Development of Non-Invasive Biosensors for Neonatal Jaundice Detection: A Review
Abstract
:1. Introduction
2. Conventional Diagnostic Techniques for Neonatal Jaundice Detection
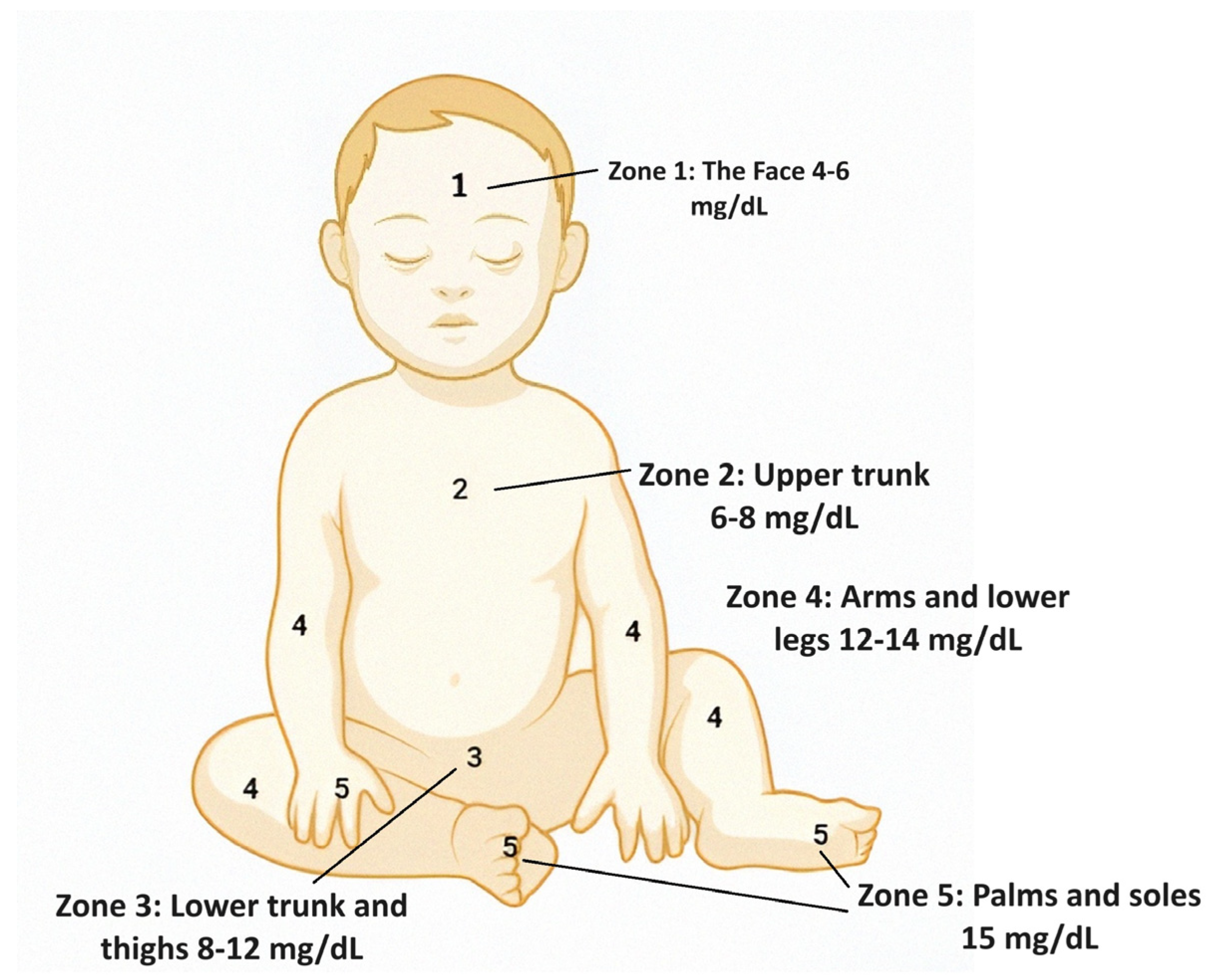
2.1. Visual Assessments
2.2. Total Serum Bilirubin
2.2.1. The Diazo Method
2.2.2. High Pressure Liquid Chromatography
2.2.3. The Enzyme-Based Method
2.2.4. Fluorescence Spectroscopy
2.3. Transcutaneous Bilirubinometer
3. Biosensors for the Detection of Neonatal Jaundice
3.1. Invasive Biosensors
3.1.1. Electrochemical Biosensors
3.1.2. Optical Biosensors
3.1.3. Piezoelectric Biosensors
3.2. Non-Invasive Biosensors
Optical Biosensors
4. Challenges and Future Scope
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| HRP | Horseradish peroxidase |
| HPLC | High-pressure liquid chromatography |
| TSB | Total serum bilirubin |
| MWCNT | Multi-walled carbon nanotubes |
| TcB | Transcutaneous bilirubinometer |
| DNA | Deoxyribonucleic acid |
| AgNP | Silver nanoparticle |
| LOD | Limit of detection |
| LED | Light-emitting diode |
References
- Nasser Bernaldo, A.J.; de Mattos Segre, C.A. Bilirubin Dosage in Cord Blood: Could It Predict Neonatal Hyperbilirubinemia? Sao Paulo Med. J. 2004, 122, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kalakonda, A.; Jenkins, B.A.; John, S. Physiology, Bilirubin. StatPearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470290/ (accessed on 8 May 2024).
- Shirzadfar, H.; Sheikhi, K.; Meschian, Z. The Epidemiologic Study of Neonatal Jaundice, Relation between Jaundice and Liver and Alternative Methods to Cure Jaundice. Clin. Pract. 2019, 16, 1117–1125. [Google Scholar] [CrossRef]
- Asefa, G.G.; Gebrewahid, T.G.; Nuguse, H.; Gebremichael, M.W.; Birhane, M.; Zereabruk, K.; Zemicheal, T.M.; Hailay, A.; Abrha, W.A.; Hadera, S.A.; et al. Determinants of Neonatal Jaundice among Neonates Admitted to Neonatal Intensive Care Unit in Public General Hospitals of Central Zone, Tigray, Northern Ethiopia, 2019: A Case-Control Study. Biomed Res. Int. 2020, 2020, 4743974. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Hamoud, A.; Stec, D.E.; Hinds, T.D., Jr. Biliverdin Reductase and Bilirubin in Hepatic Disease. Am. J. Physiol. Liver Physiol. 2018, 314, G668–G676. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Koritala, T.; Jialal, I. Unconjugated Hyperbilirubinemia. In Evidence-Based Paediatrics; Wiley: Hoboken, NJ, USA, 2023; pp. 491–498. [Google Scholar] [CrossRef]
- Wolkoff, A.W.; Berk, P.D. Bilirubin Metabolism and Jaundice. In Schiff’s Diseases of the Liver; Wiley: Hoboken, NJ, USA, 2017; pp. 103–134. ISBN 9781119251316. [Google Scholar]
- Čvorović, J.; Passamonti, S. Membrane Transporters for Bilirubin and Its Conjugates: A Systematic Review. Front. Pharmacol. 2017, 8, 308242. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, A.-R.; Weaver, L.; Stec, D.E.; Hinds, T.D. Bilirubin in the Liver–Gut Signaling Axis. Trends Endocrinol. Metab. 2018, 29, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Biliverdin-Wikipedia. Available online: https://en.wikipedia.org/wiki/Biliverdin (accessed on 8 May 2024).
- Markovic, A.P.; Stojkovic Lalosevic, M.; Mijac, D.D.; Milovanovic, T.; Dragasevic, S.; Sokic Milutinovic, A.; Krstic, M.N. Jaundice as a Diagnostic and Therapeutic Problem: A General Practitioner’s Approach. Dig. Dis. 2022, 40, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Al Sampath Kumar, K.P.; Pharma, T.; Sampath Kumar, K.P.; Bhowmik, D. The Pharma Research, a Journal Jaundice-Review of Clinical Features, Differential Diagnosis and Remedies. Res. (T. Ph. Res.) 2010, 4–6. Available online: https://www.researchgate.net/profile/Debjit-Bhowmik-3/publication/290889408_Jaundice-review_of_clinical_features_differential_diagnosis_and_remedies/links/5b34c2ec4585150d23dc9273/Jaundice-review-of-clinical-features-differential-diagnosis-and-remedies.pdf (accessed on 8 May 2024).
- Tripathi, N.; Jialal, I. Conjugated Hyperbilirubinemia. Paediatr. Child Health 2023, 18, 474–476. [Google Scholar] [CrossRef]
- Bilirubin Blood Test: Procedure, Preparation, and Risks. Available online: https://www.healthline.com/health/bilirubin-blood#procedure (accessed on 8 May 2024).
- Bilirubin Metabolism-UpToDate. Available online: https://www.uptodate.com/contents/bilirubin-metabolism (accessed on 28 August 2023).
- Conjugated Hyperbilirubinemia-StatPearls-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562172/ (accessed on 8 May 2024).
- Hansen, T.W.R. Bilirubin Brain Toxicity. J. Perinatol. 2001, 21, S48–S51. [Google Scholar] [CrossRef]
- Gallango, M.D.L.; Montiel, N.; Suinaga, R. Human Albumin from Cord Blood: Some Distinctive Characteristics. Biochem. Med. 1981, 26, 127–134. [Google Scholar] [CrossRef]
- Pathak, N.N.; Deka, A.; Arvind, P. Cord Blood Albumin, a Tool as Predictor of Neonatal Hyperbilirubinemia Requiring Intervention. New Indian J. OBGYN 2020, 7, 93–96. [Google Scholar] [CrossRef]
- Sapkota, P.; Gami, F.C. Study of Cord Blood Albumin as a Predictor of Neonatal Jaundice. Asian J. Med. Sci. 2020, 11, 58. [Google Scholar] [CrossRef]
- Meena, J.K.; Singh, S.; Verma, C.R.; Sharma, R. Utility of Cord Blood Albumin as a Predictor of Significant Neonatal Jaundice in Healthy Term Newborns. Pediatr. Oncall 2015, 12, 99–102. [Google Scholar] [CrossRef]
- Medscape Registration. Available online: https://emedicine.medscape.com/article/2054430-overview?form=fpf (accessed on 21 November 2023).
- Santhanam, P. Pediatrics 2018: Haptoglobin in Cord Blood—A Biomarker to Predict Neonatal Jaundice-Prathipa Santhanam-Brookdale University Hospital and Medical. Clin. Pediatr. Dermatol. 2018, 4, 2018. [Google Scholar]
- Haptoglobin Test: Purpose, Procedure, and Results. Available online: https://www.healthline.com/health/haptoglobin (accessed on 21 November 2023).
- Kim, E.E.; Wyckoff, H.W. Reaction Mechanism of Alkaline Phosphatase Based on Crystal Structures: Two-Metal Ion Catalysis. J. Mol. Biol. 1991, 218, 449–464. [Google Scholar] [CrossRef] [PubMed]
- What Is Alkaline Phosphatase? Function & Normal Range-SelfDecode Labs. Available online: https://labs.selfdecode.com/blog/alkaline-phosphatase-normal/ (accessed on 21 November 2023).
- Slusher, T.M.; Zipursky, A.; Bhutani, V.K. A Global Need for Affordable Neonatal Jaundice Technologies. Seminars in Perinatology 2011, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.; Kharangarh, P.R.; Dawra, S.; Tomar, M.; Gupta, V.; Pundir, C.S. A Comprehensive Review of Bilirubin Determination Methods with Special Emphasis on Biosensors. Process Biochem. 2020, 89, 165–174. [Google Scholar] [CrossRef]
- Abiha, U.; Banerjee, D.S.; Mandal, S. Demystifying Non-Invasive Approaches for Screening Jaundice in Low Resource Settings: A Review. Front. Pediatr. 2023, 11, 1292678. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P. Personalized Biosensors for Point-of-Care Diagnostics: From Bench to Bedside Applications. Nanotheranostics 2023, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.A.; Vo, G.N.L.; Vo, T.-T.T.; Doan, V.D.; Vo, V.; Le, V.T. Recent Applications and Prospects of Nanowire-Based Biosensors. Processes 2023, 11, 1739. [Google Scholar] [CrossRef]
- Yuan, M.; Long, Y.; Liu, T.; Liu, J.; Qiu, S.; Lin, T.; Xu, F.; Fang, Y. Soft Electronics for Advanced Infant Monitoring. Mater. Today 2024, 59, 332–352. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, C.V.; Vitek, L.; Coda Zabetta, C.D.; Dvořák, A.; Schenk, P.; van der Hagen, E.A.E.; Cobbaert, C.; Tiribelli, C. Screening Methods for Neonatal Hyperbilirubinemia: Benefits, Limitations, Requirements, and Novel Developments. Pediatr. Res. 2021, 90, 272–276. [Google Scholar] [CrossRef]
- Thomas, M.; Greaves, R.F.; Tingay, D.G.; Loh, T.P.; Ignjatovic, V.; Newall, F.; Oeum, M.; Tran, M.T.C.; Rajapaksa, A.E. Current and Emerging Technologies for the Timely Screening and Diagnosis of Neonatal Jaundice. Crit. Rev. Clin. Lab. Sci. 2022, 59, 332–352. [Google Scholar] [CrossRef]
- Kramer, L.I. Advancement of Dermal Icterus in the Jaundiced Newborn. Am. J. Dis. Child. 1969, 118, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Kumar, V.; Sharma, A.; Sankar, M.J.; Thukral, A.; Verma, A.; Agarwal, R. Modified Kramer’s versus Kramer’s Method for Clinical Assessment of Jaundice in Term and Near-Term Neonates. Indian J. Pediatr. 2024, 1–7. [Google Scholar] [CrossRef]
- Dizgah, M.; Bilirubin, V.M.; Hosein, M.; Reza, M.; Mirzaii-Dizgah, I.; Lachinani, H.; Dormanesh, B.; Veisizadeh, M. Bilirubin in Saliva: A Potential Biomarker for Detecting Neonatal Jaundice. Avicenna J. Dent. Res. 2019, 11, 79–82. [Google Scholar] [CrossRef]
- Puppalwar, D.P.V. Review on “Evolution of Methods of Bilirubin Estimation”. IOSR J. Dent. Med. Sci. 2012, 1, 17–28. [Google Scholar] [CrossRef]
- Ngashangva, L.; Bachu, V.; Goswami, P. Development of New Methods for Determination of Bilirubin. J. Pharm. Biomed. Anal. 2019, 162, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.T.; Wu, T.-W. The Measurement of Bilirubin Fractions in Serum. Crit. Rev. Clin. Lab. Sci. 1991, 28, 415–445. [Google Scholar] [CrossRef]
- Akmal Dzulkifli, F.; Yusoff Mashor, M.; Khalid, K. Methods for Determining Bilirubin Level in Neonatal Jaundice Screening and Monitoring: A Literature Review Automated Ki67 Counting for Brain Tumour Grading View Project Paediatrics and Child Health View Project Methods for Determining Bilirubin Level in N. J. Eng. Res. Educ. 2018, 10, 1–10. [Google Scholar]
- Mano, N.; Edembe, L. Bilirubin Oxidases in Bioelectrochemistry: Features and Recent Findings. Biosens. Bioelectron. 2013, 50, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Iyama, S.; Yamaguchi, Y.; Hayashi, S.; Yanagihara, T. Enzymatic Assay for Conjugated Bilirubin (Bc) in Serum Using Bilirubin Oxidase (BOD). J. Clin. Lab. Anal. 1999, 13, 219–223. [Google Scholar] [CrossRef]
- Wang, J.; Ozsoz, M. A Polishable Amperometric Biosensor for Bilirubin. Electroanalysis 1990, 2, 647–650. [Google Scholar] [CrossRef]
- Zucker, S.D.; Goessling, W. Mechanism of Hepatocellular Uptake of Albumin-Bound Bilirubin. Biochim. Biophys. Acta (BBA)-Biomembranes 2000, 1463, 197–208. [Google Scholar] [CrossRef]
- Dani, C.; Hulzebos, C.V.; Tiribelli, C. Transcutaneous Bilirubin Measurements: Useful, but Also Reproducible? Pediatr. Res. 2021, 89, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, S.; Singh, N.; Sitaraman, S.; Choudhary, R.; Meena, K.K.; Bairwa, G.S.; Bairwa, M.; Jeevan, A. Efficacy of Transcutaneous Bilirubinometry as Compared to Serum Bilirubin in Preterm Newborn during Phototherapy. Eur. J. Pediatr. 2021, 180, 2629–2636. [Google Scholar] [CrossRef]
- Vallinayagam, S.; Paladhi, A.G.; Pal, K.; Kyzas, G.Z. Multifunctional Biosensor Activities in Food Technology, Microbes and Toxins–A Systematic Mini Review. Process Biochem. 2022, 120, 260–264. [Google Scholar] [CrossRef]
- De Vicente, J.; Lavín, Á.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaría, B.; Quintero, S.; Hernández, A.L.; Ramírez, Y. The Uncertainty and Limit of Detection in Biosensors from Immunoassays. Meas. Sci. Technol. 2020, 31, 44004. [Google Scholar] [CrossRef]
- Ali, J.; Najeeb, J.; Asim Ali, M.; Farhan Aslam, M.; Raza, A. Biosensors: Their Fundamentals, Designs, Types and Most Recent Impactful Applications: A Review. J. Biosens. Bioelectron. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Kaur, H.; Bhosale, A. Biosensors: Classification, Fundamental Characterization and New Trends: A Review. Int. J. Health Sci. Res. 2018, 8, 315–333. [Google Scholar]
- Skládal, P. Piezoelectric Biosensors. TrAC-Trends Anal. Chem. 2016, 79, 127–133. [Google Scholar] [CrossRef]
- Dey, D.; Goswami, T. Optical Biosensors: A Revolution towards Quantum Nanoscale Electronics Device Fabrication. J. Biomed. Biotechnol. 2011, 2011, 348218. [Google Scholar] [CrossRef] [PubMed]
- Biosensors-Classification, Characterization and New Trends. Available online: https://www.researchgate.net/publication/284037145_Biosensors-classification_characterization_and_new_trends (accessed on 8 May 2024).
- Hooda, V.; Gahlaut, A.; Gothwal, A.; Hooda, V. Bilirubin Enzyme Biosensor: Potentiality and Recent Advances towards Clinical Bioanalysis. Biotechnol. Lett. 2017, 39, 1453–1462. [Google Scholar] [CrossRef]
- Narwal, V.; Batra, B.; Kalra, V.; Jalandra, R.; Ahlawat, J.; Hooda, R.; Sharma, M.; Rana, J.S. Bilirubin Detection by Different Methods with Special Emphasis on Biosensing: A Review. Sens. Bio-Sensing Res. 2021, 33, 100436. [Google Scholar] [CrossRef]
- Anzar, N.; Suleman, S.; Kumar, R.; Rawal, R.; Pundir, C.S.; Pilloton, R.; Narang, J. Electrochemical Sensor for Bilirubin Detection Using Paper-Based Screen-Printed Electrodes Functionalized with Silver Nanoparticles. Micromachines 2022, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Thangamuthu, M.; Gabriel, W.E.; Santschi, C.; Martin, O.J.F. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; Mousavi, M.P.S.; Abd El-Rahman, M.K.; Tan, E.K.W.; Homer-Vanniasinkam, S.; Whitesides, G.M. Paper-Based Potentiometric Sensing of Free Bilirubin in Blood Serum. Biosens. Bioelectron. 2019, 126, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, S.; Schulte, A.; Neugebauer, S.; Schuhmann, W. Amperometric Biosensors. Adv. Electrochem. Sci. Eng. Bioelectrochemistry 2011, 13, 1–83. [Google Scholar]
- Bollella, P. Enzyme-Based Amperometric Biosensors: 60 Years Later… Quo Vadis? Anal. Chim. Acta 2022, 1234, 340517. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Sunny; Rana, J.S.; Pundir, C.S. Construction of an Amperometric Bilirubin Biosensor Based on Covalent Immobilization of Bilirubin Oxidase onto Zirconia Coated Silica Nanoparticles/Chitosan Hybrid Film. Biosens. Bioelectron. 2013, 44, 64–69. [Google Scholar] [CrossRef]
- Li, X.; Fortuney, A.; Guilbault, G.G.; Suleiman, A.A. Determination of Bilirubin by Fiberoptic Biosensor. Anal. Lett. 1996, 29, 171–180. [Google Scholar] [CrossRef]
- Mohamad, M.; Manap, H. The Optimal Absorption of Bilirubin Using an Optical Fibre Sensor. ARPN J. Eng. Appl. Sci. 2015, 10, 8762–8764. [Google Scholar]
- Gupta, A.K.; Yadav, A.; Koch, P.; Mishra, P. Piezoelectric Biosensors: Principle, Techniques, and Their Application in Food Analysis. In Biosensors in Food Safety and Quality; CRC Press: Boca Raton, FL, USA, 2022; pp. 37–46. [Google Scholar]
- Yang, Z.; Zhang, C. Molecularly Imprinted Hydroxyapatite Thin Film for Bilirubin Recognition. Biosens. Bioelectron. 2011, 29, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, J.; Zhang, C. Piezoelectric Detection of Bilirubin Based on Bilirubin-Imprinted Titania Film Electrode. Anal. Biochem. 2012, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Resmi, P.E.; Raveendran, J.; Suneesh, P.V.; Ramanchandran, T.; Nair, B.G.; Satheesh Babu, T.G. Screen-Printed Carbon Electrode for the Electrochemical Detection of Conjugated Bilirubin. Mater. Lett. 2021, 304, 130574. [Google Scholar] [CrossRef]
- Inamori, G.; Kamoto, U.; Nakamura, F.; Isoda, Y.; Uozumi, A.; Matsuda, R.; Shimamura, M.; Okubo, Y.; Ito, S.; Ota, H. Neonatal Wearable Device for Colorimetry-Based Real-Time Detection of Jaundice with Simultaneous Sensing of Vitals. Sci. Adv. 2021, 7, eabe3793. [Google Scholar] [PubMed]
- Hashim, W.; Al-Naji, A.; Al-Rayahi, I.A.; Alkhaled, M.; Chahl, J. Neonatal Jaundice Detection Using a Computer Vision System. Designs 2021, 5, 63. [Google Scholar] [CrossRef]
- Althnian, A.; Almanea, N.; Aloboud, N. Neonatal Jaundice Diagnosis Using a Smartphone Camera Based on Eye, Skin, and Fused Features with Transfer Learning. Sensors 2021, 21, 7038. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzak, A.Y.; Mohammed, S.L.; Al-Naji, A.; Chahl, J. Real-Time Jaundice Detection in Neonates Based on Machine Learning Models. BioMedInformatics 2024, 4, 623–637. [Google Scholar] [CrossRef]
- Khanam, F.T.Z.; Al-Naji, A.; Perera, A.G.; Wang, D.; Chahl, J. Non-Invasive and Non-Contact Automatic Jaundice Detection of Infants Based on Random Forest. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2023, 11, 2516–2529. [Google Scholar] [CrossRef]
- Shoham, B.; Migron, Y.; Riklin, A.; Willner, I.; Tartakovsky, B. A Bilirubin Biosensor Based on a Multilayer Network Enzyme Electrode. Biosens. Bioelectron. 1995, 10, 341–352. [Google Scholar] [CrossRef]
- Xu, P.; Yang, H.-W.; Shi, J.-L.; Ding, B.; Zhao, X.-J.; Yang, E.-C. Efficient Detection of a Biomarker for Infant Jaundice by a Europium (Iii)-Organic Framework Luminescence Sensor. RSC Adv. 2019, 9, 37584–37593. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rosenzweig, Z. A Fiber Optic Sensor for Rapid Analysis of Bilirubin in Serum. Anal. Chim. Acta 1997, 353, 263–273. [Google Scholar] [CrossRef]
- Pita, M.; Gutierrez-Sanchez, C.; Toscano, M.D.; Shleev, S.; De Lacey, A.L. Oxygen Biosensor Based on Bilirubin Oxidase Immobilized on a Nanostructured Gold Electrode. Bioelectrochemistry 2013, 94, 69–74. [Google Scholar] [CrossRef]


| Sr. No. | Method | Types of Biosensors | Biomarker | Limit of Detection(LOD) | Reference |
|---|---|---|---|---|---|
| Invasive | Electrochemical | Bilirubin | 1 µg/mL | [58] |
| Invasive | Electrochemical | Bilirubin | [59] | |
| Invasive | Optical | Bilirubin | 4.4 × 10−7 M | [64] |
| Invasive | Optical | Bilirubin | -- | [57] |
| Invasive | Paper-based | Bilirubin | 1 g/mL | [58] |
| Invasive | Amperometric | Bilirubin | 0.1 nM | [63] |
| Invasive | Potentiometric | Serum bilirubin | 15 μL | [60] |
| Invasive | Piezoelectric | Serum bilirubin | 0.01 μM | [57] |
| Invasive | Piezoelectric | Serum bilirubin | 0.05 μM | [57] |
| Invasive | Amperometric | Bilirubin | 4 × 10−6 M | [45] |
| Invasive | Amperometric | Bilirubin | -- | [76] |
| Invasive | Luminescence sensor | Bilirubin | 1.75 μM | [77] |
| Invasive | Fibre optic | Bilirubin | 1 × 10−7 M | [75] |
| Invasive | Oxygen amperometric | Bilirubin | 6 μM | [76] |
| Non-invasive | Colorimetric | Transcutaneous bilirubin | -- | [71] |
| Non-invasive | Electrochemical | Bilirubin | 1 μM | [70] |
| Non-invasive | Computer vision system | Skin colour | 14 mg/dL | [72] |
| Non-invasive | Smartphone camera using transfer learning | Skin colour | -- | [73] |
| Non-invasive | USB webcam with machine learning technique | Skin colour | -- | [74] |
| Non-invasive | Frontal image detection based on machine learing | Skin colour | -- | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazarika, C.J.; Borah, A.; Gogoi, P.; Ramchiary, S.S.; Daurai, B.; Gogoi, M.; Saikia, M.J. Development of Non-Invasive Biosensors for Neonatal Jaundice Detection: A Review. Biosensors 2024, 14, 254. https://doi.org/10.3390/bios14050254
Hazarika CJ, Borah A, Gogoi P, Ramchiary SS, Daurai B, Gogoi M, Saikia MJ. Development of Non-Invasive Biosensors for Neonatal Jaundice Detection: A Review. Biosensors. 2024; 14(5):254. https://doi.org/10.3390/bios14050254
Chicago/Turabian StyleHazarika, Chandan Jyoti, Alee Borah, Poly Gogoi, Shrimanta S. Ramchiary, Bethuel Daurai, Manashjit Gogoi, and Manob Jyoti Saikia. 2024. "Development of Non-Invasive Biosensors for Neonatal Jaundice Detection: A Review" Biosensors 14, no. 5: 254. https://doi.org/10.3390/bios14050254
APA StyleHazarika, C. J., Borah, A., Gogoi, P., Ramchiary, S. S., Daurai, B., Gogoi, M., & Saikia, M. J. (2024). Development of Non-Invasive Biosensors for Neonatal Jaundice Detection: A Review. Biosensors, 14(5), 254. https://doi.org/10.3390/bios14050254







