PCR Independent Strategy-Based Biosensors for RNA Detection
Abstract
1. Introduction

2. Electrochemical-Based RNA Biosensors
2.1. Electrochemical Biosensors for the Ultra-Sensitive Detection of RNA
2.2. Electrochemical Biosensors for the Rapid Detection of RNA
2.3. Electrochemical Biosensors in RNA POCT
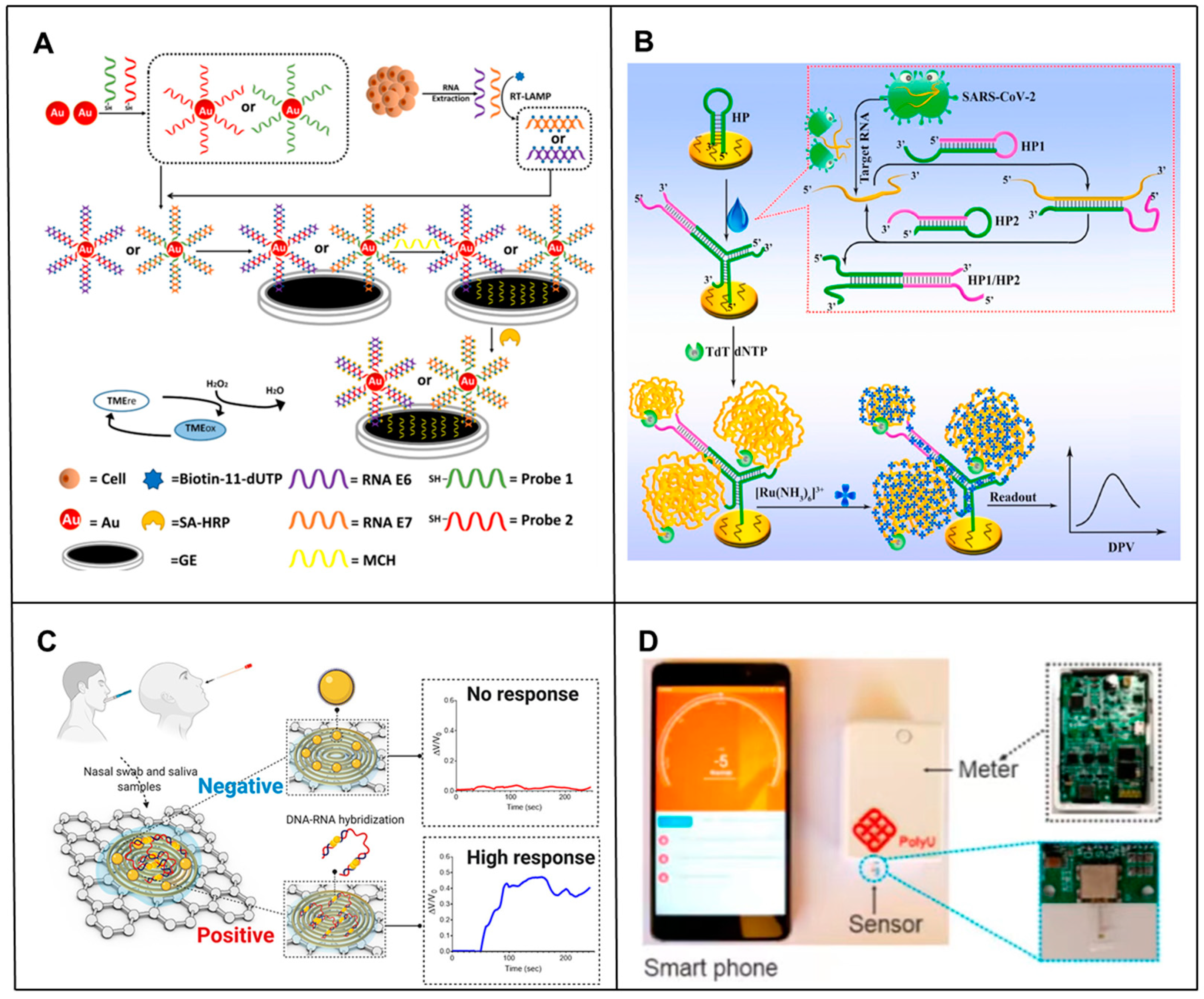
3. SPR-Based RNA Biosensors
3.1. Nanomaterial-Enhanced SPR Biosensors in RNA Detection
3.2. Signal Amplification Strategy-Enhanced SPR Biosensors in RNA Detection
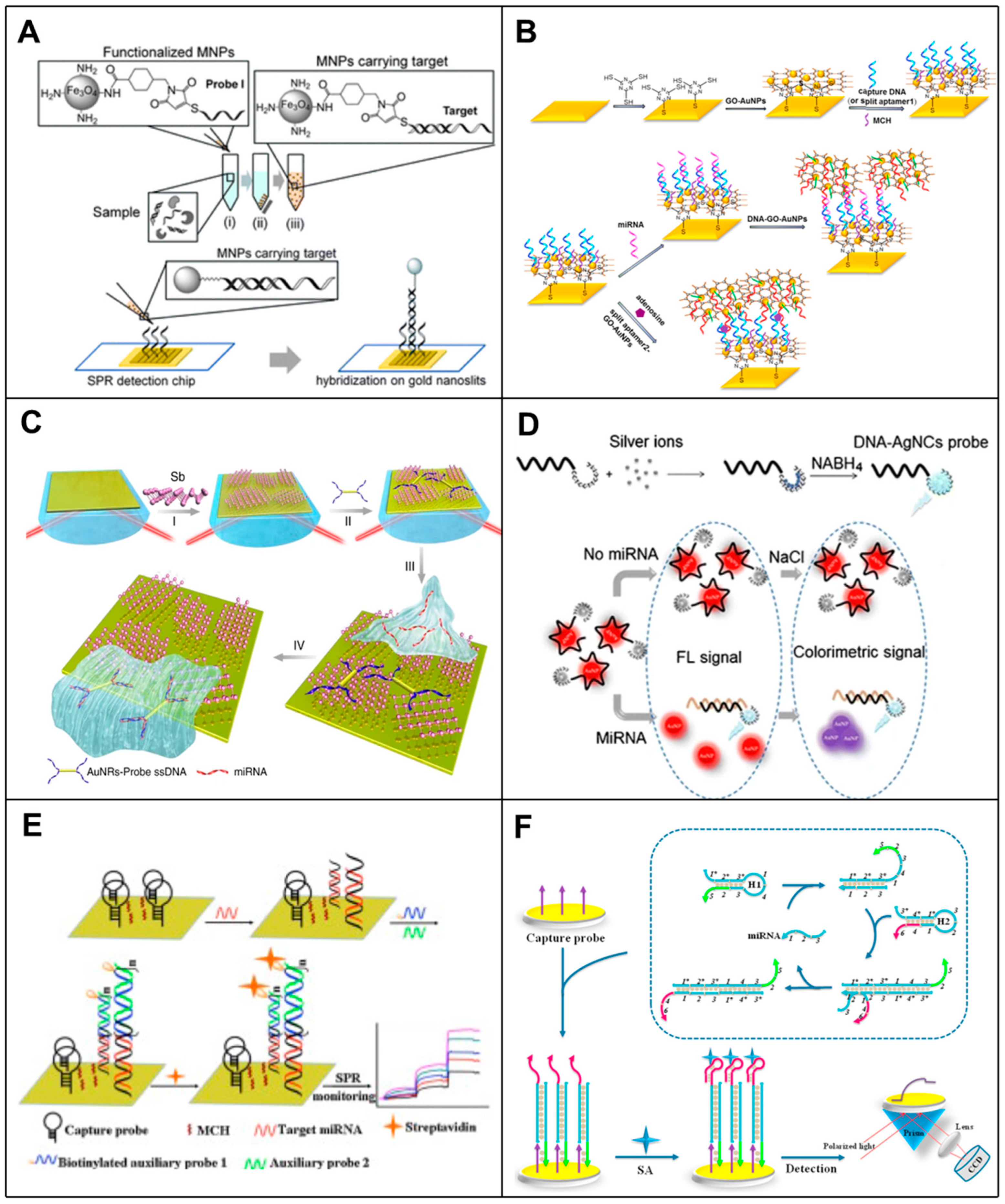
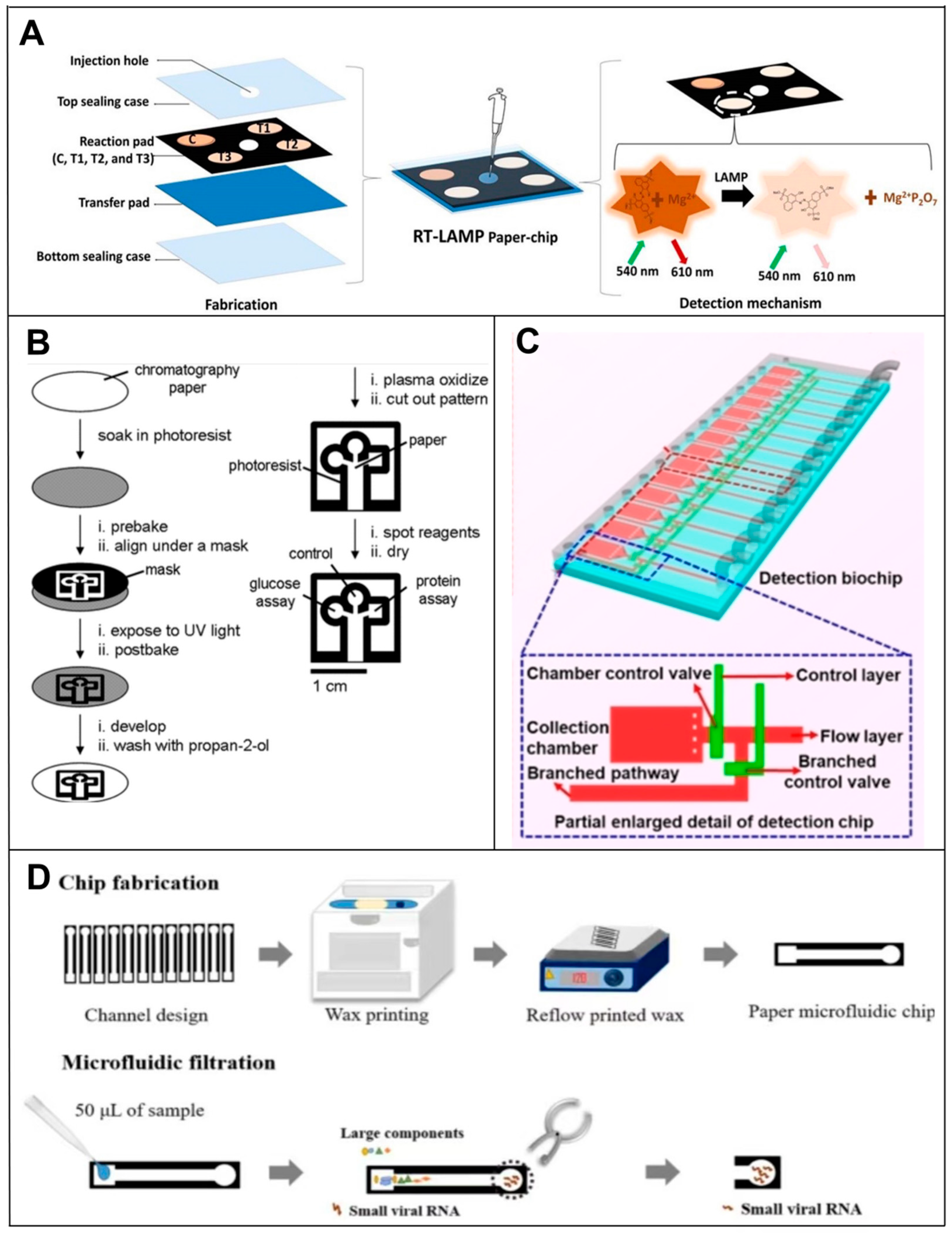
4. Microfluidic-Based RNA Biosensors
4.1. Paper-Based Microfluidic RNA Biosensors
4.2. Microchip-Based RNA Biosensors
5. Nanomaterial-Based RNA Biosensors
5.1. Graphene Oxide-Based RNA Biosensors
5.2. Carbon Nanotube-Based RNA Biosensors

6. CRISPR-Based RNA Biosensors
6.1. CRISPR-Cas9-Based RNA Biosensors
6.2. CRISPR-Cas12-Based RNA Biosensors
6.3. CRISPR-Cas13-Based RNA Biosensors
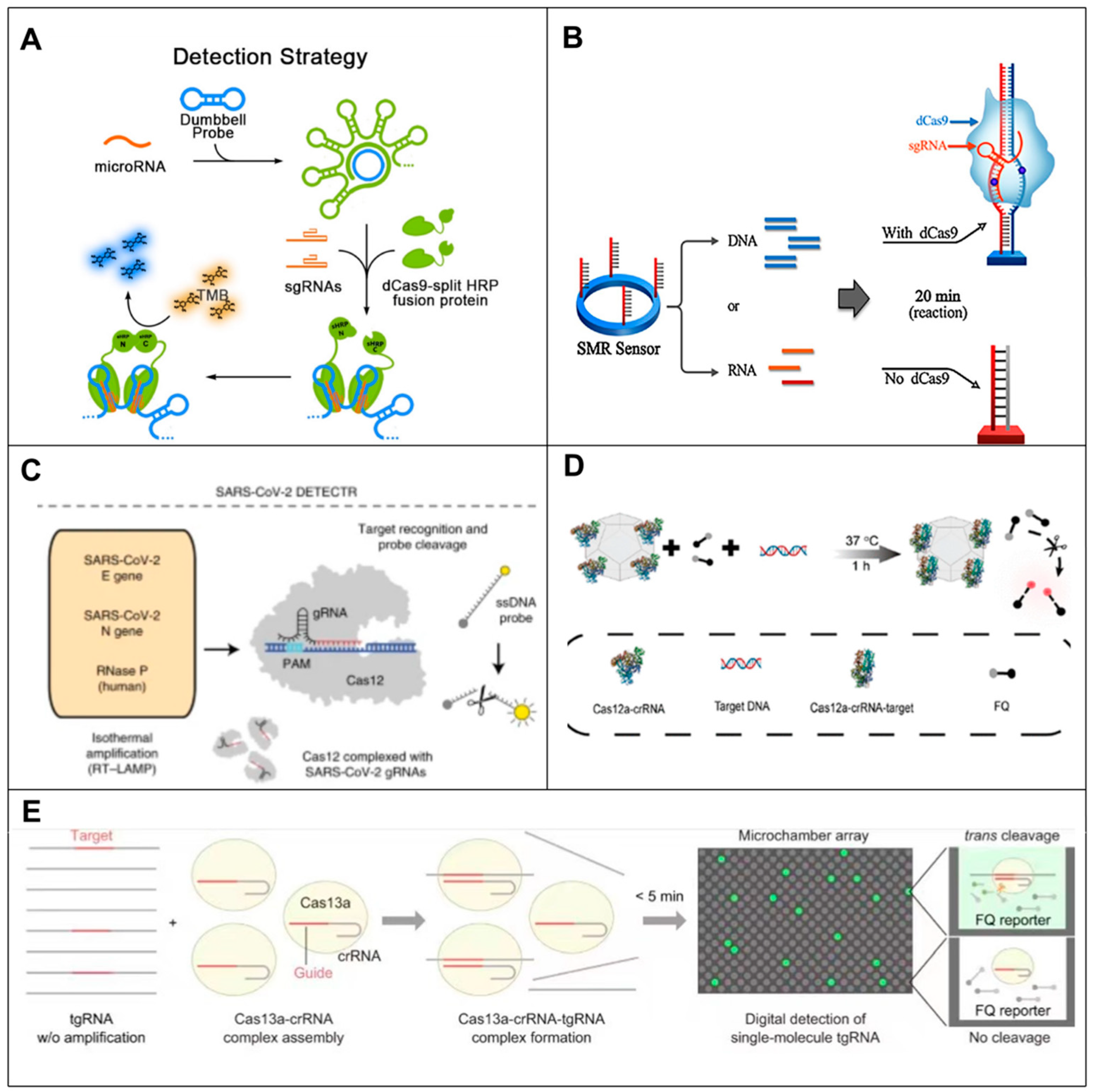
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| AuNIs | gold nanoisland |
| AuNPs | gold nanoparticles |
| AuNR | gold nanorods |
| Au-S | gold-sulfur |
| cDNA | complementary DNA |
| CHA | catalytic hairpin assembly |
| CMV | cytomegalovirus |
| CNTs | carbon nanotubes |
| COM | Cas12a-on-MAF-7 |
| CRISPR | clusters of regularly interspaced short palindromic repeats |
| crRNA | CRISPR-deriver RNA |
| CuO | copper oxide |
| DETECTR | DNA Endonuclease Targeting CRISPR Trans Reporter |
| DNA-AgNC | DNA-silver nanocluster |
| DSBs | double strand breaks |
| dsDNA | double stranded DNA |
| E-INAATs | electrochemical isothermal nucleic acid amplification tests |
| ELC | electrochemiluminescence |
| FFPE | formalin-fixed paraffin-embedded |
| G-FISH | graphene oxide-fluorescence in situ hybridization |
| GO | graphene oxide |
| gRNA | Guide RNA |
| HCR | hybridization chain reaction |
| HCV | hepatitis C virus |
| HOLMES | one-hour low-cost multipurpose highly efficient system |
| HRP | horseradish peroxidase |
| INAATs | isothermal nucleic acid amplification tests |
| LFA | lateral flow assay |
| LOD | limit of detection |
| LSPR | local surface plasmon resonance |
| MB | molecular beacons |
| miRNAs | microRNAs |
| MNPs | magnetic nanoparticles |
| MOFs | metal-organic frameworks |
| mRNA | messenger RNA |
| MTL | mass transfer restriction |
| MWCNT/AuNCs | multiwall carbon nanotube-gold nanocomposites |
| MWNTs | multi-walled carbon nanotubes |
| NC | nitrocellulose |
| N gene | nucleocapsid phosphoprotein |
| PAD | Paper-based microfludics |
| PDMS | polydimethylsiloxane |
| POC | point-of-care |
| POCT | point-of-care testing |
| Poly(A) | polyadenine |
| PPT | plasma photothermal |
| PAN | Peptide acid probe |
| rRNA | ribosomal RNA |
| RCA | rolling circle amplification |
| RdRp | RNA-dependent RNA polymerase |
| RT-LAMP | reverse transcription loop-mediated isothermal amplification |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| RT-RAA | reverse-transcription recombinase-assisted amplification |
| SA-aptamer | streptavidin aptamer |
| SCS | short complementary sequences |
| S gene | Spike protein |
| sgRNA | Small guide RNA |
| SHERLOCK | Specific High Sensitivity Enzyme Reporter Unlock |
| SNPs | single nucleotide polymorphisms |
| SPCEs | screen-printed carbon electrodes |
| SPE-Au | screen-printed gold electrodes |
| SPR | surface plasma resonance |
| ssRNA | single stranded RNA |
| SWV | Square Wave Voltammetry |
| TDT | terminal transferase |
| TMB | tetramethylbenzidine |
| tRNA | transfer RNA |
| UV | ultraviolet rays |
| μPADs | Microfluidic paper-based analytical devices |
References
- Mortimer, S.A.; Kidwell, M.A.; Doudna, J.A. Insights into RNA Structure and Function from Genome-Wide Studies. Nat. Rev. Genet. 2014, 15, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ma, R.; Cai, J.; Guo, C.; Chen, Z.; Yao, L.; Wang, Y.; Fan, R.; Wang, X.; Shi, Y. RNA Modifications: Importance in Immune Cell Biology and Related Diseases. Signal Transduct. Target. Ther. 2022, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.S.; Shtokalo, D.; Tackett, M.R.; Yang, Z.; Vyatkin, Y.; Milos, P.M.; Seilheimer, B.; McCaffrey, T.A.; Kapranov, P. On the Importance of Small Changes in RNA Expression. Methods 2013, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kellner, S.; Burhenne, J.; Helm, M. Detection of RNA Modifications. RNA Biol. 2010, 7, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Motorin, Y.; Lyko, F.; Helm, M. 5-Methylcytosine in RNA: Detection, Enzymatic Formation and Biological Functions. Nucleic Acids Res. 2010, 38, 1415–1430. [Google Scholar] [CrossRef]
- Ma, X.; Xu, J.; Zhou, F.; Ye, J.; Yang, D.; Wang, H.; Wang, P.; Li, M. Recent Advances in PCR-Free Nucleic Acid Detection for SARS-CoV-2. Front. Bioeng. Biotechnol. 2022, 10, 999358. [Google Scholar] [CrossRef]
- Calorenni, P.; Leonardi, A.A.; Sciuto, E.L.; Rizzo, M.G.; Lo Faro, M.J.; Fazio, B.; Irrera, A.; Conoci, S. PCR-Free Innovative Strategies for SARS-CoV-2 Detection. Adv. Healthc. Mater. 2023, 12, 2300512. [Google Scholar] [CrossRef]
- Shinoda, H.; Lida, T.; Makino, A.; Yoshimura, M.; Lshikawa, J.; Ando, J.; Murai, K.; Sugiyama, K.; Muramoto, Y.; Nakano, M. Automated Amplification-Free Digital RNA Detection Platform for Rapid and Sensitive SARS-CoV-2 Diagnosis. Commun. Biol. 2022, 5, 473. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Shinoda, H.; Makino, A.; Yoshimure, M.; Lida, T.; Watanabe, R. Purification/Amplification-Free RNA Detection Platform for Rapid and Multiplex Diagnosis of Plant Viral Infections. Anal. Chem. 2023, 95, 9680–9686. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Ye, F.; Zou, J.; Qu, J.; Jiang, X. An Integrated Amolification-Free Digital Crispr/Cas-Assisted Assay for Single Molecule Detection of RNA. ACS Nano 2023, 17, 7250–7256. [Google Scholar] [CrossRef]
- Tavallaie, R.; Darwish, N.; Hibbert, D.B.; Gooding, J.J. Nucleic-Acid Recognition Interfaces: How the Greater Ability of RNA Duplexes to Bend towards the Surface Influences Electrochemical Sensor Performance. Chem. Commun. 2015, 51, 16526–16529. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.R.; Ruiz, R.C.H.; Hamada, S.; Xu, C.; Yancey, K.G.; Yu, Y.; Han, W.; Luo, D. Point-of-Care Nucleic Acid Detection Using Nanotechnology. Nanoscale 2013, 5, 10141–10154. [Google Scholar] [CrossRef]
- Johnson, B.N.; Mutharasan, R. Biosensor-Based Microrna Detection: Techniques, Design, Performance, and Challenges. Analyst 2014, 139, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Mukumoto, K.; Nojima, T.; Sato, S.; Waki, M.; Takenaka, S. Direct Modification of mRNA by Ferrocenyl Carbodiimide and Its Application to Electrochemical Detection of mRNA. Anal. Sci. 2007, 23, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, P.; Cai, C.; Zhang, R.; Sang, K.; Shen, P.; Huang, Y.; Lu, Y. Triple Signal Amplification Strategy for the Ultrasensitive Electrochemical Detection of Human Papillomavirus 16 E6/E7 mRNA. Enzym. Microb. Technol. 2021, 149, 109855. [Google Scholar] [CrossRef]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid Electrochemical Detection of Coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, F.; Zhou, D.; Chen, D.; Hai, H.; Li, J. An Aptamer Biosensor for Leukemia Marker mRNA Detection Based on Polymerase-Assisted Signal Amplification and Aggregation of Illuminator. Anal. Bioanal. Chem. 2019, 411, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, Y.; Sun, Z.; Li, J.; Yi, Y.; Yang, J.; Li, G. An Electrochemical Biosensor for Sensitive Analysis of the SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 186, 113309. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, J.; Meng, X.; He, J.; Zhang, K.; Cao, Y.; Wang, D.; Dong, H.; Zhang, X. Catalytic Hairpin Assembly Gel Assay for Multiple and Sensitive Microrna Detection. Theranostics 2018, 8, 2646. [Google Scholar] [CrossRef]
- Li, P.; Wei, M.; Zhang, F.; Su, J.; Wei, W.; Zhang, Y.; Liu, S. Novel Fluorescence Switch for Microrna Imaging in Living Cells Based on Dnazyme Amplification Strategy. ACS Appl. Mater. Interfaces 2018, 10, 43405–43410. [Google Scholar] [CrossRef]
- Li, P.; Wei, M.; Zhang, F.; Su, J.; Wei, W.; Zhang, Y.; Liu, S. Application of Spectral Crosstalk Correction for Improving Multiplexed Microrna Detection Using a Single Excitation Wavelength. Anal. Chem. 2017, 89, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Feng, Y.; Yang, H.; Li, P.; Kong, L.; Wei, W.; Liu, S. Ultrasensitive and Specific Multi-Mirna Detection Based on Dual Signal Amplification. Sens. Actuators B Chem. 2021, 337, 129745. [Google Scholar] [CrossRef]
- Li, K.; Chen, T.; Wang, M.; Li, F.; Qi, X.; Song, X.; Fan, L.; Li, L. Ape1 Mediated Target-Responsive Structure Switching Electrochemical (SSE) Biosensor for RNA Detection. Sens. Actuators B Chem. 2024, 398, 134782. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Liu, S.-J.; Jiang, J.-H. Enzyme-Free Electrochemical Biosensor Based on Amplification of Proximity-Dependent Surface Hybridization Chain Reaction for Ultrasensitive mRNA Detection. Talanta 2021, 222, 121536. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, H.-S.; Tang, H.; Jiang, J.-H. Nanopore Biosensor for Sensitive and Label-Free Nucleic Acid Detection Based on Hybridization Chain Reaction Amplification. Talanta 2017, 175, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Roohizadeh, A.; Ghaffarinejad, A.; Salahandish, R.; Omidinia, E. Label-Free Rna-Based Electrochemical Nanobiosensor for Detection of Hepatitis C. Curr. Res. Biotechnol. 2020, 2, 187–192. [Google Scholar] [CrossRef]
- Kerr, E.; Farr, R.; Doeven, E.H.; Nai, Y.H.; Alexander, R.; Guijt, R.M.; Prieto-Simon, B.; Francis, P.S.; Dearnley, M.; Hayne, D.J. Amplification-Free Electrochemiluminescence Molecular Beacon-Based Microrna Sensing Using a Mobile Phone for Detection. Sens. Actuators B Chem. 2021, 330, 129261. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S.D. A Brief Review on Miniaturized Electrochemiluminescence Devices: From Fabrication to Applications. Curr. Opin. Electrochem. 2021, 30, 100800. [Google Scholar] [CrossRef]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, Ultrasensitive, and Quantitative Detection of SARS-CoV-2 Using Antisense Oligonucleotides Directed Electrochemical Biosensor Chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef]
- Ye, Y.; Ju, H. Rapid Detection of Ssdna and RNA Using Multi-Walled Carbon Nanotubes Modified Screen-Printed Carbon Electrode. Biosens. Bioelectron. 2005, 21, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Gopalan, V.; Haque, H.; Masud, M.K.; Al Hossain, S.; Yamauchi, Y.; Nguyen, N.-T.; Lam, A.K.-Y.; Shiddiky, M.J. A Pcr-Free Electrochemical Method for Messenger RNA Detection in Cancer Tissue Samples. Biosens. Bioelectron. 2017, 98, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Jamal, R.B.; Vitasovic, T.; Gosewinkel, U.; Ferapontova, E.E. Detection of E. Coli 23s Rrna by Electrocatalytic “Off-on” DNA Beacon Assay with Femtomolar Sensitivity. Biosens. Bioelectron. 2023, 228, 115214. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, N.; Yang, A.; Xu, Z.; Zhang, W.; Liu, H.; Law, H.K.-W.; Yan, F. Ultrasensitive Detection of Ribonucleic Acid Biomarkers Using Portable Sensing Platforms Based on Organic Electrochemical Transistors. Anal. Chem. 2021, 93, 14359–14364. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Gao, Q.; Jiang, C.; Tian, Q.; Ma, C.; Shi, C. Real-Time Monitoring of Isothermal Nucleic Acid Amplification on a Smartphone by Using a Portable Electrochemical Device for Home-Testing of SARS-CoV-2. Anal. Chim. Acta 2022, 1229, 340343. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, A.; Oroojalian, F.; Fathi, F.; Mokhtarzadeh, A.; de la Guardia, M. Recent Advances in Surface Plasmon Resonance Biosensors for Micrornas Detection. Biosens. Bioelectron. 2020, 169, 112599. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.-A.; Lee, N.-R.; Lee, S.K.; Ahn, J.; Shin, Y.B.; Choi, H.-S.; Lee, C.-S.; Kim, S.; Kim, M.-G. High Sensitivity Detection o 16s Rrna Using Peptide Nucleic Acid Probes and a Surface Plasmon Resonance Biosensor. Anal. Chim. Acta 2008, 630, 168–173. [Google Scholar] [CrossRef]
- Chang, S.; Liu, L.; Mu, C.; Wen, F.; Xiang, J.; Zhai, K.; Wang, B.; Wu, L.; Nie, A.; Shu, Y.; et al. An Ultrasensitive Spr Biosensor for RNA Detection Based on Robust Gep5 Nanosheets. J. Colloid Interface Sci. 2023, 651, 938–947. [Google Scholar] [CrossRef]
- Mousavi, M.Z.; Chen, H.-Y.; Wu, S.-H.; Peng, S.-W.; Lee, K.-L.; Wei, P.-K.; Cheng, J.-Y. Magnetic Nanoparticle-Enhanced Spr on Gold Nanoslits for Ultra-Sensitive, Label-Free Detection of Nucleic Acid Biomarkers. Analyst 2013, 138, 2740–2748. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Yang, X.; Wang, K.; Zhang, H.; Nie, W. High Sensitivity Surface Plasmon Resonance Biosensor for Detection of Microrna and Small Molecule Based on Graphene Oxide-Gold Nanoparticles Composites. Talanta 2017, 174, 521–526. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive Detection of Mirna with an Antimonene-Based Surface Plasmon Resonance Sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-F.; Chou, Y.-T.; Cheng, C.-Y.; Hsu, J.-F.; Su, L.-C.; Ho, J.-A.A. Amplification-Free Detection of Cytomegalovirus Mirna Using a Modification-Free Surface Plasmon Resonance Biosensor. Anal. Chem. 2021, 93, 8002–8009. [Google Scholar] [CrossRef] [PubMed]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Almuzara, C.M.; D’Auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Borghei, Y.-S.; Hosseini, M.; Ganjali, M.R.; Ju, H. Colorimetric and Energy Transfer Based Fluorometric Turn-on Method for Determination of Microrna Using Silver Nanoclusters and Gold Nanoparticles. Microchim. Acta 2018, 185, 286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, R.; Yang, X.; Wang, K.; Zhu, J.; He, L.; Li, Q. Surface Plasmon Resonance Biosensor for Enzyme-Free Amplified Microrna Detection Based on Gold Nanoparticles and DNA Supersandwich. Sens. Actuators B Chem. 2016, 223, 613–620. [Google Scholar] [CrossRef]
- Ding, X.; Yan, Y.; Li, S.; Zhang, Y.; Cheng, W.; Cheng, Q.; Ding, S. Surface Plasmon Resonance Biosensor for Highly Sensitive Detection of Microrna Based on DNA Super-Sandwich Assemblies and Streptavidin Signal Amplification. Anal. Chim. Acta 2015, 874, 59–65. [Google Scholar] [CrossRef]
- Li, J.; Lei, P.; Ding, S.; Zhang, Y.; Yang, J.; Cheng, Q.; Yan, Y. An Enzyme-Free Surface Plasmon Resonance Biosensor for Real-Time Detecting Microrna Based on Allosteric Effect of Mismatched Catalytic Hairpin Assembly. Biosens. Bioelectron. 2016, 77, 435–441. [Google Scholar] [CrossRef]
- Li, K.; An, N.; Wu, L.; Wang, M.; Li, F.; Li, L. Absolute Quantification of Micrornas Based on Mass Transport Limitation under a Laminar Flow Spr System. Biosens. Bioelectron. 2024, 244, 115776. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Mini-Thermal Platform Integrated with Microfluidic Device with on-Site Detection for Real-Time DNA Amplification. Biotechniques 2023, 74, 158–171. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Devices (UPADS)-Are a New Platform Designed for Assured. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, V.; Kumar, N.; Arun, R.K. Microfluidics-Based Nanobiosensors for Healthcare Monitoring. Mol. Biotechnol. 2024, 66, 378–401. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward Practical Application of Paper-Based Microfluidics for Medical Diagnostics: State-of-the-Art and Challenges. Lab Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, H.; Baloda, M.; Gurung, A.S.; Xu, L.-P.; Wang, T.; Zhang, X.; Liu, G. Visual Detection of Microrna with Lateral Flow Nucleic Acid Biosensor. Biosens. Bioelectron. 2014, 54, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yao, L.; Teng, J.; Yan, C.; Qin, P.; Liu, G.; Chen, W. Lateral Flow Test for Visual Detection of Multiple Micrornas. Sens. Actuators B Chem. 2018, 264, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Batule, B.S.; Seok, Y.; Kim, M.-G. Based Nucleic Acid Testing System for Simple and Early Diagnosis of Mosquito-Borne RNA Viruses from Human Serum. Biosens. Bioelectron. 2020, 151, 111998. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Linnes, J.C.; Fan, A.; Ellenson, C.K.; Pollock, N.R.; Klapperich, C.M. Based RNA Extraction, in Situ Isothermal Amplification, and Lateral Flow Detection for Low-Cost, Rapid Diagnosis of Influenza a (H1N1) from Clinical Specimens. Anal. Chem. 2015, 87, 7872–7879. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Shen, Y.; Mulchandani, A. Affordable Paper-Based Swnts Field-Effect Transistor Biosensors for Nucleic Acid Amplification-Free and Label-Free Detection of Micro RNAs. Biosens. Bioelectron. X 2023, 14, 100364. [Google Scholar] [CrossRef]
- Kaarj, K.; Akarapipad, P.; Yoon, J.-Y. Simpler, Faster, and Sensitive Zika Virus Assay Using Smartphone Detection of Loop-Mediated Isothermal Amplification on Paper Microfluidic Chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ni, D.; Fang, M.; Shi, Z.; Xu, Z. Microfluidic Ruler-Readout and Crispr Cas12a-Responded Hydrogel-Integrated Paper-Based Analytical Devices (Mreach-Pad) for Visible Quantitative Point-of-Care Testing of Invasive Fungi. Anal. Chem. 2021, 93, 16965–16973. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Qiu, J.; Wang, Y.; Wang, M.; Zhang, Y.; Han, L. Rapid and High-Throughput SARS-CoV-2 RNA Detection without RNA Extraction and Amplification by Using a Microfluidic Biochip. Chem.—A Eur. J. 2022, 28, e202104054. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Xiang, X.; Xue, L.; Cai, W.; Gao, J.; Yang, J.; Liang, Y.; Wang, L.; Chen, M.; Pang, R. Development of a Novel Raa-Based Microfluidic Chip for Absolute Quantitative Detection of Human Norovirus. Microchem. J. 2021, 164, 106050. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Liu, D.; Hu, B.; Luo, G.; Huang, Z. A Novel Microfluidic RNA Chip for Direct, Single-Nucleotide Specific, Rapid and Partially-Degraded RNA Detection. Talanta 2022, 239, 122974. [Google Scholar] [CrossRef] [PubMed]
- Burgers, P.M.J.; Kunkel, T.A. Eukaryotic DNA Replication Fork. Annu. Rev. Biochem. 2017, 86, 417–438. [Google Scholar] [CrossRef] [PubMed]
- McHenry, C.S. DNA Replicases from a Bacterial Perspective. Annu. Rev. Biochem. 2011, 80, 403–436. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Bi, Y.; Xu, X.; Zhu, Z.; Yang, C. Integrated Paper-Based Microfluidic Devices for Point-of-Care Testing. Anal. Methods 2018, 10, 3567–3581. [Google Scholar] [CrossRef]
- Basiri, A.; Heidari, A.; Nadi, M.F.; Fallahy, M.T.P.; Nezamabadi, S.S.; Sedighi, M.; Saghazadeh, A.; Rezaei, N. Microfluidic Devices for Detection of RNA Viruses. Rev. Med. Virol. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Gao, D.; Ma, Z.; Jiang, Y. Recent Advances in Microfluidic Devices for Foodborne Pathogens Detection. TrAC Trends Anal. Chem. 2022, 157, 116788. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Shi, Y.; Ping, J.; Wu, J.; Chen, H. Magnetic Particles for Integrated Nucleic Acid Purification, Amplification and Detection without Pipetting. TrAC Trends Anal. Chem. 2020, 127, 115912. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zong, N.; Ye, F.; Mei, Y.; Qu, J.; Jiang, X. Dual-Crispr/Cas12a-Assisted Rt-Raa for Ultrasensitive SARS-CoV-2 Detection on Automated Centrifugal Microfluidics. Anal. Chem. 2022, 94, 9603–9609. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mei, Y.; Jiang, X. Universal and High-Fidelity DNA Single Nucleotide Polymorphism Detection Based on a Crispr/Cas12a Biochip. Chem. Sci. 2021, 12, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Zong, N.; Gao, Y.; Chen, Y.; Luo, X.; Jiang, X. Automated Centrifugal Microfluidic Chip Integrating Pretreatment and Molecular Diagnosis for Hepatitis B Virus Genotyping from Whole Blood. Anal. Chem. 2022, 94, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mei, Y.; Zhao, X.; Jiang, X. Reagents-Loaded, Automated Assay That Integrates Recombinase-Aided Amplification and Cas12a Nucleic Acid Detection for a Point-of-Care Test. Anal. Chem. 2020, 92, 14846–14852. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.-C.; Cheng, Y.-W.; Sun, H.-Y. Rapid SARS-CoV-2 Spike Protein Detection by Carbon Nanotube-Based near-Infrared Nanosensors. Nano Lett. 2021, 21, 2272–2280. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, H.; Feder-Kubis, J.; Nguyen, D.D. Recent Advances in Nanobiosensors for Sustainable Healthcare Applications: A Systematic Literature Review. Environ. Res. 2023, 238, 117177. [Google Scholar] [CrossRef]
- Jiang, H.; Li, F.-R.; Li, W.; Lu, X.; Ling, K. Multiplexed Determination of Intracellular Messenger RNA by Using a Graphene Oxide Nanoprobe Modified with Target-Recognizing Fluorescent Oligonucleotides. Microchim. Acta 2018, 185, 552. [Google Scholar] [CrossRef]
- Hwang, D.W.; Choi, Y.R.; Kim, H.; Park, H.Y.; Kim, K.W.; Kim, M.Y.; Park, C.-K.; Lee, D. Graphene Oxide-Quenching-Based Fluorescence in Situ Hybridization (G-Fish) to Detect RNA in Tissue: Simple and Fast Tissue RNA Diagnostics. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 162–172. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; He, X.; Zhu, L.; Zhu, L.; Yang, M.; Huang, K.; Luo, H.; Xu, W. Intracellular Circrna Imaging and Signal Amplification Strategy Based on the Graphene Oxide-DNA System. Anal. Chim. Acta 2021, 1183, 338966. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, B.; Wang, M.; Li, J.; Pan, W.; Gao, X.; Li, N.; Tang, B. A Highly Sensitive Strategy for Fluorescence Imaging of Microrna in Living Cells and in Vivo Based on Graphene Oxide-Enhanced Signal Molecules Quenching of Molecular Beacon. ACS Appl. Mater. Interfaces 2018, 10, 6982–6990. [Google Scholar] [CrossRef]
- Harvey, J.D.; Jena, P.V.; Baker, H.A.; Zerze, G.H.; Williams, R.M.; Galassi, T.V.; Roxbury, D.; Mittal, J.; Heller, D.A. A Carbon Nanotube Reporter of Microrna Hybridization Events in Vivo. Nat. Biomed. Eng. 2017, 1, 0041. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoon, S.; Chang, J.; Lee, S.; Cho, H.H.; Jeong, S.H.; Jo, K.; Lee, J.H. Multifunctional Heterogeneous Carbon Nanotube Nanocomposites Assembled by DNA-Binding Peptide Anchors. Small 2020, 16, 1905821. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, S.; Chang, J.; Sundharbaabu, P.R.; Heo, J.H.; Sung, W.-K.; Ro, J.C.; Kim, K.K.; Rayappan, J.B.B.; Lee, J.H. DNA-Wrapped Cnt Sensor for Small Nucleic Acid Detection: Influence of Short Complementary Sequence. BioChip J. 2022, 16, 490–500. [Google Scholar] [CrossRef]
- Ma, H.; Xue, N.; Li, Z.; Xing, K.; Miao, X. Ultrasensitive Detection of Mirna-155 Using Multi-Walled Carbon Nanotube-Gold Nanocomposites as a Novel Fluorescence Quenching Platform. Sens. Actuators B Chem. 2018, 266, 221–227. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Atasoy, U.; Sauter, E.R. Circulating Micrornas in Breast Cancer and Healthy Subjects. BMC Res. Notes 2009, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The Oncogenic Role of Mir-155 in Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, X.; Zhang, M.; Wang, X.; Chen, Y.; Qian, C.; Wu, J.; Xu, J. Versatile Detection with Crispr/Cas System from Applications to Challenges. TrAC Trends Anal. Chem. 2021, 135, 116150. [Google Scholar] [CrossRef]
- Safari, F.; Hatam, G.; Behbahani, A.B.; Rezaei, V.; Barekati-Mowahed, M.; Petramfar, P.; Khademi, F. Crispr System: A High-Throughput Toolbox for Research and Treatment of Parkinson’s Disease. Cell. Mol. Neurobiol. 2020, 40, 477–493. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. Crispr-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded Dnase Activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.T.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 Is a Single-Component Programmable Rna-Guided Rna-Targeting Crispr Effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef]
- Wang, S.; Wei, S.; Wang, S.; Zhu, X.; Lei, C.; Huang, Y.; Nie, Z.; Yao, S. Chimeric DNA-Functionalized Titanium Carbide Mxenes for Simultaneous Mapping of Dual Cancer Biomarkers in Living Cells. Anal. Chem. 2018, 91, 1651–1658. [Google Scholar] [CrossRef]
- Wang, S.; Song, W.; Wei, S.; Zeng, S.; Yang, S.; Lei, C.; Huang, Y.; Nie, Z.; Yao, S. Functional Titanium Carbide Mxenes-Loaded Entropy-Driven RNA Explorer for Long Noncoding RNA Pca3 Imaging in Live Cells. Anal. Chem. 2019, 91, 8622–8629. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-Y.; Zhu, L.-Y.; Zhu, C.-S.; Ma, J.-X.; Hou, T.; Wu, X.-M.; Xie, S.-S.; Min, L.; Tan, D.-A.; Zhang, D.-Y.; et al. Highly Effective and Low-Cost Microrna Detection with Crispr-Cas9. ACS Synth. Biol. 2018, 7, 807–813. [Google Scholar] [CrossRef]
- Wang, X.-W.; Hu, L.-F.; Hao, J.; Liao, L.-Q.; Chiu, Y.-T.; Shi, M.; Wang, Y. A Microrna-Inducible Crispr–Cas9 Platform Serves as a Microrna Sensor and Cell-Type-Specific Genome Regulation Tool. Nat. Cell Biol. 2019, 21, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Hirosawa, M.; Fujita, Y.; Saito, H. Cell-Type-Specific Crispr Activation with Microrna-Responsive Acrlla4 Switch. ACS Synth. Biol. 2019, 8, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, X.; Zhang, K.; Deng, R.; Li, J. RNA Strand Displacement Responsive Crispr/Cas9 System for mRNA Sensing. Anal. Chem. 2019, 91, 3989–3996. [Google Scholar] [CrossRef]
- Koo, B.; Kim, D.-E.; Kweon, J.; Jin, C.E.; Kim, S.-H.; Kim, Y.; Shin, Y. Crispr/Dcas9-Mediated Biosensor for Detection of Tick-Borne Diseases. Sens. Actuators B Chem. 2018, 273, 316–321. [Google Scholar] [CrossRef]
- Marsic, T.; Ali, Z.; Tehseen, M.; Mahas, A.; Hamdan, S.; Mahfouz, M. Vigilant: An Engineered Vird2-Cas9 Complex for Lateral Flow Assay-Based Detection of SARS-CoV2. Nano Lett. 2021, 21, 3596–3603. [Google Scholar] [CrossRef]
- Aman, R.; Mahas, A.; Mahfouz, M. Nucleic Acid Detection Using Crispr/Cas Biosensing Technologies. ACS Synth. Biol. 2020, 9, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Pickar-Oliver, A.; Gersbach, C.A. The Next Generation of Crispr–Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, J.; Liu, G. Crispr/Cas Systems Towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and Portable Nucleic Acid Detection Platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic Acid Detection with Crispr-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A. Crispr-Cas12-Based Detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef]
- Mustafa, M.I.; Makhawi, A.M. Sherlock and Detectr: Crispr-Cas Systems as Potential Rapid Diagnostic Tools for Emerging Infectious Diseases. J. Clin. Microbiol. 2021, 59, 10-1128. [Google Scholar] [CrossRef]
- Li, S.-Y.; Cheng, Q.-X.; Liu, J.-K.; Nie, X.-Q.; Zhao, G.-P.; Wang, J. Crispr-Cas12a Has Both Cis-and Trans-Cleavage Activities on Single-Stranded DNA. Cell Res. 2018, 28, 491–493. [Google Scholar] [CrossRef]
- Li, S.-Y.; Cheng, Q.-X.; Wang, J.-M.; Li, X.-Y.; Zhang, Z.-L.; Gao, S.; Cao, R.-B.; Zhao, G.-P.; Wang, J. Crispr-Cas12a-Assisted Nucleic Acid Detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Li, Z.; Wang, W.; Liu, J.; Liu, L.; Zhu, G.; Karthik, L.; Wang, M.; Wang, K.-F.; Wang, Z.; et al. A Crispr-Cas12a-Derived Biosensing Platform for the Highly Sensitive Detection of Diverse Small Molecules. Nat. Commun. 2019, 10, 3672. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhou, B.; Shang, Z.; Liu, S.; Li, X.; Zhang, X.; Li, B. Active Crispr-Cas12a on Hydrophilic Metal–Organic Frameworks: A Nanobiocomposite with High Stability and Activity for Nucleic Acid Detection. Anal. Chem. 2023, 95, 10580–10587. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Shu, B.; Jiang, Y.; Ye, M.; Liu, L.; Guo, Z.; Han, Z.; Wang, Z.; Zhou, X. An Ultralocalized Cas13a Assay Enables Universal and Nucleic Acid Amplification-Free Single-Molecule RNA Diagnostics. ACS Nano 2020, 15, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhu, X.; Lu, B. Development and Application of Sensitive, Specific, and Rapid Crispr-Cas13-Based Diagnosis. J. Med. Virol. 2021, 93, 4198–4204. [Google Scholar]
- Shan, Y.; Zhou, X.; Huang, R.; Xing, D. High-Fidelity and Rapid Quantification of Mirna Combining Crrna Programmability and Crispr/Cas13a Trans-Cleavage Activity. Anal. Chem. 2019, 91, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- East-Seletsky, A.; O’Connell, M.R.; Knight, S.C.; Burstein, D.; Cate, J.H.D.; Tjian, R.; Doudna, J.A. Two Distinct Rnase Activities of Crispr-C2c2 Enable Guide-RNA Processing and RNA Detection. Nature 2016, 538, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, H.; Taguchi, Y.; Nakagawa, R.; Makino, A.; Okazaki, S.; Nakano, M.; Muramoto, Y.; Takahashi, C.; Takahashi, I.; Ando, J.; et al. Amplification-Free RNA Detection with Crispr–Cas13. Commun. Biol. 2021, 4, 476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, T.; Wang, M.; Ledesma-Amaro, R.; Lu, Y.; Hu, X.; Zhang, T.; Yang, M.; Li, Y.; Xiang, J. Crispr-Cas13a Cascade-Based Viral RNA Assay for Detecting SARS-CoV-2 and Its Mutations in Clinical Samples. Sens. Actuators B Chem. 2022, 362, 131765. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Nguyen-Duc, T.; Song, W.; Jaffrey, S.R. Fluorescence Imaging of Cellular Metabolites with RNA. Science 2012, 335, 1194. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Wu, K.Y.; Jaffrey, S.R. RNA Mimics of Green Fluorescent Protein. Science 2011, 333, 642–646. [Google Scholar] [CrossRef]
- Ying, Z.-M.; Wu, Z.; Tu, B.; Tan, W.; Jiang, J.-H. Genetically Encoded Fluorescent RNA Sensor for Ratiometric Imaging of Microrna in Living Tumor Cells. J. Am. Chem. Soc. 2017, 139, 9779–9782. [Google Scholar] [CrossRef]
- Fozouni, P.; Son, S.; de León Derby, M.D.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I. Amplification-Free Detection of SARS-CoV-2 with Crispr-Cas13a and Mobile Phone Microscopy. Cell 2021, 184, 323–333.e9. [Google Scholar] [CrossRef] [PubMed]
- Arizti-Sanz, J.; Freije, C.A.; Stanton, A.C.; Petros, B.A.; Boehm, C.K.; Siddiqui, S.; Shaw, B.M.; Adams, G.; Kosoko-Thoroddsen, T.S.-F.; Kemball, M.E. Streamlined Inactivation, Amplification, and Cas13-Based Detection of SARS-CoV-2. Nat. Commun. 2020, 11, 5921. [Google Scholar] [CrossRef] [PubMed]
- Patchsung, M.; Jantarug, K.; Pattama, A.; Aphicho, K.; Suraritdechachai, S.; Meesawat, P.; Sappakhaw, K.; Leelahakorn, N.; Ruenkam, T.; Wongsatit, T. Clinical Validation of a Cas13-Based Assay for the Detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Huang, S.; Ghalandari, B.; Li, S.; Warden, A.R.; Dang, J.; Kang, L.; Zhang, Y.; Wang, Y.; Sun, Y. Hairpin-Spacer Crrna-Enhanced Crispr/Cas13a System Promotes the Specificity of Single Nucleotide Polymorphism (SNP) Identification. Adv. Sci. 2021, 8, 2003611. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Feng, W.; Newbigging, A.M.; Le, C.; Pang, B.; Peng, H.; Cao, Y.; Wu, J.; Abbas, G.; Song, J.; Wang, D.-B.; et al. Molecular Diagnosis of COVID-19: Challenges and Research Needs. Anal. Chem. 2020, 92, 10196–10209. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ahmad, K.Z.; Warden, A.R.; Ke, Y.; Maboyi, N.; Zhi, X.; Ding, X. One-Pot Pre-Coated Interface Proximity Extension Assay for Ultrasensitive Co-Detection of Anti-SARS-CoV-2 Antibodies and Viral RNA. Biosens. Bioelectron. 2021, 193, 113535. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, S.; Ke, Y.; Chen, H.; Dang, J.; Huang, C.; Liu, W.; Cui, D.; Wang, J.; Zhi, X. A Hipad Integrated with Rgo/Mwcnts Nano-Circuit Heater for Visual Point-of-Care Testing of SARS-CoV-2. Adv. Funct. Mater. 2021, 31, 2100801. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Tao, Z.; Wan, L.; Zong, C.; Wu, J.; Tan, X.; Wang, B.; Guo, Z.; Zhang, L.; Yuan, H.; et al. Aptamer-Based Cas14a1 Biosensor for Amplification-Free Live Pathogenic Detection. Biosens. Bioelectron. 2022, 211, 114282. [Google Scholar] [CrossRef]
- Ge, H.; Wang, X.; Xu, J.; Lin, H.; Zhou, H.; Hao, T.; Wu, Y.; Guo, Z. A Crispr/Cas12a-Mediated Dual-Mode Electrochemical Biosensor for Polymerase Chain Reaction-Free Detection of Genetically Modified Soybean. Anal. Chem. 2021, 93, 14885–14891. [Google Scholar] [CrossRef]
| System | Combination | Sensitivity | Time | Target | Ref. |
|---|---|---|---|---|---|
| Electrochemical-based RNA biosensors | AuNPs/RT-LAMP/high affinity biotin-avidin system | 0.1 fmol·L−1 | ~1 h | mRNA | [6] |
| RCA | 1 copy/μL | <2 h | viral N or S genes | [7] | |
| AuNPs/polymerase-assisted signal amplification | 4.3 × 10−17 mol/L | <1 h | mRNA | [8] | |
| CHA/TDT | 26 fmol·L−1 | <1 h | SARS-CoV-2 RNA | [9] | |
| HCR | 3 fmol·L−1 | <1 h | mRNA | [14] | |
| CuO/AuNPs | 1 fmol·L−1 | ~1 h | HCV RNA | [16] | |
| MWNTs/SPE | 8.2 μg mL−1 | <5 min | tRNA | [19] | |
| SPE-Au | fmol·L−1 | <1 h | mRNA | [20] | |
| SPR-based RNA biosensors | GeP5 | 10 amol·L−1 | <1 h | SARS-CoV-2 RNA | [24] |
| MNPs/AuNPs | 7 fmol·L−1 | ~1 h | mRNA | [25] | |
| Antimonene two-dimensional nanomaterials/AuNR | amol·L−1 | ~1 h | miRNA | [27] | |
| MNP | 3 fmol·L−1 | ~2 h | miRNA | [28] | |
| DNA-AgNCs/AuNPs | fmol·L−1 | <2 h | miRNA | [31] | |
| AuNPs/DNA super-sandwich | 21 fmol·L−1 | ~1 h | miRNA | [32] | |
| DNA super-sandwich/biotin-streptavidin system | 30 pmol·L−1 | <9 min | miRNA | [33] | |
| CHA/streptavidin aptamer | 1 pmol·L−1 | <1 h | miRNA | [34] | |
| MTL | 500 fmol·L−1 | ~1 h | miRNA | [35] | |
| Microfluidic-based RNA biosensors | AuNPs | fmol·L−1 | ~2 h | miRNA | [40] |
| RT-LAMP | fmol·L−1 | <1 h | Viral RNA | [42] | |
| RT-LAMP | 160 copies/μL | <45 min | Viral RNA | [43] | |
| NoV-DID/PDMS/RT-RAA | fmol·L−1 | ~1 h | Viral RNA | [48] | |
| Nanomaterial-based RNA biosensors | GO | 0.26 nmol·L−1 | <2 h | mRNA | [61] |
| GO/CHA/HCR | 15 pmol·L−1 | <2 h | circRNA | [63] | |
| GO/MB | 30 pmol·L−1 | ~1 h | miRNA | [64] | |
| MWCNT/AuNCs | 33.4 fmol·L−1 | ~1 h | miRNA | [68] | |
| CRISPR-based RNA biosensors | RCA/CRISPR-Cas9 | fmol·L−1 | <1 h | miRNA | [77] |
| DETECTR | fmol·L−1 | 30–40 min | SARS-CoV-2 RNA | [88] | |
| HOLMES | amol·L−1 | ~1 h | Viral RNA | [90] | |
| CRISPR-Cas12/MoFs | 1 copy | ~1 h | SARS-CoV-2 RNA | [93] | |
| SATORI | 10 fmol·L−1 | <5 min | SARS-CoV-2 RNA | [97] | |
| SHERLOCK | fmol·L−1 | ~1 h | SARS-CoV-2 RNA | [86] | |
| SHERLOCKv2 | fmol·L−1 | ~1 h | SARS-CoV-2 RNA | [86] | |
| CRISPR-Cas13 | 0.216 fmol·L−1 | ~1 h | SARS-CoV-2 RNA | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, H.; Qi, X.; Ji, Y.; Li, F.; Chen, X.; Li, K.; Li, L. PCR Independent Strategy-Based Biosensors for RNA Detection. Biosensors 2024, 14, 200. https://doi.org/10.3390/bios14040200
Li X, Wang H, Qi X, Ji Y, Li F, Chen X, Li K, Li L. PCR Independent Strategy-Based Biosensors for RNA Detection. Biosensors. 2024; 14(4):200. https://doi.org/10.3390/bios14040200
Chicago/Turabian StyleLi, Xinran, Haoqian Wang, Xin Qi, Yi Ji, Fukai Li, Xiaoyun Chen, Kai Li, and Liang Li. 2024. "PCR Independent Strategy-Based Biosensors for RNA Detection" Biosensors 14, no. 4: 200. https://doi.org/10.3390/bios14040200
APA StyleLi, X., Wang, H., Qi, X., Ji, Y., Li, F., Chen, X., Li, K., & Li, L. (2024). PCR Independent Strategy-Based Biosensors for RNA Detection. Biosensors, 14(4), 200. https://doi.org/10.3390/bios14040200




