Long-Term Detection of Glycemic Glucose/Hypoglycemia by Microfluidic Sweat Monitoring Patch
Abstract
1. Introduction
2. Design and Preparation of Microfluidic Sweat Glucose Monitoring Patch
2.1. Preparation of Microfluidic Sweat Glucose Monitoring Patches
2.2. Design of Three-Dimensional Gas Valves
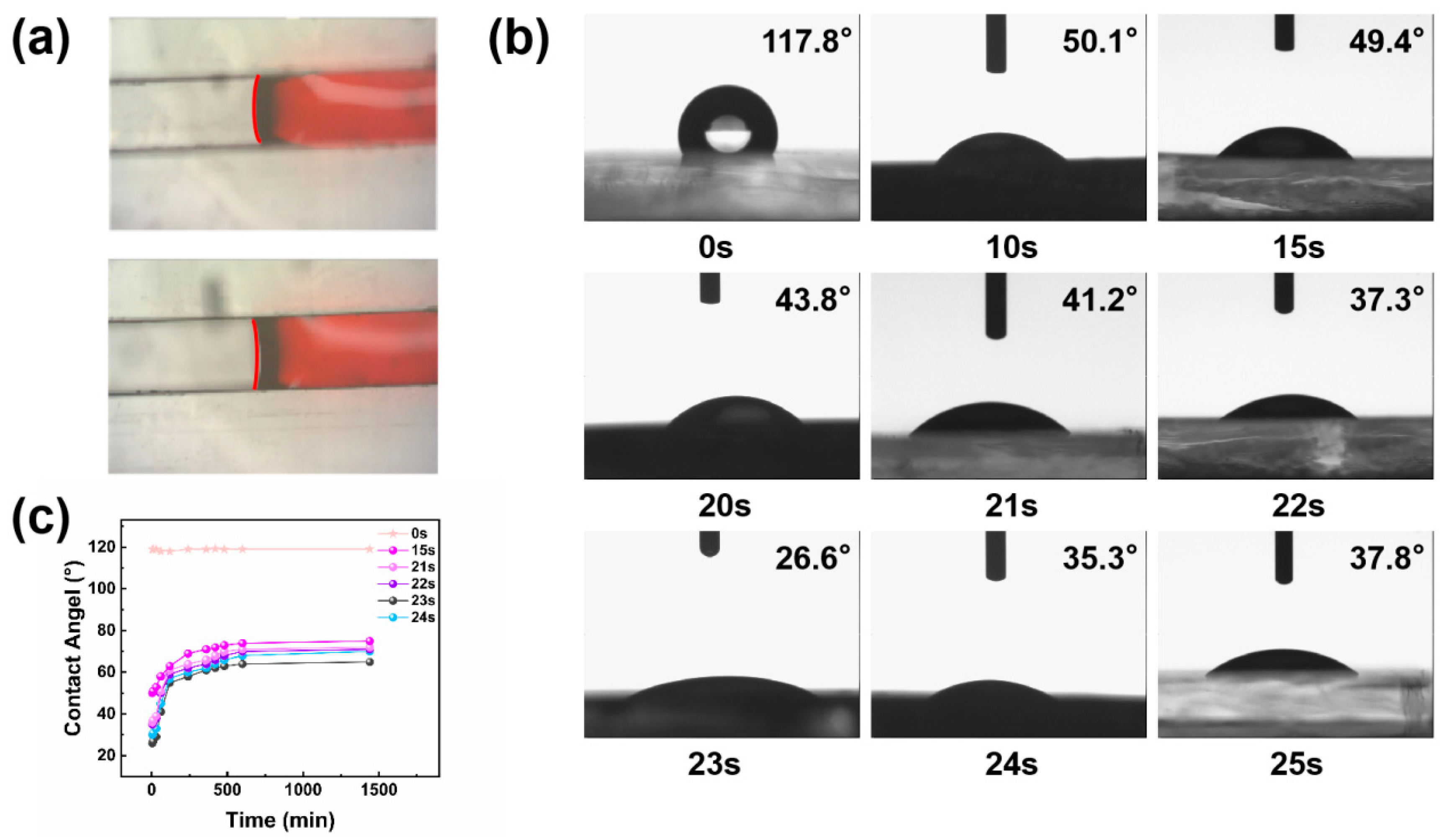
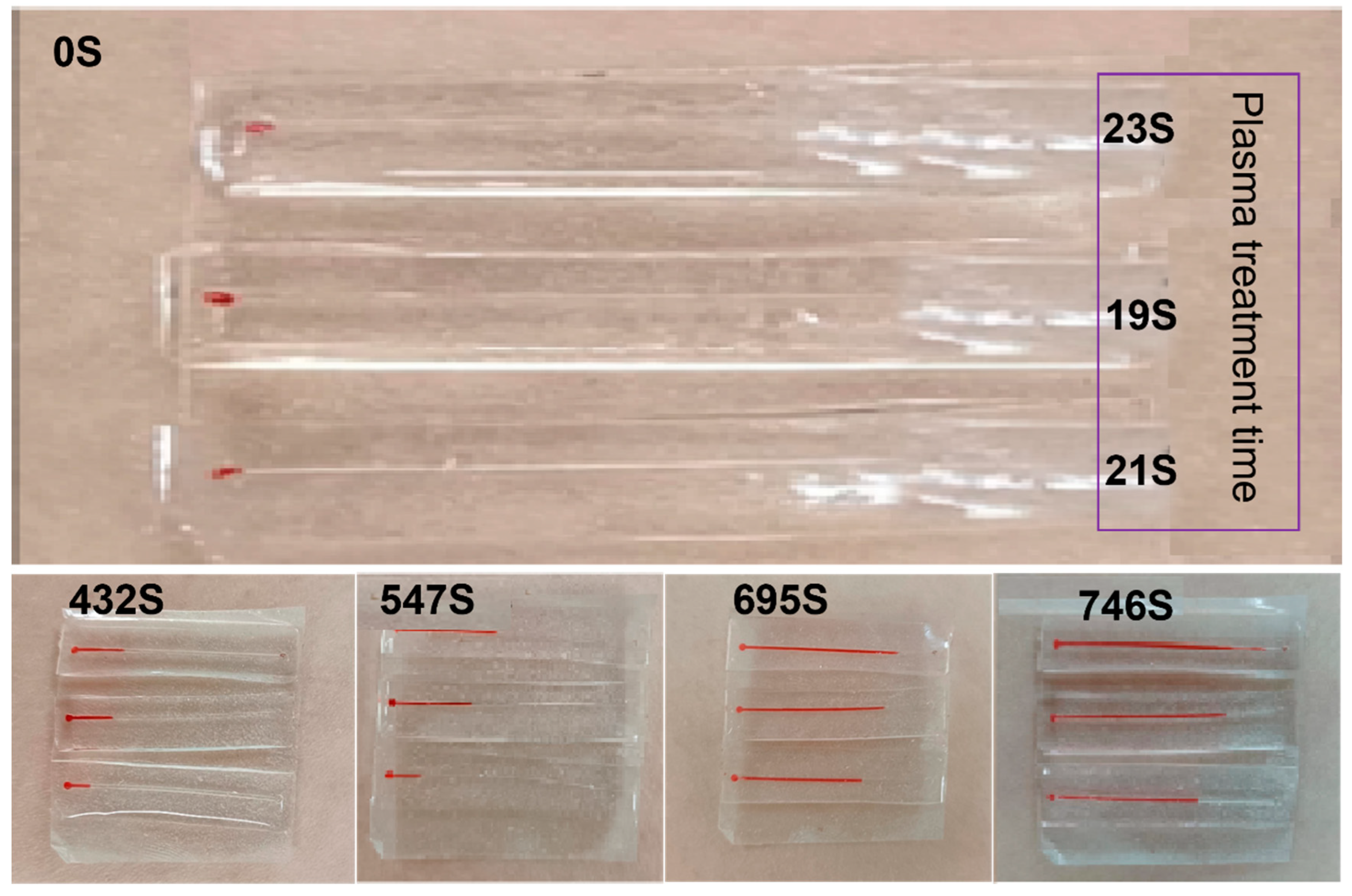
2.3. Hydrophilic Treatment of the Inner Wall of Microfluidic Channels
2.4. Microfluidic Channel Serpentine Design
3. Sweat Detection by Microfluidic Sweat Glucose Monitoring Patch
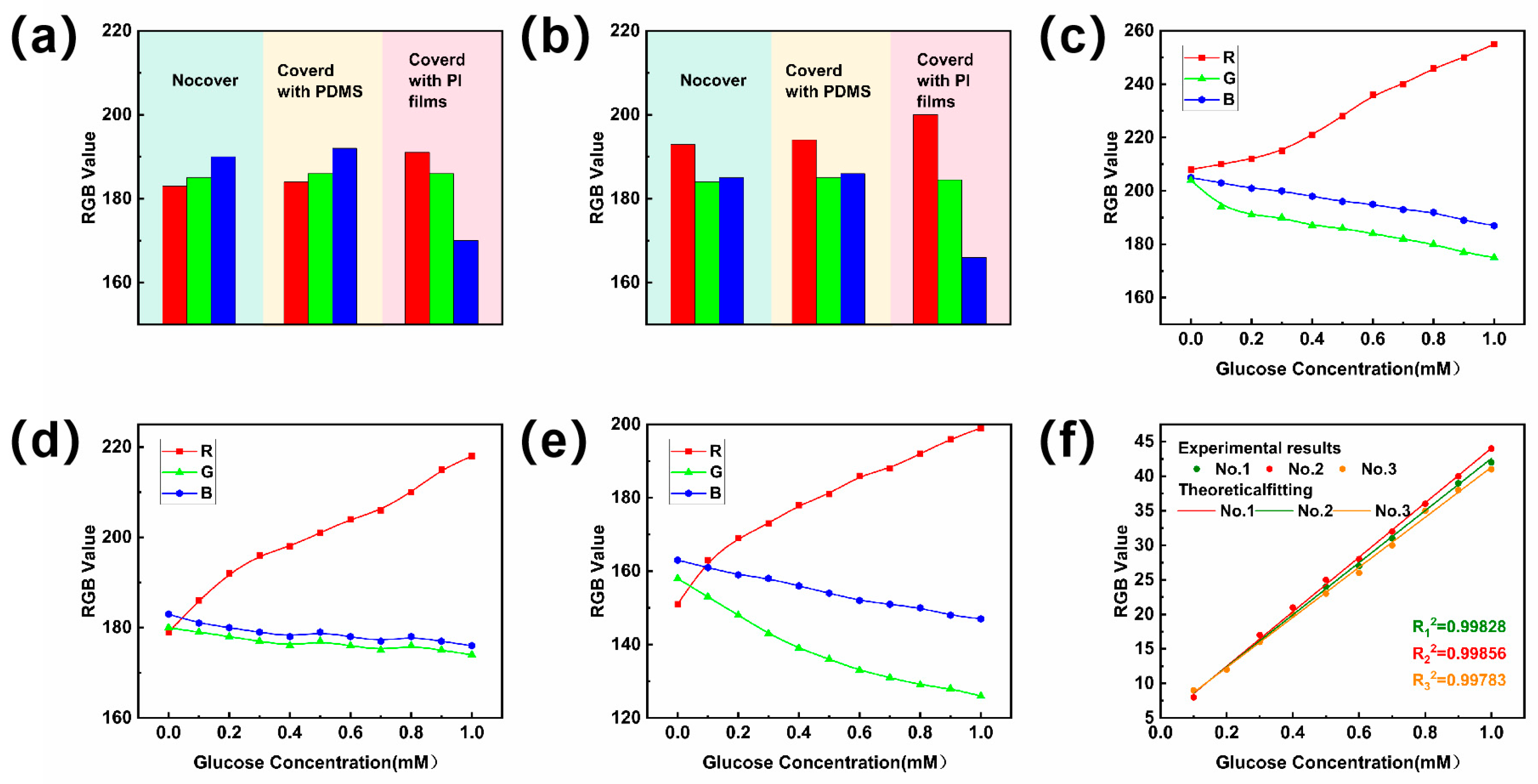
3.1. Calibration of Standard Reference Curves for Sweat Sugar Concentration Assays
3.2. Microfluidic Sweat Patch Detects Sweat Sugar in the Human Body
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dervisevic, M.; Alba, M.; Esser, L.; Tabassum, N.; Prieto-Simon, B.; Voelcker, N.H. Silicon micropillar array-based wearable sweat glucose sensor. ACS Appl. Mater. Interfaces 2022, 14, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, J.; Liu, Y.; Wong, T.; Su, J.; Yao, K.; Zhou, J.; Huang, Y.; Li, H.; Li, D.; et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 2022, 5, 201–209. [Google Scholar] [CrossRef]
- Huynh, V.L.; Trung, T.Q.; Meeseepong, M.; Lee, H.; Nguyen, T.D.; Lee, N. Hollow microfibers of elastomeric nanocomposites for fully stretchable and highly sensitive microfluidic immunobiosensor patch. Adv. Funct. Mater. 2020, 30, 2004684–2004696. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdes-Ramirez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Tai, L.C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Zhang, Y.; Won, S.M.; Bandodkar, A.J.; Sekine, Y.; Xue, Y.; Koo, J.; Harshman, S.W.; Martin, J.A.; Park, J.M.; et al. Super-absorbent polymer valves and colorimetricchemistries for time-sequenced discrete sampling and chloride analysis of sweat via skin-mounted soft microfluidics. Small 2018, 14, 1703334–1703345. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic chip-based wearable colorimetric sensor for simple and facile detection of sweat glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-worn soft microfluidic potentiometric detection system. Electroanalysis 2019, 31, 239–245. [Google Scholar] [CrossRef]
- Shi, H.; Cao, Y.; Zeng, Y.; Zhou, Y.; Wen, W.; Zhang, C.; Zhao, Y.; Chen, Z. Wearable tesla valve-based sweat collection device for sweat colorimetric analysis. Talanta 2022, 240, 123208. [Google Scholar] [CrossRef]
- Maltezos, G.; Garcia, E.; Hanrahan, G.; Gomez, F.A.; Vyawhare, S.; van Dam, R.M.; Chen, Y.; Scherer, A. Design and fabrication of chemically robust three-dimensional microfluidic valves. Lab A Chip 2007, 7, 1209–1211. [Google Scholar] [CrossRef]
- He, Y.; Wei, L.; Xu, W.; Wu, H.; Liu, A. Laser-cutting epidermal microfluidic patch with capillary bursting valves for chronological capture, storage, and colorimetric sensing of sweat. Biosensors 2023, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Klasner, S.A.; Price, A.K.; Hoeman, K.W.; Wilson, R.S.; Bell, K.J.; Culbertson, C.T. Paper-based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal. Bioanal. Chem. 2010, 397, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, H.Y.; Kang, J.Y.; Kim, T.S. How the capillary burst microvalve works. J. Colloid. Interface Sci. 2007, 306, 379–385. [Google Scholar] [CrossRef]
- Mena-Bravo, A.; Luque de Castro, M.D. Sweat: A sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 2014, 90, 139–147. [Google Scholar] [CrossRef]
- Hooton, K.; Li, L. Nonocclusive sweat collection combined with chemical isotope labeling LC-MS for human sweat metabolomics and mapping the sweat metabolomes at different skin locations. J. Anal. Chem. 2017, 89, 7847–7851. [Google Scholar] [CrossRef]
- Wei, L.; Fang, G.; Kuang, Z.; Cheng, L.; Wu, H.; Guo, D.; Liu, A. 3D-printed low-cost fabrication and facile integration of flexible epidermal microfluidics platform. Sens. Actuators B Chem. 2022, 353, 131085. [Google Scholar] [CrossRef]
- Li, T.; Liang, B.; Ye, Z.; Zhang, L.; Xu, S.; Tu, T.; Zhang, Y.; Cai, Y.; Zhang, B.; Fang, L.; et al. An integrated and conductive hydrogel-paper patch for simultaneous sensing of Chemical-Electrophysiological signals. Biosens. Bioelectron. 2022, 198, 113855. [Google Scholar] [CrossRef]
- Cheng, C.; Qiu, Y.; Tang, S.; Lin, B.; Guo, M.; Gao, B.; He, B. Artificial spider silk based programmable woven textile for efficient wound management. Adv. Funct. Mater. 2022, 32, 2107707. [Google Scholar] [CrossRef]
- Wiorek, A.; Parrilla, M.; Cuartero, M.; Crespo, G.A. Epidermal patch with glucose biosensor: pH and temperature correction toward more accurate sweat analysis during sport practice. Anal. Chem. 2020, 92, 10153–10161. [Google Scholar] [CrossRef]
- La Count, T.D.; Jajack, A.; Heikenfeld, J.; Kasting, G.B. Modeling glucose transport from systemic circulation to sweat. J. Pharm. Sci. 2019, 108, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Gu, K.; Yao, J.; Shao, Z.; Chen, X. Silk-based electrochemical sensor for the detection of glucose in sweat. Biomacromolecules 2022, 23, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-based fingertip blood-free reliable glucose monitoring: Personalized data processing for predicting blood glucose concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef]
- Noura, Z.; Shah, I.; Aziz, S.; Ahmed, A.; Jung, D.-W.; Brahim, L.; ElMostafa, R. Wearable healthcare monitoring based on a microfluidic electrochemical integrated device for sensing glucose in natural sweat. Sensors 2022, 22, 8971. [Google Scholar] [CrossRef]




| Test Scope | Duration of the Test | Sweat/Blood Glucose Level Ranging | References |
|---|---|---|---|
| 0.06–0.14 mM | 20 min | 0.001–0.02 | [23] |
| 0.06–0.8 mM | 15 min | 0.01–0.05 | [24] |
| 0.03–0.8 mM | 60 min | 0.01–0.02 | [25] |
| 0.01–1.11 mM | 10 min | 0.005–0.025 | [26] |
| 0–2 mM | 25 min | 0.01–0.11 | [27] |
| 0.01–1.11 mM | 120 min | 0.02–0.04 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Lu, L.; He, Y.; Cheng, L.; Liu, A. Long-Term Detection of Glycemic Glucose/Hypoglycemia by Microfluidic Sweat Monitoring Patch. Biosensors 2024, 14, 294. https://doi.org/10.3390/bios14060294
Xu W, Lu L, He Y, Cheng L, Liu A. Long-Term Detection of Glycemic Glucose/Hypoglycemia by Microfluidic Sweat Monitoring Patch. Biosensors. 2024; 14(6):294. https://doi.org/10.3390/bios14060294
Chicago/Turabian StyleXu, Wenjie, Lei Lu, Yuxin He, Lin Cheng, and Aiping Liu. 2024. "Long-Term Detection of Glycemic Glucose/Hypoglycemia by Microfluidic Sweat Monitoring Patch" Biosensors 14, no. 6: 294. https://doi.org/10.3390/bios14060294
APA StyleXu, W., Lu, L., He, Y., Cheng, L., & Liu, A. (2024). Long-Term Detection of Glycemic Glucose/Hypoglycemia by Microfluidic Sweat Monitoring Patch. Biosensors, 14(6), 294. https://doi.org/10.3390/bios14060294





