NIR-II Fluorescent Probes for Fluorescence-Imaging-Guided Tumor Surgery
Abstract
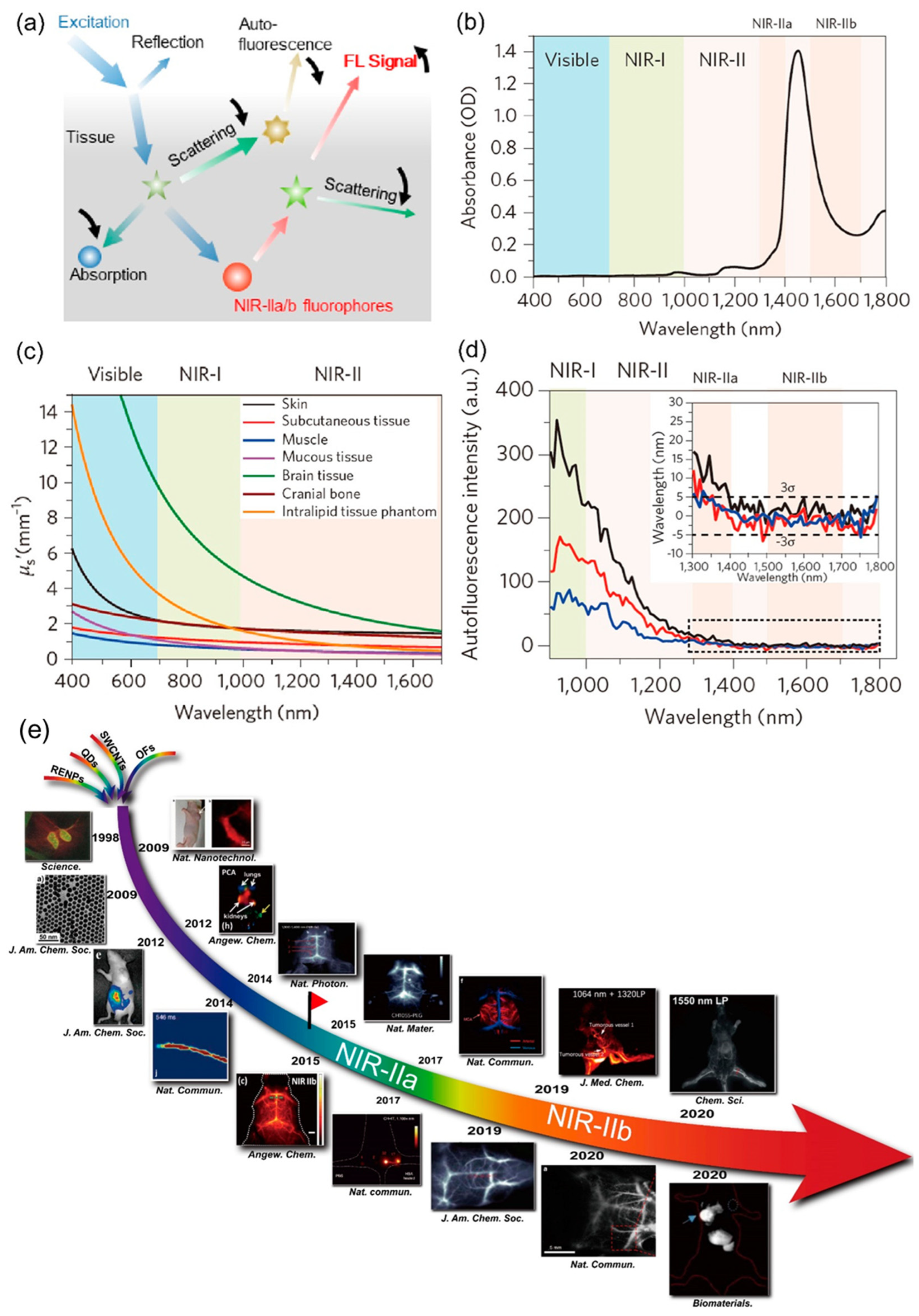
:1. Introduction
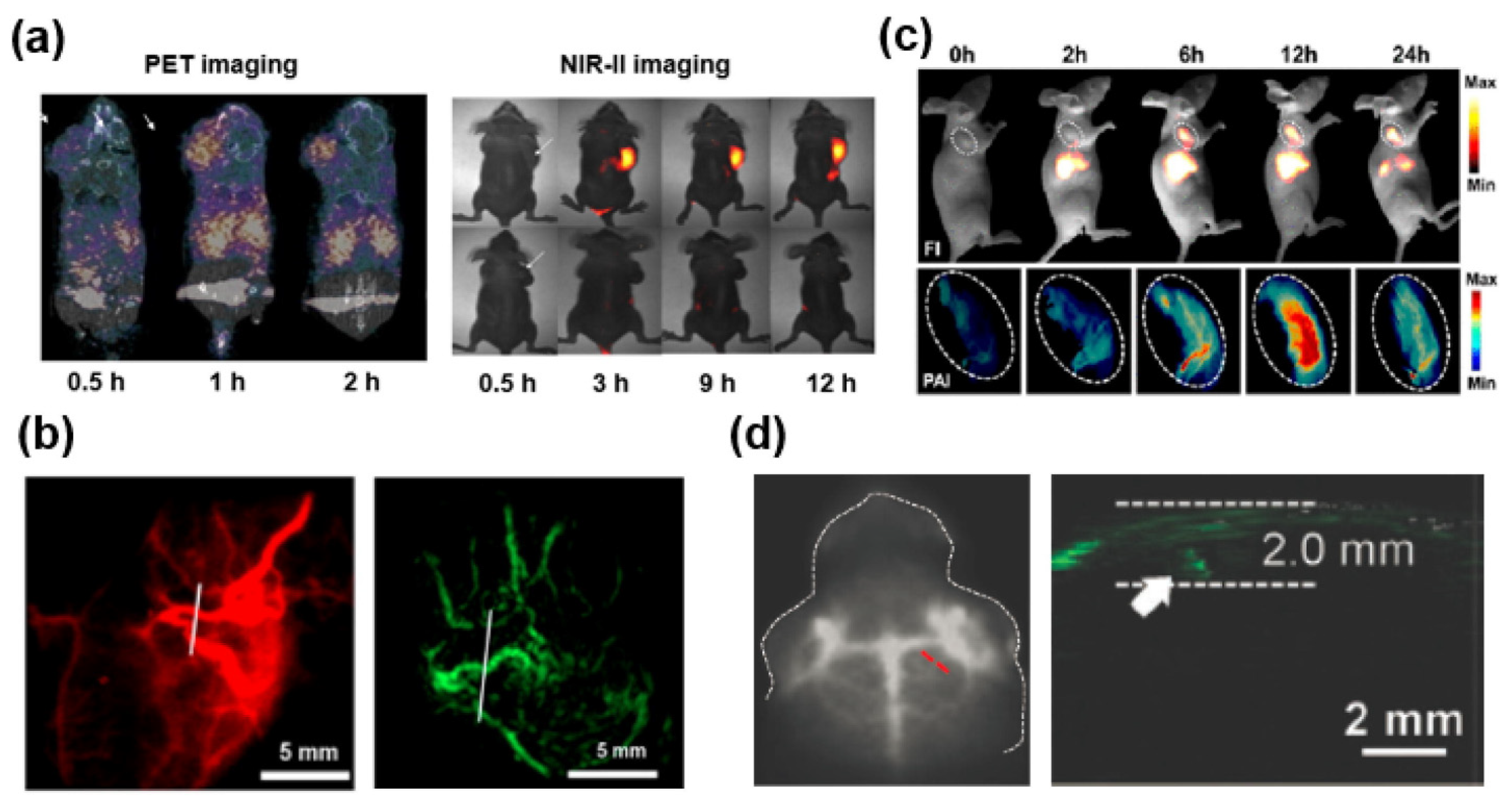
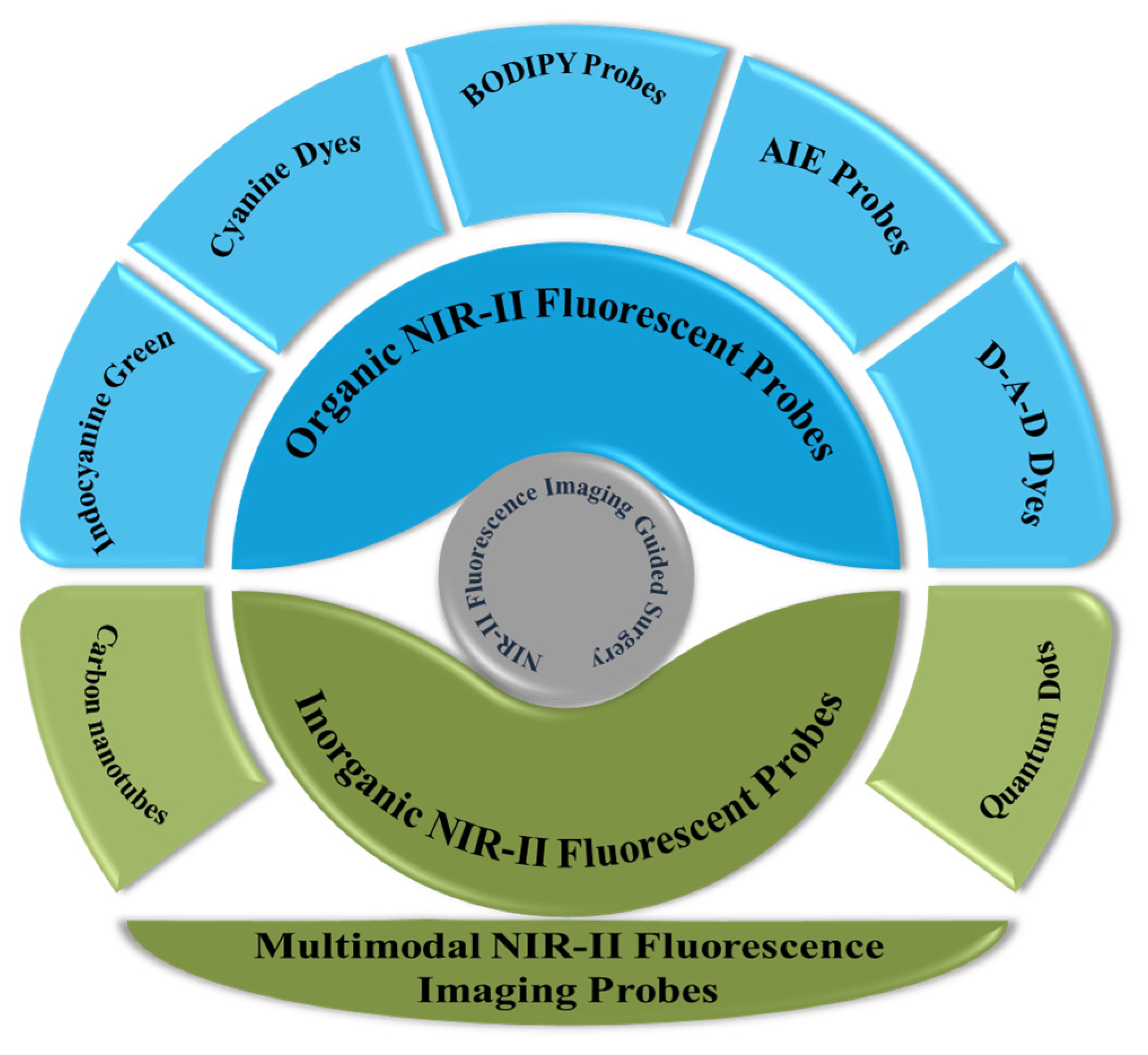
2. NIR-II Fluorescent Probes for IGS
2.1. Organic Probes
2.1.1. Indocyanine Green: Food and Drug Administration (FDA)-Approved Probe
2.1.2. Cyanine Dyes
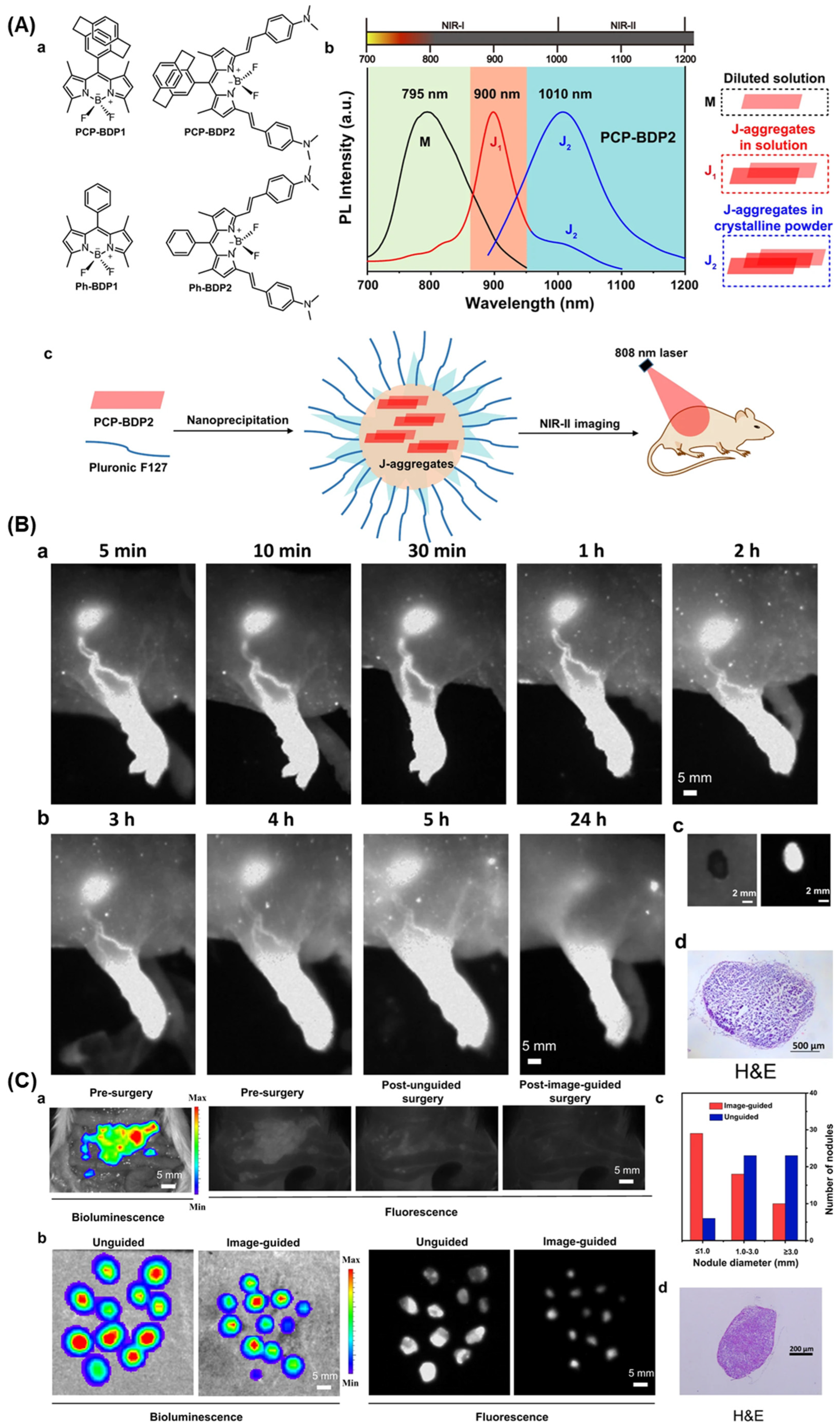
2.1.3. BODIPY Probes
2.1.4. AIE Probes
2.1.5. Donor–Acceptor–Donor Dyes
2.2. Inorganic Probes
2.2.1. Carbon Nanotubes
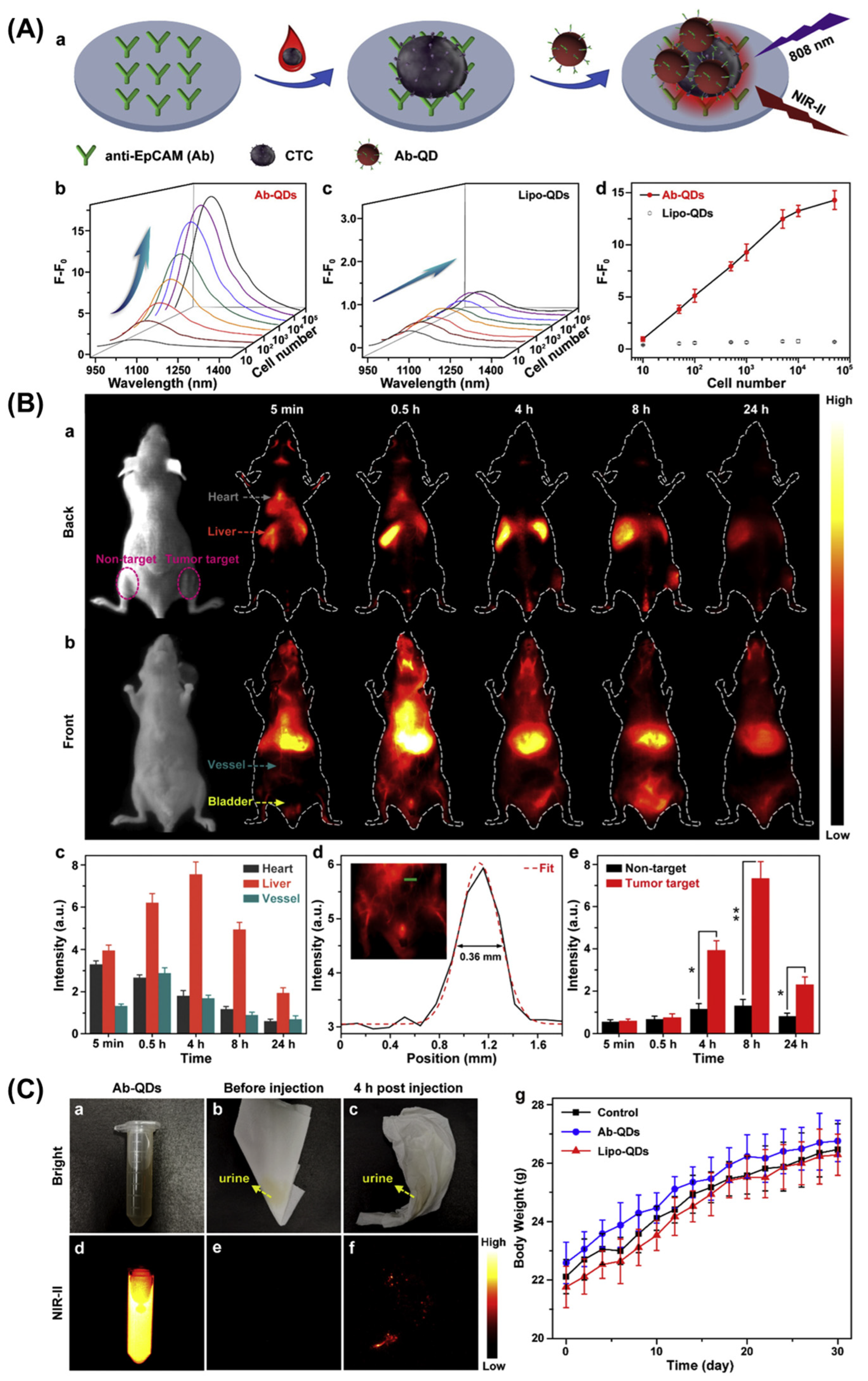
2.2.2. Quantum Dots
2.3. Multimodal NIR-II Fluorescence Imaging Probes
3. Future Prospects and Challenges
4. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Tian, J.; Chen, X. Intraoperative Imaging-Guided Cancer Surgery: From Current Fluorescence Molecular Imaging Methods to Future Multi-Modality Imaging Technology. Theranostics 2014, 4, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Chen, H.; Irfan, M.; Bajwa, S.Z.; Khan, W.S.; Baig, S.M.; Dai, Z. Fluorescence Guided Sentinel Lymph Node Mapping: From Current Molecular Probes to Future Multimodal Nanoprobes. Bioconjugate Chem. 2019, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. New Technologies for Human Cancer Imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, B.; Zhang, H.; Zhang, F. Activatable fluorescence sensors for in vivo bio-detection in the second near-infrared window. Chem. Sci. 2021, 12, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Mulder, B.G.S.; Mieog, J.S.D.; Swijnenburg, R.J.; Van De Velde, C.J.H.; Farina Sarasqueta, A.; Bonsing, B.A.; Framery, B.; Pèlegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Yim, J.J.; Bogyo, M. A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.; Van Der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Guo, Y.; Chang, X.; Ma, R.; Ye, X.; Cheng, H.; Li, Y.; Cui, H. Evaluation of a novel ovarian cancer-specific fluorescent antibody probe for targeted near-infrared fluorescence imaging. World J. Surg. Oncol. 2020, 18, 66. [Google Scholar] [CrossRef]
- Shao, W.; Chen, G.Y.; Kuzmin, A.; Kutscher, H.L.; Pliss, A.; Ohulchanskyy, T.Y.; Prasad, P.N. Tunable narrow band emissions from dye-sensitized core/shell/shell nanocrystals in the second near-infrared biological window. J. Am. Chem. Soc. 2016, 138, 16192–16195. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, Q.; Zeng, X.; Tian, T.; Zhou, W.; Cui, Y.; Wang, X.; Cheng, X.; Ding, Q.; et al. Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm. Chem. Sci. 2020, 11, 2621–2626. [Google Scholar] [CrossRef]
- Tang, Y.; Pei, F.; Lu, X.; Fan, Q.; Huang, W. Recent Advances on Activatable NIR-II Fluorescence Probes for Biomedical Imaging. Adv. Opt. Mater. 2019, 7, 1900917. [Google Scholar] [CrossRef]
- Huang, H.; Ali, A.; Liu, Y.; Xie, H.; Ullah, S.; Roy, S.; Song, Z.; Guo, B.; Xu, J. Advances in image-guided drug delivery for antibacterial therapy. Adv. Drug Deliv. Rev. 2023, 192, 114634. [Google Scholar] [CrossRef]
- Roy, S.; Bag, N.; Bardhan, S.; Hasan, I.; Guo, B. Recent progress in NIR-II fluorescence imaging-guided drug delivery for cancer theranostics. Adv. Drug Deliv. Rev. 2023, 197, 114821. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Li, Z.; Qu, L.; Guo, W. Natural flavylium-inspired far-red to NIR-II dyes and their applications as fluorescent probes for biomedical sensing. Chem. Soc. Rev. 2022, 51, 7170–7205. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2021, 122, 209–268. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, e1900321. [Google Scholar] [CrossRef]
- Li, S.; Cheng, D.; He, L.; Yuan, L. Recent Progresses in NIR-I/II Fluorescence Imaging for Surgical Navigation. Front Bioeng. Biotechnol. 2021, 9, 768698. [Google Scholar] [CrossRef]
- Yang, R.; Lou, K.; Wang, P.; Gao, Y.; Zhang, Y.; Chen, M.; Huang, W.; Zhang, G. Surgical Navigation for Malignancies Guided by Near-Infrared-II Fluorescence Imaging. Small Methods 2021, 5, e2001066. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Zhu, S. NIR-II cyanine@albumin fluorophore for deep tissue imaging and imaging-guided surgery. SmartMat 2023, e1245. [Google Scholar] [CrossRef]
- Ren, F.; Li, T.; Yao, T.; Chen, G.; Li, C.; Wang, Q. Near-Infrared-II Fluorophores for In Vivo Multichannel Biosensing. Chemosensors 2023, 11, 433. [Google Scholar] [CrossRef]
- Feng, X.; Wei, L.; Liu, Y.; Chen, X.; Tian, R. Orchestrated Strategies for Developing Fluorophores for NIR-II Imaging. Adv. Healthc. Mater. 2023, 12, e2300537. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew. Chem. Int. Ed. 2021, 60, 16294–16308. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, F.; Jiang, G.; Wang, J. Applications of indocyanine green based near-infrared fluorescence imaging in thoracic surgery. J. Thorac. Dis. 2016, 8 (Suppl. S9), S738–S743. [Google Scholar] [CrossRef]
- Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K.B.; Annapragada, A. NIR-II fluorescence imaging using indocyanine green nanoparticles. Sci. Rep. 2018, 8, 14455. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Luo, Q.; Li, Y.; Lin, X.; Fan, L.; Zhang, Y.; Liu, J.; Liu, X. Fabrication of Red Blood Cell-Based Multimodal Theranostic Probes for Second Near-Infrared Window Fluorescence Imaging-Guided Tumor Surgery and Photodynamic Therapy. Theranostics 2019, 9, 369–380. [Google Scholar] [CrossRef]
- Liu, K.; Liu, X.; Zeng, Q.; Zhang, Y.; Tu, L.; Liu, T.; Kong, X.; Wang, Y.; Cao, F.; Lambrechts, S.A.G.; et al. Covalently Assembled NIR Nanoplatform for Simultaneous Fluorescence Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano 2012, 6, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, B.; Zhao, M.; Wang, S.; Lei, Z.; Lu, L.; Zhang, H.; Feng, L.; Dou, C.; Yin, D.; et al. J-Aggregates of Cyanine Dye for NIR-II in Vivo Dynamic Vascular Imaging beyond 1500 nm. J. Am. Chem. Soc. 2019, 141, 19221–19225. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, Z.; Tian, R.; Yung, B.C.; Yang, Q.; Zhao, S.; Kiesewetter, D.O.; Niu, G.; Sun, H.; Antaris, A.L.; et al. Repurposing Cyanine NIR-I Dyes Accelerates Clinical Translation of Near-Infrared-II (NIR-II) Bioimaging. Adv. Mater. 2018, 30, e1802546. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, C.; Hu, Z.; Liu, H.; Wang, J.; Wang, Y.; Wang, X.; Ma, R.; Zhang, X.; Sun, H.; et al. High brightness NIR-II nanofluorophores based on fused-ring acceptor molecules. Nano Res. 2020, 13, 2570–2575. [Google Scholar] [CrossRef]
- Dai, H.; Shen, Q.; Shao, J.; Wang, W.; Gao, F.; Dong, X. Small Molecular NIR-II Fluorophores for Cancer Phototheranostics. Innov. 2021, 2, 100082. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sofias, A.M.; Lammers, T.; Xu, J. Image-guided drug delivery: Nanoparticle and probe advances. Adv. Drug Deliv. Rev. 2024, 206, 115188. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Feng, X.; Wei, L.; Dai, D.; Ma, Y.; Pan, H.; Ge, S.; Bai, L.; Ke, C.; Liu, Y.; et al. A genetic engineering strategy for editing near-infrared-II fluorophores. Nat. Commun. 2022, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Yalamarty SS, K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-Based Nanomedicines in Cancer Phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yan, Q.; Lv, X.; Zhu, Y.; Xin, K.; Shi, B.; Wang, R.; Chen, J.; Gao, W.; Shi, P.; et al. Imaging of Colorectal Cancers Using Activatable Nanoprobes with Second Near-Infrared Window Emission. Angew. Chem. 2018, 130, 3688–3692. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Huang, C.; Zhao, S.; Zhu, S.; Liu, R.; Zhu, H. Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules 2023, 28, 2997. [Google Scholar] [CrossRef]
- Li, K.; Duan, X.; Jiang, Z.; Chen, Y.; Zhang, G.; Liu, Z. J-aggregates of meso-[2.2]paracyclophanyl-BODIPY dye for NIR-II imaging. Nat. Commun. 2021, 12, 2376. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liu, W.; Li, H.; Sun, W.; Du, J.; Fan, J.; Peng, X. 2,1,3-Benzothiadiazole derivative AIEgens for smart phototheranostics. Coord. Chem. Rev. 2022, 473, 214803. [Google Scholar] [CrossRef]
- Alifu, N.; Zebibula, A.; Qi, J.; Zhang, H.; Sun, C.; Yu, X.; Xue, D.; Lam, J.W.Y.; Li, G.; Qian, J.; et al. Single-Molecular Near-Infrared-II Theranostic Systems: Ultrastable Aggregation-Induced Emission Nanoparticles for Long-Term Tracing and Efficient Photothermal Therapy. ACS Nano 2018, 12, 11282–11293. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xia, Q.; Zhang, Y.; Li, Y.; Feng, Z.; Zhou, J.; Qi, J.; Tang, B.Z.; Qian, J.; Lin, H. Aggregation-Induced Emission (AIE) Nanoparticles-Assisted NIR-II Fluorescence Imaging-Guided Diagnosis and Surgery for Inflammatory Bowel Disease (IBD). Adv. Healthc. Mater. 2021, 10, 2101043. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qu, C.; Chen, H.; He, M.; Tang, C.; Shou, K.; Hong, S.; Yang, M.; Jiang, Y.; Ding, B.; et al. Novel benzo-bis(1,2,5-thiadiazole) fluorophores for in vivo NIR-II imaging of cancer. Chem. Sci. 2016, 7, 6203–6207. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, X.; Li, J.; Wei, J. A short review on NIR-II organic small molecule dyes. Dye. Pigment. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Tian, X.; Liu, H.; Wei, F.; Wang, X.; Zhao, S.; Liu, C.; Tse, Y.C.; Wong, K.M.-C. A Deep-Red to Near Infrared (NIR) Fluorescent Probe Based on a Sulfur-Modified Rhodamine Derivative with Two Spirolactone Rings. ChemPlusChem 2020, 85, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Takemasa, I.; Kotake, M.; Noura, S.; Kimura, K.; Suwa, H.; Tei, M.; Takano, Y.; Munakata, K.; Matoba, S.; et al. Blood Perfusion Assessment by Indocyanine Green Fluorescence Imaging for Minimally Invasive Rectal Cancer Surgery (EssentiAL trial): A Randomized Clinical Trial. Ann. Surg. 2023, 278, e688. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Ma, H.; Zhu, S.; Lau, J.; Ma, R.; Liu, Y.; Lin, L.; Chandra, S.; Wang, S.; Zhu, X.; et al. Multiplexed NIR-II Probes for Lymph Node-Invaded Cancer Detection and Imaging-Guided Surgery. Adv. Mater. 2020, 32, e1907365. [Google Scholar] [CrossRef]
- Kosuge, H.; Sherlock, S.P.; Kitagawa, T.; Dash, R.; Robinson, J.T.; Dai, H.; McConnell, M.V. Near Infrared Imaging and Photothermal Ablation of Vascular Inflammation Using Single-Walled Carbon Nanotubes. J. Am. Heart Assoc. 2012, 1, e002568. [Google Scholar] [CrossRef]
- Mandal, A.K.; Wu, X.; Ferreira, J.S.; Kim, M.; Powell, L.R.; Kwon, H.; Groc, L.; Wang, Y.; Cognet, L. Fluorescent sp(3) Defect-Tailored Carbon Nanotubes Enable NIR-II Single Particle Imaging in Live Brain Slices at Ultra-Low Excitation Doses. Sci. Rep. 2020, 10, 5286. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Soh, H.T.; Cassell, A.M.; Quate, C.F.; Dai, H. Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers. Nature 1998, 395, 878–881. [Google Scholar] [CrossRef]
- Ceppi, L.; Bardhan, N.M.; Na, Y.; Siegel, A.; Rajan, N.; Fruscio, R.; Del Carmen, M.G.; Belcher, A.M.; Birrer, M.J. Real-Time Single-Walled Carbon Nanotube-Based Fluorescence Imaging Improves Survival after Debulking Surgery in an Ovarian Cancer Model. ACS Nano 2019, 13, 5356–5365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Huang, H.; Xie, H.; Zhang, B.; Xia, W.; Guo, B. Multifunctional nanotheranostics for near infrared optical imaging-guided treatment of brain tumors. Adv. Drug Deliv. Rev. 2022, 190, 114536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.-Y.; Li, L.; Yu, M. Doping Ag2S quantum dots with Pb yields significantly enhanced in vivo fluorescence imaging in the NIR-II window and comparable toxic effects. New J. Chem. 2023, 47, 15998–16011. [Google Scholar] [CrossRef]
- Shu, Y.; Yan, J.; Lu, Q.; Ji, Z.; Jin, D.; Xu, Q.; Hu, X. Pb ions enhanced fluorescence of Ag2S QDs with tunable emission in the NIR-II window: Facile one pot synthesis and their application in NIR-II fluorescent bio-sensing. Sens. Actuators B Chem. 2020, 307, 127593. [Google Scholar] [CrossRef]
- Ding, C.; Huang, Y.; Shen, Z.; Chen, X. Synthesis and Bioapplications of Ag2S Quantum Dots with Near-Infrared Fluorescence. Adv. Mater. 2021, 33, 2007768. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lin, Y.; Tian, Z.; Zhu, D.; Zhang, Z.; Pang, D. Ultrasmall Pb:Ag2S Quantum Dots with Uniform Particle Size and Bright Tunable Fluorescence in the NIR-II Window. Small 2018, 14, e1703296. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Tu, D.; Hu, P.; Song, X.; Gong, Z.; Chen, T.; Song, J.; Chen, Z.; Chen, X. Broadband excitable NIR-II luminescent nano-bioprobes based on CuInSe2 quantum dots for the detection of circulating tumor cells. Nano Today 2020, 35, 100943. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Liu, W.; Shan, G.; Wang, X. Biocompatible BSA-Ag2S nanoparticles for photothermal therapy of cancer. Colloids Surf. B Biointerfaces 2022, 211, 112295. [Google Scholar] [CrossRef]
- Ni, D.; Ehlerding, E.B.; Cai, W. Multimodality Imaging Agents with PET as the Fundamental Pillar. Angew. Chem. Int. Ed. 2018, 58, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zeng, X.; Xiao, Y.; Liu, C.; Zhu, H.; Zhou, H.; Chen, Z.; Xu, F.; Wang, J.; Zhu, M.; et al. Novel dual-function near-infrared II fluorescence and PET probe for tumor delineation and image-guided surgery. Chem. Sci. 2018, 9, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, H.; Tang, L.; Wang, Y.; Li, Y.; Liu, N.; Zeng, X.; Yan, Y.; Wu, J.; Chen, S.; et al. Mn-Loaded apolactoferrin dots for in vivo MRI and NIR-II cancer imaging. J. Mater. Chem. C 2019, 7, 9448–9454. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Chen, H.; Jenkins, C.H.; Zhang, G.; Zhao, W.; Zhang, Z.; Han, F.; Fung, J.; Yang, M.; Jiang, Y.; et al. Synthesis, Characterization, and Biomedical Applications of a Targeted Dual-Modal Near-Infrared-II Fluorescence and Photoacoustic Imaging Nanoprobe. ACS Nano 2017, 11, 12276–12291. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Xu, L.; Xu, Y.; Lin, Y.; Zhang, Y.; Sun, Y.; Yang, G.-F. Rational Design of a Multifunctional Molecular Dye with Single Dose and Laser for Efficiency NIR-II Fluorescence/Photoacoustic Imaging Guided Photothermal Therapy. Anal. Chem. 2019, 91, 12476–12483. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Guo, B.; Hu, D.; Xu, S.; Wu, W.; Liew, W.H.; Yao, K.; Jiang, J.; Liu, C.; Zheng, H.; et al. Bright Aggregation-Induced-Emission Dots for Targeted Synergetic NIR-II Fluorescence and NIR-I Photoacoustic Imaging of Orthotopic Brain Tumors. Adv. Mater. 2018, 30, e1800766. [Google Scholar] [CrossRef]
- Sobhana, S.; Sarathy, N.P.; Karthikeyan, L.; Shanthi, K.; Vivek, R. Ultra-small NIR-Responsive Nanotheranostic Agent for Targeted Photothermal Ablation Induced Damage-Associated Molecular Patterns (DAMPs) from Post-PTT of Tumor Cells Activate Immunogenic Cell Death. Nanotheranostics 2023, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Y.; Zhang, Y.; Kim, H.S.; Sharma, A.; Gao, J.; Yang, G.; Kim, J.S.; Sun, Y. Rational design of a multifunctional molecular dye for dual-modal NIR-II/photoacoustic imaging and photothermal therapy. Chem. Sci. 2019, 10, 8348–8353. [Google Scholar] [CrossRef]
- Cen, P.; Huang, J.; Jin, C.; Wang, J.; Wei, Y.; Zhang, H.; Tian, M. Aggregation-induced emission luminogens for in vivo molecular imaging and theranostics in cancer. Aggregate 2023, 4, e352. [Google Scholar] [CrossRef]
- Tong, S.; Xu, W.; Zhong, J.; Kang, M.; Chen, X.; Zhang, Y.; Huang, J.; Li, Z.; Zhang, C.; Gao, Z.; et al. De Novo Design of Aggregation-Induced Emission Luminogen for Three-Photon Fluorescence Imaging of Subcortical Structures Excited at Both NIR-III and NIR-IV Windows. Adv. Funct. Mater. 2023, 33, 2305521. [Google Scholar] [CrossRef]
- Kamimura, M.; Matsumoto, T.; Suyari, S.; Umezawa, M.; Soga, K. Ratiometric near-infrared fluorescence nanothermometry in the OTN-NIR (NIR II/III) biological window based on rare-earth doped β-NaYF4 nanoparticles. J. Mater. Chem. B 2017, 5, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhong, D.; Zhou, S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J. Mater. Chem. B 2018, 6, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Takahiro, S.; Yoshida, M.; Hashimoto, Y.; Fukushima, R.; Soga, K. Over-1000 nm near-infrared fluorescent biodegradable polymer nanoparticles for deep tissue in vivo imaging in the second biological window. Polym. J. 2017, 49, 799–803. [Google Scholar] [CrossRef]
- Chihara, T.; Umezawa, M.; Miyata, K.; Sekiyama, S.; Hosokawa, N.; Okubo, K.; Kamimura, M.; Soga, K. Biological Deep Temperature Imaging with Fluorescence Lifetime of Rare-Earth-Doped Ceramics Particles in the Second NIR Biological Window. Sci. Rep. 2019, 9, 12806. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, Z.; Roy, S.; Gu, J.; Ko Soe, S.; Jin, J.; Guo, B. NIR-II Fluorescent Probes for Fluorescence-Imaging-Guided Tumor Surgery. Biosensors 2024, 14, 282. https://doi.org/10.3390/bios14060282
Ullah Z, Roy S, Gu J, Ko Soe S, Jin J, Guo B. NIR-II Fluorescent Probes for Fluorescence-Imaging-Guided Tumor Surgery. Biosensors. 2024; 14(6):282. https://doi.org/10.3390/bios14060282
Chicago/Turabian StyleUllah, Zia, Shubham Roy, Jingshi Gu, Sai Ko Soe, Jian Jin, and Bing Guo. 2024. "NIR-II Fluorescent Probes for Fluorescence-Imaging-Guided Tumor Surgery" Biosensors 14, no. 6: 282. https://doi.org/10.3390/bios14060282
APA StyleUllah, Z., Roy, S., Gu, J., Ko Soe, S., Jin, J., & Guo, B. (2024). NIR-II Fluorescent Probes for Fluorescence-Imaging-Guided Tumor Surgery. Biosensors, 14(6), 282. https://doi.org/10.3390/bios14060282






