow Cytometry. Anal. Chem. 2012, 84, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Iino, T.; Okano, K.; Lee, S.W.; Yamakawa, T.; Hagihara, H.; Hong, Z.-Y.; Maeno, T.; Kasai, Y.; Sakuma, S.; Hayakawa, T.; et al. High-Speed Microparticle Isolation Unlimited by Poisson Statistics. Lab Chip 2019, 19, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.W.; Reyes, C.D.; López, G.P. Microfluidic Cell Sorting: A Review of the Advances in the Separation of Cells from Debulking to Rare Cell Isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.L.; MacKay, M.; Afshinnekoo, E.; Melnick, A.M.; Wu, S.; Mason, C.E. The Impact of Heterogeneity on Single-Cell Sequencing. Front. Genet. 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, A.R.; DeLaney, K.; Johnson, J.; Li, L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.S.; Oliver, R. Magnetic Resonance Imaging Basics. Adv. Exp. Med. Biol. 2022, 1380, 47–82. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.A.; Bhand, S.; Hayat, A.; Marty, J.L. Designed Strategies for Fluorescence-Based Biosensors for the Detection of Mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef]
| Raman Shift (cm−1) | Vibrational Mode and/or Assignments | Raman Shift (cm−1) | Vibrational Mode and/or Assignments |
|---|---|---|---|
| 407 | Carbohydrates | 1124–1128 | Cytochrome C |
| 429 | Cholesterol ester | 1168–1172 | Lipids, Tyrosine |
| 490 | Glycogen, Chitin | 1240 | Phosphate (asymmetric) |
| 538 | Cholesterol ester | 1254–1270 | Lipids, Protein, Adenine, Cytosine, Guanine |
| 573 | Cytosine, Guanine, Tryptophan | 1313–1315 | Collagen, Lipid, Guanine |
| 595 | Phosphatidylserine | 1330 | DNA, Phospholipids, Purine |
| 621 | Phenylalanine | 1361 | Guanine |
| 663–671 | Guanine, Thymine in nucleotide, Tyrosine | 1386–1389 | CH3 band |
| 726 | Adenine, Peptidoglycan | 1421 | Peptidoglycan |
| 750 | Cytochrome C | 1480–1491 | Guanine, Adenine |
| 781 | Cytosine, Uracil in nucleotide | 1573–1582 | Amide II, Nucleic acid, Peptidoglycan |
| 845–860 | Proline, Tyrosine | 1583 | Cytochrome C |
| 913–915 | Glucose, Ribose | 1601–1607 | Phenylalanine, Tyrosine |
| 1001 | Phenylalanine | 1616 | Tyrosine |
| 1082 | Carbohydrates | 1655–1676 | Protein, Lipid, Unsaturated fatty acids |
| 1102 | Phosphate (symmetric) |
| Flow Cytometry Type | Label | Throughput | Information Content | Biological Interference |
|---|---|---|---|---|
| Fluorescence Flow Cytometry |  Label Reqiured (fluorescent dye) |  100, 000 eps |  Univariate (up to 12) |  Label Dependent, Photon Dependent |
| Spontaneous Raman Flow Cytometry |  Label Free |  1eps |  Multivariate (20+) |  Photon Dependent |
| Coherent Raman Flow Cytometry |  Label Free |  100 eps |  Multivariate (15+) |  Photon Dependent |
| Sample Type | Biomolecules Detected | Applications | Raman Spectroscopy Type | Excitation Wavelength | Throughput | Reference |
|---|---|---|---|---|---|---|
| Cancer Cells | Carbon nanotubes (CNTs) | Human cervical cancer cells (HeLa) detection | Time-resolved Raman spectroscopy | 514 nm, 633 nm, and 785 nm | -- | [66] |
| Proteins (specific cell surface markers CD19, CD20, and CD45) | Malignant B cells from LY10 lymphoma cell line and primary chronic lymphocytic leukemia cells detection | SERS | 638 nm | -- | [67] | |
| Glycoprotein (neuropilin-1, NRP-1) | Cancerous and non-cancerous prostate cells detection and monitoring | SERS | 633 nm | -- | [68] | |
| Proteins, lipids, saccharides (paramylon) and pigments (chlorophyll) | Blood samples (cancerous and other cells) detection | SRS microscopy | 790 nm and 1030 nm | 140 eps | [55] | |
| Proteins, lipids, and nucleic acids | Human cancer cell lines (bladder (T24), lung (A549), renal (OSRC-2), and breast (MCF-7)) detection and sorting | Spontaneous Raman | 532 nm | 30 eps | [69] | |
| Stem Cells | Proteins, lipids, and saccharides | High-integrity pluripotent stem cells (hiPSCs) analysis and sorting | SRS microscopy | 790.6 nm and 796.6 nm | 100 eps | [58] |
| Proteins, lipids, and nucleic acids | Bone-derived mesenchymal stem cell (MSC) lines classification | Spontaneous Raman | 532 nm | -- | [70] | |
| Proteins (myofibril proteins) and saccharides (glycogen) | Cardiomyocytes derived from human embryonic stem cells (hESCs) analysis and sorting | Spontaneous Raman microscopy | 785 nm | 10 eps | [71] | |
| Microbe Cells | Lipids | Yeast cell subpopulations classification | Multiplex coherent anti-Stokes Raman scattering (MCARS) | 806 nm | 100 eps | [72] |
| Lipids (triglycerides and lipid-related molecules) | Staphylococcus aureus identification and analysis | SRS | 1040 nm Stokes beam, 680–1300 nm pump beam | 11,000 eps | [73] | |
| Saccharides (paramylon, a β-1,3-glucan) | Euglena gracilis identification and analysis | FT-CARS | 780 nm | 100 eps | [53] | |
| Pigments (chlorophyll and astaxanthin) | Haematococcus lacustris identification and analysis | FT-CARS | 780 nm | 2000 eps | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Chen, H.; Wang, C.; Ma, Y.; Song, Y. Raman Flow Cytometry and Its Biomedical Applications. Biosensors 2024, 14, 171. https://doi.org/10.3390/bios14040171
Xu J, Chen H, Wang C, Ma Y, Song Y. Raman Flow Cytometry and Its Biomedical Applications. Biosensors. 2024; 14(4):171. https://doi.org/10.3390/bios14040171
Chicago/Turabian StyleXu, Jiayang, Hongyi Chen, Ce Wang, Yuting Ma, and Yizhi Song. 2024. "Raman Flow Cytometry and Its Biomedical Applications" Biosensors 14, no. 4: 171. https://doi.org/10.3390/bios14040171
APA StyleXu, J., Chen, H., Wang, C., Ma, Y., & Song, Y. (2024). Raman Flow Cytometry and Its Biomedical Applications. Biosensors, 14(4), 171. https://doi.org/10.3390/bios14040171





