Extended Review Concerning the Integration of Electrochemical Biosensors into Modern IoT and Wearable Devices
Abstract
:1. Introduction
2. General Remarks
3. Relevant Types of Biosensors
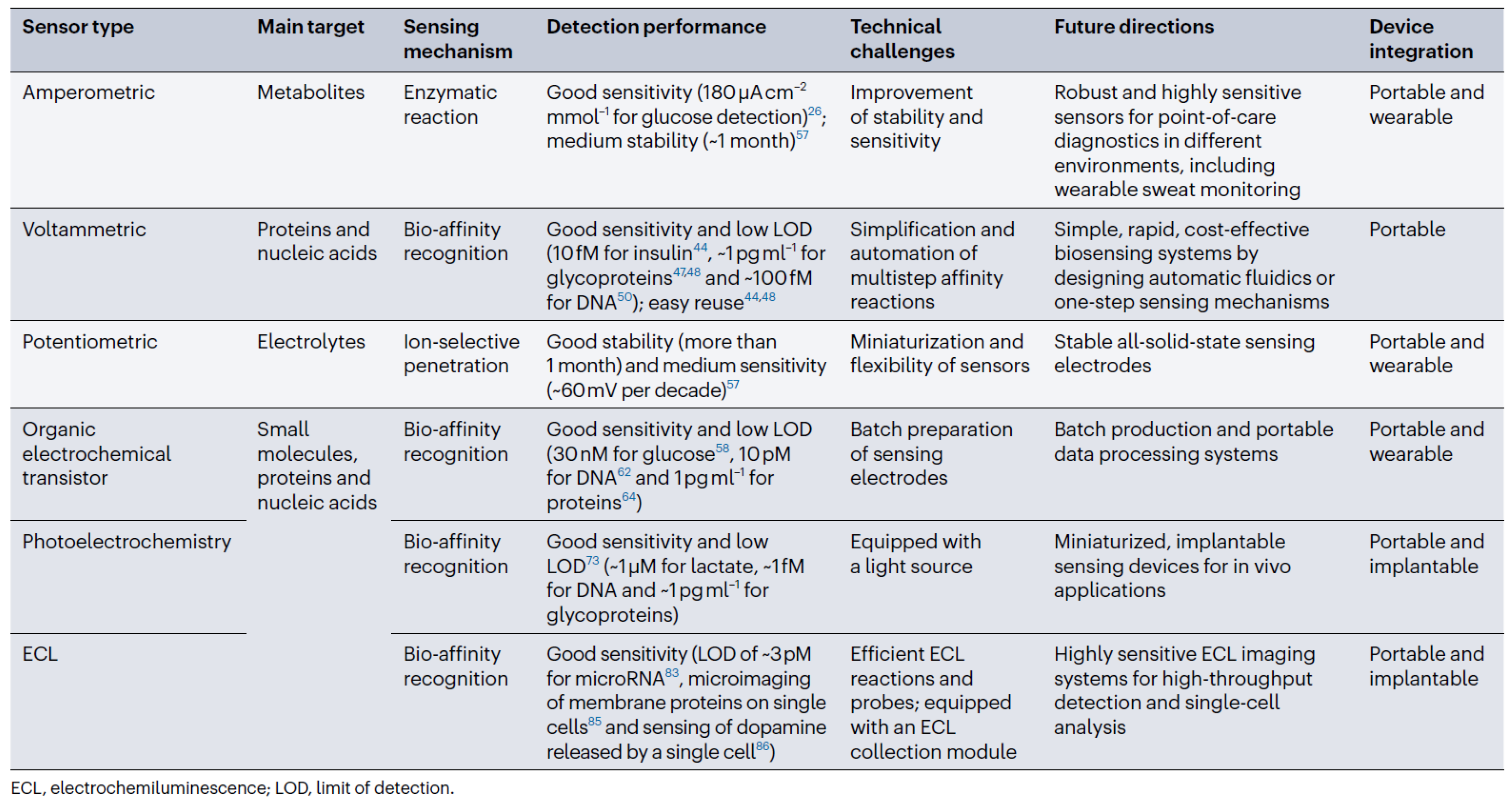
3.1. Electrochemical Detection of Biomarkers
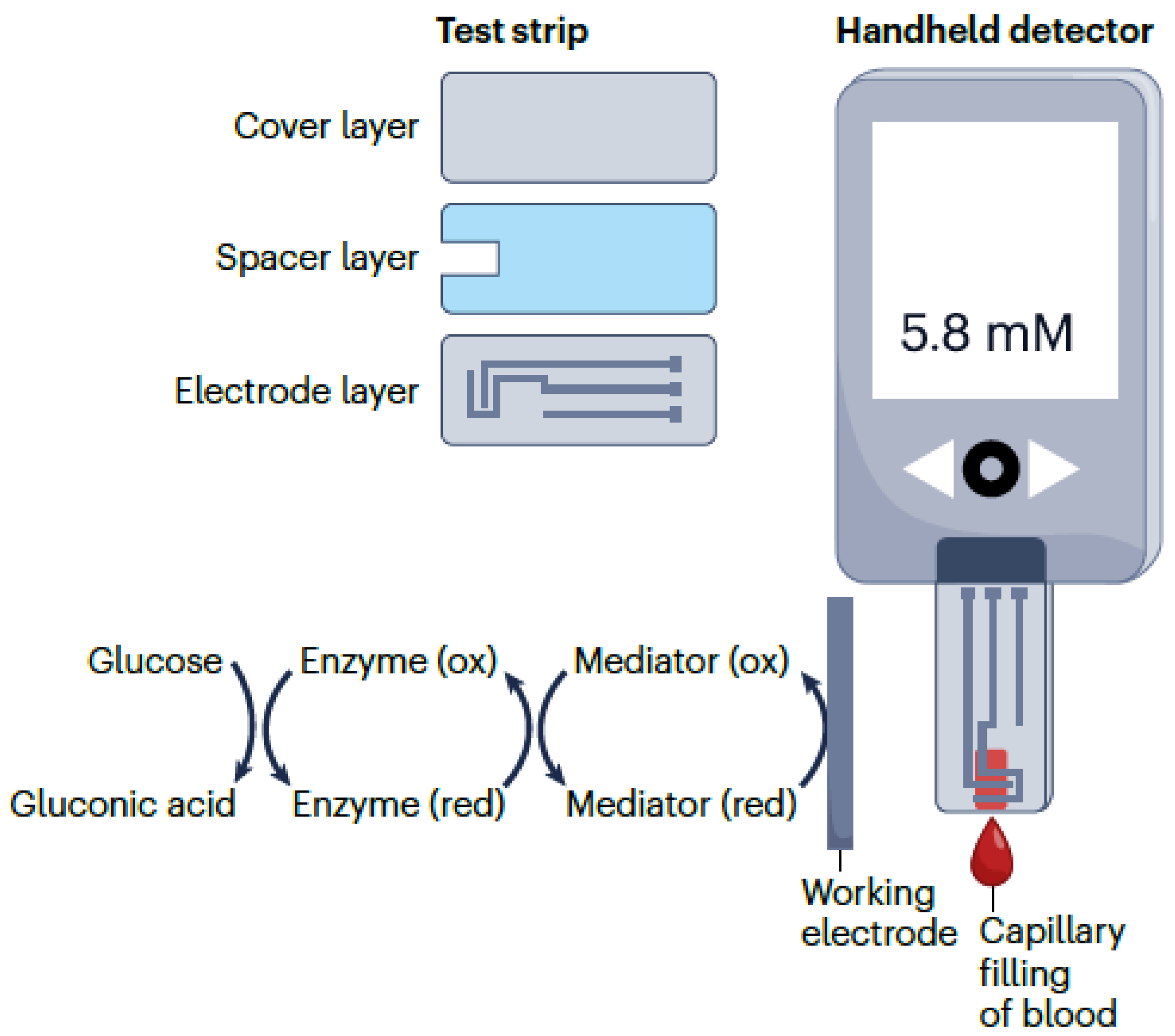
3.2. Voltammetric and Amperometric Biosensors
3.3. Potentiometric Biosensors
3.4. Organic Electrochemical Transistor Biosensors
3.5. Photoelectrochemical Biosensors
3.6. Biosensing and Bioimaging Based on Electrochemiluminescence
4. Integration into Wearable Devices
4.1. Portable Electrochemical Biosensing Devices
4.2. Relevant Wearable Biosensors Integration Scenarios
4.3. Implantable Devices
4.4. Considerations Regarding Resource-Constrained Wearable Devices
4.5. Remarks Concerning Translational Research Processes
5. Conclusions
Funding
Conflicts of Interest
References
- Clark, L.C.J.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Lambrianou, A.; Demin, S.; Hall, E.A.H. Biosensing for the 21st Century; Renneberg, R., Lisdat, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 65–95. [Google Scholar]
- Chain, C.Y.; Franchin, L.; Cisneros, J.S.; Villagra, A.P.; Labriola, C.A.; Paccagnella, A.; Bonaldo, S. Impedimetric Screen-Printed Immunosensor for the Rapid Detection of Chagas Disease. IEEE Sens. J. 2023, 24, 1167–1174. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ahommed, M.S.; Daizy, M.; Bacchu, M.S.; Ali, M.R.; Al-Mamun, M.R.; Aly, M.A.S.; Khan, M.Z.H.; Hossain, S.I. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Bonaldo, S.; Franchin, L.; Cretaio, E.; Pasqualotto, E.; Scaramuzza, M.; Paccagnella, A. Electrochemical Biosensor for the Monitoring of Phages of Lactococcus lactis in Milk-Based Samples. IEEE Sens. J. 2024, 24, 78–85. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, Z.F.; Yan, F.; Ju, H.X. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar] [CrossRef]
- Minteer, S.D. Advances in electroanalytical chemistry. J. Am. Chem. Soc. 2018, 140, 2701–2703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Xiang, Y.; Wang, M.; Basu, A.; Lu, Y. Dose-dependent response of personal glucose meters to nicotinamide coenzymes: Applications to point-of-care diagnostics of many non-glucose targets in a single step. Angew. Chem. Int. Ed. 2016, 128, 742–746. [Google Scholar] [CrossRef]
- Das, A.; Cui, X.K.; Chivukula, V.; Iyer, S.S. Detection of enzymes, viruses, and bacteria using glucose meters. Anal. Chem. 2018, 90, 11589–11598. [Google Scholar] [CrossRef]
- Gong, S.H.; Li, J.J.; Pan, W.; Li, N.; Tang, B. Duplex-specific nuclease-assisted CRISPRCas12a strategy for microRNA detection using a personal glucose meter. Anal. Chem. 2021, 93, 10719–10726. [Google Scholar] [CrossRef]
- Liu, R.; Hu, Y.S.; He, Y.; Lan, T.; Zhang, J.J. Translating daily COVID-19 screening into a simple glucose test: A proof of concept study. Chem. Sci. 2021, 12, 9022–9030. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mo, F.; Liu, Y.; Liu, Y.; Li, G.; Yu, W.; Liu, X. Portable and sensitive detection of non-glucose target by enzyme-encapsulated metal-organic-framework using personal glucose meter. Biosens. Bioelectron. 2022, 198, 113819. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.A.; Plaxco, K.W. Folding-based electrochemical biosensors: The case for responsive nucleic acid architectures. Acc. Chem. Res. 2010, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Li, F.; Dever, B.; Li, X.F.; Le, X.C. DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem. Rev. 2013, 113, 2812–2841. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.W.; Du, D.; Hua, X.; Yu, X.Y.; Lin, Y.H. Paper-based electrochemical biosensors: From test strips to paper-based microfluidics. Electroanalysis 2014, 26, 1214–1223. [Google Scholar] [CrossRef]
- Sassa, F.; Biswas, G.C.; Suzuki, H. Microfabricated electrochemical sensing devices. Lab Chip 2020, 20, 1358–1389. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Lei, J.P.; Ju, H.X. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Ju, H.X. Biosensors: Signal amplification for highly sensitive bioanalysis based on biosensors or biochips. J. Biochips Tissue Chips 2012, 2, e114. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotech. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible electrochemical bioelectronics: The rise of in situ bioanalysis. Adv. Mater. 2020, 32, 1902083. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Yang, Y.R.; Gao, W. Skin-interfaced sensors in digital medicine: From materials to applications. Matter 2020, 2, 1414–1445. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Min, J.H.; Gao, W. Wearable and implantable electronics: Moving toward precision therapy. ACS Nano 2019, 13, 12280–12286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Y.; Yin, Z. Low-power soft transistors triggering revolutionary electronics. Innovation 2024, 5, 100616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, N.; Xu, J.; Liu, Z.; Zhou, Y.; Yang, Y.; Li, S.; Huang, Y.; Jiang, S. Flexible electronics for cardiovascular healthcare monitoring. Innovation 2023, 4, 100485. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Kandeil, A.; Gomaa, M.; Mohamed El Nashar, R.; El-Sherbiny, I.M.; Hassan, R.Y. SARS-CoV-2-impedimetric biosensor: Virus-imprinted chips for early and rapid diagnosis. ACS Sens. 2021, 6, 4098–4107. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Duarte, P.A.; Ma, Y.; Savchenko, O.; Shoute, L.; Khaniani, Y.; Babiuk, S.; Zhuo, R.; Abdelrasoul, G.N.; Charlton, C.; et al. An impedimetric biosensor for COVID-19 serology test and modification of sensor performance via dielectrophoresis force. Biosen. Bioelectron. 2022, 213, 114476. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian blue-based first-generation biosensor: A sensitive amperometric electrode for glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Ju, H.X.; Zhang, X.J.; Wang, J. NanoBiosensing: Principles, Development and Application; Springer: New York, NY, USA, 2011; pp. 85–102. [Google Scholar]
- Kim, S.K.; Lee, G.H.; Jeon, C.; Han, H.H.; Kim, S.J.; Mok, J.W.; Joo, C.K.; Shin, S.; Sim, J.Y.; Myung, D.; et al. Bimetallic nanocatalysts immobilized in nanoporous hydrogels for long-term robust continuous glucose monitoring of smart contact lens. Adv. Mater. 2022, 34, 2110536. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Han, Y.K.; Sun, J.Y.; Zhang, Y.; Han, L. Dual nanocatalysts co-decorated three-dimensional, laser-induced graphene hybrid nanomaterials integrated with a smartphone portable electrochemical system for point-of-care non-enzymatic glucose diagnosis. Mater. Today Chem. 2022, 24, 100895. [Google Scholar] [CrossRef]
- Wu, J.; Tang, J.; Dai, Z.; Yan, F.; Ju, H.; El Murr, N. A disposable electrochemical immunosensor for flow injection immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 2006, 22, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, F.; Tang, J.H.; Zhai, C.; Ju, H.X. A disposable multianalyte electrochemical immunosensor array for automated simultaneous determination of tumor markers. Clin. Chem. 2007, 53, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, F.; Zhang, X.; Yan, Y.; Tang, J.; Ju, H. Disposable reagentless electrochemical immunosensor array based on a biopolymer/sol-gel membrane for simultaneous measurement of several tumor markers. Clin. Chem. 2008, 54, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-la-Villa, A.; Pozo-Ayuso, D.F.; Castano-Alvarez, M. Microfluidics and electrochemistry: An emerging tandem for next-generation analytical microsystems. Curr. Opin. Electrochem. 2019, 15, 175–185. [Google Scholar] [CrossRef]
- Kikkeri, K.; Wu, D.; Voldman, J. A sample-to-answer electrochemical biosensor system for biomarker detection. Lab Chip 2022, 22, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Koklu, A.; Wustoni, S.; Musteata, V.E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.P.; Inal, S. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-beta detection. ACS Nano 2021, 15, 8130–8141. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Luo, J.; Zhu, Y.; Kong, F.; Mao, G.; Ming, T.; Xing, Y.; Liu, J.; Dai, Y.; Yan, S.; et al. Multifunctional self-driven origami paper-based integrated microfluidic chip to detect CRP and PAB in whole blood. Biosens. Bioelectron. 2022, 208, 114225. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Su, J.; Xu, Y.; He, G.; Wang, C.; Wang, X.; Pan, T.; Ding, X.; Mi, X. DNA tetrahedron-mediated immune-sandwich assay for rapid and sensitive detection of PSA through a microfluidic electrochemical detection system. Microsyst. Nanoeng. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, J.; Kim, J.; Choi, H.S.; Kim, J.; Lee, S.; Lee, H. Single microfluidic electrochemical sensor system for simultaneous multi-pulmonary hypertension biomarker analyses. Sci. Rep. 2017, 7, 7545. [Google Scholar] [CrossRef]
- Pursey, J.P.; Chen, Y.; Stulz, E.; Park, M.K.; Kongsuphol, P. Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sens. Actuators B Chem. 2017, 251, 34–39. [Google Scholar] [CrossRef]
- Fragoso, A.; Latta, D.; Laboria, N.; von Germar, F.; Hansen-Hagge, T.E.; Kemmner, W.; Gärtner, C.; Klemm, R.; Drese, K.S.; O’Sullivan, C.K. Integrated microfluidic platform for the electrochemical detection of breast cancer markers in patient serum samples. Lab Chip 2011, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Chen, F.; Li, Q.; Wang, L.H.; Fan, C.H. Isothermal amplification of nucleic acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Simmel, F.C.; Yurke, B.; Singh, H.R. Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 2019, 119, 6326–6369. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Somoza, R.A.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the trans-cleavage activity of CRISPR-Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. Int. Ed. 2019, 58, 17399–17405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yan, Y.; Que, H.; Yang, T.; Cheng, X.; Ding, S.; Zhang, X.; Cheng, W. CRISPR/Cas12a-mediated interfacial cleaving of hairpin DNA reporter for electrochemical nucleic acid sensing. ACS Sens. 2020, 5, 557–562. [Google Scholar] [CrossRef]
- Hu, J.M.; Wang, T.Y.; Kim, J.; Shannon, C.; Easley, C.J. Quantitation of femtomolar protein levels via direct readout with the electrochemical proximity assay. J. Am. Chem. Soc. 2012, 134, 7066–7072. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, Y.; Brooks, J.C.; Godwin, L.A.; Somasundaram, S.; Torabinejad, F.; Kim, J.; Shannon, C.; Easley, C.J. A reusable electrochemical proximity assay for highly selective, real-time protein quantitation in biological matrices. J. Am. Chem. Soc. 2014, 136, 8467–8474. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.W.; Wu, J.; Yan, F.; Ju, H.X. Ratiometric electrochemical proximity assay for sensitive one-step protein detection. Sci. Rep. 2014, 4, 4360. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.W.; Wu, J.; Yan, F.; Zhang, Y.; Ju, H.X. Immunoreaction-triggered DNA assembly for one-step sensitive ratiometric electrochemical biosensing of protein biomarker. Biosens. Bioelectron. 2015, 66, 345–349. [Google Scholar] [CrossRef]
- Ren, K.W.; Wu, J.; Zhang, Y.; Yan, F.; Ju, H.X. Proximity hybridization regulated DNA biogate for sensitive electrochemical immunoassay. Anal. Chem. 2014, 86, 7494–7499. [Google Scholar] [CrossRef]
- Ren, K.W.; Wu, J.; Ju, H.X.; Yan, F. Target-driven triple-binder assembly of MNAzyme for amplified electrochemical immunosensing of protein biomarker. Anal. Chem. 2015, 87, 1694–1700. [Google Scholar] [CrossRef]
- Zhu, J.; Gan, H.Y.; Wu, J.; Ju, H.X. Molecular machine powered surface programmatic chain reaction for highly sensitive electrochemical detection of protein. Anal. Chem. 2018, 90, 5503–5508. [Google Scholar] [CrossRef]
- Man, Y.; Liu, J.; Wu, J.; Yin, L.; Pei, H.; Wu, Q.; Xia, Q.; Ju, H. An anchored monopodial DNA walker triggered by proximity hybridization for amplified amperometric biosensing of nucleic acid and protein. Anal. Chim. Acta 2020, 1107, 48–54. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef]
- Ding, J.W.; Chen, Y.; Wang, X.W.; Qin, W. Label-free and substrate-free potentiometric aptasensing using polycation-sensitive membrane electrodes. Anal. Chem. 2012, 84, 2055–2061. [Google Scholar] [CrossRef]
- Nurlely, A.M.; Heng, L.Y.; Tan, L.L. Potentiometric enzyme biosensor for rapid determination of formaldehyde based on succinimide-functionalized polyacrylate ion-selective membrane. Measurement 2021, 175, 109112. [Google Scholar] [CrossRef]
- Özbek, O.; Berkel, C.; Isildak, Ö.; Isildak, I. Potentiometric urea biosensors. Clin. Chim. Acta 2022, 524, 154–163. [Google Scholar] [CrossRef]
- Wu, J.; Chumbimuni-Torres, K.Y.; Galik, M.; Thammakhet, C.; Haake, D.A.; Wang, J. Potentiometric detection of DNA hybridization using enzyme-induced metallization and a silver ion selective electrode. Anal. Chem. 2009, 81, 10007–10012. [Google Scholar] [CrossRef]
- Ding, J.W.; Li, B.W.; Chen, L.X.; Qin, W. A three-dimensional origami paper-based device for potentiometric biosensing. Angew. Chem. Int. Ed. 2016, 55, 13033–13037. [Google Scholar] [CrossRef]
- Tang, H.; Yan, F.; Lin, P.; Xu, J.B.; Chan, H.L.W. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272. [Google Scholar] [CrossRef]
- Mak, C.H.; Liao, C.; Fu, Y.; Zhang, M.; Tang, C.Y.; Tsang, Y.H.; Chan, H.L.; Yan, F. Highly-sensitive epinephrine sensors based on organic electrochemical transistors with carbon nanomaterial modified gate electrodes. J. Mater. Chem. C 2015, 3, 6532–6538. [Google Scholar] [CrossRef]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Luo, X.T.; Hsing, I.M.; Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, N.; Yang, A.; Law, H.K.W.; Li, L.; Yan, F. Highly sensitive detection of protein biomarkers with organic electrochemical transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, N.E.; Park, J.S.; Park, I.J.; Kim, J.G.; Cho, H.J. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- He, R.X.; Zhang, M.; Tan, F.; Leung, P.H.; Zhao, X.Z.; Chan, H.L.; Yang, M.; Yan, F. Detection of bacteria with organic electrochemical transistors. J. Mater. Chem. 2012, 22, 22072–22076. [Google Scholar] [CrossRef]
- Lin, B.P.; Yan, F.; Yu, J.J.; Chan, H.L.W.; Yang, M. The application of organic electrochemical transistors in cell-based biosensors. Adv. Mater. 2010, 22, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, Y.; Wang, N.; Yang, A.; Li, Y.; Wu, J.; Ju, H.; Yan, F. Organic electrochemical transistors for the detection of cell surface glycans. ACS Appl. Mater. Interfaces 2018, 10, 18470–18477. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Wang, N.X.; Wu, J.; Yan, F.; Ju, H.X. Organic electrochemical transistor for sensing of sialic acid in serum samples. Anal. Chim. Acta 2020, 1128, 231–237. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wu, J.; Yan, F.; Ju, H.X. A facile strategy for quantitative sensing of glycans on cell surface using organic electrochemical transistors. Biosens. Bioelectron. 2021, 175, 112878. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wu, J.; Yan, F.; Ju, H.X. Monose-modified organic electrochemical transistors for cell surface glycan analysis via competitive recognition to enzyme-labeled lectin. Mikrochim. Acta 2021, 188, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 2019, 52, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Li, Y.; Yang, C.; Fu, Y.; Wang, N.; Li, L.; Yan, F. Fabric organic electrochemical transistors for biosensors. Adv. Mater. 2018, 30, 1800051. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lei, J.P.; Ju, H.X. Principles and applications of photoelectrochemical sensing strategies based on biofunctionalized nanostructures. Biosens. Bioelectron. 2017, 96, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.F.; Chen, F.Z.; Xu, Y.T.; Zhang, T.Y.; Yu, S.Y.; Zhao, W.W.; Jiang, D.; Chen, H.Y.; Xu, J.J. An integrated photoelectrochemical nanotool for intracellular drug delivery and evaluation of treatment effect. Angew. Chem. Int. Ed. 2021, 133, 25966–25969. [Google Scholar] [CrossRef]
- Hu, F.X.; Miao, J.W.; Guo, C.X.; Yang, H.B.; Liu, B. Real-time photoelectrochemical quantification of hydrogen peroxide produced by living cells. Chem. Eng. J. 2021, 407, 127203. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Kong, Y.; Dai, M.; Han, D.; Liu, Z. FRET modulated signaling: A versatile strategy to construct photoelectrochemical microsensors for in vivo analysis. Angew. Chem. Int. Ed. 2021, 60, 11774–11778. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.L.; Zhang, C.X. Electrogenerated chemiluminescence biosensing. Anal. Chem. 2020, 92, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Ding, Z.F. Highly sensitive analysis strategies of microRNAs based on electrochemiluminescence. Curr. Opin. Electrochem. 2022, 32, 100901. [Google Scholar] [CrossRef]
- Miao, W.J. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Delaney, J.L.; Hogan, C.F.; Tian, J.; Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 2011, 83, 1300–1306. [Google Scholar] [CrossRef]
- Kerr, E.; Farr, R.; Doeven, E.H.; Nai, Y.H.; Alexander, R.; Guijt, R.M.; Prieto-Simon, B.; Francis, P.S.; Dearnley, M.; Hayne, D.J.; et al. Amplification-free electrochemiluminescence molecular beacon-based microRNA sensing using a mobile phone for detection. Sens. Actuators B Chem. 2021, 330, 129261. [Google Scholar] [CrossRef]
- Deiss, F.; LaFratta, C.N.; Symer, M.; Blicharz, T.M.; Sojic, N.; Walt, D.R. Multiplexed sandwich immunoassays using electrochemiluminescence imaging resolved at the single bead level. J. Am. Chem. Soc. 2009, 131, 6088–6089. [Google Scholar] [CrossRef]
- Wang, N.N.; Chen, L.Z.; Chen, W.W.; Ju, H.X. Potential-and color-resolved electrochemiluminescence of polymer dots for array imaging of multiplex microRNAs. Anal. Chem. 2021, 93, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Cao, Y.; Gou, X.D.; Zhu, J.J. Recent progress in electrochemiluminescence sensing and imaging. Anal. Chem. 2020, 92, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, H.; Li, Y.; Li, G.; Chen, W.; Jin, Z.; Lei, J.; Wei, Q.; Ju, H. Dual intramolecular electron transfer for in situ coreactant-embedded electrochemiluminescence microimaging of membrane protein. Angew. Chem. Int. Ed. 2021, 60, 197–201. [Google Scholar] [CrossRef]
- Wang, N.; Ao, H.; Xiao, W.; Chen, W.; Li, G.; Wu, J.; Ju, H. Confined electrochemiluminescence imaging microarray for high-throughput biosensing of single cell-released dopamine. Biosens. Bioelectron. 2022, 201, 113959. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Nagata, R.; Yokoyama, K.; Clark, S.A.; Karube, I. A glucose sensor fabricated by the screen printing technique. Biosens. Bioelectron. 1995, 10, 261–267. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose meters: A review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Karon, B.S.; Boyd, J.C.; Klee, G.G. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin. Chem. 2010, 56, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 2011, 3, 697–703. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y. Biocomputing for portable, resettable, and quantitative point-of-care diagnostics: Making the glucose meter a logic-gate responsive device for measuring many clinically relevant targets. Angew. Chem. Int. Ed. 2018, 57, 9702–9706. [Google Scholar] [CrossRef] [PubMed]
- Amalfitano, E.; Karlikow, M.; Norouzi, M.; Jaenes, K.; Cicek, S.; Masum, F.; Sadat Mousavi, P.; Guo, Y.; Tang, L.; Sydor, A.; et al. A glucose meter interface for point-of-care gene circuit-based diagnostics. Nat. Commun. 2021, 12, 724. [Google Scholar] [CrossRef]
- Zhang, X.; Dhawane, A.N.; Sweeney, J.; He, Y.; Vasireddi, M. Electrochemical assay to detect influenza viruses and measure drug susceptibility. Angew. Chem. Int. Ed. 2015, 54, 5929–5932. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Park, K.S.; Park, H.G. A personal glucose meter for label-free and washing-free biomolecular detection. Anal. Chem. 2018, 90, 11340–11343. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Ray, P.; Carlin, A.F.; Magallanes, C.; Morgan, S.C.; Laurent, L.C.; Aronoff-Spencer, E.S.; Hall, D.A. Hitting the diagnostic sweet spot: Point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer. Biosens. Bioelectron. 2021, 180, 113111. [Google Scholar] [CrossRef]
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Chen, Y.; Ho, N.R.; Sundah, N.R.; Natalia, A.; Liu, Y.; Miow, Q.H.; Wang, Y.; Tambyah, P.A.; et al. Accessible detection of SARS-CoV-2 through molecular nanostructures and automated microfluidics. Biosens. Bioelectron. 2021, 194, 113629. [Google Scholar] [CrossRef]
- Gong, M.M.; Sinton, D. Turning the page: Advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiang, Y.; Lu, Y.; Crooks, R.M. Aptamer-based origami paper analytical device for electrochemical detection of adenosine. Angew. Chem. Int. Ed. 2012, 51, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, S.; Dou, Y.; Zhao, Z.; Jia, X.; Ding, X.; Song, S. Smartphone-based electrochemical biosensors for directly detecting serum-derived exosomes and monitoring their secretion. Anal. Chem. 2022, 94, 3235–3244. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H. Smartphone-powered electrochemical biosensing dongle for emerging medical IoTs application. IEEE T. Ind. Inform. 2018, 14, 2592–2597. [Google Scholar] [CrossRef]
- Ainla, A.; Mousavi, M.P.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernandez-Abedul, M.T.; Whitesides, G.M. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Avila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, K.; Chen, D.; Shen, G.Z. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy 2019, 58, 624–632. [Google Scholar] [CrossRef]
- Bocu, R.; Costache, C. A homomorphic encryption-based system for securely managing personal health metrics data. IBM J. Res. Dev. 2018, 62, 1:1–1:10. [Google Scholar] [CrossRef]
- Quinton, P.M. Sweating and its disorders. Annu. Rev. Med. 1983, 34, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kang, D.; Han, S.; Kim, S.B.; Rogers, J.A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 2017, 6, 1601355. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Yin, L.; Nathan, A.; Wang, J.; Dahiya, R. Energy autonomous sweat-based wearable systems. Adv. Mater. 2021, 33, 2100899. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, L.L.; Shen, G.Z. Recent advances in smart wearable sensing systems. Adv. Mater. Technol. 2018, 3, 1800444. [Google Scholar] [CrossRef]
- Fu, K.; Zhou, J.; Wu, H.U.; Su, Z.Q. Fibrous self-powered sensor with high stretchability for physiological information monitoring. Nano Energy 2021, 88, 106258. [Google Scholar] [CrossRef]
- Jia, W.; Valdes-Ramirez, G.; Bandodkar, A.J.; Windmiller, J.R.; Wang, J. Epidermal biofuel cells: Energy harvesting from human perspiration. Angew. Chem. Int. Ed. 2013, 52, 7233–7236. [Google Scholar] [CrossRef]
- Falk, M.; Andoralov, V.; Silow, M.; Toscano, M.D.; Shleev, S. Miniature biofuel cell as a potential power source for glucose-sensing contact lenses. Anal. Chem. 2013, 85, 6342–6348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xi, J.; Huang, W.; Yuen, M.M.F. Stretchable conductive elastomer for wireless wearable communication applications. Sci. Rep. 2017, 7, 10958. [Google Scholar] [CrossRef] [PubMed]
- Lukocius, R.; Vaitkunas, M.; Virbalis, J.A.; Dosinas, A.; Vegys, A. Physiological parameters monitoring system for occupational safety. Elektron. Elektrotech. 2014, 20, 57–60. [Google Scholar] [CrossRef]
- Bujes-Garrido, J.; Arcos-Martínez, M.J. Development of a wearable electrochemical sensor for voltammetric determination of chloride ions. Sens. Actuators B Chem. 2017, 240, 224–228. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for continuous glucose monitoring: Current problems and future promises. J. Diabetes Sci. Technol. 2010, 4, 1540–1562. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, T.; Schuhmann, W. Long-term implantable glucose biosensors. Curr. Opin. Electrochem. 2018, 10, 112–119. [Google Scholar] [CrossRef]
- Dalrymple, A.N. Implanted devices: The importance of both electrochemical performance and biological acceptance. Neural Regen. Res. 2021, 16, 1188–1189. [Google Scholar] [CrossRef]
- Kotanen, C.N.; Moussy, F.G.; Carrara, S.; Guiseppi-Elie, A. Implantable enzyme amperometric biosensors. Biosens. Bioelectron. 2012, 35, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Tennen, H.; Wolpert, H. Continuous glucose monitoring: A review for behavioral researchers. Psychosom. Med. 2012, 74, 356–365. [Google Scholar] [CrossRef]
- McGarraugh, G. The chemistry of commercial continuous glucose monitors. Diabetes Technol. Ther. 2009, 11, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian-Ardakani, Z.; Hosu, O.; Cristea, C.; Mazloum-Ardakani, M.; Marrazza, G. Latest trends in electrochemical sensors for neurotransmitters: A review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef] [PubMed]
- Seaton, B.T.; Heien, M.L. Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo. Anal. Bioanal. Chem. 2021, 413, 6689–6701. [Google Scholar] [CrossRef]
- Wickramasinghe, Y.; Yang, Y.; Spencer, S.A. Current problems and potential techniques in in vivo glucose monitoring. J. Fluoresc. 2004, 14, 513–520. [Google Scholar] [CrossRef]
- Nichols, S.P.; Koh, A.; Storm, W.L.; Shin, J.H.; Schoenfisch, M.H. Biocompatible materials for continuous glucose monitoring devices. Chem. Rev. 2013, 113, 2528–2549. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Merricks, E.P.; Wimsey, L.E.; Nichols, T.C.; Schoenfisch, M.H. Long-term accurate continuous glucose biosensors via extended nitric oxide release. ACS Sens. 2019, 4, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Ben Amar, A.; Kouki, A.B.; Cao, H. Power approaches for implantable medical devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Barbosa, A.I.; Correlo, V.M.; Reis, R.L. An outlook on implantable biosensors for personalized medicine. Engineering 2021, 7, 1696–1699. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.J.; Liu, J. Suitability of a thermoelectric power generator for implantable medical electronic devices. J. Phys. D Appl. Phys. 2007, 40, 5790–5800. [Google Scholar] [CrossRef]
- Simons, P.; Schenk, S.A.; Gysel, M.A.; Olbrich, L.F.; Rupp, J.L.M. A ceramic-electrolyte glucose fuel cell for implantable electronics. Adv. Mater. 2022, 34, 2109075. [Google Scholar] [CrossRef] [PubMed]
- Scholten, K.; Meng, E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 2018, 544, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef]
- Kutner, N.; Kunduru, K.R.; Rizik, L.; Farah, S. Recent advances for improving functionality, biocompatibility, and longevity of implantable medical devices and deliverable drug delivery systems. Adv. Funct. Mater. 2021, 31, 2010929. [Google Scholar] [CrossRef]
- Hsieh, K.; Ferguson, B.S.; Eisenstein, M.; Plaxco, K.W.; Soh, H.T. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015, 48, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, T.; Lu, Y. Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. Trends Anal. Chem. 2020, 124, 115782. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, C.; Tang, Y.T.; Ho, C.; Parameswaran, M.A.; Yu, H.Z. A USB-based electrochemical biosensor prototype for point-of-care diagnosis. Sens. Actuators B Chem. 2012, 161, 908–913. [Google Scholar] [CrossRef]
- Tu, J.B.; Torrente-Rodriguez, R.M.; Wang, M.Q.; Gao, W. The era of digital health: A review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- Gordon, W.J.; Stern, A.D. Challenges and opportunities in software-driven medical devices. Nat. Biomed. Eng. 2019, 3, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, C. Wearable electrochemical sensors for noninvasive monitoring of health-a perspective. Curr. Opin. Electrochem. 2020, 23, 42–46. [Google Scholar] [CrossRef]
- Dixon, A.M.; Allstot, E.G.; Gangopadhyay, D.; Allstot, D.J. Compressed sensing system considerations for ECG and EMG wireless biosensors. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 156–166. [Google Scholar] [CrossRef]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lin, P.; Chan, H.L.W.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563. [Google Scholar] [CrossRef]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.K.; et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ni, X.; Lee, J.Y.; Arafa, H.; Pe, D.J.; Xu, S.; Avila, R.; Irie, M.; Lee, J.H.; Easterlin, R.L.; et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 2020, 4, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lee, G.H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.R.; Park, S. From diagnosis to treatment: Recent advances in patient-friendly biosensors and implantable devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Dong, H.; Cao, X.; Luong, J.H.; Zhang, X. Implantable electrochemical sensors for biomedical and clinical applications: Progress, problems, and future possibilities. Curr. Med. Chem. 2007, 14, 937–951. [Google Scholar] [PubMed]
- Xu, J.; Cheng, C.; Li, X.; Lu, Y.; Hu, S.; Liu, G.; Zhu, L.; Wang, N.; Wang, L.; Cheng, P.; et al. Implantable platinum nanotree microelectrode with a battery-free electrochemical patch for peritoneal carcinomatosis monitoring. Biosens. Bioelectron. 2021, 185, 113265. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandey, S.K.; Singh, J.; Srivastava, S.; Sachan, S.; Singh, S.K. Biomedical perspective of electrochemical nanobiosensor. Nanomicro Lett. 2016, 8, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.M.; Bode, B.W.; Einhorn, D.; Kayne, D.M.; Reed, J.H.; White, N.H.; Mastrototaro, J.J. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol. Ther. 2000, 2, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.J.; Sandberg, S.G.; Wanat, M.J.; Gan, J.O.; Horne, E.A.; Hart, A.S.; Akers, C.A.; Parker, J.G.; Willuhn, I.; Martinez, V.; et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 2010, 7, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Goud, K.Y.; Raza, N.; Ballesteros, E.; Lee, S.E.; Hong, J.; Deep, A.; Kim, K.H. Nanomaterial-based electrochemical sensors for the detection of neurochemicals in biological matrices. Trends Anal. Chem. 2019, 110, 15–34. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Yuan, L.; Zhang, B.; Bishop, E.S.; Wang, K.; Tang, J.; Zheng, Y.Q.; Xu, W.; Niu, S.; et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 2022, 606, 94–101. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, B.; Fan, F.; Wang, X.; Yan, L.; Yuan, W.; Wang, S.; Liu, H.; Li, Z.; Wang, Z.L. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator. Adv. Mater. 2014, 26, 5851–5856. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.T.; Park, H.; Lee, J.H.; Oh, S.; Park, K.I.; Byun, M.; Ahn, G.; Jeong, C.K.; No, K.; Kwon, H.; et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv. Mater. 2014, 26, 4880–4887. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocu, R. Extended Review Concerning the Integration of Electrochemical Biosensors into Modern IoT and Wearable Devices. Biosensors 2024, 14, 214. https://doi.org/10.3390/bios14050214
Bocu R. Extended Review Concerning the Integration of Electrochemical Biosensors into Modern IoT and Wearable Devices. Biosensors. 2024; 14(5):214. https://doi.org/10.3390/bios14050214
Chicago/Turabian StyleBocu, Razvan. 2024. "Extended Review Concerning the Integration of Electrochemical Biosensors into Modern IoT and Wearable Devices" Biosensors 14, no. 5: 214. https://doi.org/10.3390/bios14050214
APA StyleBocu, R. (2024). Extended Review Concerning the Integration of Electrochemical Biosensors into Modern IoT and Wearable Devices. Biosensors, 14(5), 214. https://doi.org/10.3390/bios14050214




