Enhancing Sensitivity in SARS-CoV-2 Rapid Antigen Testing through Integration of a Water-Soluble Polymer Wall
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipments
2.2. Preparation of AuNPs and AuNP–Antibody Conjugates
2.3. Fabrication of LFIA Test Strips
2.4. Statistics and Analysis of Results
3. Results and Discussion
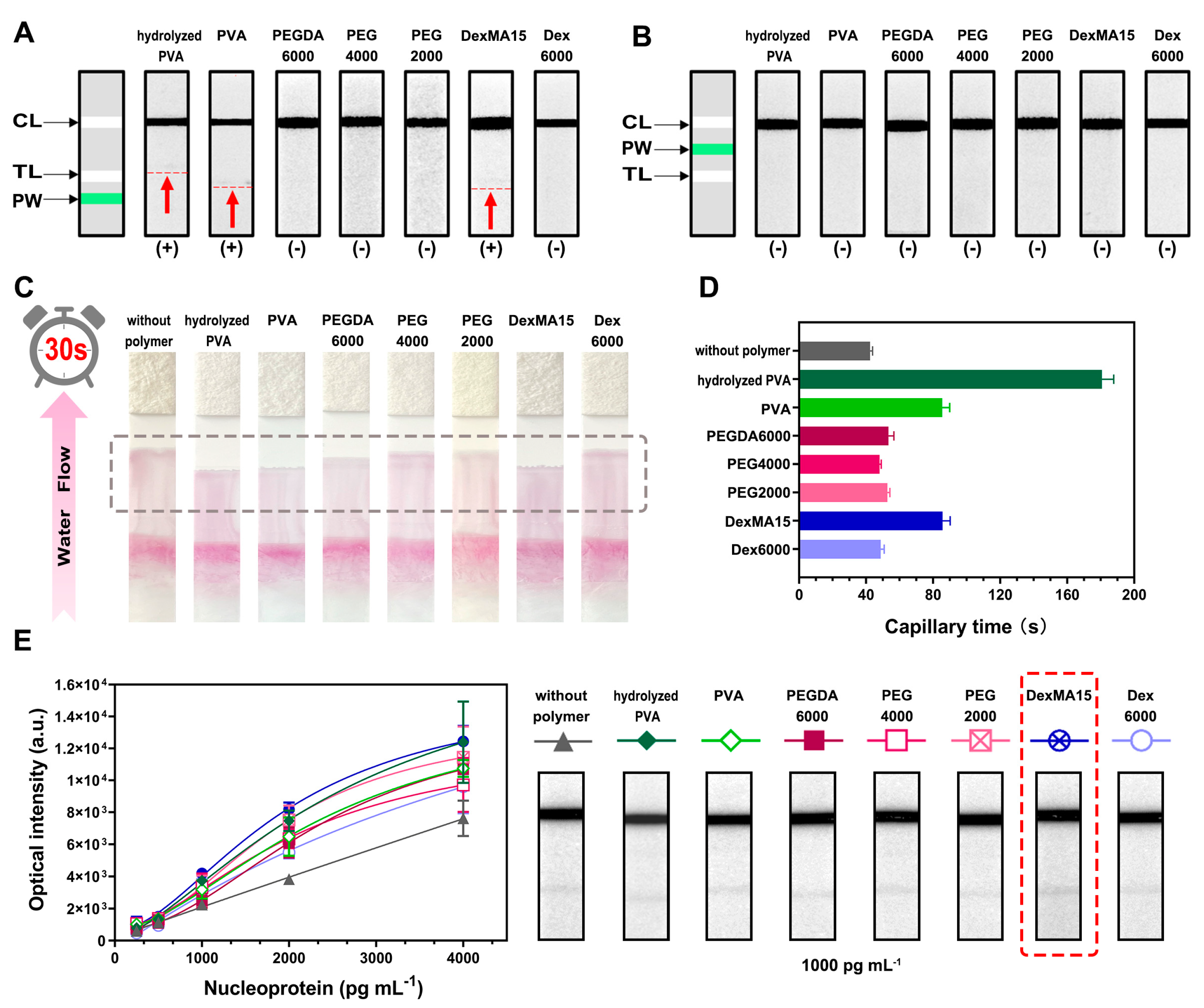
3.1. Design of the WSPW-LFIA Assay
3.2. Construction of WSPW-LFIA
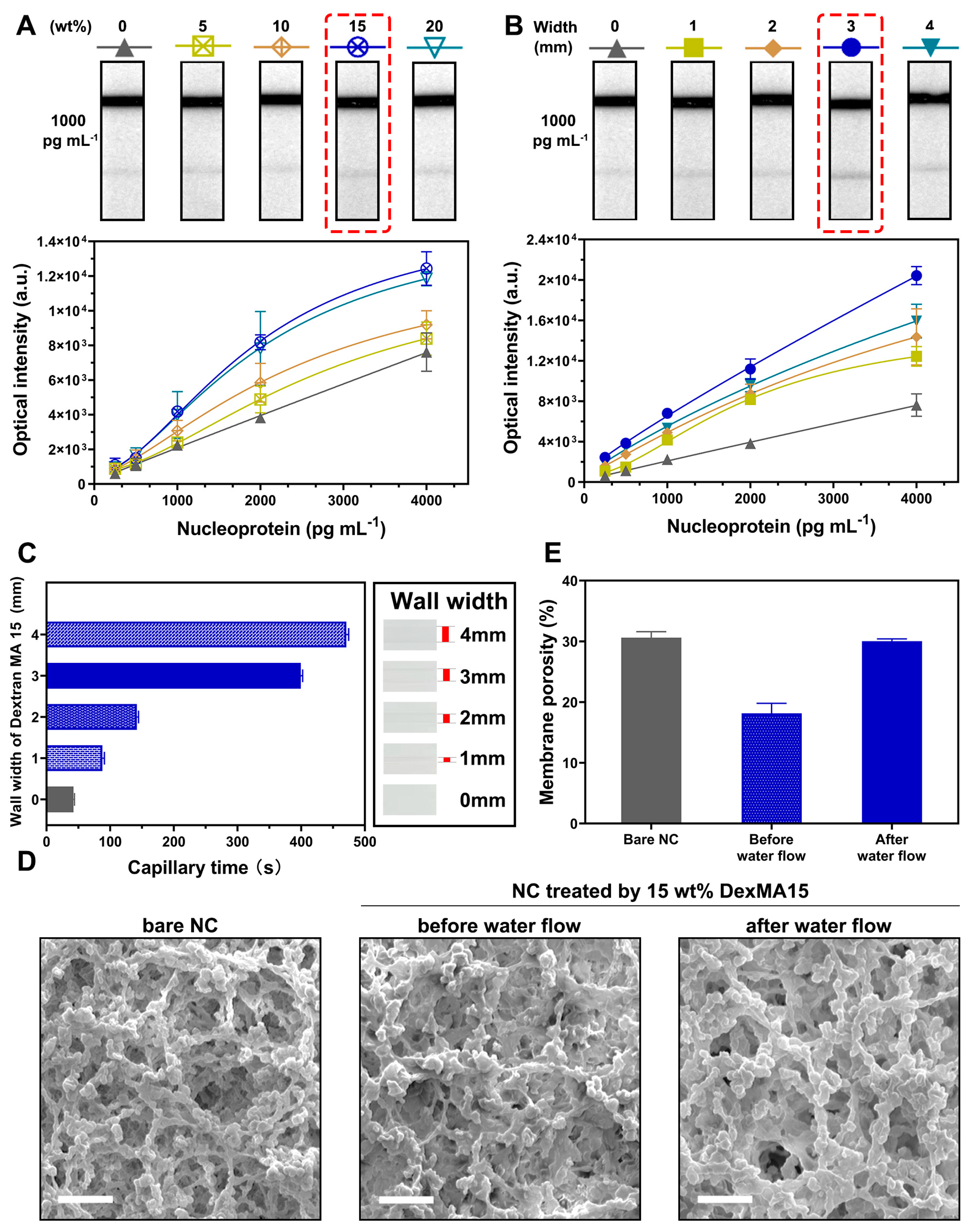
3.3. Optimization of WSPW-LFIA with DexMA15
3.4. Detection of WSPW-LFIA with DexMA15
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Bishop, J.D.; Hsieh, H.V.; Gasperino, D.J.; Weigl, B.H. Sensitivity enhancement in lateral flow assays: A systems perspective. Lab Chip 2019, 19, 2486–2499. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Ranjan, P.; Sanghi, S.K.; Srivastava, A.K.; Khan, R. Point-of-Care Biosensor-Based Diagnosis of COVID-19 Holds Promise to Combat Current and Future Pandemics. ACS Appl. Bio Mater. 2020, 3, 7326–7343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef] [PubMed]
- Rosella, L.C.; Agrawal, A.; Gans, J.; Goldfarb, A.; Sennik, S.; Stein, J. Large-scale implementation of rapid antigen testing system for COVID-19 in workplaces. Sci. Adv. 2022, 8, eabm3608. [Google Scholar] [CrossRef] [PubMed]
- Gremmels, H.; Winkel, B.M.; Schuurman, R.; Rosingh, A.; Rigter, N.A.; Rodriguez, O.; Ubijaan, J.; Wensing, A.M.; Bonten, M.J.; Hofstra, L.M. Real-life validation of the PanbioTM COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine 2021, 31, 100677. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Woo, K.; Kim, J.M.; Jo, H.J.; Jeong, Y.; Lee, K.H. SARS-CoV-2 Variant Screening Using a Virus-Receptor-Based Electrical Biosensor. Nano Lett. 2022, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Huang, C.C.; Wu, C.C.; Chen, P.H.; Tripathi, A.; Wang, Y.L. Saliva-based COVID-19 detection: A rapid antigen test of SARS-CoV-2 nucleocapsid protein using an electrical-double-layer gated field-effect transistor-based biosensing system. Sens. Actuators B Chem. 2022, 357, 131415. [Google Scholar] [CrossRef]
- Gamage, S.S.; Pahattuge, T.N.; Wijerathne, H.; Childers, K.; Vaidyanathan, S.; Athapattu, U.S.; Zhang, L.; Zhao, Z.; Hupert, M.L.; Muller, R.M.; et al. Microfluidic affinity selection of active SARS-CoV-2 virus particles. Sci. Adv. 2022, 8, eabn9665. [Google Scholar] [CrossRef]
- Chen, J.; Ho, W.K.; Yin, B.; Zhang, Q.; Li, C.; Yan, J.; Huang, Y.; Hao, J.; Yi, C.; Zhang, Y.; et al. Magnetic-responsive upconversion luminescence resonance energy transfer (LRET) biosensor for ultrasensitive detection of SARS-CoV-2 spike protein. Biosens. Bioelectron. 2024, 248, 115969. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shim, S.; Cho, W.W.; Cho, S.; Baek, H.; Lee, S.M.; Shin, D.S. Electrochemical Impedance-Based Biosensors for the Label-Free Detection of the Nucleocapsid Protein from SARS-CoV-2. ACS Sens. 2022, 7, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- Białobrzeska, W.; Ficek, M.; Dec, B.; Osella, S.; Trzaskowski, B.; Jaramillo-Botero, A.; Pierpaoli, M.; Rycewicz, M.; Dashkevich, Y.; Łęga, T.; et al. Performance of electrochemical immunoassays for clinical diagnostics of SARS-CoV-2 based on selective nucleocapsid N protein detection: Boron-doped diamond, gold and glassy carbon evaluation. Biosens. Bioelectron. 2022, 209, 114222. [Google Scholar] [CrossRef] [PubMed]
- Su, W.Y.; Ho, T.S.; Tsai, T.C.; Du, P.X.; Tsai, P.S.; Keskin, B.B.; Shizen, M.A.; Lin, P.C.; Lin, W.H.; Shih, H.C.; et al. Developing magnetic barcode bead fluorescence assay for high throughput analyzing humoral responses against multiple SARS-CoV-2 variants. Biosens. Bioelectron. 2023, 241, 115709. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.E.; Ryu, E.; Kim, J.; Shin, C.J.; Kwon, O.S. Fluorophore-encapsulated nanobeads for on-site, rapid, and sensitive lateral flow assay. Sens. Actuators B Chem. 2023, 381, 133364. [Google Scholar] [CrossRef] [PubMed]
- Korenkov, M.; Poopalasingam, N.; Madler, M.; Vanshylla, K.; Eggeling, R.; Wirtz, M.; Fish, I.; Dewald, F.; Gieselmann, L.; Lehmann, C.; et al. Evaluation of a rapid antigen test to detect sars-cov-2 infection and identify potentially infectious individuals. J. Clin. Microbiol. 2021, 59, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, S.; Suter-Riniker, F.; Jent, P.; Bittel, P.; Nagler, M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings: Antigen tests in real-life clinical settings. Int. J. Infect. Dis. 2021, 109, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Woloshin, S.; Patel, N.; Kesselheim, A.S. False Negative Tests for SARS-CoV-2 Infection—Challenges and Implications. N. Engl. J. Med. 2020, 383, e38. [Google Scholar] [CrossRef]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef]
- Parolo, C.; Medina-Sánchez, M.; de La Escosura-Muñiz, A.; Merkoçi, A. Simple paper architecture modifications lead to enhanced sensitivity in nanoparticle based lateral flow immunoassays. Lab Chip 2013, 13, 386–390. [Google Scholar] [CrossRef]
- Tang, R.; Yang, H.; Gong, Y.; Liu, Z.; Li, X.; Wen, T.; Qu, Z.; Zhang, S.; Mei, Q.; Xu, F. Improved Analytical Sensitivity of Lateral Flow Assay using Sponge for HBV Nucleic Acid Detection. Sci. Rep. 2017, 7, 1360. [Google Scholar] [CrossRef]
- Fu, E.; Lutz, B.; Kauffman, P.; Yager, P. Controlled reagent transport in disposable 2D paper networks. Lab Chip 2010, 10, 918–920. [Google Scholar] [CrossRef]
- Lutz, B.; Liang, T.; Fu, E.; Ramachandran, S.; Kauffman, P.; Yager, P. Dissolvable fluidic time delays for programming multi-step assays in instrument-free paper diagnostics. Lab Chip 2013, 13, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Z.; Yang, Y.; Li, L.; Wang, L.; Li, A.; Qu, Z.; Xu, F. Sensitivity Enhancement of Nucleic Acid Lateral Flow Assays through a Physical-Chemical Coupling Method: Dissoluble Saline Barriers. ACS Sens. 2019, 4, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Medina-Sánchez, M.; de La Escosura-Muñiz, A.; Merkoçi, A. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip 2014, 14, 4406–4414. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Ngo, D.B.; Parolo, C.; Hu, L.; Álvarez-Diduk, R.; Bergua, J.F.; Rosati, G.; Surareungchai, W.; Merkoçi, A. Lateral flow assay modified with time-delay wax barriers as a sensitivity and signal enhancement strategy. Biosens. Bioelectron. 2020, 168, 112559. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Pollitt, M.J.; Buckton, G.; Piper, R.; Brocchini, S. Measuring antibody coatings on gold nanoparticles by optical spectroscopy. RSC Adv. 2015, 5, 24521–24527. [Google Scholar] [CrossRef]
- Keeling-Tucker, T.; Brennan, J.D. Fluorescent probes as reporters on the local structure and dynamics in sol-gel-derived nanocomposite materials. Chem. Mater. 2001, 13, 3331–3350. [Google Scholar] [CrossRef]
- Tsai, H.C.; Doong, R.A. Preparation and characterization of urease-encapsulated biosensors in poly (vinyl alcohol)-modified silica sol-gel materials. Biosens. Bioelectron. 2007, 23, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.B.; He, D.; Zhang, C.L.; Yu, S.H. Facile fabrication of gold nanoparticles-poly (vinyl alcohol) electrospun water-stable nanofibrous mats: Efficient substrate materials for biosensors. ACS Appl. Mater. Interfaces 2012, 4, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, S.; Kitano, K.; Ogino, I.; Mukai, S.R. New method for introducing mesopores into carbon microhoneycombs using dextran. Microporous Mesoporous Mater. 2016, 231, 171–177. [Google Scholar] [CrossRef]
- Szleifer, I. Polymers and proteins: Interactions at interfaces. Curr. Opin. Solid State Mater. Sci. 1997, 2, 337–344. [Google Scholar] [CrossRef]
- Inoue, Y.; Nakanishi, T.; Ishihara, K. Elastic repulsion from polymer brush layers exhibiting high protein repellency. Langmuir 2013, 29, 10752–10758. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, Y.; Jie, H.; Liu, S.; Shen, C.; Liu, Q. Enhancing Sensitivity in SARS-CoV-2 Rapid Antigen Testing through Integration of a Water-Soluble Polymer Wall. Biosensors 2024, 14, 305. https://doi.org/10.3390/bios14060305
Wang X, Wang Y, Jie H, Liu S, Shen C, Liu Q. Enhancing Sensitivity in SARS-CoV-2 Rapid Antigen Testing through Integration of a Water-Soluble Polymer Wall. Biosensors. 2024; 14(6):305. https://doi.org/10.3390/bios14060305
Chicago/Turabian StyleWang, Xiuzhen, Yu Wang, Huiyang Jie, Sidi Liu, Chenguang Shen, and Qian Liu. 2024. "Enhancing Sensitivity in SARS-CoV-2 Rapid Antigen Testing through Integration of a Water-Soluble Polymer Wall" Biosensors 14, no. 6: 305. https://doi.org/10.3390/bios14060305
APA StyleWang, X., Wang, Y., Jie, H., Liu, S., Shen, C., & Liu, Q. (2024). Enhancing Sensitivity in SARS-CoV-2 Rapid Antigen Testing through Integration of a Water-Soluble Polymer Wall. Biosensors, 14(6), 305. https://doi.org/10.3390/bios14060305





