Fluorescence Multi-Detection Device Using a Lensless Matrix Addressable microLED Array
Abstract
1. Introduction
2. Materials and Methods
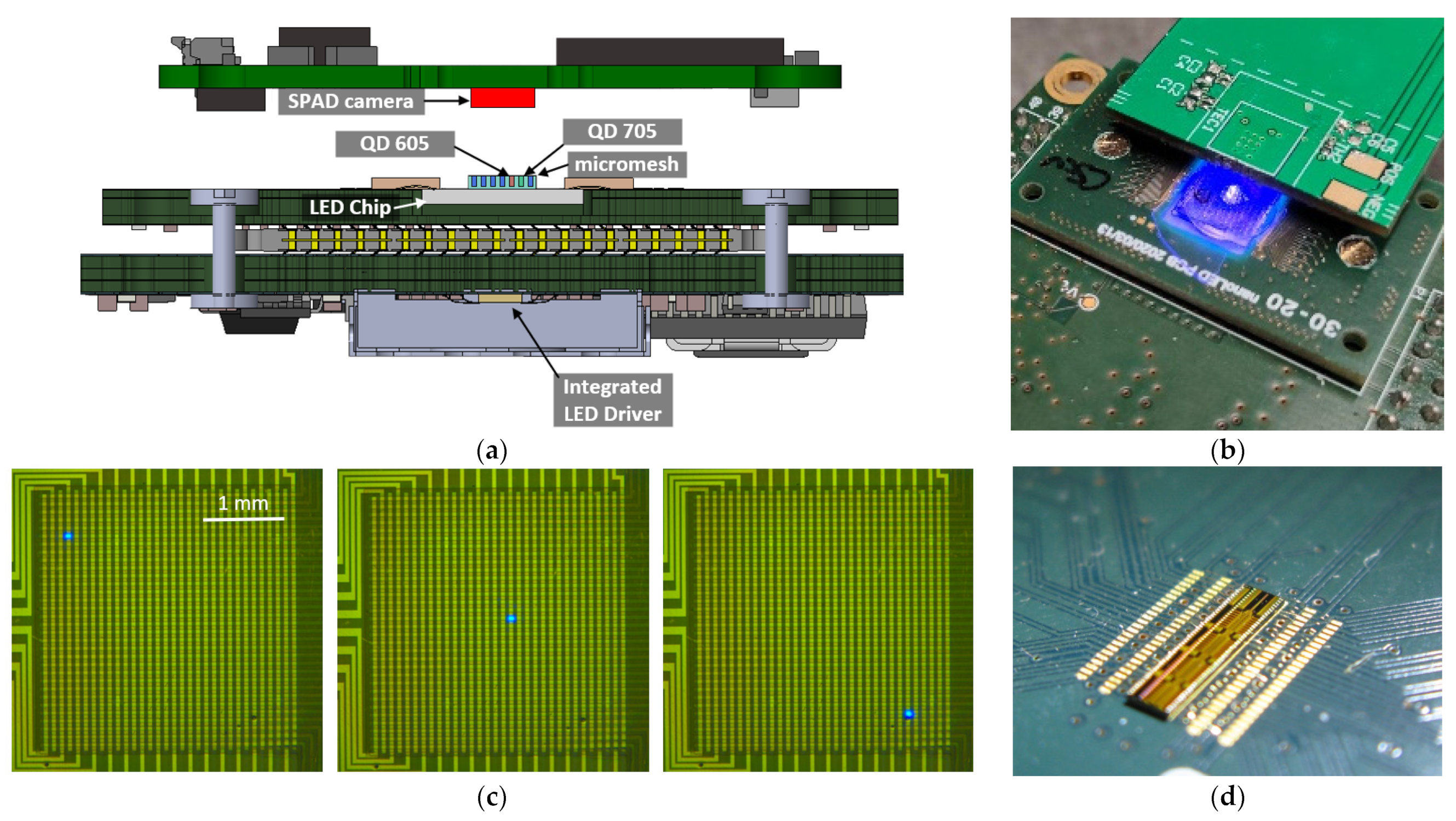
2.1. Instrument
2.2. microLED Array
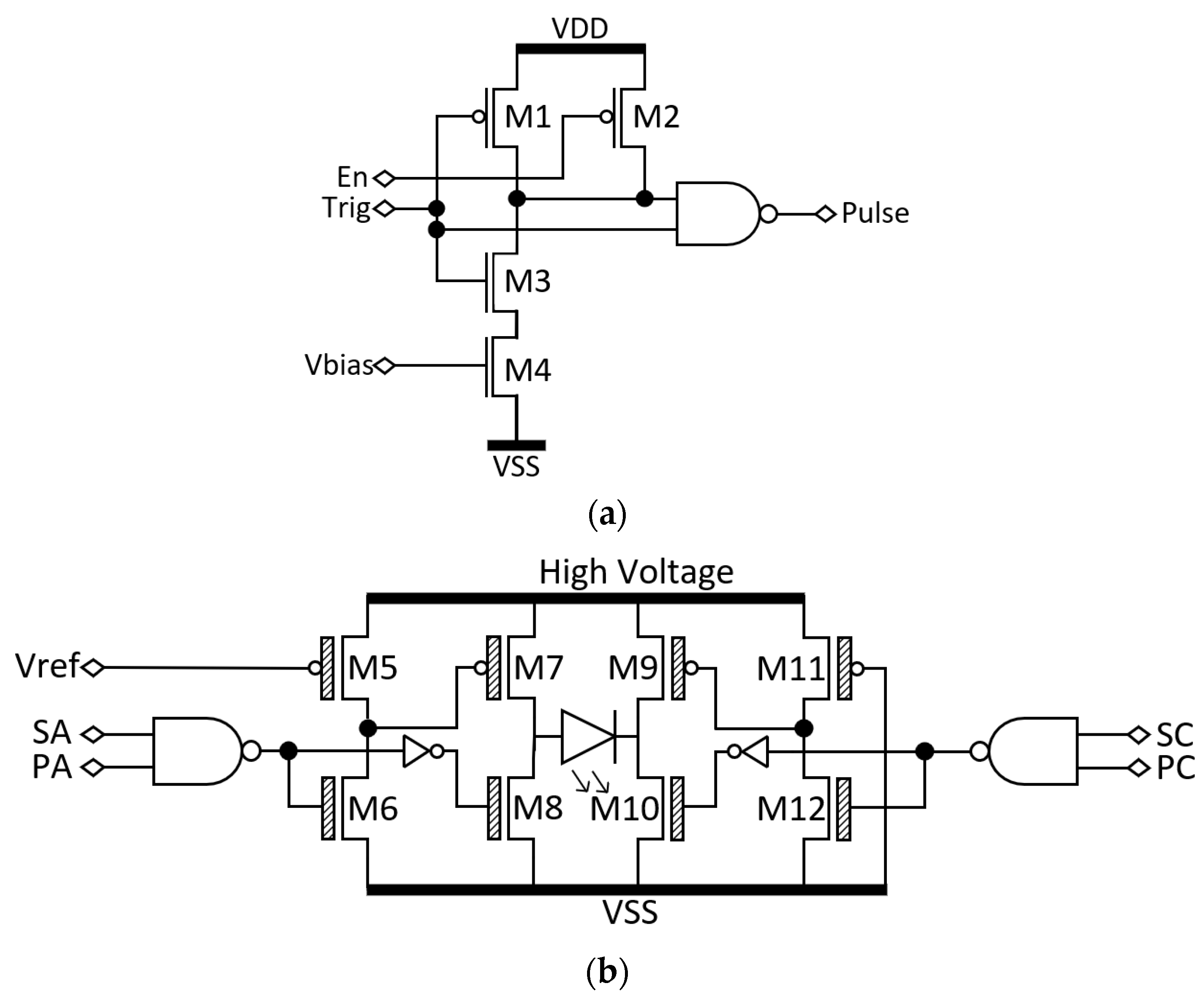
2.3. Custom Driver Chip
2.4. SPAD Camera
2.5. Fluorescent Particles
2.6. Micromesh
3. Results
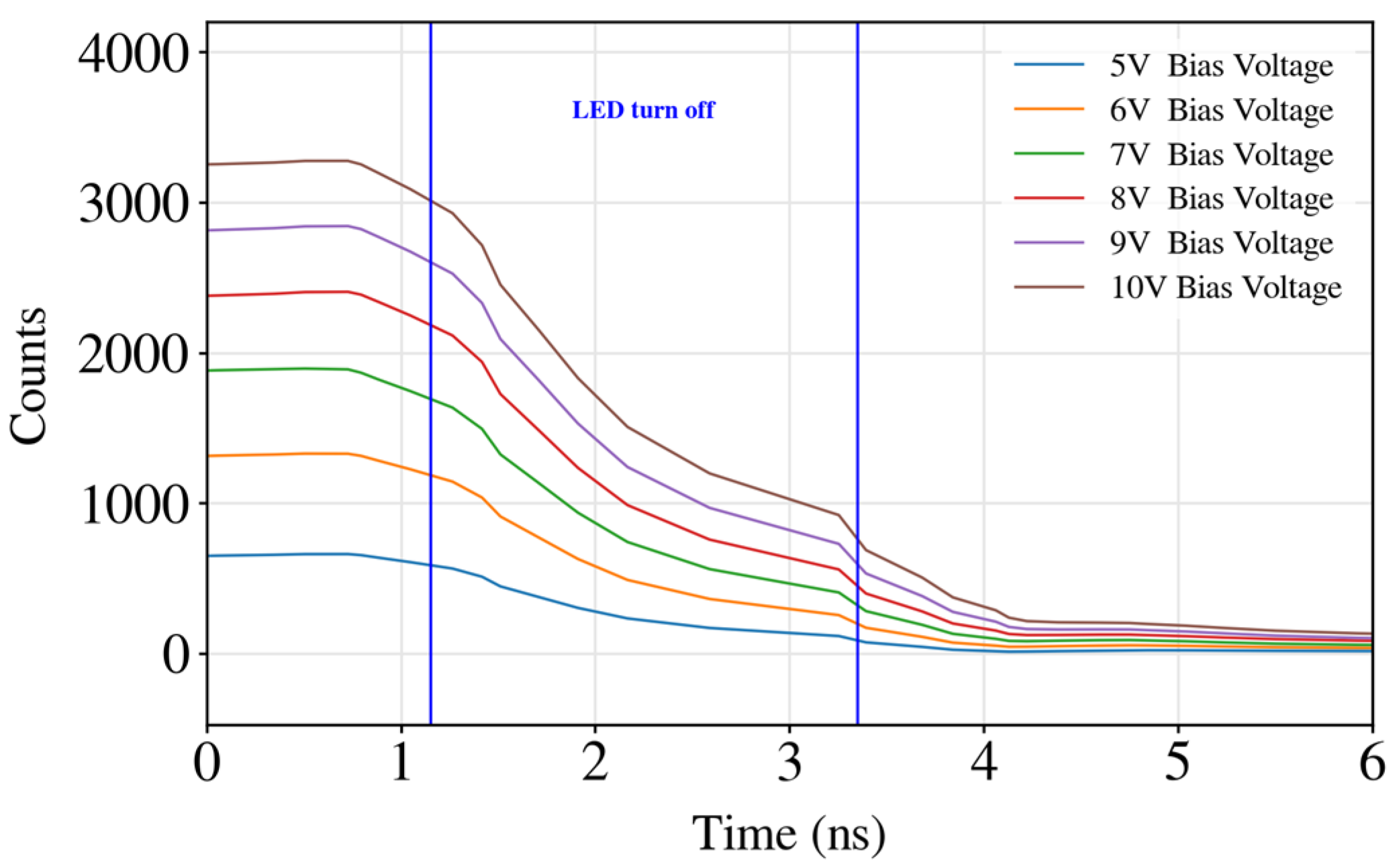
3.1. Intensity of Fluorescence Measurements
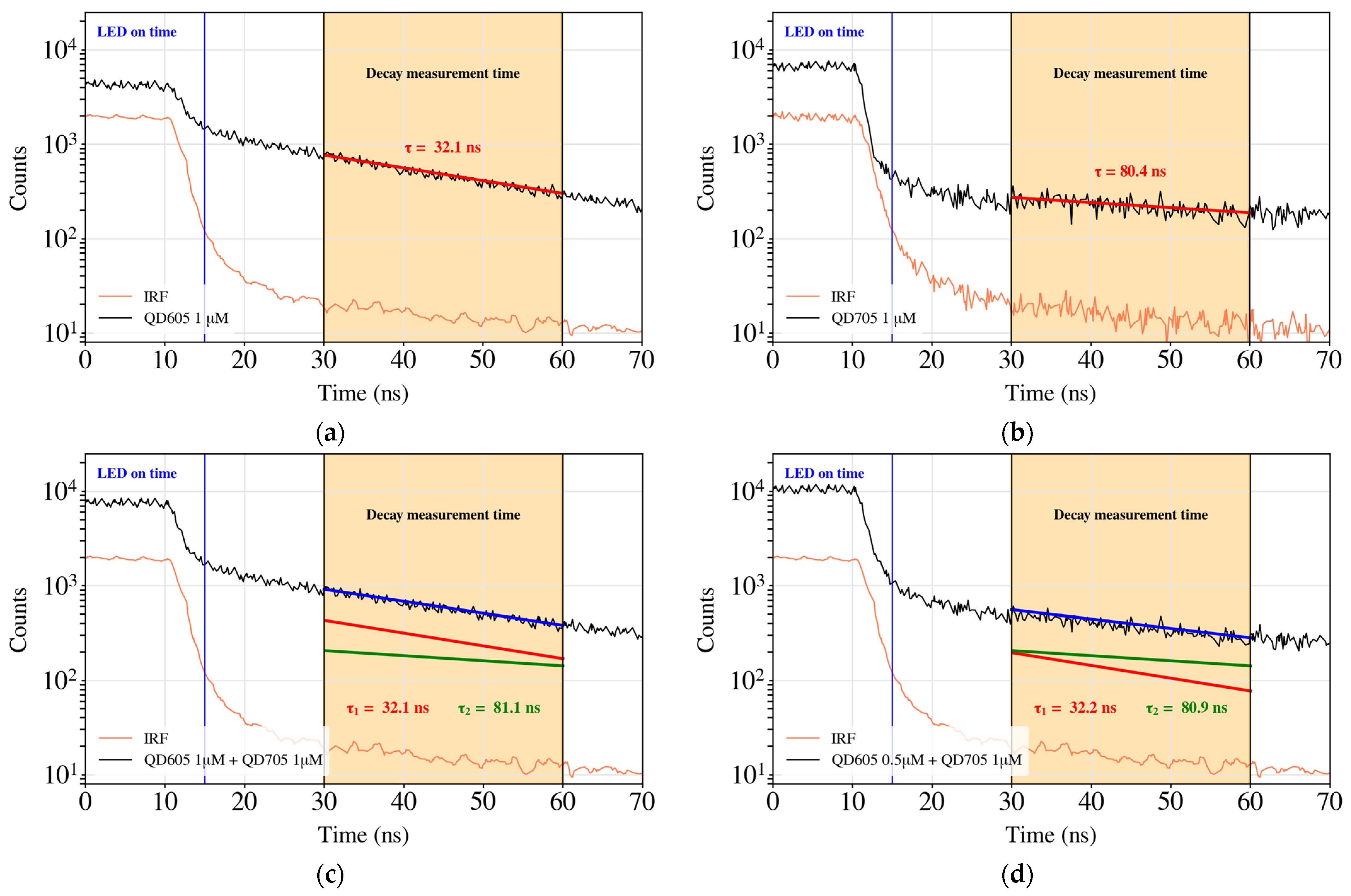
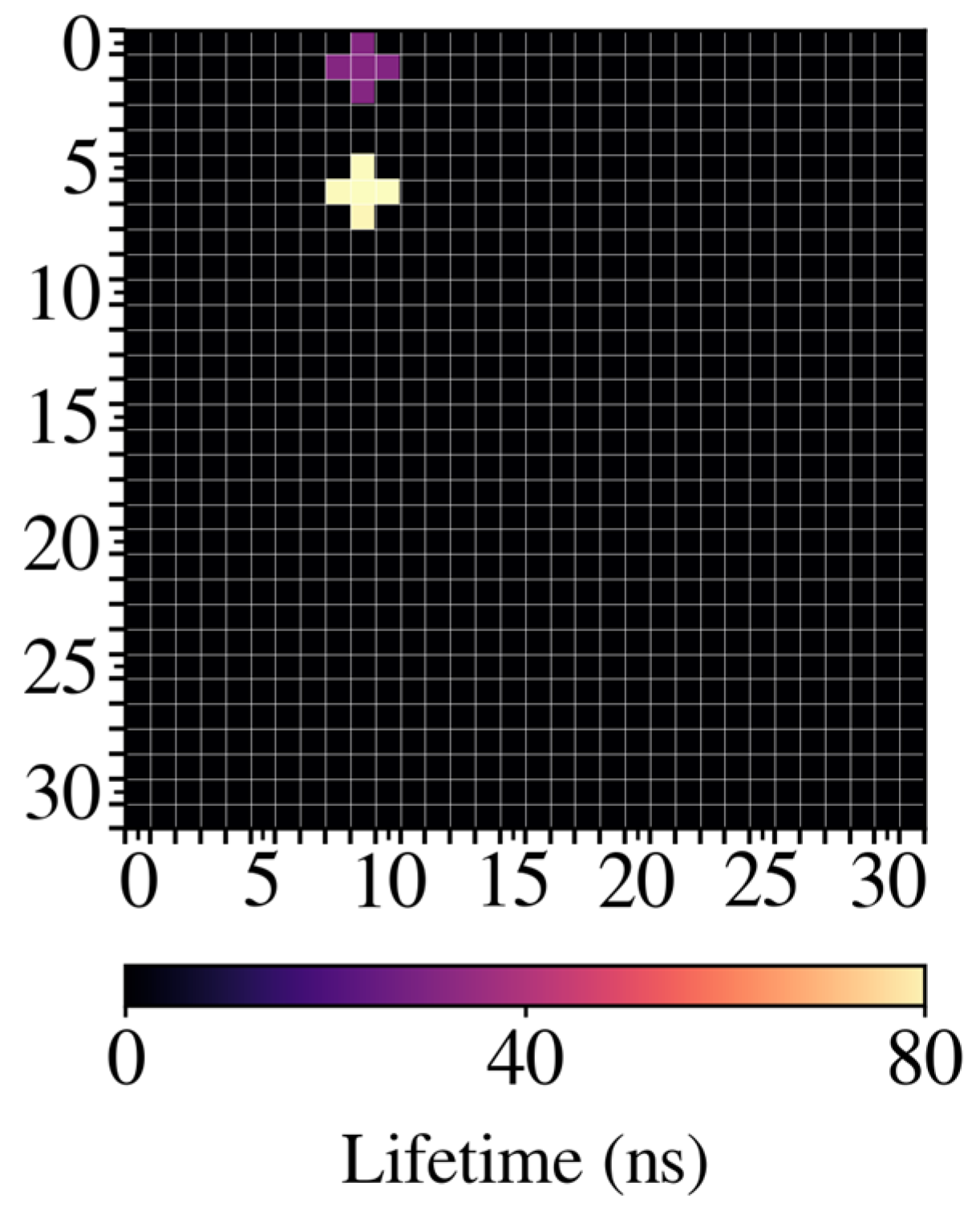
3.2. Time-Correlated Fluorescence Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 8 March 2024).
- Mills, A. Health Care Systems in Low- and Middle-Income Countries. N. Engl. J. Med. 2014, 370, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O’Kennedy, R. Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Boniface, R.; Moshabela, M.; Zulliger, R.; MacPherson, P.; Nyasulu, P. Correlates of Delayed Diagnosis among Human Immunodeficiency Virus-Infected Pulmonary Tuberculosis Suspects in a Rural HIV Clinic, South Africa. Tuberc. Res. Treat. 2012, 2012, 827148. [Google Scholar] [CrossRef] [PubMed]
- Mashamba-Thompson, T.P.; Sartorius, B.; Drain, P.K. Operational Assessment of Point-of-Care Diagnostics in Rural Primary Healthcare Clinics of KwaZulu-Natal, South Africa: A Cross-Sectional Survey. BMC Health Serv. Res. 2018, 18, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Miller, B.L. Immunosensor-Based Label-Free and Multiplex Detection of Influenza Viruses: State of the Art. Biosens. Bioelectron. 2019, 141, 111476. [Google Scholar] [CrossRef] [PubMed]
- Hojat, K.; Duppenthaler, A.; Aebi, C. Impact of the Availability of an Influenza Virus Rapid Antigen Test on Diagnostic Decision Making in a Pediatric Emergency Department. Pediatr. Emerg. Care 2013, 29, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-Chip Devices for Global Health: Past Studies and Future Opportunities. Lab. Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.T. Point-of-Care Testing in Microbiology: A Mechanism for Improving Patient Outcomes. Clin. Chem. 2020, 66, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Vashist, S.K. Point-of-Care Diagnostics: Recent Advances and Trends. Biosensors 2017, 7, 62. [Google Scholar] [CrossRef]
- Kozel, T.R.; Burnham-Marusich, A.R. Point-of-Care Testing for Infectious Diseases: Past, Present, and Future. J. Clin. Microbiol. 2017, 55, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Global HIV Hepatitis and STIs Programmes (HHS); Sexual and Reproductive Health and Research (SRH); World Health Organization. The Diagnostics Landscape for Sexually Transmitted Infections; WHO: Geneva, Switzerland, 2023; ISBN 9789240077126. [Google Scholar]
- Qin, J.; Wang, W.; Gao, L.; Yao, S.Q. Emerging Biosensing and Transducing Techniques for Potential Applications in Point-of-Care Diagnostics. Chem. Sci. 2022, 13, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Pruksaphon, K.; Intaramat, A.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Diagnostic Laboratory Immunology for Talaromycosis (Penicilliosis): Review from the Bench-Top Techniques to the Point-of-Care Testing. Diagn. Microbiol. Infect. Dis. 2020, 96, 114959. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Tu, D.; Tong, L.; Sarwar, M.; Bhimaraj, A.; Li, C.; Coté, G.L.; Di Carlo, D. A Review of Biosensor Technologies for Blood Biomarkers toward Monitoring Cardiovascular Diseases at the Point-of-Care. Biosens. Bioelectron. 2021, 171, 112621. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Fu, Y.; Chang, K.H.; Lin, K.J.; Lu, Y.J.; Cheng, C.M. Point-of-Care Devices Using Disease Biomarkers to Diagnose Neurodegenerative Disorders. Trends Biotechnol. 2018, 36, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Sandbhor Gaikwad, P.; Banerjee, R. Advances in Point-of-Care Diagnostic Devices in Cancers. Analyst 2018, 143, 1326–1348. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.L.; Rossi, J.M. Point of Care Whole Blood Microfluidics for Detecting and Managing Thrombotic and Bleeding Risks. Lab. Chip 2021, 21, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Stedtfeld, R.D.; Tourlousse, D.M.; Seyrig, G.; Stedtfeld, T.M.; Kronlein, M.; Price, S.; Ahmad, F.; Gulari, E.; Tiedje, J.M.; Hashsham, S.A. Gene-Z: A Device for Point of Care Genetic Testing Using a Smartphone. Lab. Chip 2012, 12, 1454–1462. [Google Scholar] [CrossRef]
- Clerc, O.; Greub, G. Routine Use of Point-of-Care Tests: Usefulness and Application in Clinical Microbiology. Clin. Microbiol. Infect. 2010, 16, 1054–1061. [Google Scholar] [CrossRef]
- Cholkar, K.; Hirani, N.D.; Natarajan, C. Nanotechnology-Based Medical and Biomedical Imaging for Diagnostics. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 355–374. ISBN 9780323429979. [Google Scholar]
- Pennathur, S.; Fygenson, D.K. Improving Fluorescence Detection in Lab on Chip Devices. Lab. Chip 2008, 8, 649–652. [Google Scholar] [PubMed]
- Canals, J.; Franch, N.; Alonso, O.; Vilà, A.; Diéguez, A. A Point-of-Care Device for Molecular Diagnosis Based on CMOS SPAD Detectors with Integrated Microfluidics. Sensors 2019, 19, 445. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Boryczka, J.; Wu, N. Visible-Light and near-Infrared Fluorescence and Surface-Enhanced Raman Scattering Point-of-Care Sensing and Bio-Imaging: A Review. Chem. Soc. Rev. 2022, 51, 329–375. [Google Scholar] [CrossRef]
- Berner, M.; Hilbig, U.; Schubert, M.B.; Gauglitz, G. Laser-Induced Fluorescence Detection Platform for Point-of-Care Testing. Meas. Sci. Technol. 2017, 28, 085701. [Google Scholar] [CrossRef]
- Jin, B.; Li, Z.; Zhao, G.; Ji, J.; Chen, J.; Yang, Y.; Xu, R. Upconversion Fluorescence-Based Paper Disc for Multiplex Point-of-Care Testing in Water Quality Monitoring. Anal. Chim. Acta 2022, 1192, 339388. [Google Scholar] [CrossRef]
- He, Q.; Yu, D.; Bao, M.; Korensky, G.; Chen, J.; Shin, M.; Kim, J.; Park, M.; Qin, P.; Du, K. High-Throughput and All-Solution Phase African Swine Fever Virus (ASFV) Detection Using CRISPR-Cas12a and Fluorescence Based Point-of-Care System. Biosens. Bioelectron. 2020, 154, 112068. [Google Scholar] [CrossRef]
- Mukunda, D.C.; Joshi, V.K.; Mahato, K.K. Light Emitting Diodes (LEDs) in Fluorescence-Based Analytical Applications: A Review. Appl. Spectrosc. Rev. 2022, 57, 1–38. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Liu, H.; Su, X.; Chen, Z.; Li, S.; He, N. A Highly Integrated and Diminutive Fluorescence Detector for Point-of-Care Testing: Dual Negative Feedback Light-Emitting Diode (LED) Drive and Photoelectric Processing Circuits Design and Implementation. Biosensors 2022, 12, 764. [Google Scholar] [CrossRef]
- Mohanan, S.M.P.C.; Russell, K.; Duncan, S.; Kiang, A.; Lochenie, C.; Duffy, E.; Kennedy, S.; Prajna, N.V.; Williams, R.L.; Dhaliwal, K.; et al. FluoroPi Device with SmartProbes: A Frugal Point-of-Care System for Fluorescent Detection of Bacteria from a Pre-Clinical Model of Microbial Keratitis. Transl. Vis. Sci. Technol. 2023, 12, 1. [Google Scholar] [CrossRef]
- Lian, C.; Young, D.; Randall, R.E.; Samuel, I.D.W. Organic Light-Emitting Diode Based Fluorescence-Linked Immunosorbent Assay for SARS-CoV-2 Antibody Detection. Biosensors 2022, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, C.; Saqib, M.; Hao, R. Single-Molecule Fluorescence Methods for Protein Biomarker Analysis. Anal. Bioanal. Chem. 2023, 415, 3655–3669. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Kanani, S.S.; Tsai, H.Y.; Algar, W.R. Quantitative and Multiplexed Chopper-Based Time-Gated Imaging for Bioanalysis on a Smartphone. Anal. Chem. 2023, 95, 13258–13265. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xu, J.; Cardoso Dos Santos, M.; Hildebrandt, N. Multiplexed Biosensing and Bioimaging Using Lanthanide-Based Time-Gated Förster Resonance Energy Transfer. Acc. Chem. Res. 2022, 55, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, P.; Lu, Y.; Wang, R.; Zhou, L.; Zheng, X.; Li, X.; Piper, J.A.; Zhang, F. Lifetime-Engineered NIR-II Nanoparticles Unlock Multiplexed In Vivo Imaging. Nat. Nanotechnol. 2018, 13, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Obahiagbon, U.; Smith, J.T.; Zhu, M.; Katchman, B.A.; Arafa, H.; Anderson, K.S.; Blain Christen, J.M. A Compact, Low-Cost, Quantitative and Multiplexed Fluorescence Detection Platform for Point-of-Care Applications. Biosens. Bioelectron. 2018, 117, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Henrikson, R.H.; Xu, L.; Lee, L.P. A Point-of-Care Instrument for Rapid Multiplexed Pathogen Genotyping. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: New York, NY, USA, 2011. ISBN 9781424441228. [Google Scholar]
- Smith, J.T.; Obahiagbon, U.; Ewaisha, R.; Katchman, B.A.; Kaftanoglu, K.; Arafa, H.M.; Kullman, D.E.; Anderson, K.S. Low-Cost, Disposable Fluorescence-Based Biorecognition System for Multiplexed Point-of-Care Molecular Diagnostics. In Proceedings of the 2016 IEEE Healthcare Innovation Point-Of-Care Technologies Conference (HI-POCT), Cancun, Mexico, 9–11 November 2016. [Google Scholar]
- Manzanas, C.; Alam, M.M.; Loeb, J.C.; Lednicky, J.A.; Wu, C.Y.; Fan, Z.H. A Valve-Enabled Sample Preparation Device with Isothermal Amplification for Multiplexed Virus Detection at the Point-of-Care. ACS Sens. 2021, 6, 4176–4184. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Lin, L.; Wu, B.; Huang, E.; Wang, Y.; Li, Z.; He, H.; Lei, X.; Xu, B.; Liu, D. A Pocket-Sized Device Automates Multiplexed Point-of-Care RNA Testing for Rapid Screening of Infectious Pathogens. Biosens. Bioelectron. 2021, 181, 113145. [Google Scholar] [CrossRef] [PubMed]
- Wasisto, H.S.; Prades, J.D.; Gülink, J.; Waag, A. Beyond Solid-State Lighting: Miniaturization, Hybrid Integration, and Applications of GaN Nano-and Micro-LEDs. Appl. Phys. Rev. 2019, 6, 041315. [Google Scholar] [CrossRef]
- Tian, P.; McKendry, J.J.D.; Gong, Z.; Zhang, S.; Watson, S.; Zhu, D.; Watson, I.M.; Gu, E.; Kelly, A.E.; Humphreys, C.J.; et al. Characteristics and Applications of Micro-Pixelated GaN-Based Light Emitting Diodes on Si Substrates. J. Appl. Phys. 2014, 115, 033112. [Google Scholar] [CrossRef]
- Rae, B.R.; Yang, J.B.; McKendry, J.; Gong, Z.; Renshaw, D.; Girkin, J.M.; Gu, E.; Dawson, M.D.; Henderson, R.K. A Vertically Integrated CMOS Microsystem for Time-Resolved Fluorescence Analysis. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 437–444. [Google Scholar] [CrossRef]
- Xie, E.; Bian, R.; He, X.; Islim, M.S.; Chen, C.; Mckendry, J.J.D.; Gu, E.; Haas, H.; Dawson, M.D. Over 10 Gbps VLC for Long-Distance Applications Using a GaN-Based Series-Biased Micro-LED Array. IEEE Photonics Technol. Lett. 2020, 32, 499–502. [Google Scholar] [CrossRef]
- Vilella, E.; Diéguez, A. A Gated Single-Photon Avalanche Diode Array Fabricated in a Conventional CMOS Process for Triggered Systems. Sens. Actuators A Phys. 2012, 186, 163–168. [Google Scholar] [CrossRef]
- Vilella, E.; Diéguez, A. Dynamic Range Extension of SiPM Detectors with the Time-Gated Operation. Opt. Express 2014, 22, 12007. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Rae, B.R.; Henderson, R.K.; Gong, Z.; McKendry, J.; Gu, E.; Dawson, M.D.; Turnbull, G.A.; Samuel, I.D.W. Ultra-Portable Explosives Sensor Based on a CMOS Fluorescence Lifetime Analysis Micro-System. AIP Adv. 2011, 1, 032115. [Google Scholar] [CrossRef]
- Bornemann, S.; Gulink, J.; Moro, V.; Canals, J.; Wolter, S.; Schottler, G.; Bezshlyakh, D.; Prades, J.; Dieguez, A.; Waag, A. Processing and Characterization of Monolithic Passive-Matrix GaN-Based MicroLED Arrays with Pixel Sizes from 5 to 50 Μm. IEEE Photonics J. 2021, 13, 8200209. [Google Scholar] [CrossRef]
- Lim, S.G.; Lee, K.; Kim, Y.J. Mobile AMOLED Display Power Model Considering I-R Drop in Smartphones. IEEE Trans. Ind. Electron. 2021, 68, 2694–2702. [Google Scholar] [CrossRef]
- Seong, J.; Jang, J.; Lee, J.; Lee, M. CMOS Backplane Pixel Circuit with Leakage and Voltage Drop Compensation for an Micro-LED Display Achieving 5000 PPI or Higher. IEEE Access 2020, 8, 49467–49476. [Google Scholar] [CrossRef]
- Canals, J.; Franch, N.; Moro, V.; Moreno, S.; Prades, J.D.; Romano-Rodríguez, A.; Bornemann, S.; Bezshlyakh, D.D.; Waag, A.; Vogelbacher, F.; et al. A Novel Approach for a Chip-Sized Scanning Optical Microscope. Micromachines 2021, 12, 527. [Google Scholar] [CrossRef]
- Bezshlyakh, D.D.; Spende, H.; Weimann, T.; Hinze, P.; Bornemann, S.; Gülink, J.; Canals, J.; Prades, J.D.; Dieguez, A.; Waag, A. Directly Addressable GaN-Based Nano-LED Arrays: Fabrication and Electro-Optical Characterization. Microsyst. Nanoeng. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Franch Masdeu, N. Development of a Nano-Illumination Microscope; University of Barcelona: Barcelona, Spain, 2022; Available online: http://hdl.handle.net/2445/189889 (accessed on 15 March 2024).
- Gulinatti, A.; Maccagnani, P.; Rech, I.; Ghioni, M.; Cova, S. 35 Ps Time Resolution at Room Temperature with Large Area Single Photon Avalanche Diodes. Electron. Lett. 2005, 41, 272–274. [Google Scholar] [CrossRef]
- Acerbi, F.; Ferri, A.; Gola, A.; Cazzanelli, M.; Pavesi, L.; Zorzi, N.; Piemonte, C. Characterization of Single-Photon Time Resolution: From Single SPAD to Silicon Photomultiplier. IEEE Trans. Nucl. Sci. 2014, 61, 2678–2686. [Google Scholar] [CrossRef]
- ZedBoard Zynq-7000 ARM/FPGA SoC Development Board. Available online: https://digilent.com/shop/zedboard-zynq-7000-arm-fpga-soc-development-board/ (accessed on 21 March 2024).
- Franch, N.; Alonso, O.; Canals, J.; Vila, A.; Dieguez, A. A Low Cost Fluorescence Lifetime Measurement System Based on SPAD Detectors and FPGA Processing. J. Instrum. 2017, 12, C02070. [Google Scholar] [CrossRef]
- QdotTM 605 ITKTM Streptavidin Conjugate Kit. Available online: https://www.thermofisher.com/order/catalog/product/Q10001MP?SID=srch-srp-Q10001MP (accessed on 8 March 2024).
- QdotTM 705 ITKTM Amino (PEG) Quantum Dots. Available online: https://www.thermofisher.com/order/catalog/product/Q21561MP?SID=srch-srp-Q21561MP (accessed on 8 March 2024).
- Bhuckory, S.; Lefebvre, O.; Qiu, X.; Wegner, K.D.; Hildebrandt, N. Evaluating Quantum Dot Performance in Homogeneous FRET Immunoassays for Prostate Specific Antigen. Sensors 2016, 16, 197. [Google Scholar] [CrossRef]
- tebu-bio Micromesh Array, Well Size 250 um, 8 mm Array. Available online: https://www.tebubio.com/es-eur/micromesh-array-well-size-250um-8mm-array-10-arrays-per-p/5637365732.p (accessed on 8 March 2024).
- The Principle of Time-Correlated Single Photon Counting. Available online: https://www.picoquant.com/images/uploads/page/files/7253/technote_tcspc.pdf (accessed on 10 May 2024).
- Giraud, G.; Schulze, H.; Bachmann, T.T.; Campbell, C.J.; Mount, A.R.; Ghazal, P.; Khondoker, M.R.; Ember, S.W.J.; Ciani, I.; Tlili, C.; et al. Solution State Hybridization Detection Using Time-Resolved Fluorescence Anisotropy of Quantum Dot-DNA Bioconjugates. Chem. Phys. Lett. 2010, 484, 309–314. [Google Scholar] [CrossRef]
- Bückers, J.; Wildanger, D.; Vicidomini, G.; Kastrup, L.; Hell, S.W. Simultaneous Multi-Lifetime Multi-Color STED Imaging for Colocalization Analyses. Opt. Express 2011, 19, 3130–3143. [Google Scholar] [CrossRef]
- Sipior, J.; Carter, G.M.; Lakowicz, J.R.; Rao, G. Blue Light-Emitting Diode Demonstrated as an Ultraviolet Excitation Source for Nanosecond Phase-Modulation Fluorescence Lifetime Measurements. Rev. Sci. Instrum. 1997, 68, 2666–2670. [Google Scholar] [CrossRef]
- Araki, T.; Misawa, H. Light Emitting Diode-Based Nanosecond Ultraviolet Light Source for Fluorescence Lifetime Measurements. Rev. Sci. Instrum. 1995, 66, 5469–5472. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Cho, N.; Sung, Y.; Yeom, G. Effect of GaN Microlens Array on Efficiency of GaN-Based Blue-Light-Emitting Diodes. Jpn. J. Appl. Phys. Part 2 Lett. 2005, 44, L18. [Google Scholar] [CrossRef]
- Burri, S.; Maruyama, Y.; Michalet, X.; Regazzoni, F.; Bruschini, C.; Charbon, E. Architecture and Applications of a High Resolution Gated SPAD Image Sensor. Opt. Express 2014, 22, 17573. [Google Scholar] [CrossRef]
- Léonard, J.; Dumas, N.; Caussé, J.P.; Maillot, S.; Giannakopoulou, N.; Barre, S.; Uhring, W. High-Throughput Time-Correlated Single Photon Counting. Lab. A Chip—Miniaturisation Chem. Biol. 2014, 14, 4338–4343. [Google Scholar] [CrossRef]
- Griffin, C.; Gu, E.; Choi, H.W.; Jeon, C.W.; Rolinski, O.J.; Birch, D.J.S.; Girkin, J.M.; Dawson, M.D. Fluorescence Excitation and Lifetime Measurements Using GaN/InGaN Micro LED Arrays. In Proceedings of the The 17th Annual Meeting of the IEEELasers and Electro-Optics Society, 2004. LEOS 2004, Rio Grande, PR, USA, 11 November 2004. [Google Scholar]
- Rae, B.R.; Muir, K.R.; Gong, Z.; McKendry, J.; Girkin, J.M.; Gu, E.; Renshaw, D.; Dawson, M.D.; Henderson, R.K. A CMOS Time-Resolved Fluorescence Lifetime Analysis Micro-System. Sensors 2009, 9, 9255–9274. [Google Scholar] [CrossRef]
- Ou, F.; Chong, W.C.; Xu, Q.; Chen, Y.; Li, Q.; Zhang, L. Monochromatic Active Matrix Micro-LED Micro-Displays with >5,000 Dpi Pixel Density Fabricated Using Monolithic Hybrid Integration Process. Dig. Tech. Pap. 2018, 49, 1677–1680. [Google Scholar] [CrossRef]
- Canals, J.; Moro, V.; Schöttler, G.; Bornemann, S.; Waag, A.; Prades, J.D.; Diéguez, A. A 9 Kfps 1411 PPI GaN-Based ΜLED Display CMOS Backplane. Dig. Tech. Pap. 2023, 54, 125–128. [Google Scholar] [CrossRef]
- Bani Hassan, N.; Dehkhoda, F.; Xie, E.; Herrnsdorf, J.; Strain, M.J.; Henderson, R.; Dawson, M.D. Ultrahigh Frame Rate Digital Light Projector Using Chip-Scale LED-on-CMOS Technology. Photonics Res. 2022, 10, 2434. [Google Scholar] [CrossRef]
- Jade Bird Display, “0.22” MicroLED|JBD|10000PPI MicroLED”. Available online: https://www.jb-display.com/weixianshiping/3.html (accessed on 10 May 2024).
- Poher, V.; Grossman, N.; Kennedy, G.T.; Nikolic, K.; Zhang, H.X.; Gong, Z.; Drakakis, E.M.; Gu, E.; Dawson, M.D.; French, P.M.W.; et al. Micro-LED Arrays: A Tool for Two-Dimensional Neuron Stimulation. J. Phys. D Appl. Phys. 2008, 41, 094014. [Google Scholar] [CrossRef]
- Moreno, S.; Canals, J.; Moro, V.; Franch, N.; Vilà, A.; Romano, A.; Prades, J.D.; Bezshlyakh, D.D.; Waag, A.; Diéguez, A. Nano-Illumination Microscopy as a Fast Low-Cost Chip-Sized Technique to Face Pandemics. In Proceedings of the Biosensors for Pandemics, online, 6 May 2020. [Google Scholar]
- Canals, J.; Moro, V.; Franch, N.; Moreno, S.; Alonso, O.; Vilà, A.; Prades, J.D.; Gülink, J.; Bezshlyakh, D.D.; Waag, A.; et al. A Shadow Image Microscope Based on an Array of NanoLEDs. Proc. SPIE 2020, 11351, 113510D. [Google Scholar]
- Vilà, A.; Moreno, S.; Canals, J.; Diéguez, A. A Compact Raster Lensless Microscope Based on a Microdisplay. Sensors 2021, 21, 5941. [Google Scholar] [CrossRef]
- Vila, A.; Moreno, S.; Canals, J.; Moro, V.; Franch, N.; Wartenberg, P.; Dieguez, A. Ultra-Compact and Large Field-of-View Nano-Illumination Light Microscope Based on an Array of Organic Light-Emitting Diodes. Proc. SPIE 2021, 11693, 1169308. [Google Scholar]
- Prajapati, E.; Kumar, S.; Kumar, S. Muscope: A Miniature on-Chip Lensless Microscope. Lab. Chip 2021, 21, 4357–4363. [Google Scholar] [CrossRef]
- Franch, N.; Canals, J.; Moro, V.; Vilà, A.; Romano-Rodríguez, A.; Prades, J.D.; Guelink, J.; Bezshlyakh, D.; Waag, A.; Kluczyk, K.; et al. Nano-Illumination Microscopy: A Technique Based on Scanning with an Array of Individually Addressable NanoLEDs. Opt. Express 2020, 28, 19044–19057. [Google Scholar] [CrossRef] [PubMed]





| Reference | [77] | [76] | [78] | [79] | [74] | This Work |
|---|---|---|---|---|---|---|
| Driver type | in-pixel | in-pixel | in-pixel | MA | DA | MA |
| Application | display | display | display | neuron stimulation | fluorescence | fluorescence |
| Resolution | 128 × 128 | 512 × 512 | 1920 × 1080 | 64 × 64 | 8 × 8 | 32 × 32 |
| Pixel pitch | 50 µm | 18 µm | 2.5 µm | 40 µm | 200 µm | 100 µm |
| Pixel density | 508 PPI | 1411 PPI | 10,000 PPI | 635 PPI | 127 PPI | 254 PPI |
| Switch speed | 83 kfps | 1 MHz–9.15 kfps | n.a. | 600 fps | 1.28 GHz | 500 MHz |
| Max. LED current | 87 µA | 120 µA | 1.6 µA | 10 mA | n. a. | 20 mA |
| LED bias voltage | 5 V | up to 5V | n.a. V | up to 4V | up to 5V | up to 10 V |
| CMOS Tech. Node | 0.18 µm | 0.18 µm | n.a. | n.a. | 0.35 µm | 0.35 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, V.; Canals, J.; Moreno, S.; Higgins-Wood, S.; Alonso, O.; Waag, A.; Prades, J.D.; Dieguez, A. Fluorescence Multi-Detection Device Using a Lensless Matrix Addressable microLED Array. Biosensors 2024, 14, 264. https://doi.org/10.3390/bios14060264
Moro V, Canals J, Moreno S, Higgins-Wood S, Alonso O, Waag A, Prades JD, Dieguez A. Fluorescence Multi-Detection Device Using a Lensless Matrix Addressable microLED Array. Biosensors. 2024; 14(6):264. https://doi.org/10.3390/bios14060264
Chicago/Turabian StyleMoro, Victor, Joan Canals, Sergio Moreno, Steffen Higgins-Wood, Oscar Alonso, Andreas Waag, J. Daniel Prades, and Angel Dieguez. 2024. "Fluorescence Multi-Detection Device Using a Lensless Matrix Addressable microLED Array" Biosensors 14, no. 6: 264. https://doi.org/10.3390/bios14060264
APA StyleMoro, V., Canals, J., Moreno, S., Higgins-Wood, S., Alonso, O., Waag, A., Prades, J. D., & Dieguez, A. (2024). Fluorescence Multi-Detection Device Using a Lensless Matrix Addressable microLED Array. Biosensors, 14(6), 264. https://doi.org/10.3390/bios14060264







