Coffee Ring Effect Enhanced Surface Plasmon Resonance Imaging Biosensor via 2-λ Fitting Detection Method
Abstract
:1. Introduction
2. The Principle of the 2-λ Fitting SPRi Detection Method
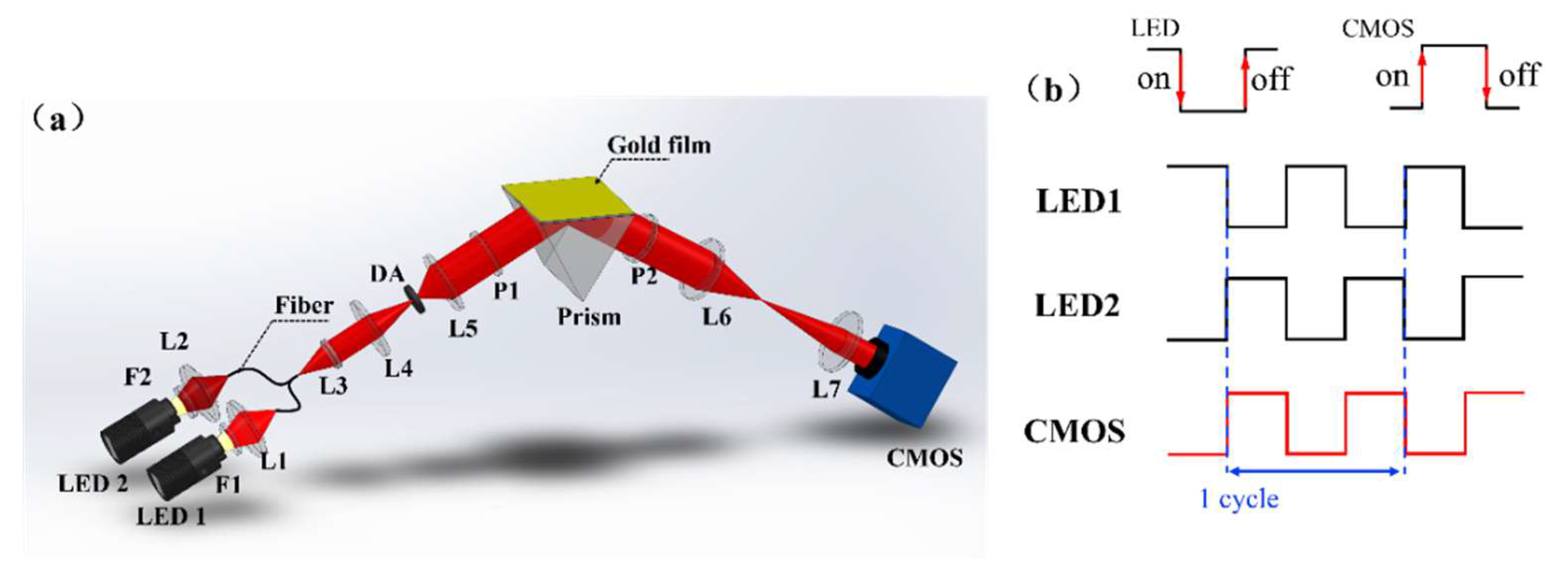
3. Optical Setup
4. Results and Discussion
4.1. Chemicals and Materials
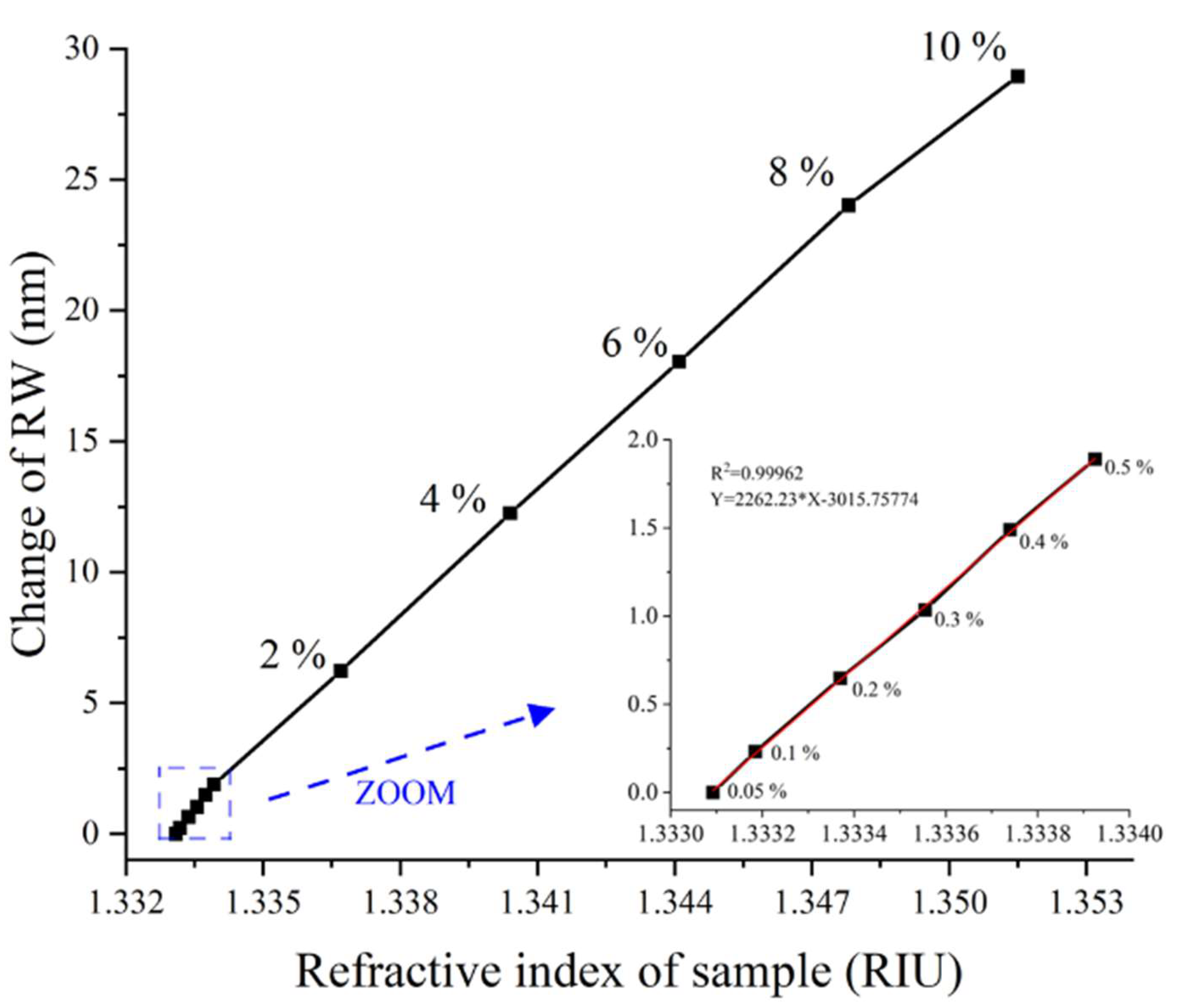
4.2. RIR and Dynamic Range of WSPRi Measurement
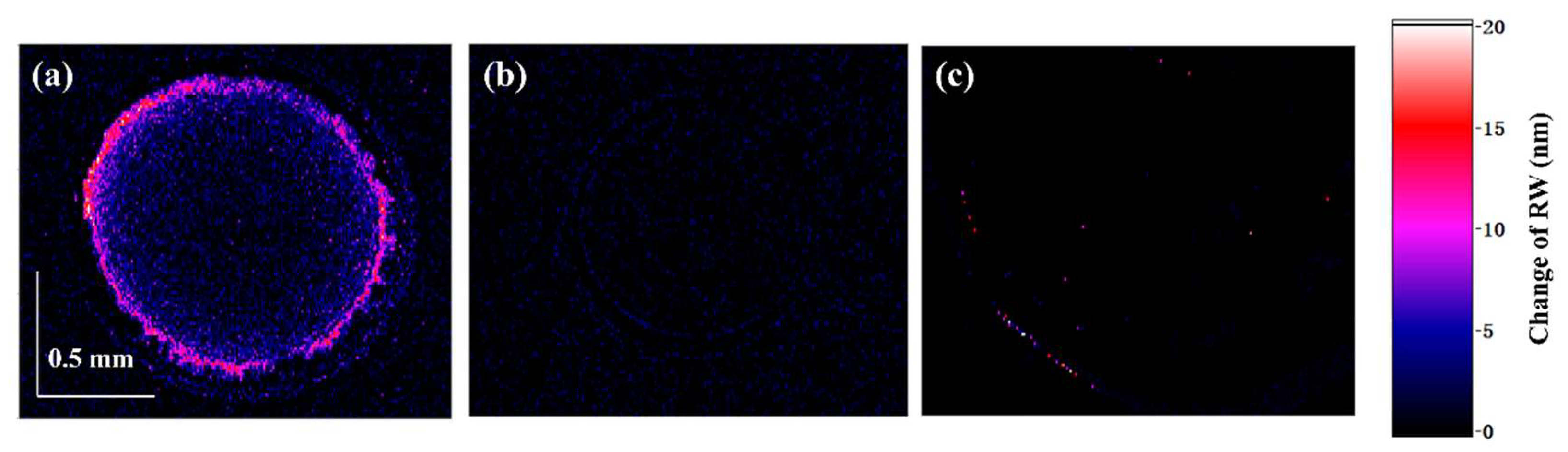
4.3. CRE SPR Enhancement
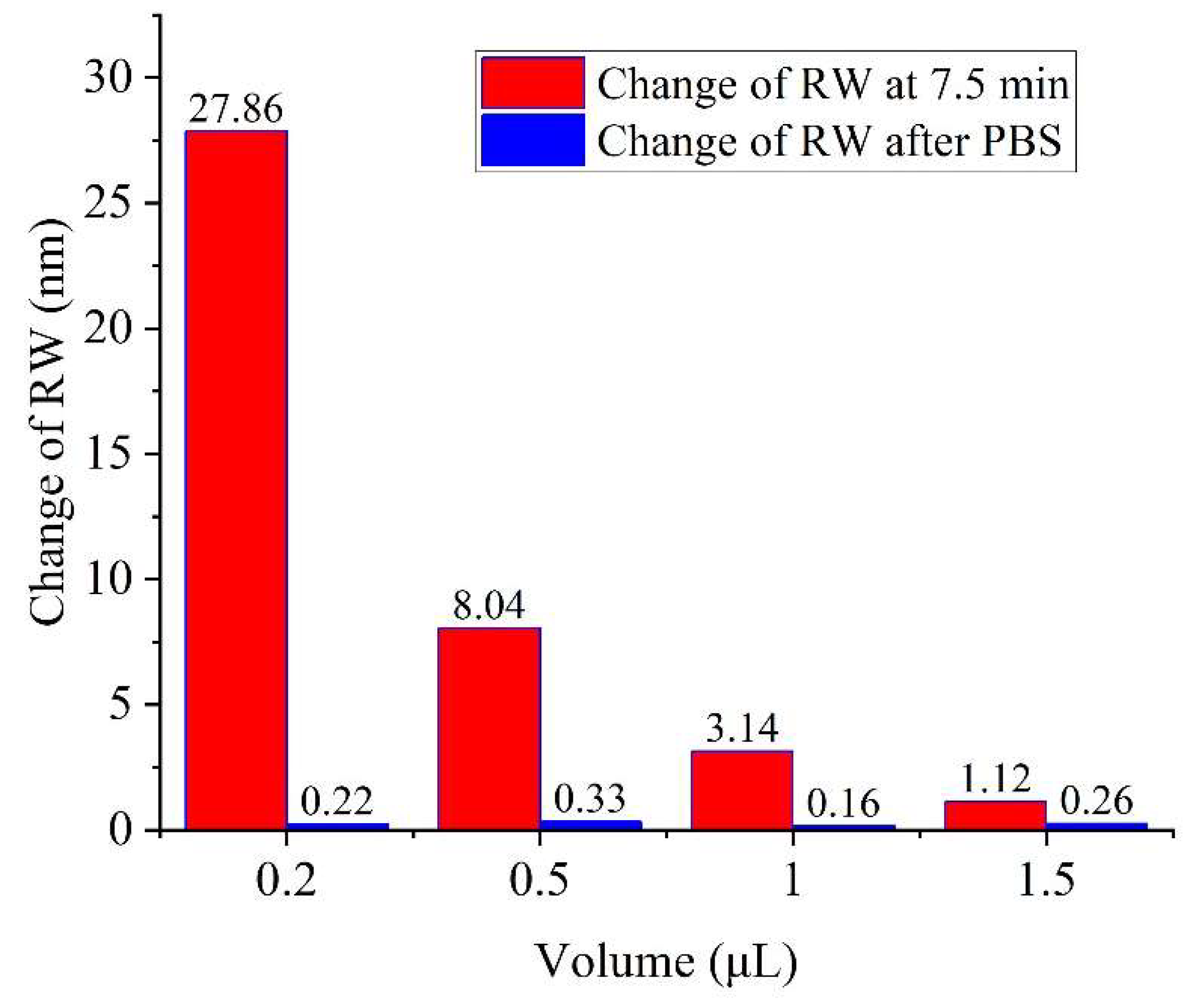
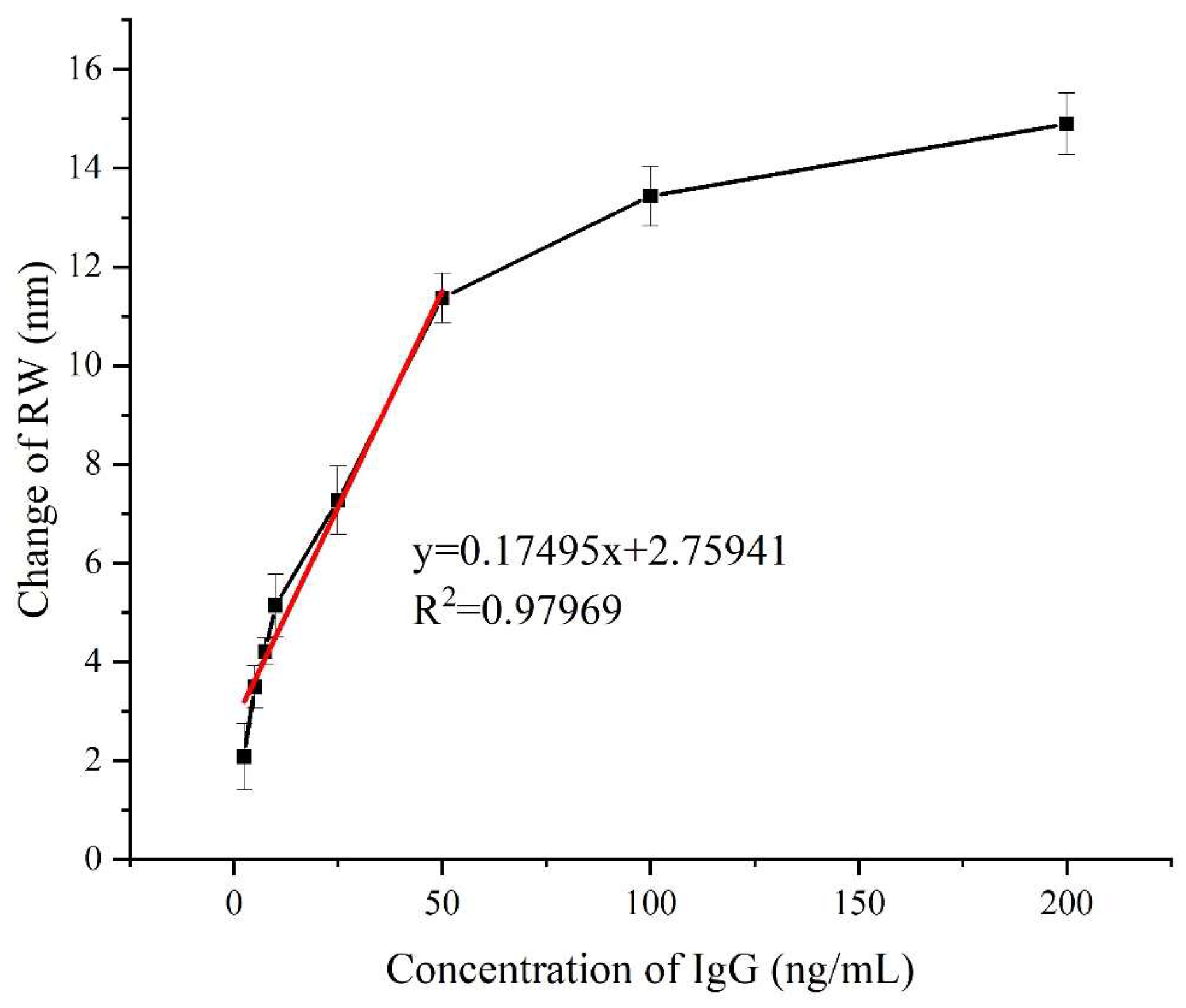
4.4. Antibody-Antigen Binding Enhancement with CRE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azzouz, A.; Hejji, L.; Kim, K.H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in surface plasmon resonance-based biosensor technologies for cancer biomarker detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.H.; Zhu, X.; Liang, T.; Wang, K.H.; Rittenhouse, A.G.; Akinde, O.; Eby, Y.; Ruff, J.E.; Blankson, J.N.; Abedon, A.T.; et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am. J. Transplant. 2022, 22, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Gordan, S.; Lux, A. FcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015, 36, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Beketov, G.; Shynkarenko, O.; Apatska, M. Towards improving ELISA surfaces: SPR assessment of polystyrene modification efficiency for promoting immobilization of biomolecules. Anal. Biochem. 2021, 618, 114101. [Google Scholar] [CrossRef]
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153. [Google Scholar] [CrossRef]
- Shen, C.L.; Lou, Q.; Liu, K.K.; Dong, L.; Shan, C.X. Chemiluminescent carbon dots: Synthesis, properties, and applications. Nano Today 2020, 35, 23. [Google Scholar] [CrossRef]
- Bockova, M.; Slaby, J.; Springer, T.; Homola, J. Advances in Surface Plasmon Resonance Imaging and Microscopy and Their Biological Applications. Annu. Rev. Anal. Chem. 2019, 12, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of surface plasmon resonance biosensors in cancer detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef] [PubMed]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.L.; Chen, J.J.; Zhou, J.; Zeng, Y.J.; Ho, H.P.; Shao, Y.H. Passively and actively enhanced surface plasmon resonance sensing strategies towards single molecular detection. Nano Res. 2022, 15, 8367–8388. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.L.; Chen, J.J.; Zeng, Y.J.; Gu, D.Y.; Gao, B.Z.; Shao, Y.H. Polymeric microsphere enhanced surface plasmon resonance imaging immunosensor for occult blood monitoring. Sens. Actuator B-Chem. 2022, 350, 8. [Google Scholar] [CrossRef]
- Escobedo, C.; Brolo, A.G.; Gordon, R.; Sinton, D. Optofluidic concentration: Plasmonic nanostructure as concentrator and sensor. Nano Lett. 2012, 12, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Luan, J.; Wang, Z.; Jiang, Q.; Gupta, R.; Cao, S.; Liu, K.K.; Morrissey, J.J.; Kharasch, E.D.; Naik, R.R.; et al. Gold Nanorod Size-Dependent Fluorescence Enhancement for Ultrasensitive Fluoroimmunoassays. ACS Appl. Mater. Interfaces 2021, 13, 11414–11423. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, Y.; Zhou, J.; Wang, X.; Jia, B.; Miyan, R.; Zhang, T.; Sang, W.; Wang, Y.; Qiu, H.; et al. Optothermophoretic flipping method for biomolecule interaction enhancement. Biosens. Bioelectron. 2022, 204, 114084. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.; Tarafdar, S.; Tarasevich, Y.Y.; Dutta Choudhury, M.; Dutta, T. Evaporation of a Droplet: From physics to applications. Phys. Rep. 2019, 804, 1–56. [Google Scholar] [CrossRef]
- Devineau, S.; Anyfantakis, M.; Marichal, L.; Kiger, L.; Morel, M.; Rudiuk, S.; Baigl, D. Protein Adsorption and Reorganization on Nanoparticles Probed by the Coffee-Ring Effect: Application to Single Point Mutation Detection. J. Am. Chem. Soc. 2016, 138, 11623–11632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, B.; Chen, Y.; Liu, H. Converting colour to length based on the coffee-ring effect for quantitative immunoassays using a ruler as readout. Lab Chip 2018, 18, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, W.; Wang, S.; Liu, W.; Liu, N.; Zhang, D. A visual cardiovascular biomarker detection strategy based on distance as readout by the coffee-ring effect on microfluidic paper. Biochem. Eng. J. 2021, 176, 108176. [Google Scholar] [CrossRef]
- Dak, P.; Ebrahimi, A.; Swaminathan, V.; Duarte-Guevara, C.; Bashir, R.; Alam, M.A. Droplet-based Biosensing for Lab-on-a-Chip, Open Microfluidics Platforms. Biosensors 2016, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kanok, R.; Ciprian, D.; Hlubina, P. Surface Plasmon Resonance-Based Sensing Utilizing Spatial Phase Modulation in an Imaging Interferometer. Sensors 2020, 20, 1616. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, O.R.; Live, L.S.; Masson, J.F. High-resolution surface plasmon resonance sensors based on a dove prism. Talanta 2009, 77, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, D.; Zeng, J.; Deng, J.; Hu, N.; Yang, J. Multi-channel surface plasmon resonance biosensor using prism-based wavelength interrogation. Opt. Express 2020, 28, 14007–14017. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xia, G.; Jin, S.; Bai, L.; Wang, J.; Chen, Q.; Cai, X. Effect of Spectral Signal-to-Noise Ratio on Resolution Enhancement at Surface Plasmon Resonance. Sensors 2021, 21, 641. [Google Scholar] [CrossRef] [PubMed]
- Puiu, M.; Bala, C. SPR and SPR Imaging: Recent Trends in Developing Nanodevices for Detection and Real-Time Monitoring of Biomolecular Events. Sensors 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, W.S.; Song, D.Q.; Cao, Y.B.; Liu, X.; Zhang, H.Q.; Xu, H.Y. Studies on AOTF as the wavelength selector for SPR sensor. Chem. J. Chin. Univ. -Chin. 2004, 25, 441–444. [Google Scholar]
- Huo, Z.; Li, Y.; Chen, B.; Zhang, W.; Yang, X.; Yang, X. Recent advances in surface plasmon resonance imaging and biological applications. Talanta 2023, 255, 124213. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, L.; Geng, Z.; Shum, P.P.; Ma, X.; Wang, J. Refractometric Imaging and Biodetection Empowered by Nanophotonics. Laser Photonics Rev. 2023, 17, 2200814. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, X.; Zhou, J.; Miyan, R.; Qu, J.; Ho, H.P.; Zhou, K.; Gao, B.Z.; Chen, J.; Shao, Y. High-throughput imaging surface plasmon resonance biosensing based on ultrafast two-point spectral-dip tracking scheme. Opt. Express 2020, 28, 20624–20633. [Google Scholar] [CrossRef] [PubMed]
- Sereda, A.; Moreau, J.; Canva, M.; Maillart, E. High performance multi-spectral interrogation for surface plasmon resonance imaging sensors. Biosens. Bioelectron. 2014, 54, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sereda, A.; Moreau, J.; Boulade, M.; Olivéro, A.; Canva, M.; Maillart, E. Compact 5-LEDs illumination system for multi-spectral surface plasmon resonance sensing. Sens. Actuators B Chem. 2015, 209, 208–211. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, X.; Wang, Y.; Li, M.; Zhou, K.; Zhang, L.; Tan, Y. Surface plasmon resonance biosensor with laser heterodyne feedback for highly-sensitive and rapid detection of COVID-19 spike antigen. Biosens. Bioelectron. 2022, 15, 114163. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Loo, F.C.; Cong, H.; Lin, W.; Kong, S.K.; Yam, Y.; Chen, S.C.; Ho, H.P. Real-time multi-channel SPR sensing based on DMD-enabled angular interrogation. Opt. Express 2018, 26, 24627–24636. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Kai, D.; Niu, Z.; Nie, Z.; Wang, Y.; Shao, Y.; Ma, L.; Zhang, F.; Liu, G.; Chen, J. Coffee Ring Effect Enhanced Surface Plasmon Resonance Imaging Biosensor via 2-λ Fitting Detection Method. Biosensors 2024, 14, 195. https://doi.org/10.3390/bios14040195
Zeng Y, Kai D, Niu Z, Nie Z, Wang Y, Shao Y, Ma L, Zhang F, Liu G, Chen J. Coffee Ring Effect Enhanced Surface Plasmon Resonance Imaging Biosensor via 2-λ Fitting Detection Method. Biosensors. 2024; 14(4):195. https://doi.org/10.3390/bios14040195
Chicago/Turabian StyleZeng, Youjun, Dongyun Kai, Zhenxiao Niu, Zhaogang Nie, Yuye Wang, Yonghong Shao, Lin Ma, Fangteng Zhang, Guanyu Liu, and Jiajie Chen. 2024. "Coffee Ring Effect Enhanced Surface Plasmon Resonance Imaging Biosensor via 2-λ Fitting Detection Method" Biosensors 14, no. 4: 195. https://doi.org/10.3390/bios14040195
APA StyleZeng, Y., Kai, D., Niu, Z., Nie, Z., Wang, Y., Shao, Y., Ma, L., Zhang, F., Liu, G., & Chen, J. (2024). Coffee Ring Effect Enhanced Surface Plasmon Resonance Imaging Biosensor via 2-λ Fitting Detection Method. Biosensors, 14(4), 195. https://doi.org/10.3390/bios14040195





