Rapid Determination of Cr3+ and Mn2+ in Water Using Laser-Induced Breakdown Spectroscopy Combined with Filter Paper Modified with Gold Nanoclusters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Preparation of AuNCs and AuNPs Modified Filter Paper
2.3. LIBS-FP-AuNCs Sensor for Cr3+ and Mn2+ Detection
3. Results and Discussion
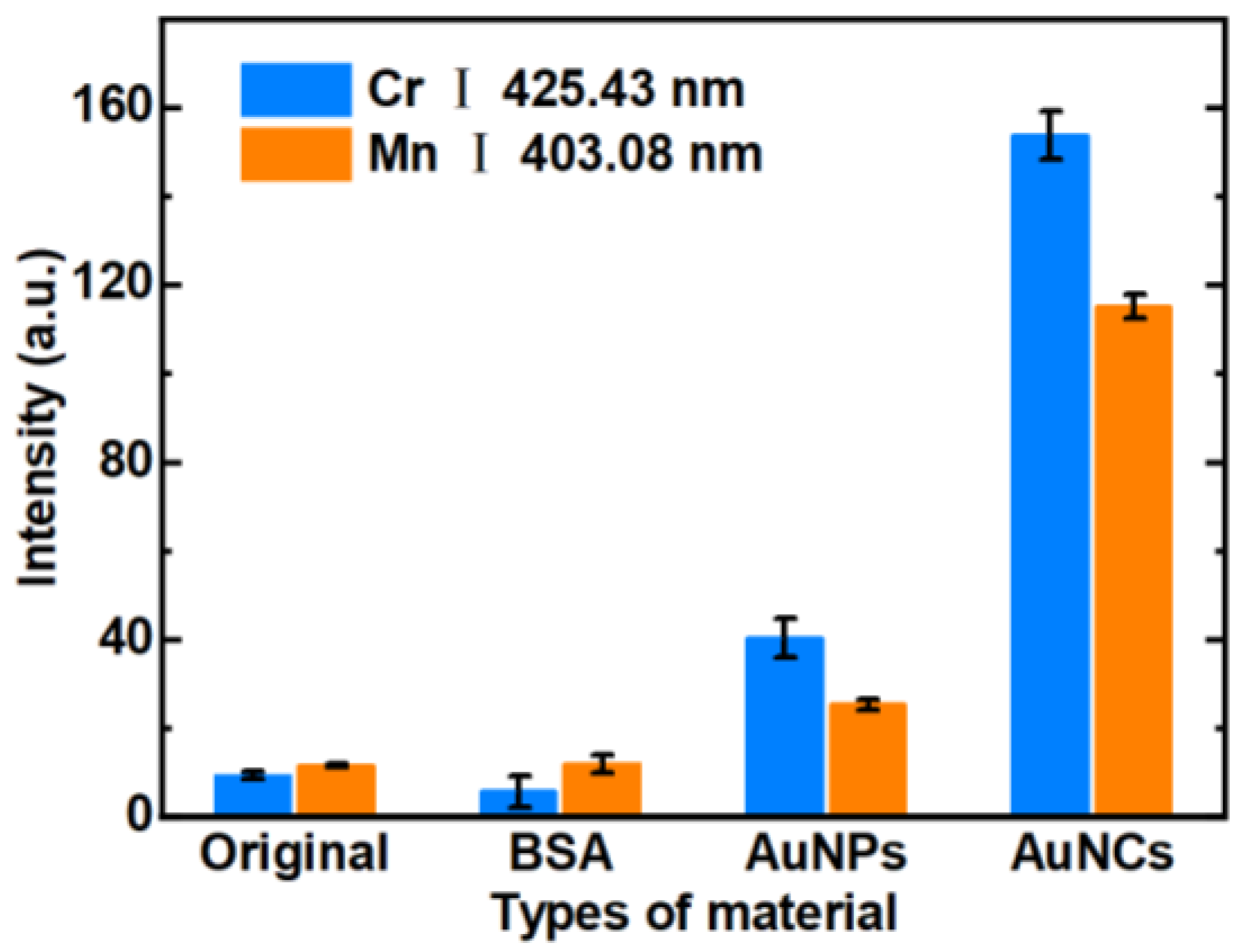
3.1. Comparison of Adsorption Capabilities of Filter Papers Modified with Different Materials
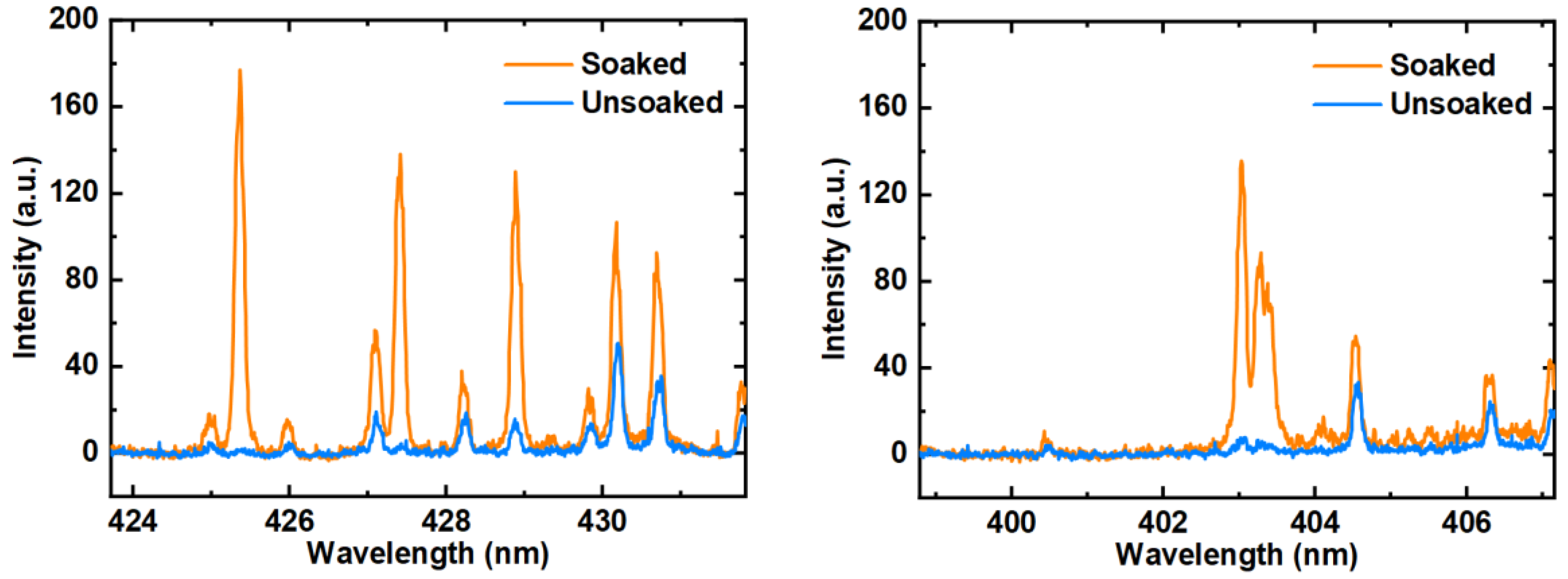
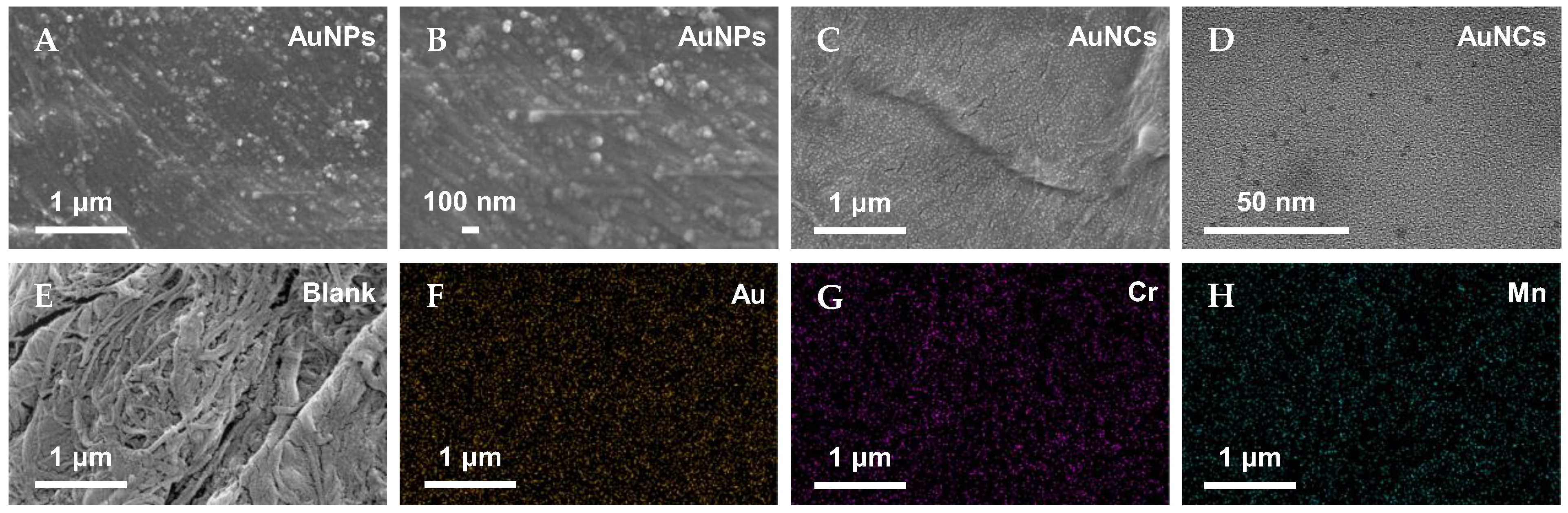
3.2. Characterization of AuNCs-Modified Filter Paper
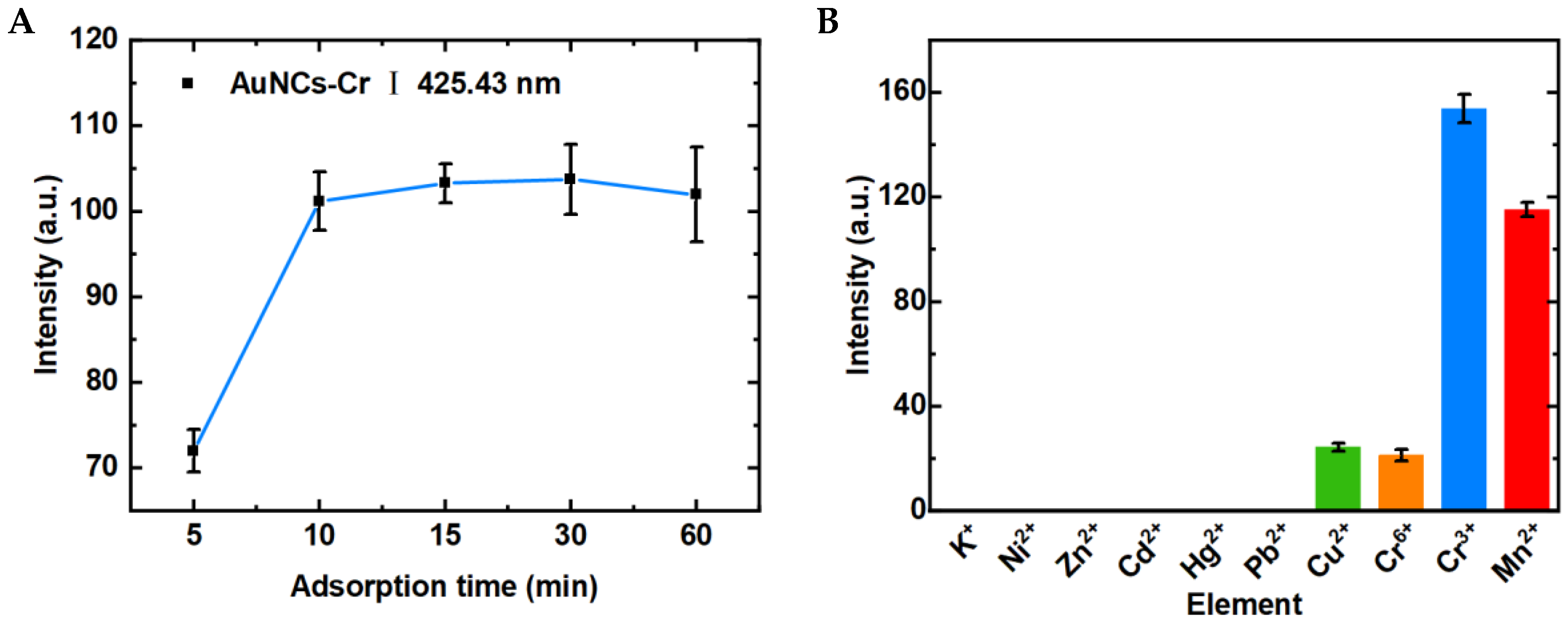
3.3. Optimization of Experimental Conditions
3.4. LIBS-FP-AuNCs for Multi-Element Detection
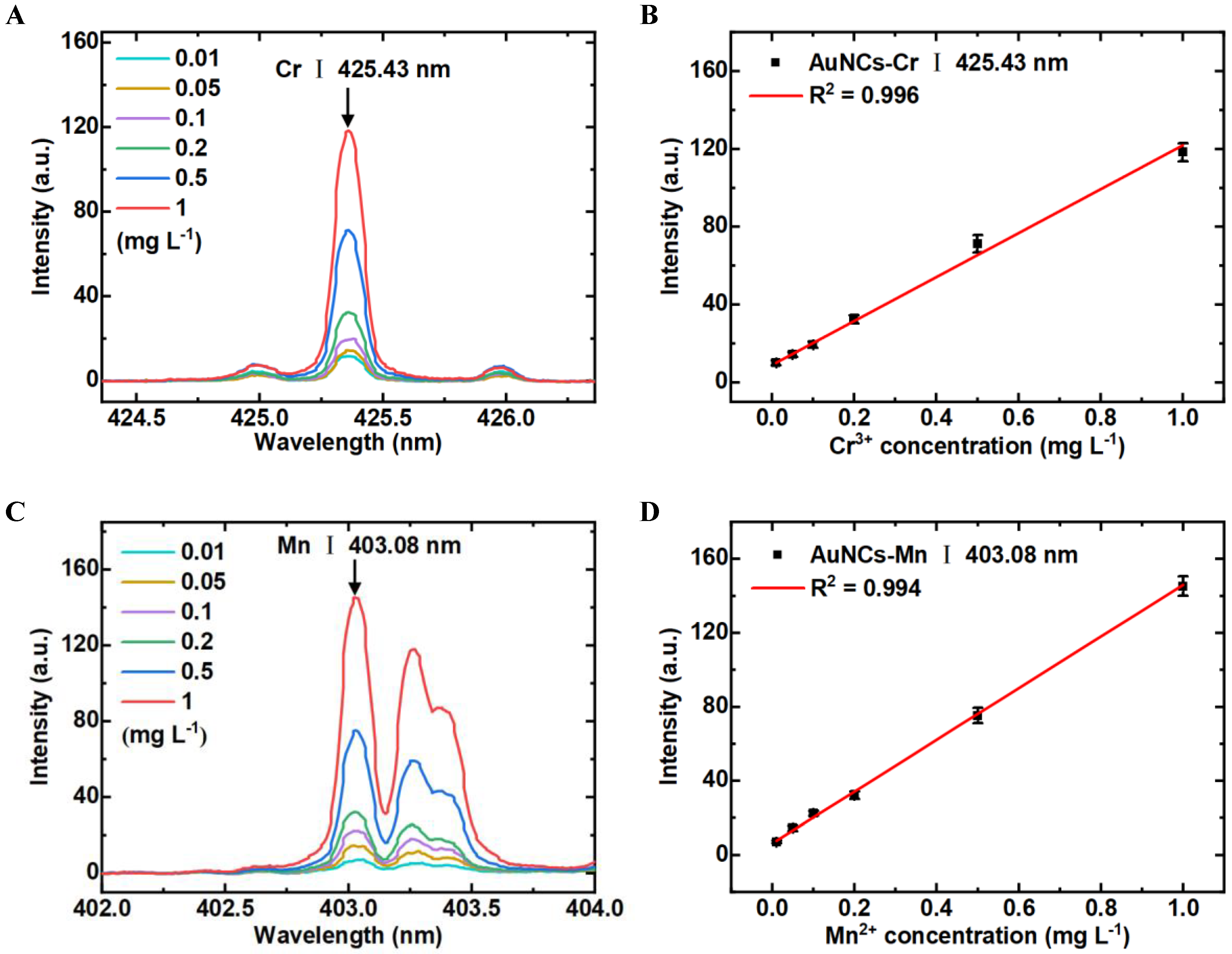
3.5. Detection Sensitivity of LIBS-FP-AuNCs for Cr3+ and Mn2+ Analysis
3.6. Determination of Cr3+ and Mn2+ Ions in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manage. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z.; Qian, W. Impacts of Human Activities and Sampling Strategies on Soil Heavy Metal Distribution in a Rapidly Developing Region of China. Ecotoxicol. Environ. Saf. 2014, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Singh Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global Evaluation of Heavy Metal Content in Surface Water Bodies: A Meta-Analysis Using Heavy Metal Pollution Indices and Multivariate Statistical Analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, X.; Zeng, G.; Li, J. Impacts of Human Activity Modes and Climate on Heavy Metal “Spread” in Groundwater Are Biased. Chemosphere 2016, 152, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, S.; Han, Y.; Bate, B.; Ke, H.; Chen, Y. Soil Heavy Metal Pollution of Industrial Legacies in China and Health Risk Assessment. Sci. Total Environ. 2022, 816, 151632. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of Heavy Metal Pollution and Human Health Risk in Urban Soils of Steel Industrial City (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human Health Risk Assessment of Heavy Metals in Soil and Food Crops in the Pearl River Delta Urban Agglomeration of China. Food Chem. 2020, 316, 126213. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Chartrand, M.S.; Aaseth, J. Manganese Exposure and Neurotoxic Effects in Children. Environ. Res. 2017, 155, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Krum, B.N.; Queirós, L.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Aschner, M. Manganese in the Diet: Bioaccessibility, Adequate Intake, and Neurotoxicological Effects. J. Agric. Food Chem. 2020, 68, 12893–12903. [Google Scholar] [CrossRef]
- Mortada, W.I.; El-Naggar, A.; Mosa, A.; Palansooriya, K.N.; Yousaf, B.; Tang, R.; Wang, S.; Cai, Y.; Chang, S.X. Biogeochemical Behaviour and Toxicology of Chromium in the Soil-Water-Human Nexus: A Review. Chemosphere 2023, 331, 138804. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of Heavy Metals in Soil, Mushroom and Plant Samples by Atomic Absorption Spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. Multi-Element Coprecipitation for Separation and Enrichment of Heavy Metal Ions for Their Flame Atomic Absorption Spectrometric Determinations. J. Hazard. Mater. 2009, 162, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, R.; Rucandio, I.; Gómez-Pinilla, I.; Borlaf, F.; García, F.; Larrea, M.T. Evaluation of Different Digestion Systems for Determination of Trace Mercury in Seaweeds by Cold Vapour Atomic Fluorescence Spectrometry. J. Food Compos. Anal. 2015, 38, 7–12. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Liu, B.; Liu, Y.; Li, S.; Lu, J.; Tian, J.; Zhao, W.; Yang, Z. Simultaneous Speciation of Inorganic Arsenic and Antimony in Water Samples by Hydride Generation-Double Channel Atomic Fluorescence Spectrometry with on-Line Solid-Phase Extraction Using Single-Walled Carbon Nanotubes Micro-Column. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 74–80. [Google Scholar] [CrossRef]
- Pereira, J.S.F.; Moraes, D.P.; Antes, F.G.; Diehl, L.O.; Santos, M.F.P.; Guimarães, R.C.L.; Fonseca, T.C.O.; Dressler, V.L.; Flores, É.M.M. Determination of Metals and Metalloids in Light and Heavy Crude Oil by ICP-MS after Digestion by Microwave-Induced Combustion. Microchem. J. 2010, 96, 4–11. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş. Determination of Trace Elements in Commonly Consumed Medicinal Herbs by ICP-MS and Multivariate analysisICP-MS. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Sharma, B.; Chawla, P.A.; Bhatia, R. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): A Powerful Analytical Technique for Elemental Analysis. Food Anal. Methods 2022, 15, 666–688. [Google Scholar] [CrossRef]
- Bozorgzadeh, E.; Pasdaran, A.; Ebrahimi-Najafabadi, H. Determination of Toxic Heavy Metals in Fish Samples Using Dispersive Micro Solid Phase Extraction Combined with Inductively Coupled Plasma Optical Emission Spectroscopy. Food Chem. 2021, 346, 128916. [Google Scholar] [CrossRef] [PubMed]
- Fortes, F.J.; Moros, J.; Lucena, P.; Cabalín, L.M.; Laserna, J.J. Laser-Induced Breakdown Spectroscopy. Anal. Chem. 2013, 85, 640–669. [Google Scholar] [CrossRef]
- Cao, F.; Jiao, F.; Ma, S.; Dong, D. Laser-Induced Breakdown Spectroscopy Mediated Amplification Sensor for Copper (II) Ions Detection Using Click Chemistry. Sens. Actuators B Chem. 2022, 371, 132594. [Google Scholar] [CrossRef]
- Cremers, D.A.; Chinni, R.C. Laser-Induced Breakdown Spectroscopy—Capabilities and Limitations. Appl. Spectrosc. Rev. 2009, 44, 457–506. [Google Scholar] [CrossRef]
- Yi, R.; Yang, X.; Zhou, R.; Li, J.; Yu, H.; Hao, Z.; Guo, L.; Li, X.; Lu, Y.; Zeng, X. Determination of Trace Available Heavy Metals in Soil Using Laser-Induced Breakdown Spectroscopy Assisted with Phase Transformation Method. Anal. Chem. 2018, 90, 7080–7085. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tang, Z.; Li, Q.; Zhou, R.; Lv, J.; Zhang, W.; Zhan, K.; Li, X.; Zeng, X. Lead of Detection in Rhododendron Leaves Using Laser-Induced Breakdown Spectroscopy Assisted by Laser-Induced Fluorescence. Sci. Total Environ. 2020, 738, 139402. [Google Scholar] [CrossRef] [PubMed]
- Bilge, G.; Sezer, B.; Boyaci, I.H.; Eseller, K.E.; Berberoglu, H. Performance Evaluation of Laser Induced Breakdown Spectroscopy in the Measurement of Liquid and Solid Samples. Spectrochim. Acta Part B At. Spectrosc. 2018, 145, 115–121. [Google Scholar] [CrossRef]
- Babushok, V.I.; DeLucia, F.C.; Gottfried, J.L.; Munson, C.A.; Miziolek, A.W. Double Pulse Laser Ablation and Plasma: Laser Induced Breakdown Spectroscopy Signal Enhancement. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 999–1014. [Google Scholar] [CrossRef]
- Díaz Pace, D.M.; D’Angelo, C.A.; Bertuccelli, D.; Bertuccelli, G. Analysis of Heavy Metals in Liquids Using Laser Induced Breakdown Spectroscopy by Liquid-to-Solid Matrix Conversion. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 929–933. [Google Scholar] [CrossRef]
- Zhang, J.R.; Zeng, A.L.; Luo, H.Q.; Li, N.B. Fluorescent Silver Nanoclusters for Ultrasensitive Determination of Chromium(VI) in Aqueous Solution. J. Hazard. Mater. 2016, 304, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Lu, M.; Du, Y.; Chen, M.; Meng, S.; Ji, W.; Sun, C.; Peng, W. Near-Infrared Band Gold Nanoparticles-Au Film “Hot Spot” Model Based Label-Free Ultratrace Lead (II) Ions Detection via Fiber SPR DNAzyme Biosensor. Sens. Actuators B Chem. 2021, 337, 129816. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M.; Ju, H. Smartphone-Enabled Colorimetric Visual Quantification of Highly Hazardous Trivalent Chromium Ions in Environmental Waters and Catalytic Reduction of p-Nitroaniline by Thiol-Functionalized Gold Nanoparticles. Chemosphere 2023, 340, 139838. [Google Scholar] [CrossRef]
- Ma, S.; Cao, F.; Wen, X.; Xu, F.; Tian, H.; Fu, X.; Dong, D. Detection of Heavy Metal Ions Using Laser-Induced Breakdown Spectroscopy Combined with Filter Paper Modified with PtAg Bimetallic Nanoparticles. J. Hazard. Mater. 2023, 443, 130188. [Google Scholar] [CrossRef]
- Skotadis, E.; Tsekenis, G.; Chatzipetrou, M.; Patsiouras, L.; Madianos, L.; Bousoulas, P.; Zergioti, I.; Tsoukalas, D. Heavy Metal Ion Detection Using DNAzyme-Modified Platinum Nanoparticle Networks. Sens. Actuators B Chem. 2017, 239, 962–969. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, L.; Niu, Q.; Liu, X.; Luo, L.; Jiang, H.; You, T. Highly Fluorescent Magnetic ATT-AuNCs@ZIF-8 for All-in-One Detection and Removal of Hg2+: An Ultrasensitive Probe to Evaluate Its Removal Efficiency. Inorg. Chem. 2023, 62, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hu, B.; Ge, S.; Chi, B.; Yan, X.; Zheng, X. Facile Preparation of Bimetallic Au-Cu Nanoclusters as Fluorescent Nanoprobes for Sensitive Detection of Cr3+ and S2O82− Ions. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2023, 301, 122855. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cao, F.; Zheng, W.; Tian, Y.; Xianyu, Y.; Xu, P.; Zhang, W.; Wang, Z.; Deng, K.; Jiang, X. Detection of the Nanomolar Level of Total Cr[(III) and (VI)] by Functionalized Gold Nanoparticles and a Smartphone with the Assistance of Theoretical Calculation Models. Nanoscale 2015, 7, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, G.; Wang, Z.; Ren, W.; Zhang, Y.; Shen, Z.; Li, T.; Wu, A. Selective Colorimetric Detection of Cr(III) and Cr(VI) Using Gallic Acid Capped Gold Nanoparticles. Dalton Trans. 2016, 45, 8347–8354. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Mehta, V.N.; Bhamore, J.R.; Rao, P.T.; Park, T.-J.; Kailasa, S.K. Diaminodiphenyl Sulfone as a Novel Ligand for Synthesis of Gold Nanoparticles for Simultaneous Colorimetric Assay of Three Trivalent Metal Cations (Al3+, Fe3+ and Cr3+). J. Mol. Liq. 2020, 312, 113409. [Google Scholar] [CrossRef]
- Manjubaashini, N.; Daniel Thangadurai, T.; Bharathi, G.; Nataraj, D. Rhodamine Capped Gold Nanoparticles for the Detection of Cr3+ Ion in Living Cells and Water Samples. J. Lumin. 2018, 202, 282–288. [Google Scholar] [CrossRef]
- Ejeta, S.Y.; Imae, T. Selective Colorimetric and Electrochemical Detections of Cr(III) Pollutant in Water on 3-Mercaptopropionic Acid-Functionalized Gold Plasmon Nanoparticles. Anal. Chim. Acta 2021, 1152, 338272. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dong, C.; Li, Y.; Wang, Z.; Gao, Y.; Shen, Z.; Wu, A. A Novel AgNPs-Based Colorimetric Sensor for Rapid Detection of Cu2+ or Mn2+ via pH Control. RSC Adv. 2015, 5, 20595–20602. [Google Scholar] [CrossRef]
- Annadhasan, M.; Muthukumarasamyvel, T.; Sankar Babu, V.R.; Rajendiran, N. Green Synthesized Silver and Gold Nanoparticles for Colorimetric Detection of Hg2+, Pb2+, and Mn2+ in Aqueous Medium. ACS Sustain. Chem. Eng. 2014, 2, 887–896. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Singhal, R.K.; Saha, S.; Kailasa, S.K. Ultra-Small Two Dimensional MXene Nanosheets for Selective and Sensitive Fluorescence Detection of Ag+ and Mn2+ Ions. Colloids Surf. Physicochem. Eng. Asp. 2019, 565, 70–77. [Google Scholar] [CrossRef]
- George, J.M.; Priyanka, R.N.; Mathew, B. Bimetallic Ag–Au Nanoparticles as pH Dependent Dual Sensing Probe for Mn(II) Ion and Ciprofloxacin. Microchem. J. 2020, 155, 104686. [Google Scholar] [CrossRef]



| Real Sample | Sample No. | Target Ions (Original Concentration) | Added (mg L−1) | Founded (mg L−1) | Recovery |
|---|---|---|---|---|---|
| Tea broth | 1 | Cr (5.20 μg L−1) | 0 | - | - |
| 2 | 0.02 | 0.026 ±0.005 | 102.1% | ||
| 3 | 0.06 | 0.065 ±0.017 | 99.7% | ||
| 4 | 0.3 | 0.288 ±0.018 | 94.6% | ||
| River water | 5 | Mn (0.147 μg L−1) | 0 | - | - |
| 6 | 0.02 | 0.020 ±0.005 | 98.8% | ||
| 7 | 0.06 | 0.063 ±0.007 | 104.6% | ||
| 8 | 0.3 | 0.316 ±0.025 | 105.1% |
| Material | Existing Form | Target Ions | Detection Method | LOD | Ref. |
|---|---|---|---|---|---|
| GA-AuNPs | Liquid | Cr3+ | Colorimetric | 1.5 μM | [36] |
| DDS-AuNPs | Liquid | Cr3+ | UV–vis spectra | 0.78 μM | [37] |
| Rh6G-AuNPs | Liquid | Cr3+ | Fluorescence | 9.28 µM | [38] |
| AuNPs@3-mpa | Liquid | Cr3+ | Electrochemistry | 5.36 µM | [39] |
| AgNPs | Liquid | Mn2+ | Colorimetric | 0.5 µM | [40] |
| SiNPs | Liquid | Mn2+ | UV–vis spectra | 0.54 μM | [41] |
| Ti3C2 MXene nanosheets | Liquid | Mn2+ | Fluorescence | 0.11 μM | [42] |
| (Ag-Au)mixNPs | Liquid | Mn2+ | Electrochemistry | 8.42 μM | [43] |
| AuNCs | Solid | Cr3+ | LIBS | 0.15 μM | This work |
| Mn2+ | 0.17 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Song, C.; Ma, S.; Cao, F.; Dong, D. Rapid Determination of Cr3+ and Mn2+ in Water Using Laser-Induced Breakdown Spectroscopy Combined with Filter Paper Modified with Gold Nanoclusters. Biosensors 2024, 14, 267. https://doi.org/10.3390/bios14060267
Dai X, Song C, Ma S, Cao F, Dong D. Rapid Determination of Cr3+ and Mn2+ in Water Using Laser-Induced Breakdown Spectroscopy Combined with Filter Paper Modified with Gold Nanoclusters. Biosensors. 2024; 14(6):267. https://doi.org/10.3390/bios14060267
Chicago/Turabian StyleDai, Xuan, Changbo Song, Shixiang Ma, Fengjing Cao, and Daming Dong. 2024. "Rapid Determination of Cr3+ and Mn2+ in Water Using Laser-Induced Breakdown Spectroscopy Combined with Filter Paper Modified with Gold Nanoclusters" Biosensors 14, no. 6: 267. https://doi.org/10.3390/bios14060267
APA StyleDai, X., Song, C., Ma, S., Cao, F., & Dong, D. (2024). Rapid Determination of Cr3+ and Mn2+ in Water Using Laser-Induced Breakdown Spectroscopy Combined with Filter Paper Modified with Gold Nanoclusters. Biosensors, 14(6), 267. https://doi.org/10.3390/bios14060267




