Development of a Tetherless Bioimpedance Device That Uses Morphologic Changes to Predict Blood Flow Restrictions Mimicking Peripheral Artery Disease Progression
Abstract
:1. Introduction
2. Materials and Methods
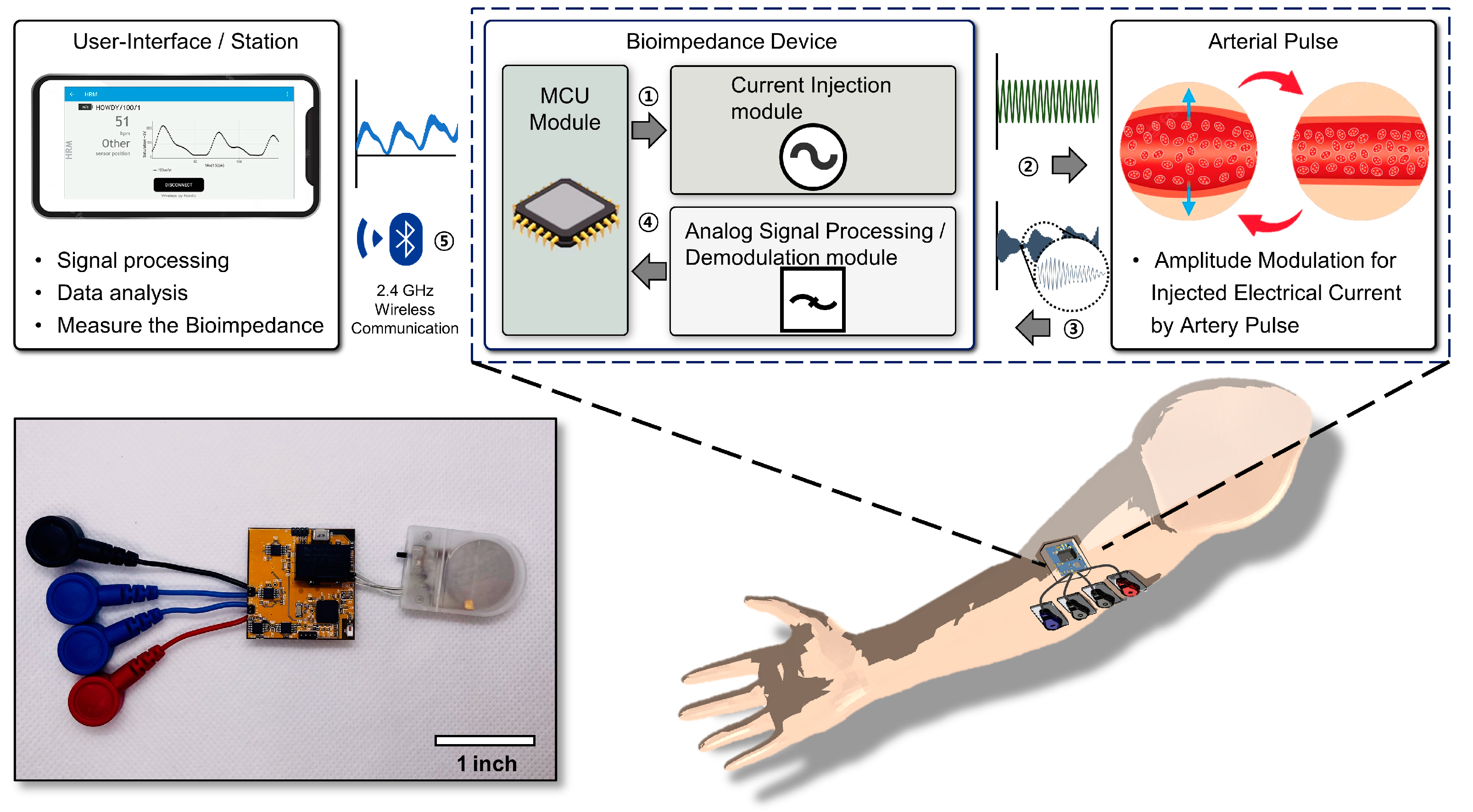
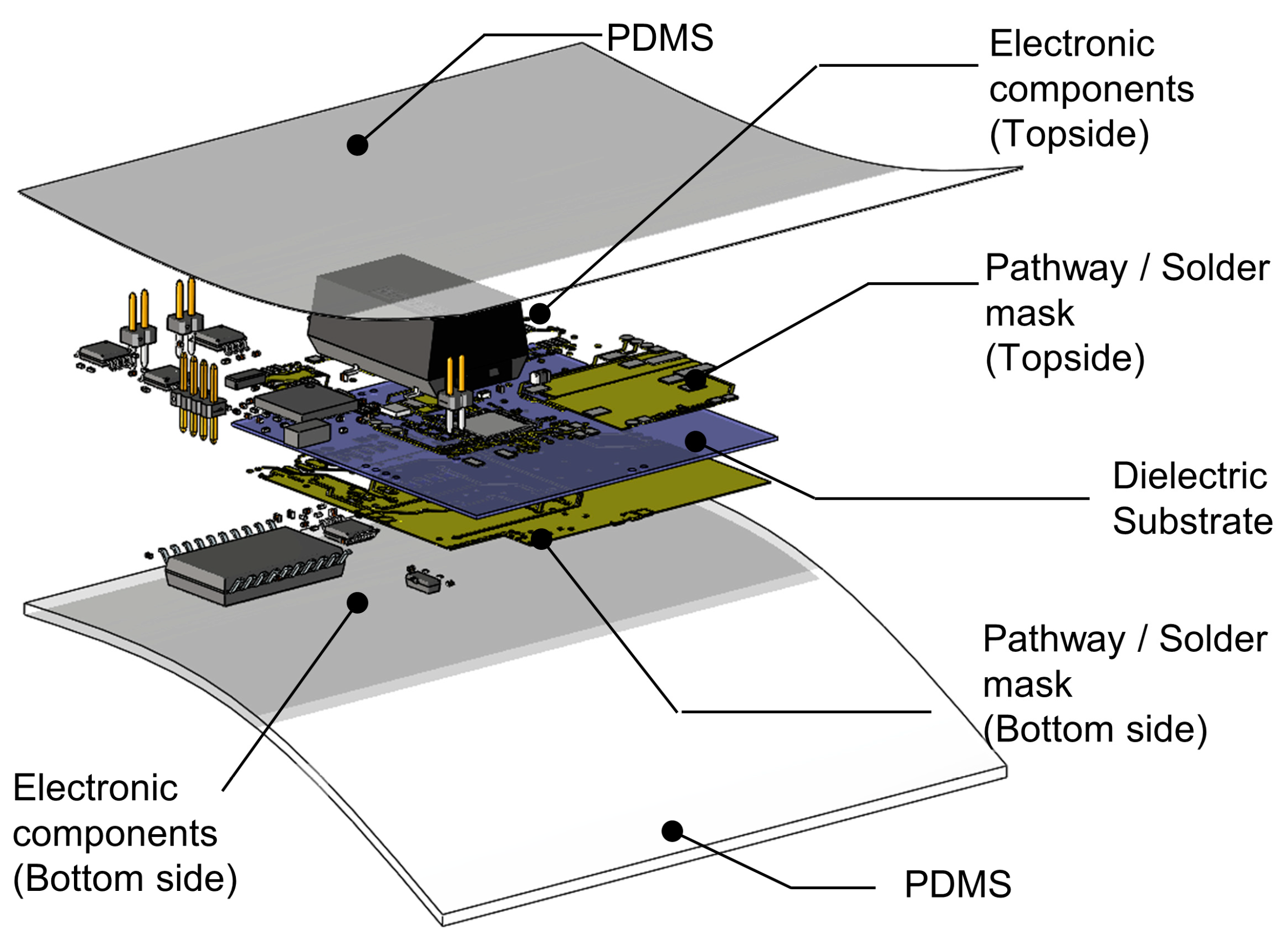
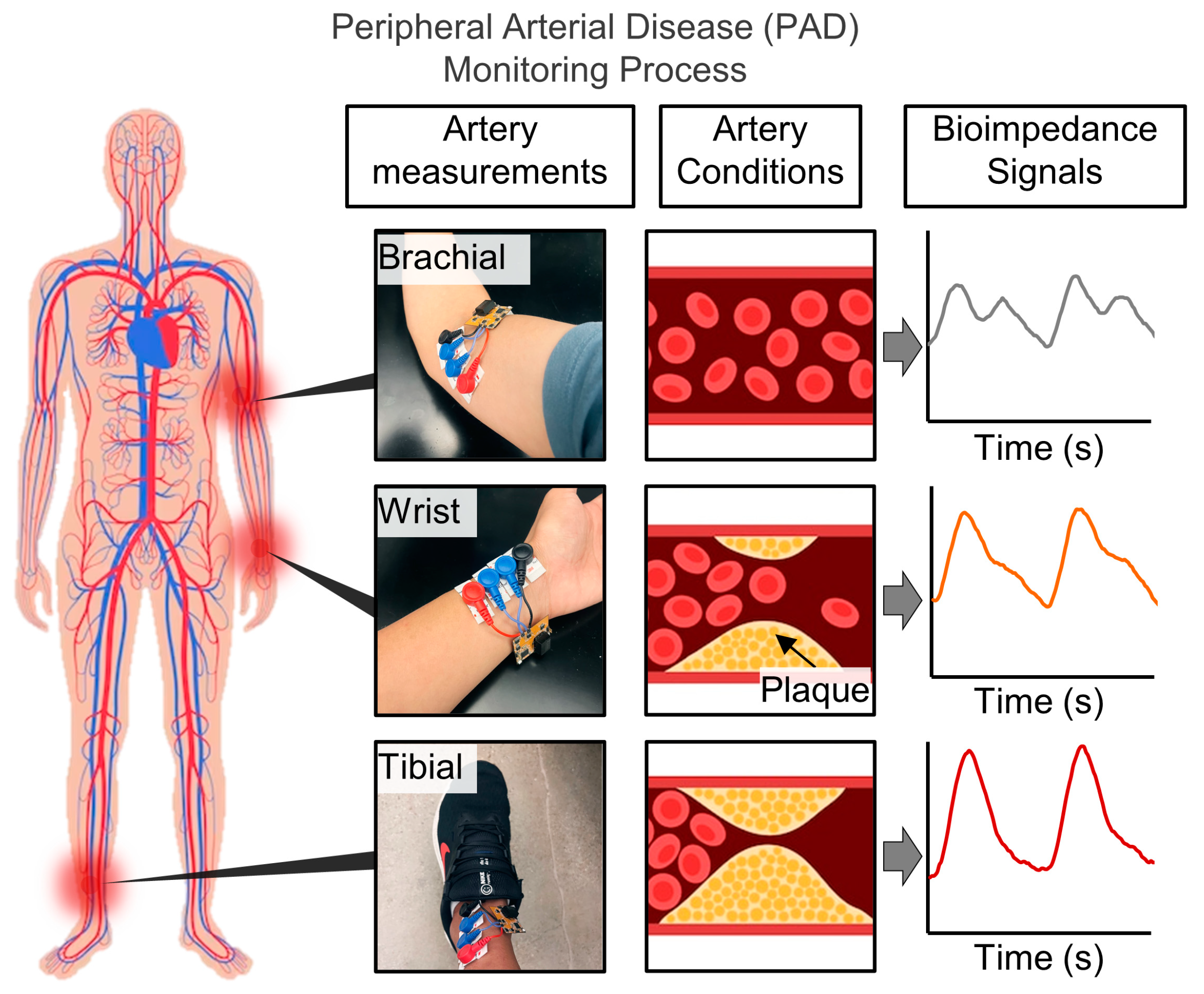
2.1. System Illustration and Target Locations on the Body
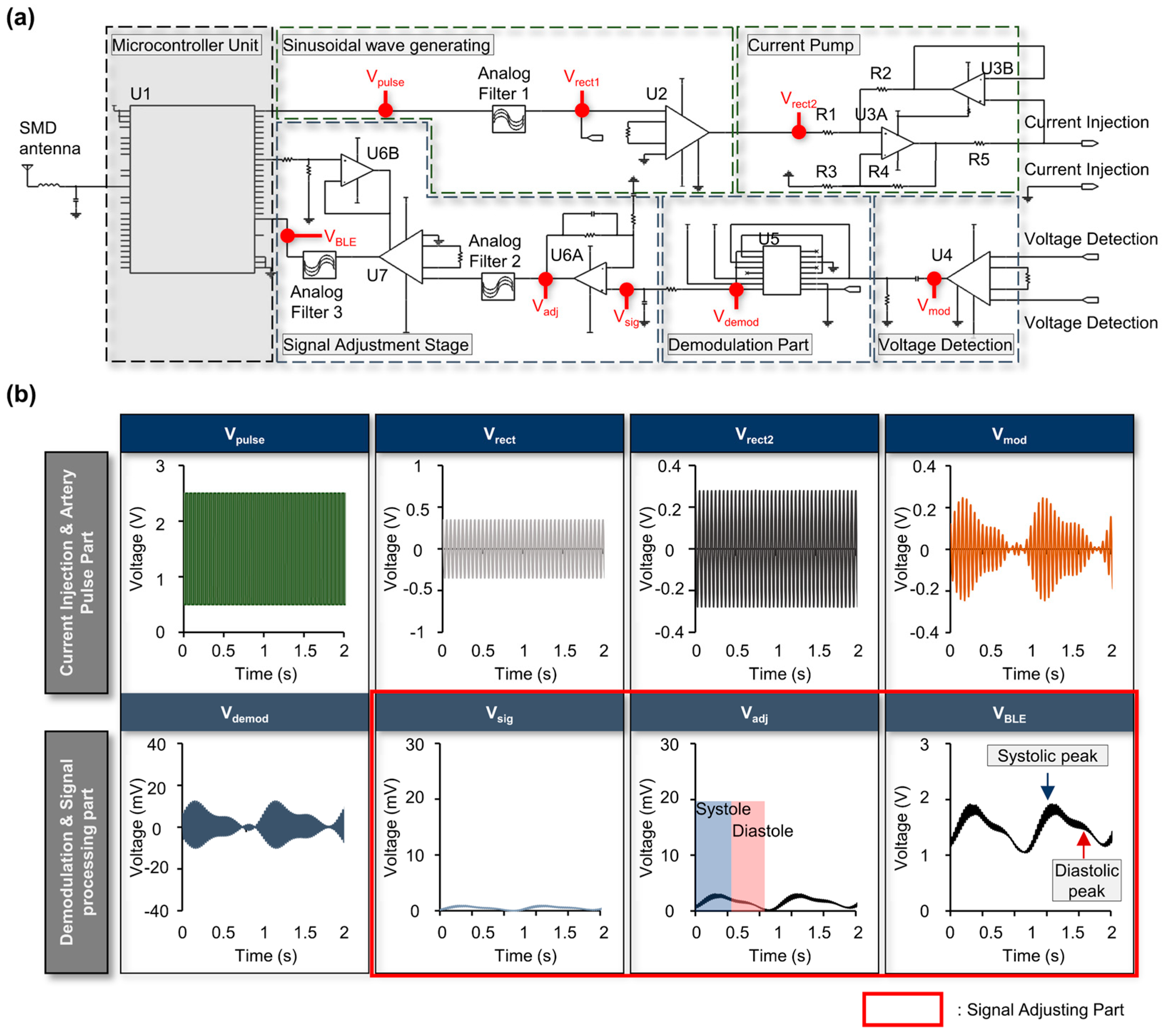
2.2. Bioimpedance Circuit and Node-by-Node Signal Examples
3. Results
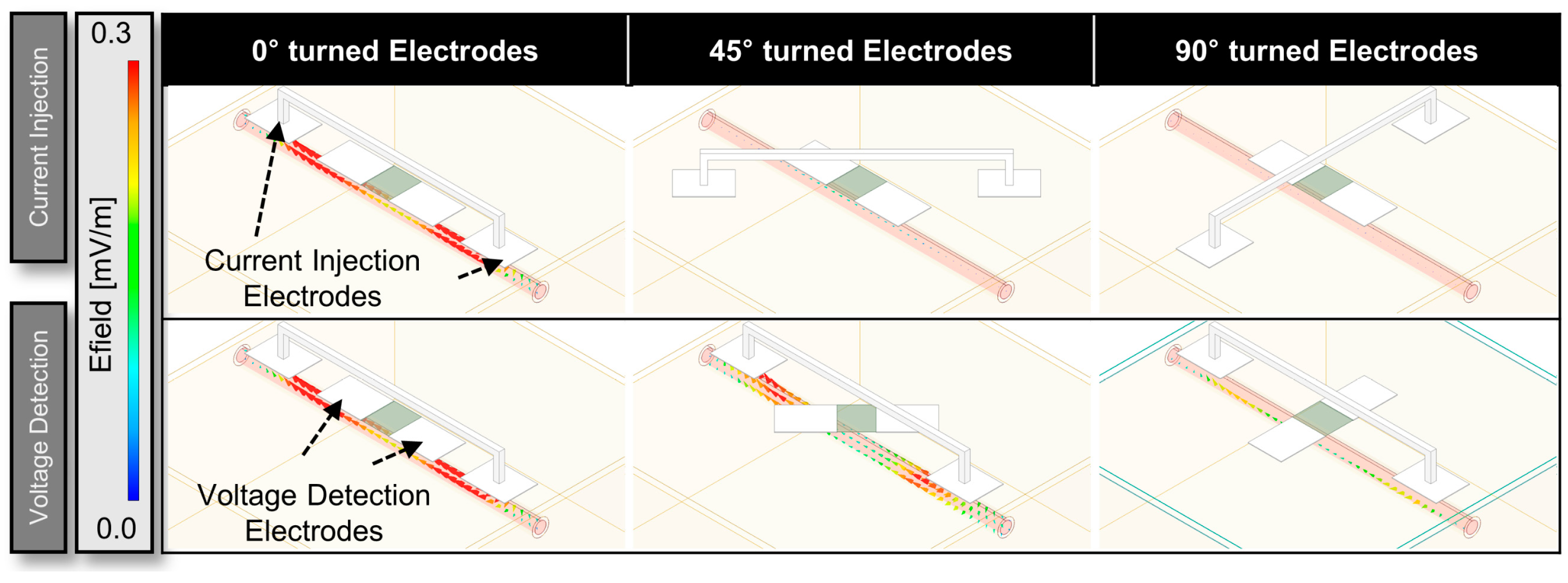
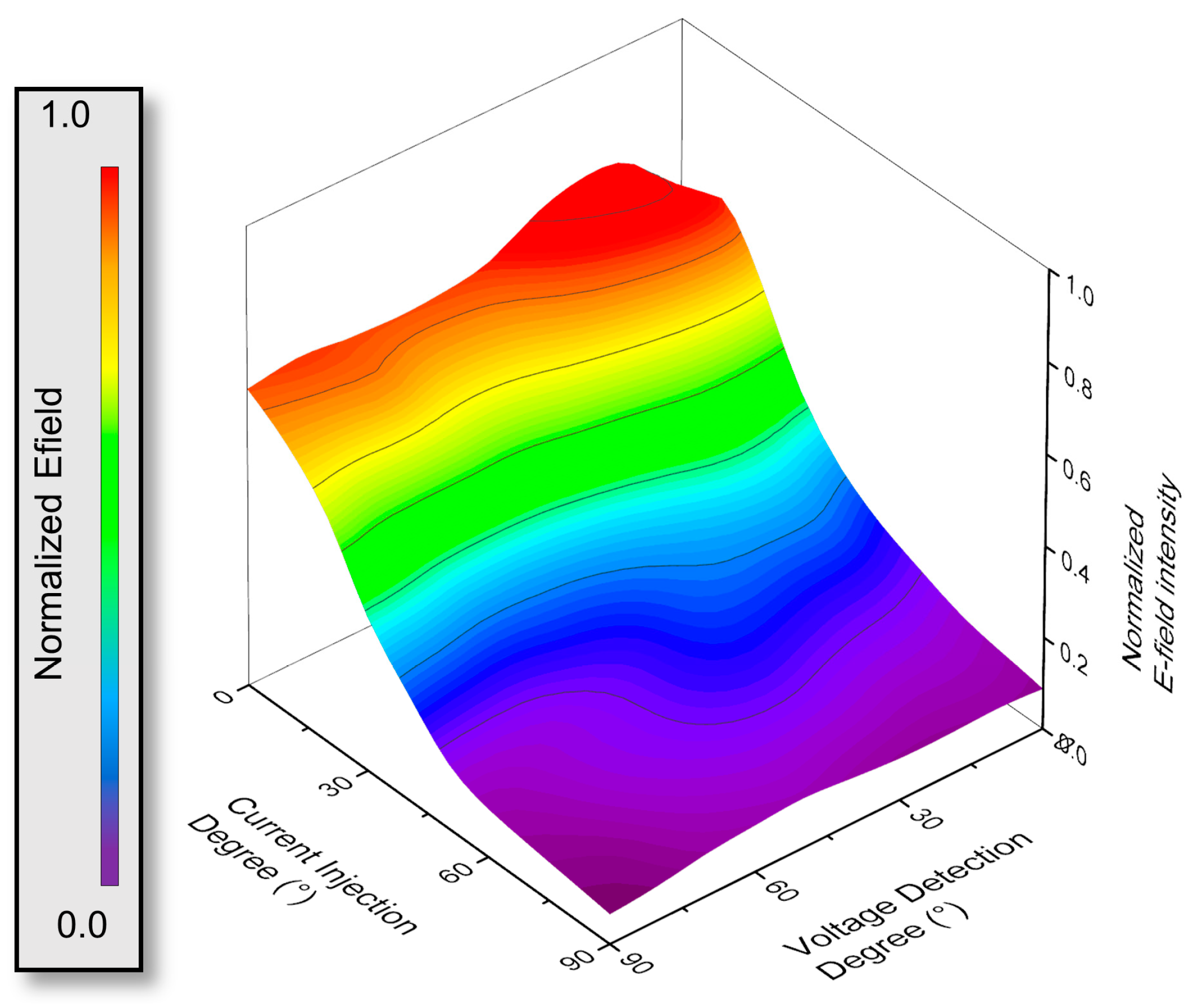
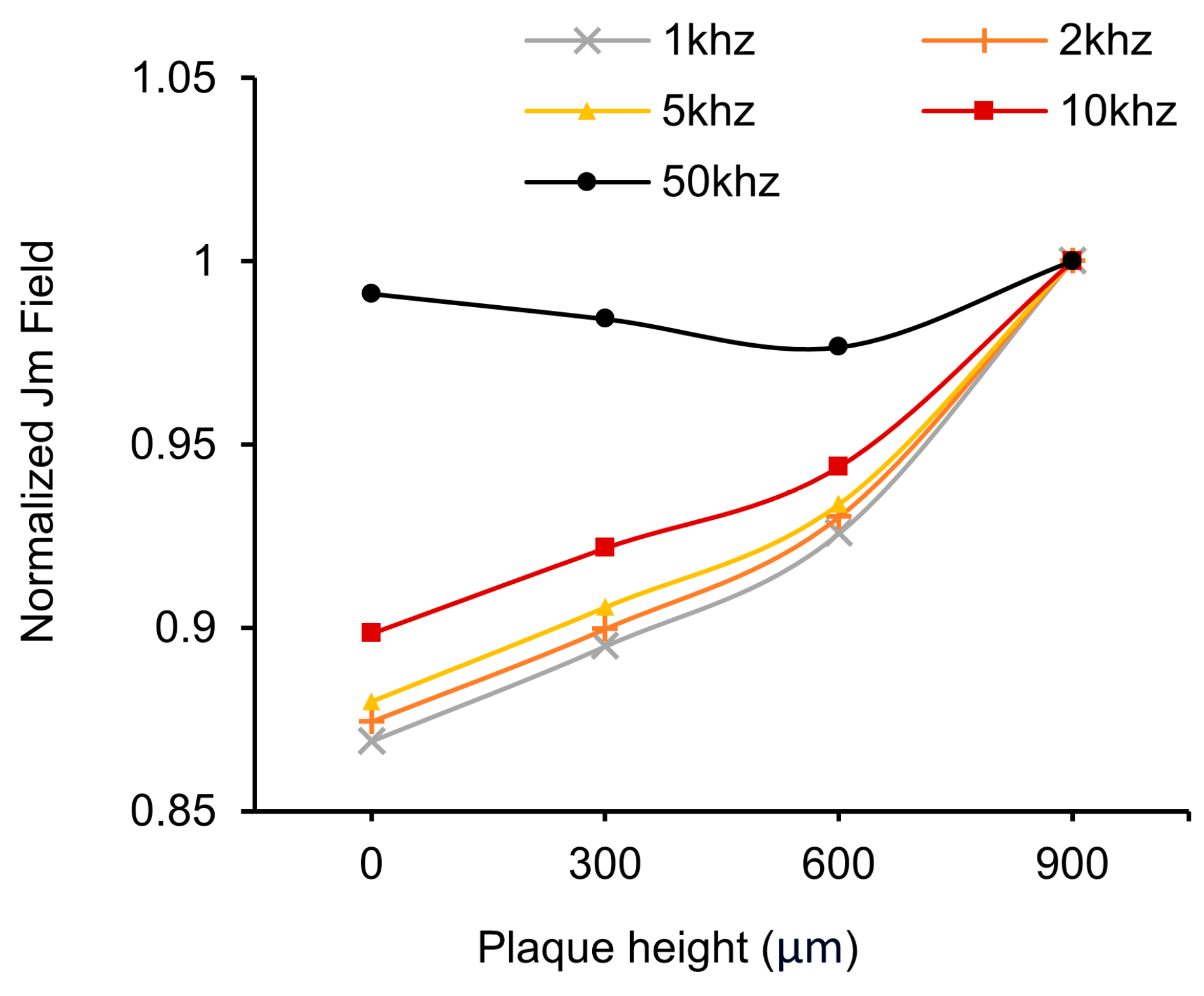
3.1. HFSS Simulation for Optimization
3.2. System Illustration and Target Locations on the Body
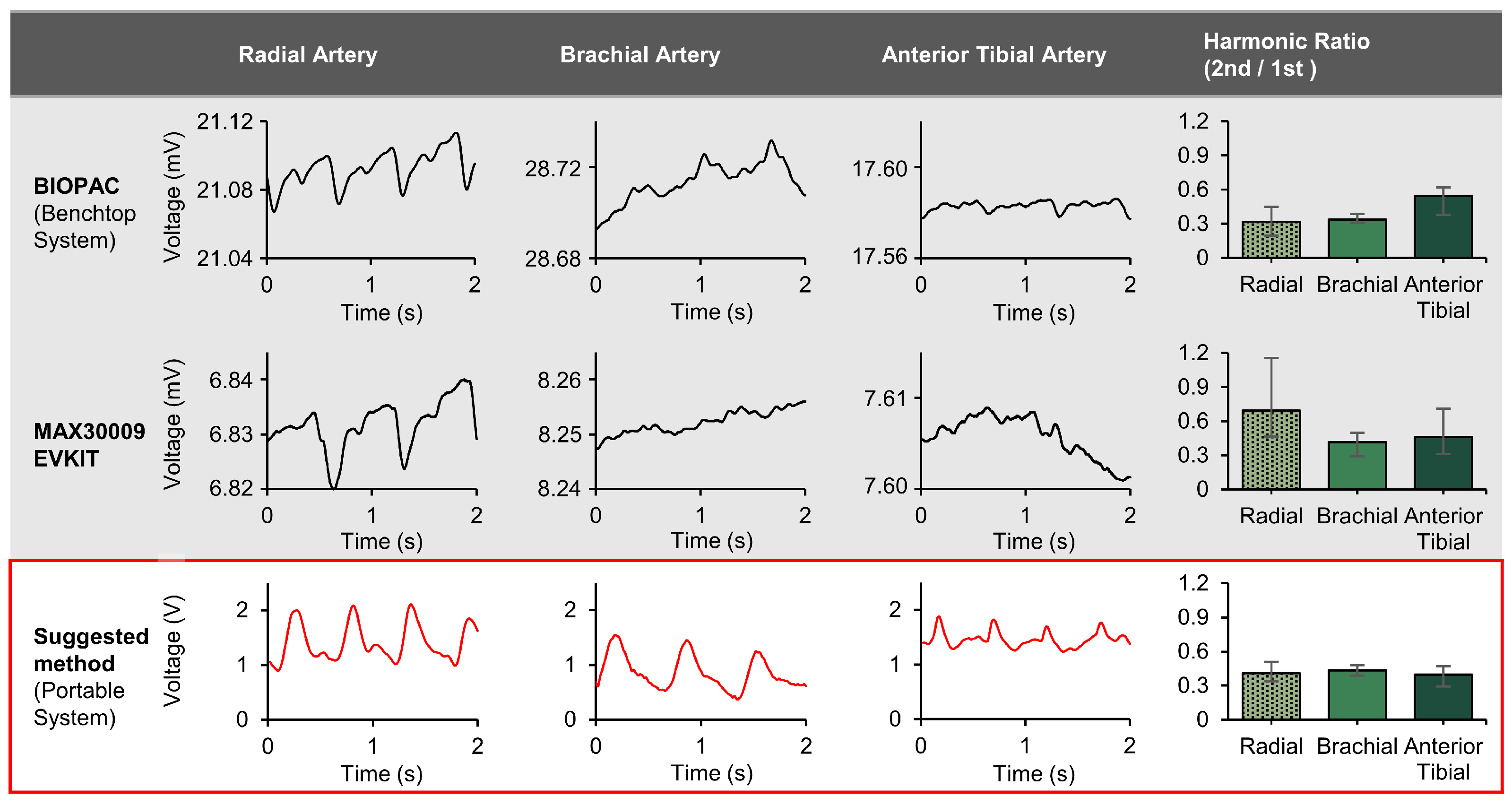
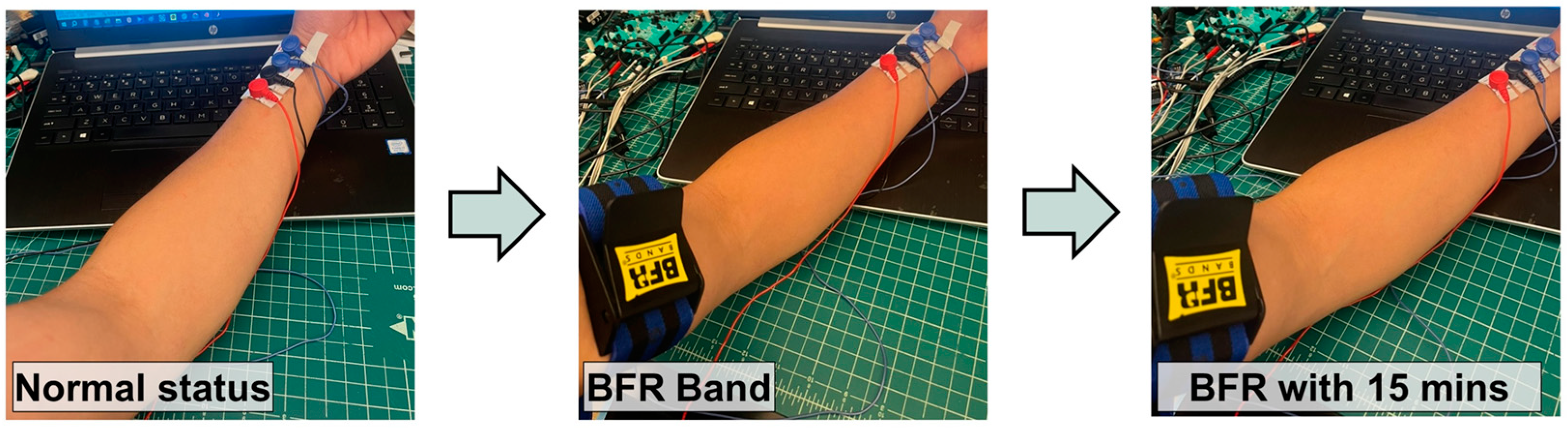
3.3. Bioimpedance Circuit and Node-by-Node Signal Examples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiatt, W.R.; Goldstone, J.; Smith Jr, S.C.; McDermott, M.; Moneta, G.; Oka, R.; Newman, A.B.; Pearce, W.H.; American Heart Association Writing Group 1. Atherosclerotic peripheral vascular disease symposium II: Nomenclature for vascular diseases. Circulation 2008, 118, 2826–2829. [Google Scholar] [CrossRef] [PubMed]
- Soor, G.S.; Vukin, I.; Leong, S.W.; Oreopoulos, G.; Butany, J. Peripheral vascular disease: Who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology 2008, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Jiang, N.; Li, Z.; Xu, S. Burden of peripheral artery disease and its attributable risk factors in 204 countries and territories from 1990 to 2019. Front. Cardiovasc. Med. 2022, 9, 868370. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.; Tikhomirov, A.; Myasishcheva, G.; Shaheen, Z.; Volkov, A.; Briko, A.; Shchukin, S. Multi-channel bioimpedance system for detecting vascular tone in human limbs: An approach. Sensors 2021, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S. Determination of Arterial Compliance Using Electrical Peripheral Bioimpedance. IEEJ Trans. Electr. Electron. Eng. 2023, 18, 436–441. [Google Scholar] [CrossRef]
- Lindholm, D.; Fukaya, E.; Leeper, N.J.; Ingelsson, E. Bioimpedance and New-Onset Heart Failure: A Longitudinal Study of >500 000 Individuals From the General Population. J. Am. Heart Assoc. 2018, 7, e008970. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.H.; Jafari, R.; Chung, W.-Y. A Robust Bioimpedance Structure for Smartwatch-Based Blood Pressure Monitoring. Sensors 2018, 18, 2095. [Google Scholar] [CrossRef] [PubMed]
- Shash, Y.H.; Eldosoky, M.A.A.; Elwakad, M.T. The effect of vascular diseases on bioimpedance measurements: Mathematical modeling. Biomed. Res. Ther. 2018, 5, 2414–2431. [Google Scholar] [CrossRef]
- Metshein, M.; Abdullayev, A.; Gautier, A.; Larras, B.; Frappe, A.; Cardiff, B.; Annus, P.; Land, R.; Märtens, O. Sensor-Location-Specific Joint Acquisition of Peripheral Artery Bioimpedance and Photoplethysmogram for Wearable Applications. Sensors 2023, 23, 7111. [Google Scholar] [CrossRef]
- Ouwendijk, R.; de Vries, M.; Pattynama, P.M.T.; van Sambeek, M.R.H.M.; de Haan, M.W.; Stijnen, T.; van Engelshoven, J.M.A.; Hunink, M.G.M. Imaging peripheral arterial disease: A randomized controlled trial comparing contrast-enhanced MR angiography and multi–detector row CT angiography. Radiology 2005, 236, 1094–1103. [Google Scholar] [CrossRef]
- Cooke, J.P.; Wilson, A.M. Biomarkers of peripheral arterial disease. J. Am. Coll. Cardiol. 2010, 55, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.J.W.M.; Willems, S.A.; Goncalves, L.N.; Hamming, J.F.; Schepers, A. Reliability of Bedside Tests for Diagnosing Peripheral Arterial Disease in Patients Prone to Medial Arterial Calcification: A Systematic Review. EClinicalMedicine 2022, 50, 101532. [Google Scholar] [CrossRef] [PubMed]
- Wald, L.L.; McDaniel, P.C.; Witzel, T.; Stockmann, J.P.; Cooley, C.Z. Low-cost and portable MRI. J. Magn. Reson. Imaging 2020, 52, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Samian, S.; Mazli, M.Z.; Amrizal, M.N.; Aljunid, S.M. Cost of magnetic resonance imaging (MRI) and computed tomography (CT) scan in UKMMC. BMC Health Serv. Res. 2012, 12, P11. [Google Scholar] [CrossRef]
- McLane, H.C.; Berkowitz, A.L.; Patenaude, B.N.; McKenzie, E.D.; Wolper, E.; Wahlster, S.; Fink, G.; Mateen, F.J. Availability, accessibility, and affordability of neurodiagnostic tests in 37 countries. Neurology 2015, 85, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Groenemeyer, D.H.W.; Seibel, R.M.M. Interventional CT and MRI: A challenge for safety and cost reduction in the health care system. In Proceedings of the Health Care Technology Policy II: The Role of Technology in the Cost of Health Care: Providing the Solutions, Arlington, VA, USA, 10–12 May 1995; Volume 2499, pp. 132–148. [Google Scholar]
- Richardson, L. Radiation exposure and diagnostic imaging. J. Am. Acad. Nurse Pract. 2010, 22, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Semelka, R.C.; Armao, D.M.; Elias, J.; Huda, W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J. Magn. Reson. Imaging 2007, 25, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.; Palmer, L. Risk of radiation and choice of imaging. Dig. Dis. 2009, 27, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Georgakopoulos, C.; Koutagiar, I.; Tousoulis, D. Diagnostic modalities in peripheral artery disease. Curr. Opin. Pharmacol. 2018, 39, 68–76. [Google Scholar] [CrossRef]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. Lower extremity peripheral artery disease: Contemporary epidemiology, management gaps, and future directions: A scientific statement from the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef]
- Koivunen, V.; Juonala, M.; Venermo, M.; Laivuori, M.; Jalkanen, J.M.; Hakovirta, H.H. Toe pressure and toe brachial index are predictive of cardiovascular mortality regardless of the most diseased arterial segment in symptomatic lower-extremity artery disease—A retrospective cohort study. PLoS ONE 2021, 16, e0259122. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Masaki, H.; Kojima, K.; Tanemoto, K. Toe-brachial index in the second toe: Substitutability to toe-brachial index in the great toe and ankle-brachial index. Ann. Vasc. Dis. 2016, 9, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kamath, T.P.; Prasad, R.; Allison, M.A.; Criqui, M.C.; Ix, J.H.; Rifkin, D.E.; Garimella, P.S. Association of Ankle-Brachial and toe-Brachial Indexes with Mortality in patients with CKD. Kidney Med. 2020, 2, 68–75. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Seward, J.B. Central arterial pressure and arterial pressure pulse: New views entering the second century after Korotkov. Mayo Clin. Proc. 2006, 81, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Arain, F.A.; Cooper, L.T., Jr. Peripheral arterial disease: Diagnosis and management. Mayo Clin. Proc. 2008, 83, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.I.; Lewis, G.F.; Porges, S.W. The Physiocam: A novel non-contact sensor to Measure heart rate Variability in clinical and Field applications. Front. Public Health 2017, 5, 300. [Google Scholar] [CrossRef] [PubMed]
- Hedayatipour, A.; Aslanzadeh, S.; Hesari, S.H.; Haque, M.A.; McFarlane, N. A wearable CMOS impedance to frequency sensing system for non-invasive impedance measurements. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.-J.; Rafi, A.K.; Cheon, S.-I.; Je, M.; Ha, S. On-chip sinusoidal signal generators for electrical impedance spectroscopy: Methodological review. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 337–360. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Coté, G. Arterial Pulse Wave Velocity Signal Reconstruction Using Low Sampling Rates. Biosensors 2024, 14, 92. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Jafari, R. Non-Invasive Cardiac and Respiratory Activity Assessment from Various Human Body Locations Using Bioimpedance. IEEE Open J. Eng. Med. Biol. 2021, 2, 210–217. [Google Scholar] [CrossRef]
- Schaumann, R.; Xiao, H.; Mac, V.V. Design of Analog Filters, 2nd ed.; Oxford University Press, Inc.: Oxford, UK, 2009; ISBN 0195373944. [Google Scholar]
- Cornish, B.H.; Ward, L.C.; Thomas, B.J.; Jebb, S.A.; Elia, M. Evaluation of Multiple Frequency Bioelectrical Impedance and Cole-Cole Analysis for the Assessment of Body Water Volumes in Healthy Humans. Eur. J. Clin. Nutr. 1996, 50, 159–164. [Google Scholar] [PubMed]
- Fu, B.; Freeborn, T.J. Cole-impedance parameters representing biceps tissue bioimpedance in healthy adults and their alterations following eccentric exercise. J. Adv. Res. 2020, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-W.; Chen, W.-X.; Chu, H.-W.; Lin, S.-F. Single-channel bioimpedance measurement for wearable continuous blood pressure monitoring. IEEE Trans. Instrum. Meas. 2020, 70, 4001909. [Google Scholar] [CrossRef]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; Defense Technical Information Center: Fort Belvoir, VA, USA, 1996.
- Vogel, J.; Niederer, D.; Engeroff, T.; Vogt, L.; Troidl, C.; Schmitz-Rixen, T.; Banzer, W.; Troidl, K. Effects on the Profile of Circulating miRNAs after Single Bouts of Resistance Training with and without Blood Flow Restriction—A Three-Arm, Randomized Crossover Trial. Int. J. Mol. Sci. 2019, 20, 3249. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Niederer, D.; Jung, G.; Troidl, K. Exercise-Induced Vascular Adaptations under Artificially Versus Pathologically Reduced Blood Flow: A Focus Review with Special Emphasis on Arteriogenesis. Cells 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Hernando, F.J.S.; Conejero, A.M. Peripheral artery disease: Pathophysiology, diagnosis and treatment. Rev. Esp. Cardiol. 2007, 60, 969. [Google Scholar]
- Gamero, M.; Kim, W.S.; Hong, S.; Vorobiev, D.; Morgan, C.D.; Park, S. Il Multimodal sensing capabilities for the detection of shunt failure. Sensors 2021, 21, 1747. [Google Scholar] [CrossRef] [PubMed]
- Maundy, B.J.; Elwakil, A.S.; Gift, S.J.G. Enhancing the improved Howland circuit. Int. J. Circuit Theory Appl. 2019, 47, 532–541. [Google Scholar] [CrossRef]
- Pallas-Areny, R.; Webster, J.G. AC instrumentation amplifier for bioimpedance measurements. IEEE Trans. Biomed. Eng. 1993, 40, 830–833. [Google Scholar] [CrossRef]
- Min, M.; Parve, T.; Ronk, A.; Annus, P.; Paavle, T. Synchronous sampling and demodulation in an instrument for multifrequency bioimpedance measurement. IEEE Trans. Instrum. Meas. 2007, 56, 1365–1372. [Google Scholar] [CrossRef]
- Morales, I.; González-Landaeta, R.; Simini, F. Pressure sensors used as bioimpedance plantar electrodes: A feasibility study. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lausanne, Switzerland, 23–25 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Kim, S.H.; Moon, J.-H.; Kim, J.H.; Jeong, S.M.; Lee, S.-H. Flexible, stretchable and implantable PDMS encapsulated cable for implantable medical device. Biomed. Eng. Lett. 2011, 1, 199–203. [Google Scholar] [CrossRef]
- Hong, S.; Kim, W.S.; Han, Y.; Cherukuri, R.; Jung, H.; Campos, C.; Wu, Q.; Park, S. Il Optogenetic Targeting of Mouse Vagal Afferents Using an Organ-specific, Scalable, Wireless Optoelectronic Device. Bio-Protocol 2022, 12, e4341. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Annus, P.; Kõiv, H.; Krivošei, A.; Uuetoa, T.; Lamp, J. Bioimpedance sensing—A viable alternative for tonometry in non-invasive assessment of central aortic pressure. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 373–378. [Google Scholar]
- Xu, D.; Zou, L.; Xing, Y.; Hou, L.; Wei, Y.; Zhang, J.; Qiao, Y.; Hu, D.; Xu, Y.; Li, J. Diagnostic value of ankle-brachial index in peripheral arterial disease: A meta-analysis. Can. J. Cardiol. 2013, 29, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Showkat, I.; Khanday, F.A.; Beigh, M.R. A review of bio-impedance devices. Med. Biol. Eng. Comput. 2023, 61, 927–950. [Google Scholar] [CrossRef] [PubMed]
- Dutt, A.G.; Verling, M.; Karlen, W. Wearable bioimpedance for continuous and context-aware clinical monitoring. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3985–3988. [Google Scholar]
- Farre Romera, M.D. Design, Implementation and Test of a Low-Cost Electrical Impedance Spectroscopy System Based on an AD5940. Bachelor’s Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. [Google Scholar]
- Scagliusi, S.F.; Pérez, P.; Martín, D.; Huertas, G.; Olmo, A.; Yúfera, A.; Delano, M. Enhancing the Precision of AD5940 Segmental Bioimpedance Measurements through Self-Calibration. In Proceedings of the 2023 IEEE BioSensors Conference (BioSensors), London, UK, 30 July–1 August 2023; pp. 1–4. [Google Scholar]
- Critcher, S.; Freeborn, T.J. Residual Impedance Impact on MAX30001 Accuracy for Bioimpedance Applications. In Proceedings of the 2021 IEEE 12th Latin America Symposium on Circuits and System (LASCAS), Arequipa, Peru, 21–24 February 2021; pp. 1–4. [Google Scholar]
- Crandall, H.; Burt, A.; Sanchez, B. Characterization of the Analog Device Inc (ADI) MAX30009 Bioimpedance Analog Front End Chip. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 2502–2505. [Google Scholar]
- Kassanos, P. Bioimpedance Sensors: A Tutorial. IEEE Sens. J. 2021, 21, 22190–22219. [Google Scholar] [CrossRef]
- Haykin, S.; Van Veen, B. Signals and Systems; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 8126512652. [Google Scholar]
- Razavi, B.; Behzad, R. RF Microelectronics; Prentice Hall: New York, NY, USA, 2012; Volume 2. [Google Scholar]
- Razavi, B. Fundamentals of Microelectronics; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 1119695147. [Google Scholar]
- Jaiswal, B. Design of a Sine Wave Inverter Using Arm LPC1768 Controller. Ph.D. Thesis, Maulana Abul Kalam Azad University of Technology, West Bengal, India, 2019. [Google Scholar]
- Carlosena, A.; Martinez, P.; Porta, S. Wein-bridge oscillators with opamp independent oscillation frequency. IEEE Trans. Instrum. Meas. 1991, 40, 644–646. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, X.; Wang, M.; Zhang, W.; Li, G.; Lin, L. The differential Howland current source with high signal to noise ratio for bioimpedance measurement system. Rev. Sci. Instrum. 2014, 85, 055111. [Google Scholar] [CrossRef] [PubMed]
- Behrouzirad, A.; Sugrue, P.; Todorovic, M.; McCarthy, K.G.; Galvin, P. Analysis and Application of a Buffered Feedback Path Improved Howland Current Source in BioImpedance Measurements. In Proceedings of the 2021 32nd Irish Signals and Systems Conference (ISSC), Athlone, Ireland, 10–11 June 2021; pp. 1–6. [Google Scholar]
- Mahnam, A.; Yazdanian, H.; Mosayebi Samani, M. Comprehensive study of Howland circuit with non-ideal components to design high performance current pumps. Measurement 2016, 82, 94–104. [Google Scholar] [CrossRef]
- Fedotov, A.A. Baseline drift filtering for an arterial pulse signal. Meas. Tech. 2014, 57, 91–96. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical impedance methods for noninvasive health monitoring: A review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Ye, X.; Wu, L.; Mao, K.; Feng, Y.; Li, J.; Ning, L.; Chen, J. Bioimpedance measurement of knee injuries using bipolar electrode configuration. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Nøvik, S.; Drageseth, M.F.; Grøndalen, M.B.; Nilsen, O.; Krauss, S.J.K.; Martinsen, Ø.G.; Häfliger, P.D. A CMOS Multi-Electrode Array for Four-Electrode Bioimpedance Measurements. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S. Electric impedance of suspensions of spheres. J. Gen. Physiol. 1928, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, W.; Park, S. Design of an inductive spiral-loop loaded unit cell in a mushroom-shaped high impedance surface for sub-ghz applications. Prog. Electromagn. Res. M 2021, 100, 1–11. [Google Scholar] [CrossRef]
- Al-Harosh, M.; Yangirov, M.; Kolesnikov, D.; Shchukin, S. Bio-impedance sensor for real-time artery diameter waveform assessment. Sensors 2021, 21, 8438. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H. Clinical assessment of central blood pressure. Curr. Hypertens. Rev. 2012, 8, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W.W. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1444128787. [Google Scholar]
- Bereza, T.; Skrzat, J.; Mróz, I.; Pasternak, A.; Tomaszewski, K.; Mizia, E.; Kielczewski, S.; Pawlicki, D.; Kurzydło, W.; Bachul, P. Blood vessels of the shin—Anterior tibial artery—Anatomy and embryology—Own studies and review of the literature. Folia Med. Cracov. 2016, 56, 33–47. [Google Scholar]
- Paarmann, L.D. Design and Analysis of Analog Filters: A Signal Processing Perspective; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; Volume 617, ISBN 0306480123. [Google Scholar]
- Williams, A.B. Analog Filter and Circuit Design Handbook; McGraw-Hill Education: New York, NY, USA, 2014; ISBN 0071816712. [Google Scholar]
- de la O Serna, J.A. Taylor–Fourier analysis of blood pressure oscillometric waveforms. IEEE Trans. Instrum. Meas. 2013, 62, 2511–2518. [Google Scholar] [CrossRef]
- Van De Water, J.M.; Dove, G.B.; Mount, B.E.; Linton, L.A. Application of bioelectric impedance to the measurement of arterial flow. J. Surg. Res. 1973, 15, 22–29. [Google Scholar] [CrossRef]




| Method | Objective Measurements | Cost Efficiency | Portable for POC | Ionizing Radiation |
|---|---|---|---|---|
| X-rays | Yes | No | No | Yes |
| MRI | Yes | No | No | No |
| CT | Yes | No | No | Yes |
| Blood test | Yes | No | No | No |
| Ankle-brachial index | No | Yes | Yes | No |
| Toe-branchial index | No | Yes | Yes | No |
| Toe pressure | No | Yes | Yes | No |
| Our Technology | Yes | Yes | Yes | No |
| Method | BIOPAC | MAX30009 EVKIT | Suggested Method |
|---|---|---|---|
| Injection Frequency | 12.5 kHz | 9.984 kHz | 10 kHz |
| Injection Current amount | 400 μA | 96 μA | 84.7 μA |
| Digital Filter Type | Low Pass Filter | Low Pass Filter | N/A |
| Cutoff Frequency | 10 Hz | 6.24 Hz | N/A |
| Measurement Time | 30 s | 30 s | 30 s |
| 1st Harmonic | 2nd Harmonic | Harmonic Ratio (2nd/1st) | |
|---|---|---|---|
| Normal | 24.73 dB (±1.36 dB) | 17.43 dB (±2.53 dB) | 0.43 (±0.06) |
| BFR | 30.03 dB (±0.83 dB) | 19.43 dB (±2.53 dB) | 0.30 (±0.05) |
| BFR 15 mins | 30.03 dB (±0.83 dB) | 17.17 dB (±3.36 dB) | 0.23 (±0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Coté, G. Development of a Tetherless Bioimpedance Device That Uses Morphologic Changes to Predict Blood Flow Restrictions Mimicking Peripheral Artery Disease Progression. Biosensors 2024, 14, 286. https://doi.org/10.3390/bios14060286
Hong S, Coté G. Development of a Tetherless Bioimpedance Device That Uses Morphologic Changes to Predict Blood Flow Restrictions Mimicking Peripheral Artery Disease Progression. Biosensors. 2024; 14(6):286. https://doi.org/10.3390/bios14060286
Chicago/Turabian StyleHong, Sungcheol, and Gerard Coté. 2024. "Development of a Tetherless Bioimpedance Device That Uses Morphologic Changes to Predict Blood Flow Restrictions Mimicking Peripheral Artery Disease Progression" Biosensors 14, no. 6: 286. https://doi.org/10.3390/bios14060286





