Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications
Abstract
:1. Introduction
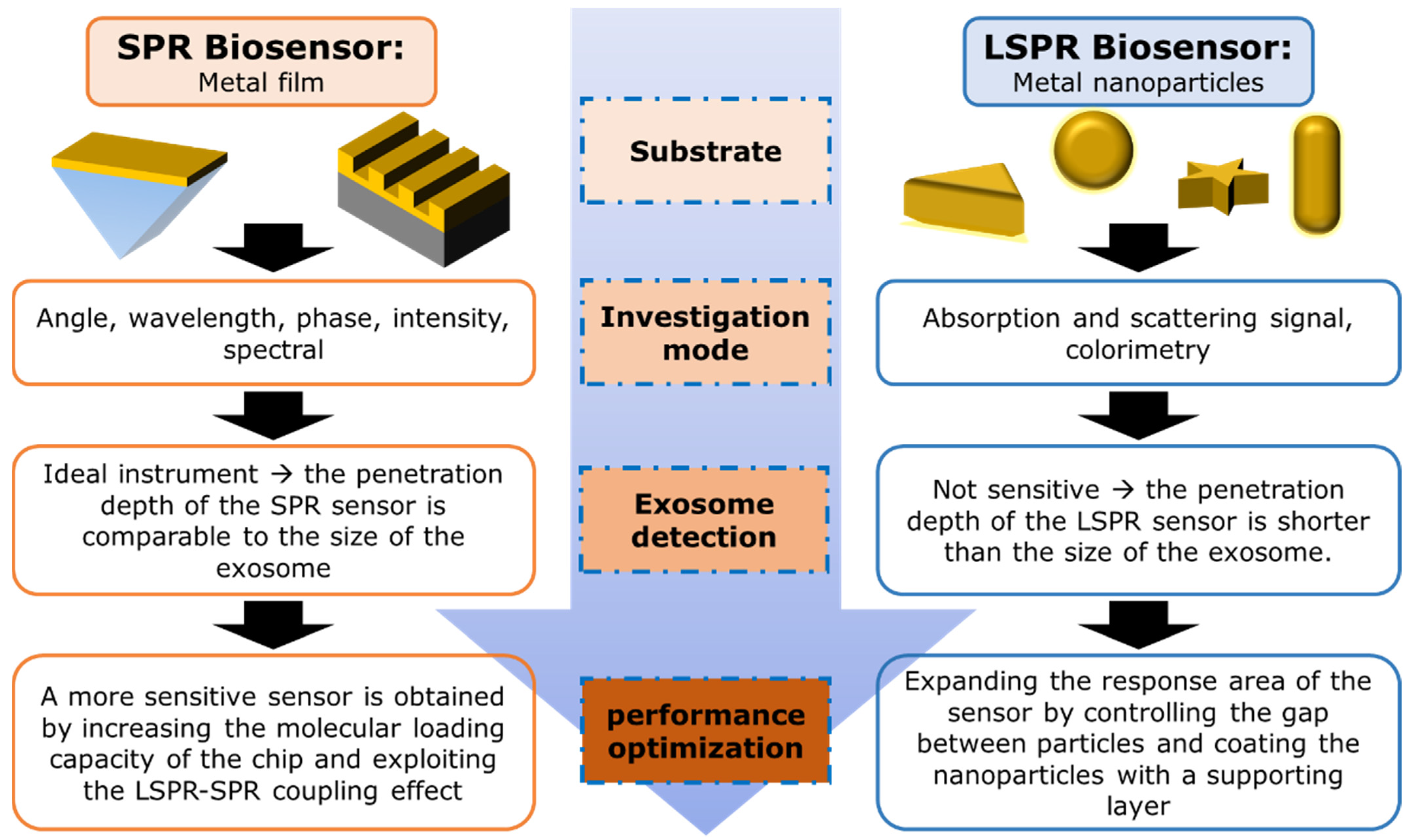
2. Working Principles of Nanoplasmonic Biosensors
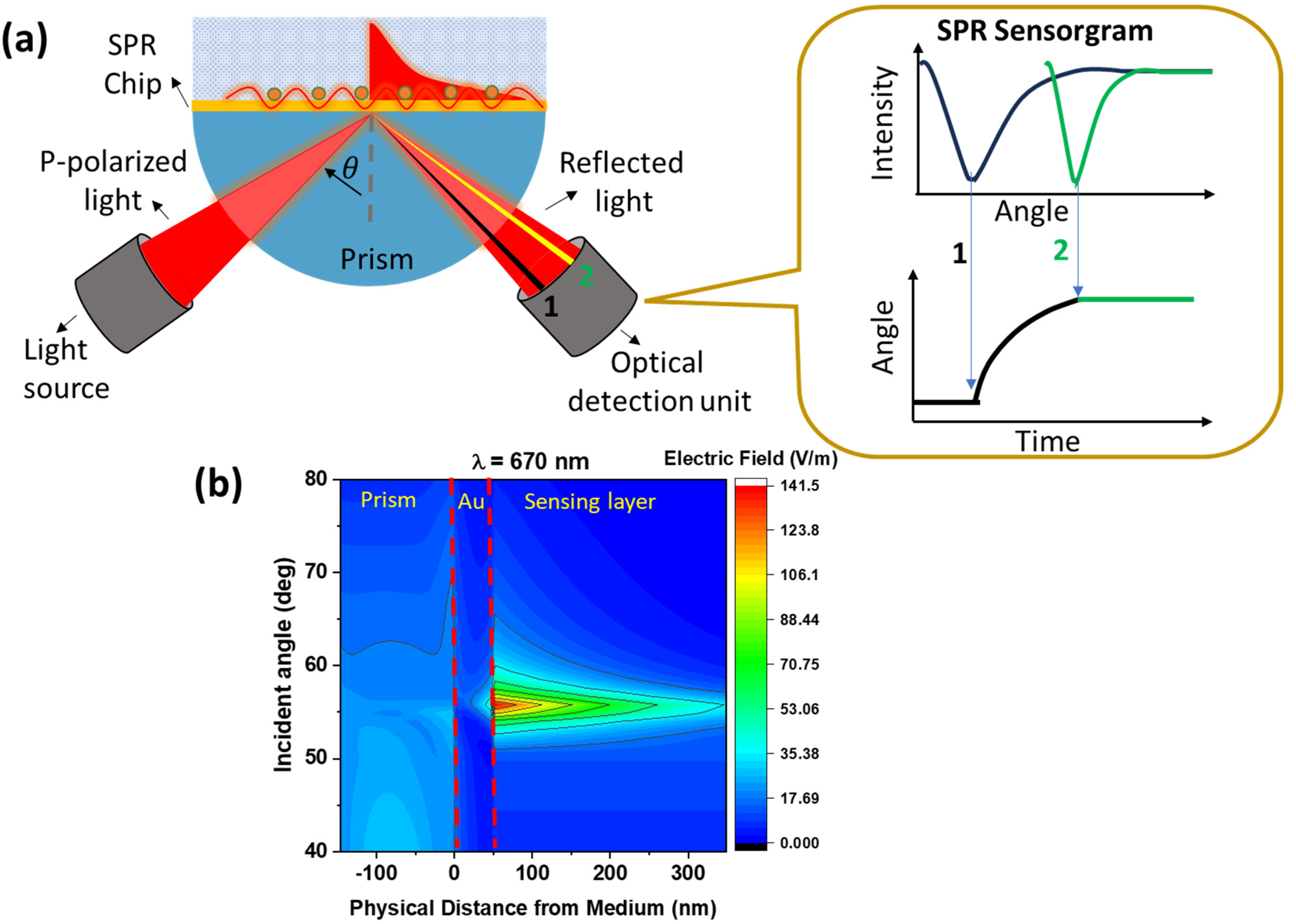
2.1. SPR-Based Biosensor
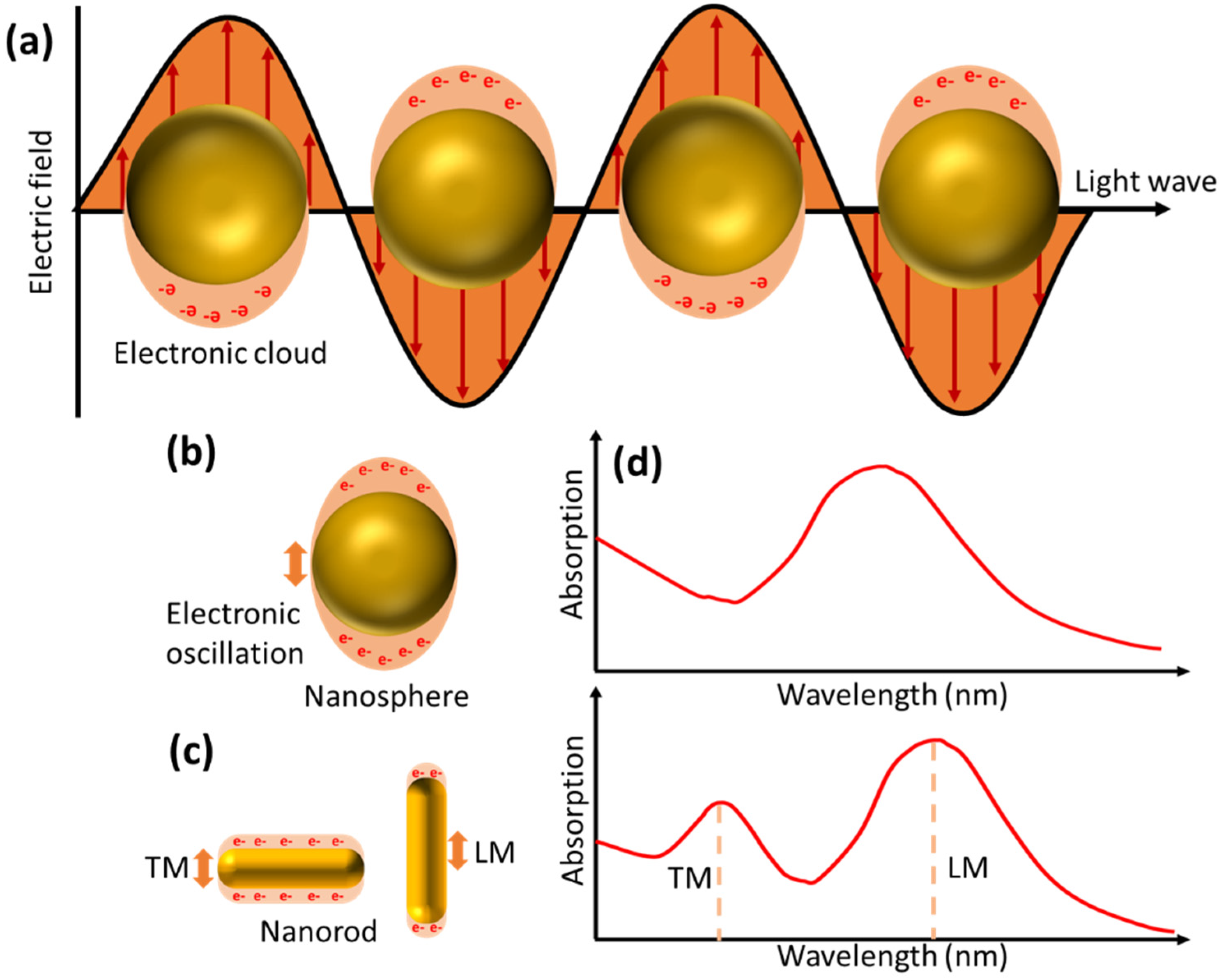
2.2. LSPR-Based Biosensor
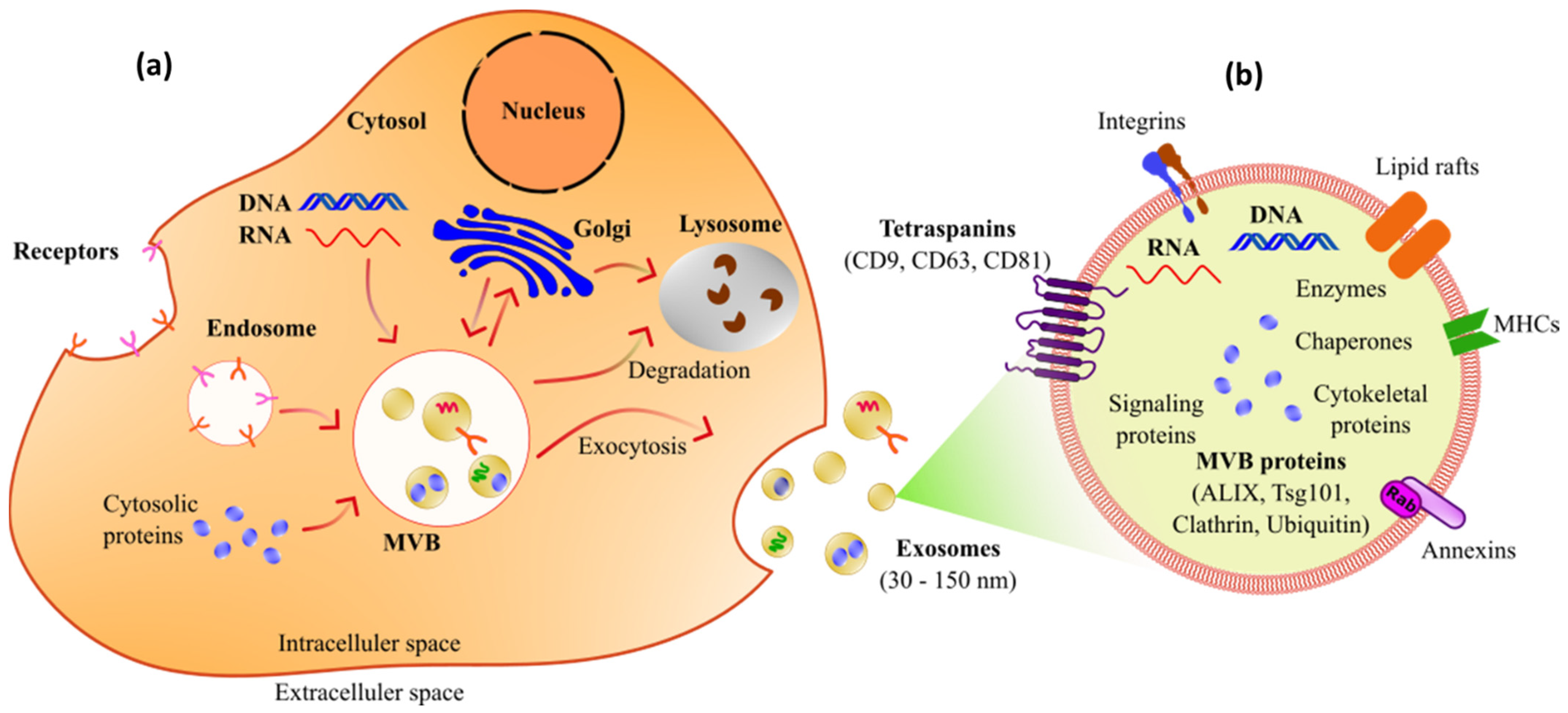
3. Potential Use of Exosomes as Biomarkers
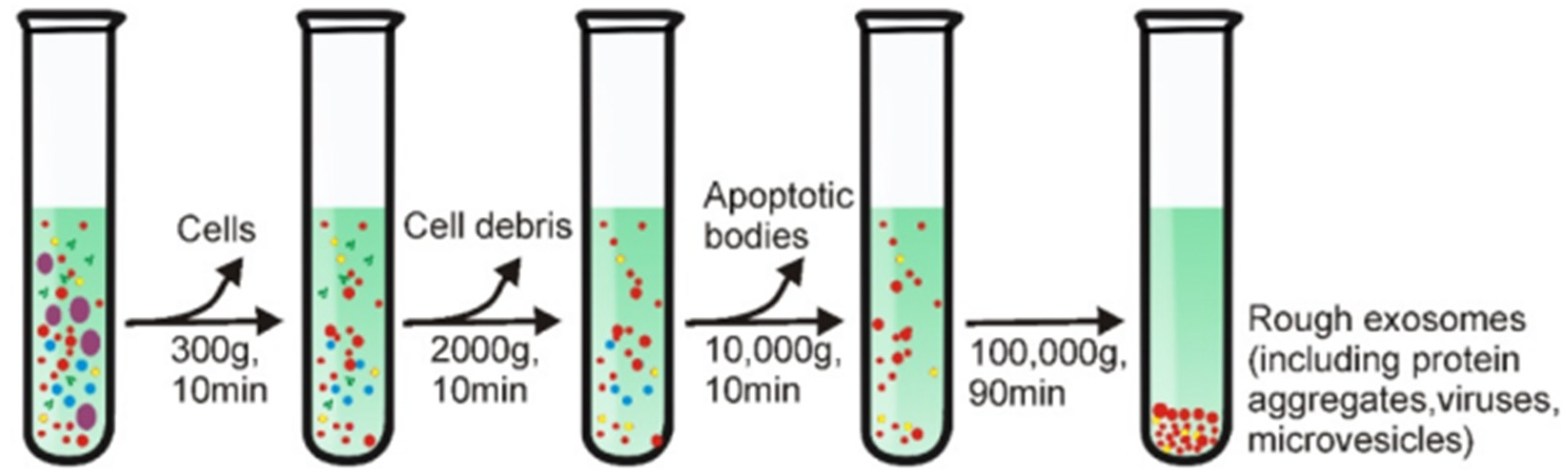
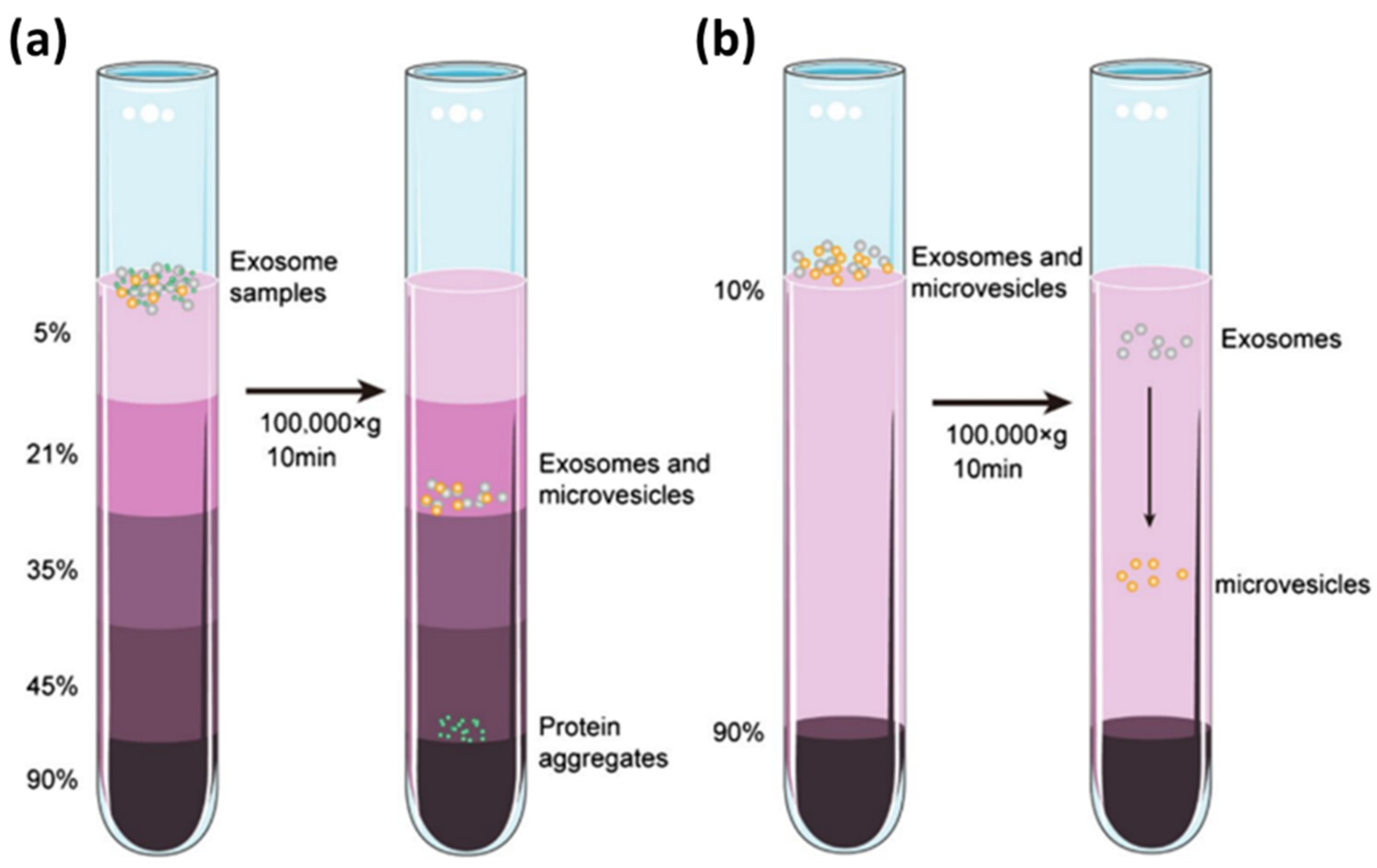
4. Isolation of Exosomes
5. Development of SPR Biosensor for Exosome Detection
5.1. SPR Biosensor with Conventional Structure
5.2. SPR Biosensor Modified with 2D Material
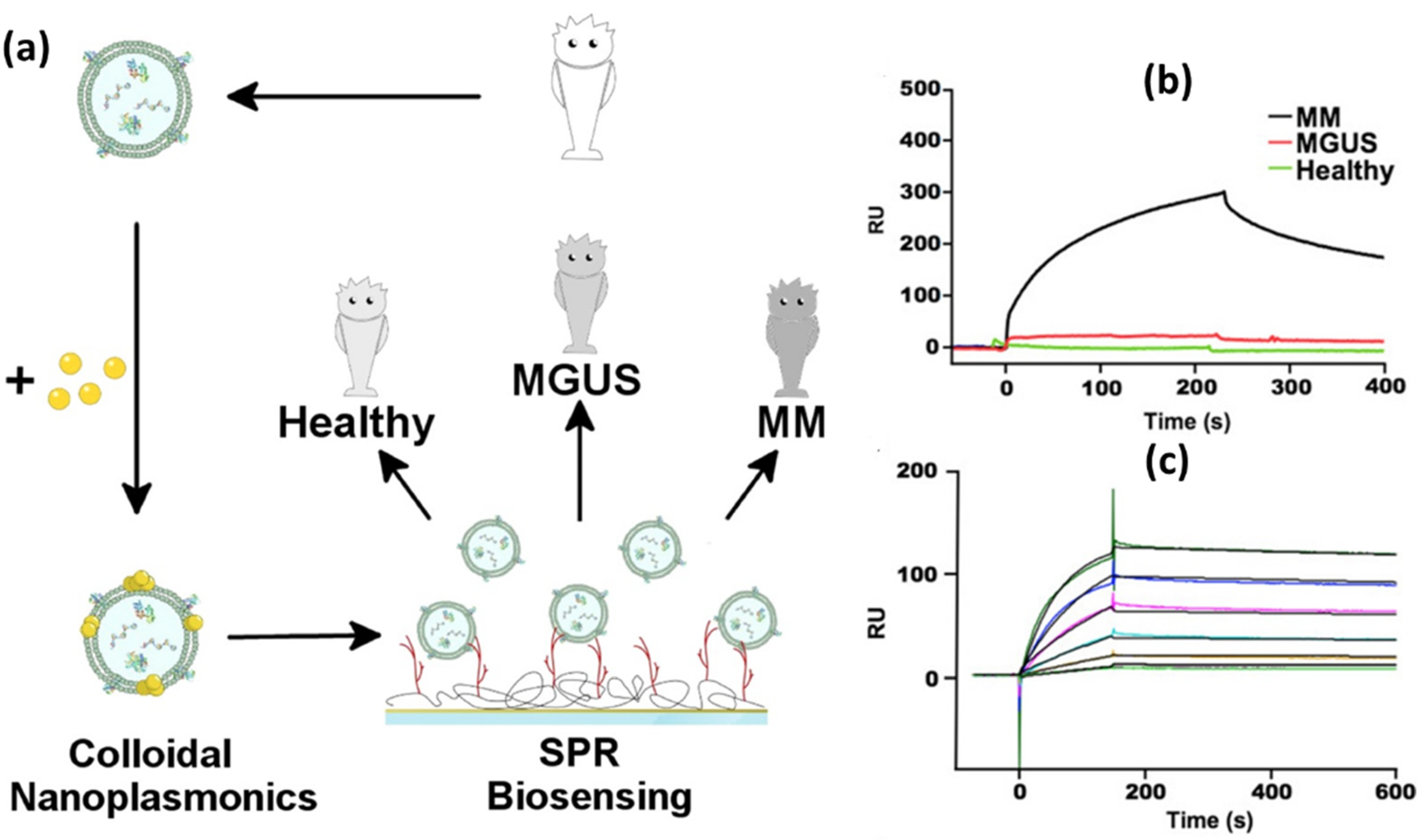
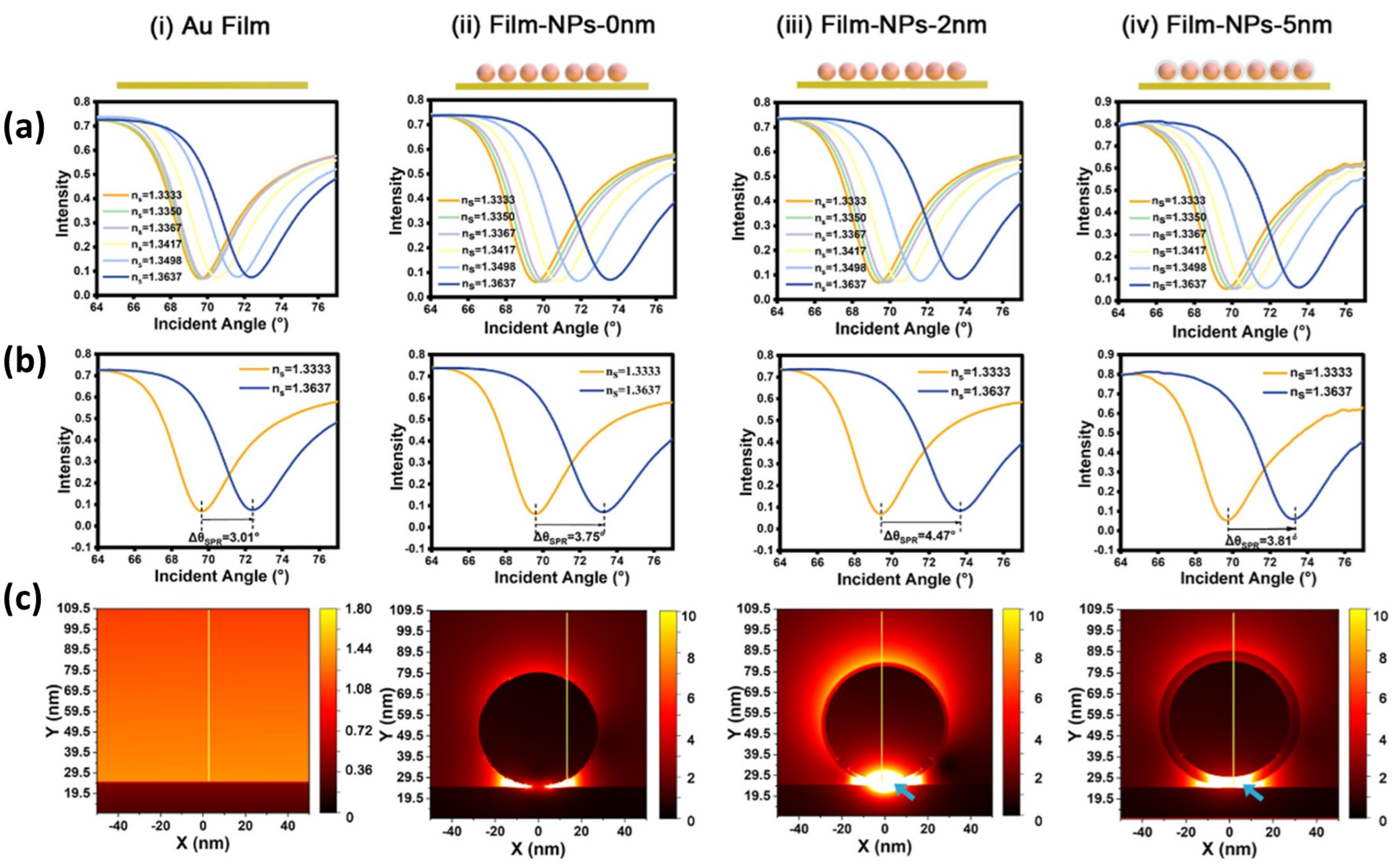
5.3. SPR Biosensor Modified with Metal Nanoparticles
| Recognition Element | Specific Target | Developed Biosensor System | Detection Limit | Ref. | |
|---|---|---|---|---|---|
(Exosomes/mL) | |||||
| Conventional SPR Biosensors | |||||
| Aptamer | PD-L1 exosomes | Exosome detection was carried out by utilizing the interaction of streptavidin and biotin using a conventional SPR chip | 44.50 pM | 2.68 × 1010 | [121] |
| Anti-HER2 | HER2 (+) Exosome | Conventional SPR chip was functionalized with anti-HER2 | 0.828 × 104 exosomes/μL | 8.28 × 106 | [122] |
| anti-EGFR | EGFR exosomes | Conventional SPR chip was functionalized with anti-EGFR | 3.5 × 109 exosomes/mL | 3.5 × 109 | [120] |
| Biotinylated antibody | EGFR variant-III | SPR Chip based on Titanium nitride (TiN) | 2.75 × 10−3 µg/mL | 1.99 × 1027 | [147] |
| SPR Biosensors Modified with 2D Materials | |||||
| Peptide | PD-L1 exosomes | Gold-based SPR chips deposited with graphene | 20 exosomes/mL | 20 | [134] |
| peptide | PD-L1 exosomes | Sensitivity-enhanced SPR biosensor with MXene@MOF heterostructure | 5.24 exosomes/mL | 5.24 | [135] |
| Peptide | PD-L1 exosomes | SPR chip was deposited with a 2D metal–organic framework (MOF) | 16.7 exosomes/mL | 16.7 | [137] |
| peptide | PD-L1 exosomes | Enhancing the sensitivity of the SPR biosensor is carried out by utilizing the large surface area properties of single-walled carbon nanowires | 75.23 exosomes/mL | 75.23 | [138] |
| Antibody | Anti-CD81 | The sensitivity of the Goos–Hanchen (GH) shift-based SPR biosensor is enhanced with a thin layer of Ge2Sb2Te5 (GST) | 104 exosomes/mL | 104 | [136] |
| SPR Biosensors Modified with Metal Nanoparticles | |||||
| Heparin | multiple myeloma | The SPR signal was amplified with Au NPs | 0.06 nM | 3.61 × 1010 | [139] |
| Aptamer | hepatic carcinoma SMMC-7721 | The SPR signal was amplified using AuNPs coated with polydopamine | 5.6 × 105 exosomes/mL | 5.6 × 105 | [148] |
| molecular aptamer beacon (MAB) | HER2-positive exosomes | The SPR signal was amplified with AuNPs coated with tyramine | 1 × 104 exosomes/mL | 104 | [149] |
| DNA | MCF-7 breast cancer cells | SPR biosensor with dual AuNP-assisted signal amplification | 5 × 103 exosomes/mL | 5 × 103 | [141] |
| aptamer-DNA linker | LNCaP | SPRi with signal amplification with hydrogel-AuNP supramolecular sphere | 1 × 105 exosomes/mL | 105 | [150] |
| peptide | PD-L1 exosomes | Enhanced SPR sensitivity is due to the substantial increase in the electromagnetic field generated at the tips of the gold nanoflowers with multi-tip tiny petals | 4.95 exosomes/mL | 4.95 | [151] |
6. Development of LSPR Biosensor for Exosome Detection
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uyar, R.; Özçelikay-Akyıldız, G.; Kaya, S.İ.; Bereketoğlu Nergis, S.; Beşbinar, Ö.; Ünal, M.A.; Yilmazer, A.; Özkan, S.A. Early Cancer Detection Based on Exosome Biosensors in Biological Samples. Sens. Actuators B Chem. 2024, 400, 134886. [Google Scholar] [CrossRef]
- Bari, S.M.I.; Hossain, F.B.; Nestorova, G.G. Advances in Biosensors Technology for Detection and Characterization of Extracellular Vesicles. Sensors 2021, 21, 7645. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Garssen, J.; Movassaghi, M.; Mirsaeidi, M.; Adcock, I.M. Exosomes and Exosomal MiRNA in Respiratory Diseases. Mediators Inflamm. 2016, 2016, 5628404. [Google Scholar] [CrossRef]
- Sałaga-Zaleska, K.; Kuchta, A.; Bzoma, B.; Chyła-Danił, G.; Safianowska, A.; Płoska, A.; Kalinowski, L.; Dębska-Ślizień, A.; Jankowski, M. Nanoparticle Tracking Analysis of Urinary Extracellular Vesicle Proteins as a New Challenge in Laboratory Medicine. Int. J. Mol. Sci. 2023, 24, 12228. [Google Scholar] [CrossRef]
- Morales-Kastresana, A.; Jones, J.C. Flow Cytometric Analysis of Extracellular Vesicles. Methods Mol. Biol. 2017, 1545, 215–225. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Liu, C.; Qie, Y.; Qin, W.; Zhao, K.; Zhu, J.; Zhao, L.; Li, M.; Guo, L.H. Emerging Immunoassay Technologies for the Rapid Detection of Exosomes. Sens. Actuators B Chem. 2021, 345, 130336. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Li, S.; Liu, H.; Jin, L.; Chen, Z.; Zhao, Y.; Li, Z.; Deng, Y.; He, N. A Simple Fluorescence Aptasensor for Gastric Cancer Exosome Detection Based on Branched Rolling Circle Amplification. Nanoscale 2020, 12, 2445–2451. [Google Scholar] [CrossRef]
- Luo, S.; Wu, Y.; Pan, W.; Zhong, G.; Situ, B.; Li, B.; Ye, X.; Jiang, X.; Li, W.; Zhang, Y.; et al. An Integrated Magneto-Fluorescent Nanosensor for Rapid and Sensitive Detection of Tumor-Derived Exosomes. Sens. Actuators B Chem. 2023, 374, 132792. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, W.S.; Guo, Y.; Peng, H.; Zhu, M.; Miao, D.; Su, G. Engineering of Exosome-Triggered Enzyme-Powered DNA Motors for Highly Sensitive Fluorescence Detection of Tumor-Derived Exosomes. Biosens. Bioelectron. 2020, 167, 112482. [Google Scholar] [CrossRef]
- Zhu, N.; Li, G.; Zhou, J.; Zhang, Y.; Kang, K.; Ying, B.; Yi, Q.; Wu, Y. A Light-up Fluorescence Resonance Energy Transfer Magnetic Aptamer-Sensor for Ultra-Sensitive Lung Cancer Exosome Detection. J. Mater. Chem. B 2021, 9, 2483–2493. [Google Scholar] [CrossRef]
- Jin, Y.; Du, N.; Huang, Y.; Shen, W.; Tan, Y.; Chen, Y.Z.; Dou, W.T.; He, X.P.; Yang, Z.; Xu, N.; et al. Fluorescence Analysis of Circulating Exosomes for Breast Cancer Diagnosis Using a Sensor Array and Deep Learning. ACS Sens. 2022, 7, 1524–1532. [Google Scholar] [CrossRef]
- Pan, H.; Dong, Y.; Gong, L.; Zhai, J.; Song, C.; Ge, Z.; Su, Y.; Zhu, D.; Chao, J.; Su, S.; et al. Sensing Gastric Cancer Exosomes with MoS2-Based SERS Aptasensor. Biosens. Bioelectron. 2022, 215, 114553. [Google Scholar] [CrossRef]
- Han, Z.; Peng, X.; Yang, Y.; Yi, J.; Zhao, D.; Bao, Q.; Long, S.; Yu, S.X.; Xu, X.X.; Liu, B.; et al. Integrated Microfluidic-SERS for Exosome Biomarker Profiling and Osteosarcoma Diagnosis. Biosens. Bioelectron. 2022, 217, 114709. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.-H.; Gao, Z.; Shen, J.; He, J.; MacLachlan, A.; Ma, C.; Chang, Y.; Yang, W.; Cai, Y.; et al. Nanoplasmonic Sandwich Immunoassay for Tumor-Derived Exosome Detection and Exosomal PD-L1 Profiling. ACS Sens. 2021, 6, 3308–3319. [Google Scholar] [CrossRef]
- Lv, X.; Geng, Z.; Su, Y.; Fan, Z.; Wang, S.; Fang, W.; Chen, H. Label-Free Exosome Detection Based on a Low-Cost Plasmonic Biosensor Array Integrated with Microfluidics. Langmuir 2019, 35, 9816–9824. [Google Scholar] [CrossRef]
- Hammond, J.L.; Bhalla, N.; Rafiee, S.D.; Estrela, P. Localized Surface Plasmon Resonance as a Biosensing Platform for Developing Countries. Biosensors 2014, 4, 172–188. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Satish, L.; Kushmaro, A.; Shvalya, V.; Cvelbar, U.; Abdulhalim, I. Engineering the Penetration Depth of Nearly Guided Wave Surface Plasmon Resonance towards Application in Bacterial Cells Monitoring. Sens. Actuators B Chem. 2021, 345, 130338. [Google Scholar] [CrossRef]
- Pal, S.; Verma, A.; Prajapati, Y.K.; Saini, J.P. Figure of Merit Enhancement of Surface Plasmon Resonance Biosensor Using Ga-Doped Zinc Oxide in Near Infrared Range. Photonic Sens. 2020, 10, 340–352. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Yang, R.; Liu, W.; Sun, S. A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film. Sensors 2017, 17, 2777. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Xie, J.; Han, Y.; Huang, Y.; Zhang, H. Exosomal Analysis: Advances in Biosensor Technology. Clin. Chim. Acta 2021, 518, 142–150. [Google Scholar] [CrossRef]
- Xu, L.; Shoaie, N.; Jahanpeyma, F.; Zhao, J.; Azimzadeh, M.; Al−Jamal, K.T. Optical, Electrochemical and Electrical (Nano)Biosensors for Detection of Exosomes: A Comprehensive Overview. Biosens. Bioelectron. 2020, 161, 112222. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.F. Interaction Studies of Localized Surface Plasmon Resonance Immunosensor Based on Gold Nanoparticles. IEEE Sens. J. 2023, 23, 19262–19271. [Google Scholar] [CrossRef]
- Farooq, S.; Wali, F.; Zezell, D.M.; de Araujo, R.E.; Rativa, D. Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis. Polymers 2022, 14, 1592. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Kotsifaki, D.G. Plasmonic and Metamaterial Biosensors: A Game-Changer for Virus Detection. Sens. Diagn. 2023, 2, 600–619. [Google Scholar] [CrossRef]
- Song, S.; Lee, J.U.; Jeon, M.J.; Kim, S.; Sim, S.J. Detection of Multiplex Exosomal MiRNAs for Clinically Accurate Diagnosis of Alzheimer’s Disease Using Label-Free Plasmonic Biosensor Based on DNA-Assembled Advanced Plasmonic Architecture. Biosens. Bioelectron. 2022, 199, 113864. [Google Scholar] [CrossRef]
- Bonyár, A. Maximizing the Surface Sensitivity of Lspr Biosensors through Plasmon Coupling—Interparticle Gap Optimization for Dimers Using Computational Simulations. Biosensors 2021, 11, 527. [Google Scholar] [CrossRef]
- Farooq, S.; Rativa, D.; de Araujo, R.E. Optimizing the Sensing Performance of SiO2-Au Nanoshells. Plasmonics 2019, 14, 1519–1526. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Critical Overview on the Application of Sensors and Biosensors for Clinical Analysis. TrAC Trends Anal. Chem. 2016, 85, 36–60. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.F.; Tai, M.J.; Nurrohman, D.T.; Lin, T.L.; Wang, Y.H.; Chen, C.Y. Immunoassay-Amplified Responses Using a Functionalized Mos2-Based Spr Biosensor to Detect Papp-A2 in Maternal Serum Samples to Screen for Fetal down’s Syndrome. Int. J. Nanomed. 2021, 16, 2715–2733. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Wang, Y.-H.; Chiu, N.-F. Exploring Graphene and MoS2 Chips Based Surface Plasmon Resonance Biosensors for Diagnostic Applications. Front. Chem. 2020, 8, 728. [Google Scholar] [CrossRef]
- Fathi, F.; Jalili, R.; Amjadi, M.; Rashidi, M.R. SPR Signals Enhancement by Gold Nanorods for Cell Surface Marker Detection. BioImpacts 2019, 9, 71–78. [Google Scholar] [CrossRef]
- Kumar, R.; Agarwal, S.; Pal, S.; Verma, A.; Kumar Prajapati, Y. Refractive Index Sensing Using MXene Mediated Surface Plasmon Resonance Sensor in Visible to near Infrared Regime. Measurement 2024, 224, 113682. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.K.; Kushwaha, A.S.; Srivastava, S.K. A Comparative Study among WS2, MoS2 and Graphene Based Surface Plasmon Resonance (SPR) Sensor. Sens. Actuators Rep. 2020, 2, 100015. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A Comprehensive Review on Plasmonic-Based Biosensors Used in Viral Diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lin, H.-B.; Wang, X.-Z.; Hu, S.-Q.; Luo, Y.-H. Enhanced Sensitivity of a Surface Plasmon Resonance Biosensor Utilizing Au/ITO Hyperbolic Metamaterial. Results Phys. 2023, 49, 106522. [Google Scholar] [CrossRef]
- Kurt, H.; Pishva, P.; Pehlivan, Z.S.; Arsoy, E.G.; Saleem, Q.; Bayazıt, M.K.; Yüce, M. Nanoplasmonic Biosensors: Theory, Structure, Design, and Review of Recent Applications. Anal. Chim. Acta 2021, 1185, 338842. [Google Scholar] [CrossRef]
- Long, S.; Cao, J.; Wang, Y.; Gao, S.; Xu, N.; Gao, J.; Wan, W. Grating Coupled SPR Sensors Using off the Shelf Compact Discs and Sensitivity Dependence on Grating Period. Sens. Actuators Rep. 2020, 2, 100016. [Google Scholar] [CrossRef]
- Joseph, S.; Sarkar, S.; Joseph, J. Grating-Coupled Surface Plasmon-Polariton Sensing at a Flat Metal-Analyte Interface in a Hybrid-Configuration. ACS Appl. Mater. Interfaces 2020, 12, 46519–46529. [Google Scholar] [CrossRef]
- Ji, L.; Yang, S.; Shi, R.; Fu, Y.; Su, J.; Wu, C. Polymer Waveguide Coupled Surface Plasmon Refractive Index Sensor: A Theoretical Study. Photonic Sens. 2020, 10, 353–363. [Google Scholar] [CrossRef]
- Walter, J.G.; Eilers, A.; Alwis, L.S.M.; Roth, B.W.; Bremer, K. Spr Biosensor Based on Polymer Multi-Mode Optical Waveguide and Nanoparticle Signal Enhancement. Sensors 2020, 20, 2889. [Google Scholar] [CrossRef]
- Ansari, M.T.I.; Raghuwanshi, S.K.; Kumar, S. Recent Advancement in Fiber-Optic-Based SPR Biosensor for Food Adulteration Detection—A Review. IEEE Trans. Nanobioscience 2023, 22, 978–988. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Z.; Zhang, Y.; Li, S.; Zhang, Y.; Yang, X.; Zhang, J.; Yuan, L. Specialty Optical Fibers and 2D Materials for Sensitivity Enhancement of Fiber Optic SPR Sensors: A Review. Opt. Laser Technol. 2022, 152, 108167. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, R.-J.; Xia, F.; Peng, Y. Current Status of Optical Fiber Biosensor Based on Surface Plasmon Resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research Advances on Surface Plasmon Resonance Biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef]
- Fontana, E.; Kim, J.-M.; Llamas-Garro, I.; Cavalcanti, G.O. Microfabricated Otto Chip Device for Surface Plasmon Resonance-Based Optical Sensing. Appl. Opt. 2015, 54, 9200. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sharma, A.K. Advancements in Grating Nanostructure Based Plasmonic Sensors in Last Two Decades: A Review. IEEE Sens. J. 2021, 21, 12633–12644. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Kong, S.K.; Kabashin, A.V. Phase-Sensitive Surface Plasmon Resonance Biosensors: Methodology, Instrumentation and Applications. Ann. Phys. 2012, 524, 637–662. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. SPR and SPR Imaging: Recent Trends in Developing Nanodevices for Detection and Real-Time Monitoring of Biomolecular Events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef]
- Cai, H.; Wang, M.; Liu, J.; Wang, X. Theoretical and Experimental Study of a Highly Sensitive SPR Biosensor Based on Au Grating and Au Film Coupling Structure. Opt. Express 2022, 30, 26136. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sharma, A.K.; Marques, C. On The Application of SiO2/SiC Grating on Ag for High-Performance Fiber Optic Plasmonic Sensing of Cortisol Concentration. Materials 2020, 13, 1623. [Google Scholar] [CrossRef]
- Sarcina, L.; Macchia, E.; Loconsole, G.; D’Attoma, G.; Saldarelli, P.; Elicio, V.; Palazzo, G.; Torsi, L. Surface Plasmon Resonance Assay for Label-Free and Selective Detection of Xylella Fastidiosa. Adv. NanoBiomed Res. 2021, 1, 2100043. [Google Scholar] [CrossRef]
- Sang, W.; Huang, S.; Chen, J.; Dai, X.; Liu, H.; Zeng, Y.; Zhang, T.; Wang, X.; Qu, J.; Ho, H.-P.; et al. Wavelength Sequential Selection Technique for High-Throughput Multi-Channel Phase Interrogation Surface Plasmon Resonance Imaging Sensing. Talanta 2023, 258, 124405. [Google Scholar] [CrossRef]
- Yang, C.-H.; Wu, T.-H.; Chang, C.-C.; Lo, H.-Y.; Liu, H.-W.; Huang, N.-T.; Lin, C.-W. Biosensing Amplification by Hybridization Chain Reaction on Phase-Sensitive Surface Plasmon Resonance. Biosensors 2021, 11, 75. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Tai, J.; Dai, X.; Liu, X.; Wang, Y.; Chen, J.; Qu, J.; Ho, H.P.; Shao, Y. Optimizing Surface Plasmon Resonance Spectral Imaging through AOTF-Calibrated Light Sources and Image Feedback. Opt. Laser Technol. 2024, 176, 111021. [Google Scholar] [CrossRef]
- Yi, R.-M.; Zhang, Z.; Liu, C.-X.; Qi, Z.-M. Gold-Silver Alloy Film Based Surface Plasmon Resonance Sensor for Biomarker Detection. Mater. Sci. Eng. C 2020, 116, 111126. [Google Scholar] [CrossRef]
- Chen, S.; Chu, S.; Song, Y.; Wu, H.; Liu, Y.; Peng, W. Near-Infrared Surface Plasmon Resonance Sensor with a Graphene-Gold Surface Architecture for Ultra-Sensitive Biodetection. Anal. Chim. Acta 2022, 1205, 339692. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zeng, J.; Deng, J.; Hu, N.; Yang, J. Multi-Channel Surface Plasmon Resonance Biosensor Using Prism-Based Wavelength Interrogation. Opt. Express 2020, 28, 14007. [Google Scholar] [CrossRef]
- Sarapukdee, P.; Spenner, C.; Schulz, D.; Palzer, S. Optimizing Stability and Performance of Silver-Based Grating Structures for Surface Plasmon Resonance Sensors. Sensors 2023, 23, 6743. [Google Scholar] [CrossRef]
- Bijalwan, A.; Rastogi, V. Sensitivity Enhancement of a Conventional Gold Grating Assisted Surface Plasmon Resonance Sensor by Using a Bimetallic Configuration. Appl. Opt. 2017, 56, 9606. [Google Scholar] [CrossRef]
- Bijalwan, A.; Rastogi, V. Gold–Aluminum-Based Surface Plasmon Resonance Sensor with a High Quality Factor and Figure of Merit for the Detection of Hemoglobin. Appl. Opt. 2018, 57, 9230. [Google Scholar] [CrossRef]
- Cao, K.; Wu, M.; Wang, E.; Liu, C.; Zhu, H.; Ma, C.; Cao, J. Dual-Mode SPR/SERS Biosensor Utilizing Metal Nanogratings Fabricated via Wet Etching-Assisted Direct Laser Interference Patterning. Appl. Surf. Sci. 2024, 655, 159621. [Google Scholar] [CrossRef]
- Kotlarek, D.; Vorobii, M.; Ogieglo, W.; Knoll, W.; Rodriguez-Emmenegger, C.; Dostálek, J. Compact Grating-Coupled Biosensor for the Analysis of Thrombin. ACS Sens. 2019, 4, 2109–2116. [Google Scholar] [CrossRef]
- Du, W.; Zhao, F. Silicon Carbide Based Surface Plasmon Resonance Waveguide Sensor with a Bimetallic Layer for Improved Sensitivity. Mater. Lett. 2017, 186, 224–226. [Google Scholar] [CrossRef]
- Akowuah, E.K.; Gorman, T.; Haxha, S.; Oliver, J.V. Dual Channel Planar Waveguide Surface Plasmon Resonance Biosensor for an Aqueous Environment. Opt. Express 2010, 18, 24412. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. Highly Selective SPR Based Fiber Optic Sensor for the Detection of Hydrogen Peroxide. Sens. Actuators B Chem. 2021, 329, 129062. [Google Scholar] [CrossRef]
- Arjmand, M.; Saghafifar, H.; Alijanianzadeh, M.; Soltanolkotabi, M. A Sensitive Tapered-Fiber Optic Biosensor for the Label-Free Detection of Organophosphate Pesticides. Sens. Actuators B Chem. 2017, 249, 523–532. [Google Scholar] [CrossRef]
- Chiu, N.F.; Yang, C.-D.; Chen, C.C.; Kuo, C.T. Stepwise Control of Reduction of Graphene Oxide and Quantitative Real-Time Evaluation of Residual Oxygen Content Using EC-SPR for a Label-Free Electrochemical Immunosensor. Sens. Actuators B Chem. 2018, 258, 981–990. [Google Scholar] [CrossRef]
- Wang, D.S.; Fan, S.K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef]
- Xiao, C.; Eriksson, J.; Suska, A.; Filippini, D.; Mak, W.C. Print-and-Stick Unibody Microfluidics Coupled Surface Plasmon Resonance (SPR) Chip for Smartphone Imaging SPR (Smart-ISRP). Anal. Chim. Acta 2022, 1201, 339606. [Google Scholar] [CrossRef]
- Kim, D.M.; Park, J.S.; Jung, S.-W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. [Google Scholar] [CrossRef]
- Farooq, S.; de Araujo, R.E. Engineering a Localized Surface Plasmon Resonance Platform for Molecular Biosensing. Open J. Appl. Sci. 2018, 8, 126–139. [Google Scholar] [CrossRef]
- Pellas, V.; Hu, D.; Mazouzi, Y.; Mimoun, Y.; Blanchard, J.; Guibert, C.; Salmain, M.; Boujday, S. Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications. Biosensors 2020, 10, 146. [Google Scholar] [CrossRef]
- Bansal, A.; Sekhon, J.S.; Verma, S.S. Scattering Efficiency and LSPR Tunability of Bimetallic Ag, Au, and Cu Nanoparticles. Plasmonics 2014, 9, 143–150. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Minopoli, A.; Acunzo, A.; Della Ventura, B.; Velotta, R. Nanostructured Surfaces as Plasmonic Biosensors: A Review. Adv. Mater. Interfaces 2022, 9, 2101133. [Google Scholar] [CrossRef]
- Jeon, H.-B.; Tsalu, P.V.; Ha, J.W. Shape Effect on the Refractive Index Sensitivity at Localized Surface Plasmon Resonance Inflection Points of Single Gold Nanocubes with Vertices. Sci. Rep. 2019, 9, 13635. [Google Scholar] [CrossRef]
- Jing, L.; Xie, C.; Li, Q.; Yang, M.; Li, S.; Li, H.; Xia, F. Electrochemical Biosensors for the Analysis of Breast Cancer Biomarkers: From Design to Application. Anal. Chem. 2022, 94, 269–296. [Google Scholar] [CrossRef]
- Bang, C.; Thum, T. Exosomes: New Players in Cell-Cell Communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome Engineering in Cell Therapy and Drug Delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered Exosomes from Different Sources for Cancer-Targeted Therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Wu, Y. Recent Advances in Exosomal Protein Detection Via Liquid Biopsy Biosensors for Cancer Screening, Diagnosis, and Prognosis. AAPS J. 2018, 20, 41. [Google Scholar] [CrossRef]
- Cheng, N.; Du, D.; Wang, X.; Liu, D.; Xu, W.; Luo, Y.; Lin, Y. Recent Advances in Biosensors for Detecting Cancer-Derived Exosomes. Trends Biotechnol. 2019, 37, 1236–1254. [Google Scholar] [CrossRef]
- Greenwel, P.; Tanaka, S.; Penkov, D.; Zhang, W.; Olive, M.; Moll, J.; Vinson, C.; Di Liberto, M.; Ramirez, F. Tumor Necrosis Factor Alpha Inhibits Type I Collagen Synthesis through Repressive CCAAT/Enhancer-Binding Proteins. Mol. Cell. Biol. 2000, 20, 912–918. [Google Scholar] [CrossRef]
- Martínez-Reza, I.; Díaz, L.; García-Becerra, R. Preclinical and Clinical Aspects of TNF-α and Its Receptors TNFR1 and TNFR2 in Breast Cancer. J. Biomed. Sci. 2017, 24, 90. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, S.; Crunteanu, A.; Xie, Z.; Humbert, G.; Ma, L.; Wei, Y.; Brunel, A.; Bessette, B.; Orlianges, J.C.; et al. Targeted Sub-Attomole Cancer Biomarker Detection Based on Phase Singularity 2D Nanomaterial-Enhanced Plasmonic Biosensor. Nano-Micro Lett. 2021, 13, 96. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Yang, X.X.; Malichewe, C.Y.; Li, Y.S.; Guo, X.L. Exosomal MicroRNAs-Mediated Intercellular Communication and Exosome-Based Cancer Treatment. Int. J. Biol. Macromol. 2020, 158, 530–541. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Biava, P.M. Exosomes and Cell Communication: From Tumour-Derived Exosomes and Their Role in Tumour Progression to the Use of Exosomal Cargo for Cancer Treatment. Cancers 2021, 13, 822. [Google Scholar] [CrossRef]
- Decastro, J.; Littig, J.; Chou, P.P.; Mack-Onyeike, J.; Srinivasan, A.; Conboy, M.J.; Conboy, I.M.; Aran, K. The Microfluidic Toolbox for Analyzing Exosome Biomarkers of Aging. Molecules 2021, 26, 535. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Wise, P.M.; Neviani, P.; Riwaldt, S.; Corydon, T.J.; Wehland, M.; Braun, M.; Krüger, M.; Infanger, M.; Grimm, D. Changes in Exosomal Mirna Composition in Thyroid Cancer Cells after Prolonged Exposure to Real Microgravity in Space. Int. J. Mol. Sci. 2021, 22, 12841. [Google Scholar] [CrossRef]
- Kurian, T.K.; Banik, S.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Mol. Biotechnol. 2021, 63, 249–266. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Yi, X.; Chen, J.; Huang, D.; Feng, S.; Yang, T.; Li, Z.; Wang, X.; Zhao, M.; Wu, J.; Zhong, T. Current Perspectives on Clinical Use of Exosomes as Novel Biomarkers for Cancer Diagnosis. Front. Oncol. 2022, 12, 966981. [Google Scholar] [CrossRef]
- Huda, M.N.; Nafiujjaman, M.; Deaguero, I.G.; Okonkwo, J.; Hill, M.L.; Kim, T.; Nurunnabi, M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2106–2149. [Google Scholar] [CrossRef]
- Tan, J.; Wen, Y.; Li, M. Emerging Biosensing Platforms for Quantitative Detection of Exosomes as Diagnostic Biomarkers. Coord. Chem. Rev. 2021, 446, 214111. [Google Scholar] [CrossRef]
- Haizan, I.; Park, D.H.; Choi, M.Y.; Lee, H.; Choi, J.H. Nanomaterials-Based Exosomes for the Diagnostics and Drug Deliveries of Central Nervous System Diseases. Biochip J. 2023, 17, 293–307. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent Developments in Isolating Methods for Exosomes. Front. Bioeng. Biotechnol. 2023, 10, 1100892. [Google Scholar] [CrossRef]
- Martins, T.S.; Vaz, M.; Henriques, A.G. A Review on Comparative Studies Addressing Exosome Isolation Methods from Body Fluids. Anal. Bioanal. Chem. 2023, 415, 1239–1263. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular Organelles Important in Intercellular Communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. BioMed Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, Opportunity, and Perspective on Exosome Isolation—Efforts for Efficient Exosome-Based Theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Z.; Ma, Z.-J.; Kang, X.-W.Z.; Ma, Z.-J.; Kang, X.-W. Current Status and Outlook of Advances in Exosome Isolation. Anal. Bioanal. Chem. 2022, 414, 7123–7141. [Google Scholar] [CrossRef]
- Wang, J.; Ma, P.; Kim, D.H.; Liu, B.-F.; Demirci, U. Towards Microfluidic-Based Exosome Isolation and Detection for Tumor Therapy. Nano Today 2021, 37, 101066. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.C.; Wu, H.J.; Ugaz, V.M. Microfluidics for Exosome Isolation and Analysis: Enabling Liquid Biopsy for Personalized Medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and Technologies for Exosome Isolation and Characterization. Small Methods 2018, 2, 1800021. [Google Scholar] [CrossRef]
- Guan, S.; Guan, S.; Yu, H.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Characterization of Urinary Exosomes Purified with Size Exclusion Chromatography and Ultracentrifugation. J. Proteome Res. 2020, 19, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-Step Isolation of Extracellular Vesicles by Size-Exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Hong, C.; Ludwig, S.; Azambuja, J.H.; Sharma, P.; Theodoraki, M.; Whiteside, T.L. Isolation and Analysis of Tumor-Derived Exosomes. Curr. Protoc. Immunol. 2019, 127, e91. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Kong, F.; Zhang, Q.; Xiao, J.; Zhang, Y.; Yan, B. Tango of Dual Nanoparticles: Interplays between Exosomes and Nanomedicine. Bioeng. Transl. Med. 2022, 7, e10269. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.-F. The Current Status and Future Promise of SPR Biosensors. Biosensors 2022, 12, 933. [Google Scholar] [CrossRef]
- Hsu, C.C.; Yang, Y.; Kannisto, E.; Zeng, X.; Yu, G.; Patnaik, S.K.; Dy, G.K.; Reid, M.E.; Gan, Q.; Wu, Y. Simultaneous Detection of Tumor Derived Exosomal Protein-MicroRNA Pairs with an Exo-PROS Biosensor for Cancer Diagnosis. ACS Nano 2023, 17, 8108–8122. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Guan, M.; Liu, Y.; Lv, M.; Zhang, C.; Zhang, H.; Zhang, Z. Isolation of Circulating Exosomes and Identification of Exosomal PD-L1 for Predicting Immunotherapy Response. Nanoscale 2022, 14, 8995–9003. [Google Scholar] [CrossRef] [PubMed]
- Sina, A.A.I.; Vaidyanathan, R.; Wuethrich, A.; Carrascosa, L.G.; Trau, M. Label-Free Detection of Exosomes Using a Surface Plasmon Resonance Biosensor. Anal. Bioanal. Chem. 2019, 411, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jiang, J.; Zhou, X.; Kolay, J.; Wang, R.; Wan, Z.; Wang, S. Label-Free Imaging and Biomarker Analysis of Exosomes with Plasmonic Scattering Microscopy. Chem. Sci. 2022, 367, 12760–12768. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, D.; Bakhshpour, M.; Göktürk, I.; Aşır, S.; Yılmaz, F.; Denizli, A. Selective Dopamine Detection by SPR Sensor Signal Amplification Using Gold Nanoparticles. New J. Chem. 2021, 45, 18296–18306. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-N.; Zheng, W.; Li, X.; Zhao, Y. Optical Fiber SPR Biosensor Based on Gold Nanoparticle Amplification for DNA Hybridization Detection. Talanta 2022, 247, 123599. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Spadavecchia, J.; Boukherroub, R. Surface Plasmon Resonance: Signal Amplification Using Colloidal Gold Nanoparticles for Enhanced Sensitivity. Rev. Anal. Chem. 2014, 33, 153–164. [Google Scholar] [CrossRef]
- Das, C.M.; Guo, Y.; Yang, G.; Kang, L.; Xu, G.; Ho, H.P.; Yong, K.T. Gold Nanorod Assisted Enhanced Plasmonic Detection Scheme of COVID-19 SARS-CoV-2 Spike Protein. Adv. Theory Simul. 2020, 3, 2000185. [Google Scholar] [CrossRef] [PubMed]
- Nurrohman, D.T.; Chiu, N.-F. Surface Plasmon Resonance Biosensor Performance Analysis on 2D Material Based on Graphene and Transition Metal Dichalcogenides. ECS J. Solid State Sci. Technol. 2020, 9, 115023. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, Y.; Jiang, M.; Jiang, S.; Liu, R.; Lu, J.; Du, L.; Li, P.; Wang, C. Design and Fabrication of an Ultra-Sensitive Ta2C MXene/Au-Coated Tilted Grating Sensor. Sens. Actuators B Chem. 2022, 369, 132391. [Google Scholar] [CrossRef]
- Gao, J.; Yang, W.; Liu, R.; Feng, J.; Li, Y.; Jiang, M.; Jiang, S. A Reliable Gold Nanoparticle/Cu-TCPP 2D MOF/Gold/D-Shaped Fiber Sensor Based on SPR and LSPR Coupling for Dopamine Detection. Appl. Surf. Sci. 2024, 655, 159523. [Google Scholar] [CrossRef]
- Chung, K.; Rani, A.; Lee, J.E.; Kim, J.E.; Kim, Y.; Yang, H.; Kim, S.O.; Kim, D.; Kim, D.H. Systematic Study on the Sensitivity Enhancement in Graphene Plasmonic Sensors Based on Layer-by-Layer Self-Assembled Graphene Oxide Multilayers and Their Reduced Analogues. ACS Appl. Mater. Interfaces 2015, 7, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Peng, X.; Zhou, Y.; Liu, Y.; Koh, K.; Chen, H. Review of Interface Modification Based on 2D Nanomaterials for Surface Plasmon Resonance Biosensors. ACS Photonics 2022, 9, 3807–3823. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhao, J.; Chen, J.; Hu, X.; Koh, K.; Chen, H. A Simple and Direct SPR Platform Combining Three-in-One Multifunctional Peptides for Ultra-Sensitive Detection of PD-L1 Exosomes. Sens. Actuators B Chem. 2021, 346, 130496. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Zhou, Y.; Lee, J.; Chen, Q.; Chen, H. Interface-Engineered 2D Heterojunction with Photoelectric Dual Gain: Mxene@MOF-Enhanced SPR Spectroscopy for Direct Sensing of Exosomes. Small 2023, 20, e2308897. [Google Scholar] [CrossRef] [PubMed]
- Hedhly, M.; Wang, Y.; Brunel, A.; Beffara, F.; Akil, H.; Verdier, M.; Bessette, B.; Crunteanu, A.; Ho, H.P.; Humbert, G.; et al. Ultra-Sensitive Real-Time Detection of Cancer-Derived Exosomes Directly from Cell Supernatants by a Large Goos–Hänchen Signal Generation on Plasmonic Sensing Interface. Biosens. Bioelectron. X 2023, 15, 100391. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Chen, Q.; Koh, K.; Hu, X.; Chen, H. Rapid and Sensitive Detection of PD-L1 Exosomes Using Cu-TCPP 2D MOF as a SPR Sensitizer. Biosens. Bioelectron. 2022, 201, 113954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chang, W.; Liu, H.; Wang, Y.; Zhao, X.; Chen, H. Single-Walled Carbon Nanowires-Integrated SPR Biosensors: A Facile Approach for Direct Detection of Exosomal PD-L1. Sens. Actuators B Chem. 2024, 399, 134795. [Google Scholar] [CrossRef]
- Di Noto, G.; Bugatti, A.; Zendrini, A.; Mazzoldi, E.L.; Montanelli, A.; Caimi, L.; Rusnati, M.; Ricotta, D.; Bergese, P. Merging Colloidal Nanoplasmonics and Surface Plasmon Resonance Spectroscopy for Enhanced Profiling of Multiple Myeloma-Derived Exosomes. Biosens. Bioelectron. 2016, 77, 518–524. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Zhu, H.; Lu, Y.; Song, H.; Niu, J.; Chen, H. Cancer Cell Membrane Functionalized Gold Nanoparticles: Natural Receptor Tenascin-C as Biomimetic Probe for Sensitive Detection of Circulating Exosomes. Sens. Actuators B Chem. 2022, 372, 132673. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct Quantification of Cancerous Exosomes via Surface Plasmon Resonance with Dual Gold Nanoparticle-Assisted Signal Amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Wang, Y.; Hu, X.; Koh, K.; Chen, H. Tunable Au@SiO2/Au Film Metasurface as Surface Plasmon Resonance Enhancer for Direct and Ultrasensitive Detection of Exosomes. Anal. Chem. 2023, 95, 9663–9671. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zheng, W.; Hu, S.; Peng, X.; Luo, Y.; Lee, J.; Chen, H. Multifunctional DNA Scaffold Mediated Gap Plasmon Resonance: Application to Sensitive PD-L1 Sensor. Biosens. Bioelectron. 2024, 247, 115938. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, X.; Li, F.; Xie, Y.; Shen, J.; Wang, X.; Huang, Y.; Lin, S.; Chen, J.; Zhang, L.; et al. Label-Free Plasmonic Metasensing of PSA and Exosomes in Serum for Rapid High-Sensitivity Diagnosis of Early Prostate Cancer. Biosens. Bioelectron. 2023, 235, 115380. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Zeng, X.; Todd, A.R.; Barnes, L.F.; Winstone, J.M.A.; Trinidad, J.C.; Novotny, M.V.; Jarrold, M.F.; Clemmer, D.E. Charge Detection Mass Spectrometry Measurements of Exosomes and Other Extracellular Particles Enriched from Bovine Milk. Anal. Chem. 2020, 92, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Jarrold, M.F. Applications of Charge Detection Mass Spectrometry in Molecular Biology and Biotechnology. Chem. Rev. 2022, 122, 7415–7441. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Thakur, A.; Xu, C.; Ng, S.; Lee, Y.; Wu, C.L. Detection of Glioma-Derived Exosomes with the Biotinylated Antibody-Functionalized Titanium Nitride Plasmonic Biosensor. Adv. Funct. Mater. 2019, 29, 1806761. [Google Scholar] [CrossRef]
- Liao, G.; Liu, X.; Yang, X.; Wang, Q.; Geng, X.; Zou, L.; Liu, Y.; Li, S.; Zheng, Y.; Wang, K. Surface Plasmon Resonance Assay for Exosomes Based on Aptamer Recognition and Polydopamine-Functionalized Gold Nanoparticles for Signal Amplification. Microchim. Acta 2020, 187, 251. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Cheng, W.; Wu, T.; Li, J.; Li, X.; Liu, L.; Bai, H.; Ding, S.; Li, X.; et al. Surface Plasmon Resonance Biosensor for Exosome Detection Based on Reformative Tyramine Signal Amplification Activated by Molecular Aptamer Beacon. J. Nanobiotechnology 2021, 19, 450. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Wei, X.; Fan, Y.; Qian, H.; Li, S.; Xiang, Y.; Ding, S. Surface Plasmon Resonance Biosensor Using Hydrogel-AuNP Supramolecular Spheres for Determination of Prostate Cancer-Derived Exosomes. Microchim. Acta 2020, 187, 590. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Xia, J.; Zhu, Z.; Koh, K.; Chen, H. Controllable Synthesis of Multi-Tip Spatial Gold Nanostructures to Facilitate SPR Enhancement for Exosomal PD-L1 Assay. Chem. Eng. J. 2023, 481, 148137. [Google Scholar] [CrossRef]
- Tang, L.; Li, J. Plasmon-Based Colorimetric Nanosensors for Ultrasensitive Molecular Diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhao, J.; Weng, G.-J.; Li, J.-J.; Li, X.; Zhu, J.; Zhao, J.-W. A Colorimetric/SERS Dual-Mode Sensing Method for the Detection of Mercury(Ii) Based on Rhodanine-Stabilized Gold Nanobipyramids. J. Mater. Chem. C 2018, 6, 12283–12293. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Z. Enzyme-Catalysed Deposition of Ultrathin Silver Shells on Gold Nanorods: A Universal and Highly Efficient Signal Amplification Strategy for Translating Immunoassay into a Litmus-Type Test. Chem. Commun. 2015, 51, 6928–6931. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhao, X.; Xu, Z. Plasmonic Colorimetric Biosensor for Visual Detection of Telomerase Activity Based on Horseradish Peroxidase-Encapsulated Liposomes and Etching of Au Nanobipyramids. Sens. Actuators B Chem. 2019, 296, 126646. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, J.; Wei, Y.; Wang, D.; Yang, C.; Xu, Z. Plasmonic Colorimetric Biosensor for Sensitive Exosome Detection via Enzyme-Induced Etching of Gold Nanobipyramid@MnO2 Nanosheet Nanostructures. Anal. Chem. 2020, 92, 15244–15252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Yue, S.; Lu, Y.; Yang, C.; Fang, J.; Xu, Z. Sensitive Multicolor Visual Detection of Exosomes via Dual Signal Amplification Strategy of Enzyme-Catalyzed Metallization of Au Nanorods and Hybridization Chain Reaction. ACS Sens. 2019, 4, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Huang, W.X.; Zhang, P.J.; Chen, L.; Lio, C.K.; Zhou, H.; Qing, L.-S.; Luo, P. Colorimetric Determination of the Early Biomarker Hypoxia-Inducible Factor-1 Alpha (HIF-1α) in Circulating Exosomes by Using a Gold Seed-Coated with Aptamer-Functionalized Au@Au Core-Shell Peroxidase Mimic. Microchim. Acta 2020, 187, 61. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Qiu, G.; NG, S.P.; Guan, J.; Yue, J.; Lee, Y.; Wu, C.M.L. Direct Detection of Two Different Tumor-Derived Extracellular Vesicles by SAM-AuNIs LSPR Biosensor. Biosens. Bioelectron. 2017, 94, 400–407. [Google Scholar] [CrossRef]
- Li, H.; Huang, T.; Lu, L.; Yuan, H.; Zhang, L.; Wang, H.; Yu, B. Ultrasensitive Detection of Exosomes Using an Optical Microfiber Decorated with Plasmonic MoSe2-Supported Gold Nanorod Nanointerfaces. ACS Sens. 2022, 7, 1926–1935. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, L.; Li, S.; Qi, R.; Chen, X.; Li, W. Highly Sensitive Refractive Index Detection Based on Compact HSC-SPR Structure in a Microfluidic Chip. Sens. Actuators A Phys. 2019, 297, 111558. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A Label-Free Ultrasensitive Microfluidic Surface Plasmon Resonance Biosensor for Aflatoxin B1 Detection Using Nanoparticles Integrated Gold Chip. Food Chem. 2020, 307, 125530. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Rahim Ferhan, A.; Cho, N.J. Nanoplasmonic Sensors for Biointerfacial Science. Chem. Soc. Rev. 2017, 46, 3615–3660. [Google Scholar] [CrossRef] [PubMed]






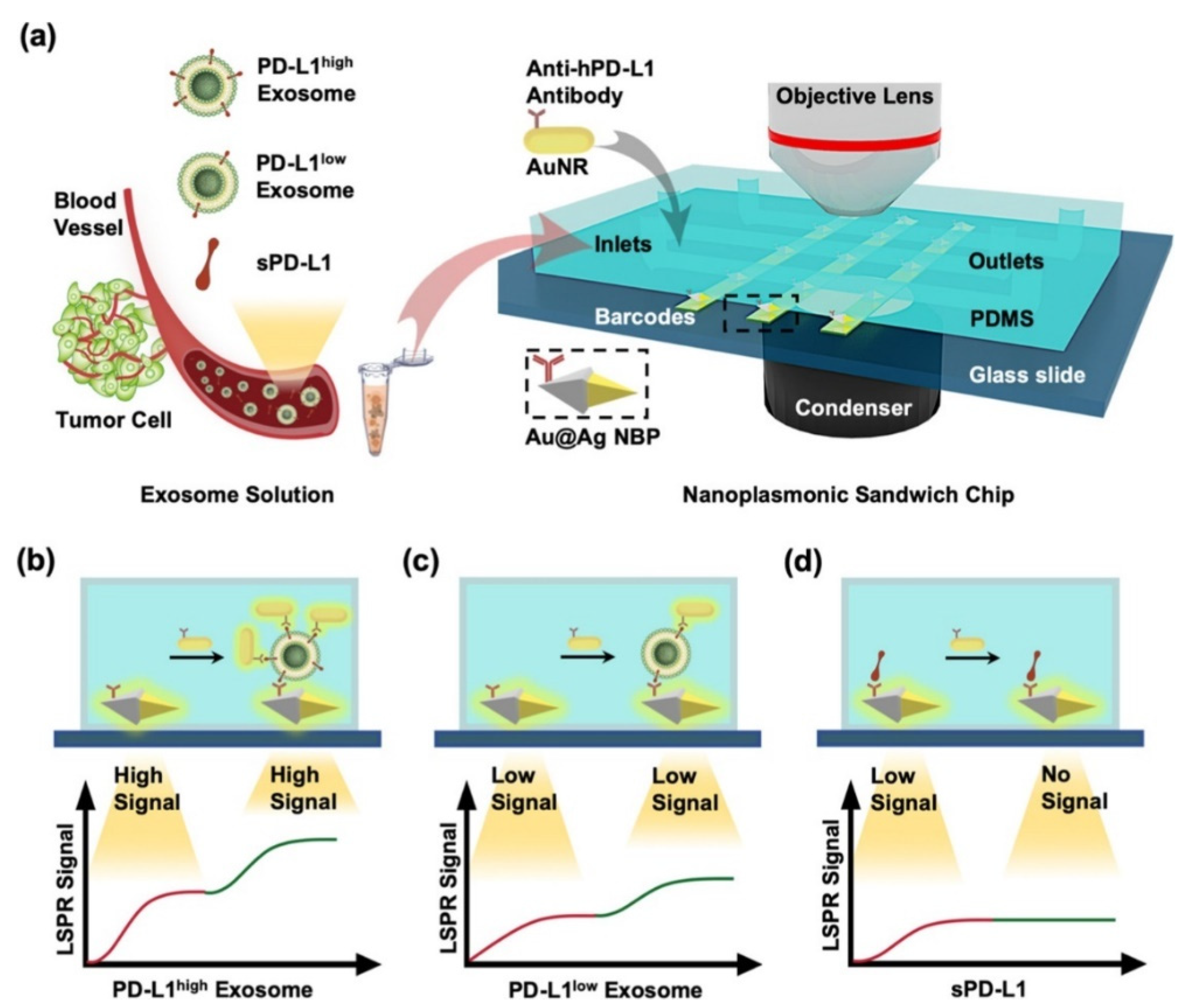
| Optical Chip for SPR Sensor | Chip Used | Investigation Modes | Sensitivity | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Prism | Conventional SPR chips based on gold | Angle | 1.9 × 106°/M | 4.1 nM | [53] |
| Conventional SPR chips based on gold | Angle | 141.1°/RIU | - | [51] | |
| Conventional SPR chips based on gold | Phase | - | 14.02 ng/mL | [54] | |
| SPR biosensor with signal amplified using Hybridization Chain Reaction | Phase | - | 7.5 × 10−7 RIU | [55] | |
| Conventional SPR chips based on gold | Intensity | - | - | [56] | |
| SPR biosensor based on Au-Ag alloy film | Wavelength | 5676.9 nm/RIU | - | [57] | |
| Near-infrared SPR sensor based on graphene-AuNPs architecture | Wavelength | 39,160 nm/RIU | 7.2 fg/mL | [58] | |
| Conventional SPR chips based on gold | Wavelength | 1032 nm/RIU | - | [59] | |
| Grating | Ag-based grating | Angle | 128.85°/RIU | - | [60] |
| Enhancement of the SPR sensitivity with Ag-Au bimetallic grating | Angle | 346°/RIU | - | [61] | |
| Enhancement of the SPR sensitivity with Au-Al bimetallic grating | Angle | 245.2°/RIU | - | [62] | |
| Au nanograting on silicon substrate | Wavelength | 751 nm/RIU | 23.5 nM | [63] | |
| Periodically corrugated gold film is coated with a thin antifouling polymer layer | Wavelength | - | 1.1 nM | [64] | |
| Waveguide | SiC waveguide-based SPR sensor is deposited with an Au-Ag bimetallic layer | Wavelength | 2581 nm/RIU | - | [65] |
| Polymer waveguide-based SPR sensor | Wavelength | 4518 nm/RIU | 2.2 × 10−7 RIU | [41] | |
| Dual channel planar waveguide-based SPR sensor | Wavelength | 1500 nm/RIU. | - | [66] | |
| Optical fiber | SPR fiber optic biosensor enhanced in sensitivity with graphene oxide | Wavelength | 2471 nm/RIU | 55 μM | [67] |
| SPR fiber optic biosensor based on Au | Wavelength | 1699 nm/RIU | - | [67] | |
| SPR biosensor based on tapered fiber optics | Wavelength | 2100 nm/RIU | 2.4 × 10−10 M | [68] |
| Isolation Method | Principle | Time | Purity | Yield | Cost | Ref. |
|---|---|---|---|---|---|---|
| differential ultracentrifugation | Size and density | >4 h | Medium | Low | expensive equipment | [110,111,112] |
| gradient density ultracentrifugation | Size and density | >16 h | High | Low | high | [113,114] |
| Size-exclusion chromatography | Size | Less than 20 min | high | high | Medium to high | [115,116] |
| Immunoaffinity capture | Specific binding | 4–20 h | high | medium | Expensive antibodies functionalization | [109,117] |
| Ultrafiltration | size and molecular weight | 0.5 h | low | medium | medium | [117] |
| Precipitation | Solubility | 0.25–12 h | low | high | low | [118] |
| microfluidics | Specific binding, size, and density | 0.5 h | high | Low to medium | high | [118] |
| Recognition Element | Specific Target | Developed Biosensor System | Detection Limit | Ref. | |
|---|---|---|---|---|---|
(Exosomes/mL) | |||||
| CD63 aptamer | CD63 | Colorimetric biosensor where exosome quantification is based on metallization of Au NRs and hybridization chain reaction (HCR) | 1.6 × 102 exosomes/mL | 1.6 × 102 | [157] |
| anti-CD63 | exosome transmembrane protein CD63 | LSPR biosensor based on gold nano-ellipsoid arrays integrated with microfluidics | 1 ng/mL | 7.245 × 1026 | [17] |
| HIF-1α- aptamer | HIF-1α | Au NPs with a diameter of 13 nm were functionalized with aptamer. The bond between the ligand and the analyte results in changes in the absorbance intensity. | 0.2 ng/L | 1.449 × 1026 | [158] |
| - | A-549 and SH-SY5Y cells | LSPR biosensor with self-assembly gold nanoislands (SAM-AuNIs) | 0.194 µg/mL | 1.41 × 1029 | [159] |
| locked nucleic acid (LNA) | exo-miR-125b | DNA-assembled advanced plasmonic architecture (DAPA)-based plasmonic biosensor | 10.54 aM | 6.344 × 103 | [27] |
| anti-hPD-L1 antibody | PD-L1 exosomes | nanoplasmonic sandwich composed of Au@Ag core-shell nanobipyramid (NBP) and AuNR | 1.2 × 103 exosomes/μL | 1.2 × 106 | [16] |
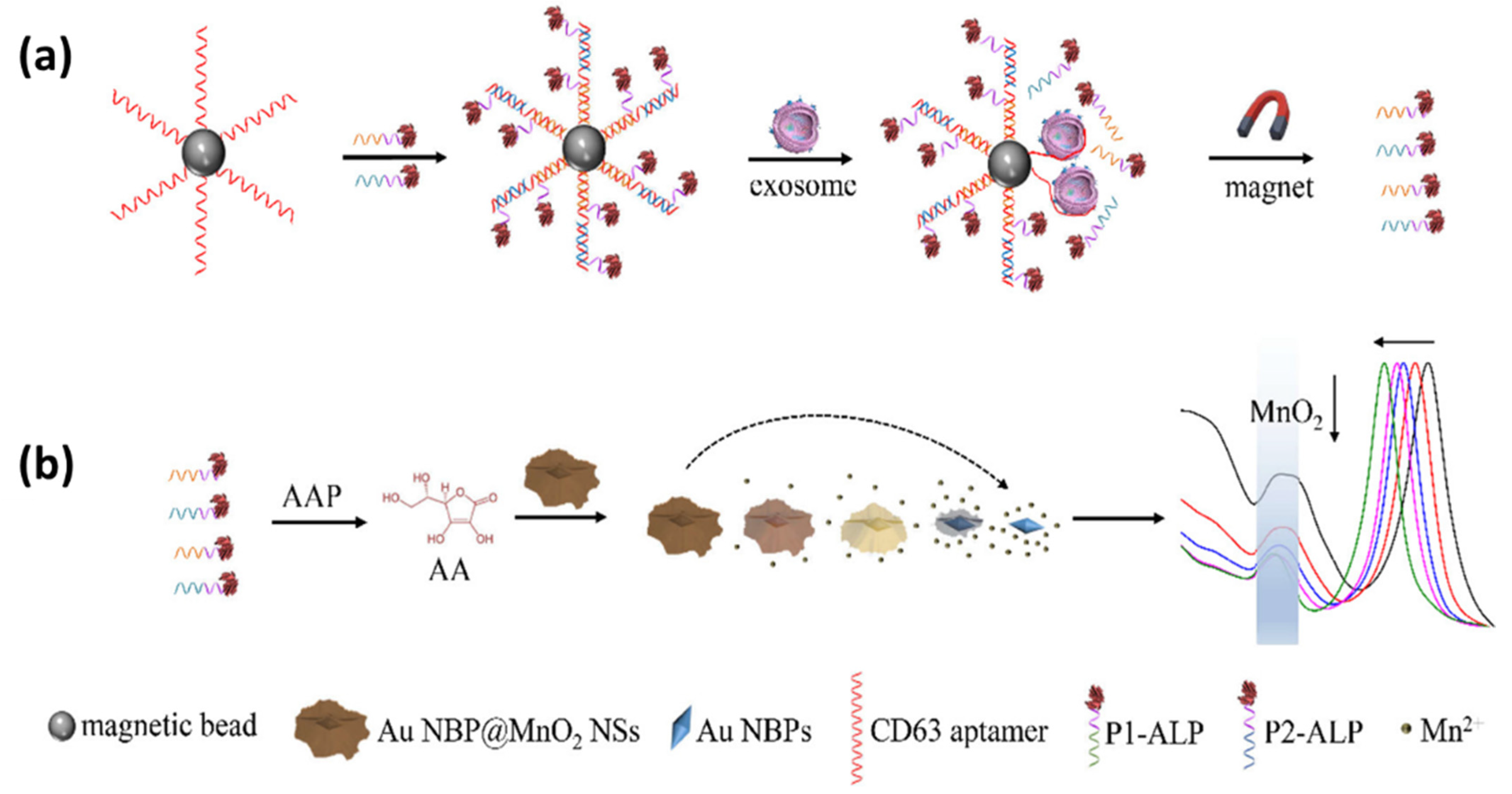
| CD63 aptamer | CD63 | colorimetric biosensors based on Au NBP@MnO2 nanostructures | 1.35 × 102 exosomes/μL | 1.35 × 105 | [152] |
| CA9 Aptamer | Clear-Cell Renal Cancer Exosome | optical microfiber integrated with -supported Au NRs | 9.32 exosomes/mL | 9.32 | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurrohman, D.T.; Chiu, N.-F.; Hsiao, Y.-S.; Lai, Y.-J.; Nanda, H.S. Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications. Biosensors 2024, 14, 307. https://doi.org/10.3390/bios14060307
Nurrohman DT, Chiu N-F, Hsiao Y-S, Lai Y-J, Nanda HS. Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications. Biosensors. 2024; 14(6):307. https://doi.org/10.3390/bios14060307
Chicago/Turabian StyleNurrohman, Devi Taufiq, Nan-Fu Chiu, Yu-Sheng Hsiao, Yun-Ju Lai, and Himansu Sekhar Nanda. 2024. "Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications" Biosensors 14, no. 6: 307. https://doi.org/10.3390/bios14060307
APA StyleNurrohman, D. T., Chiu, N.-F., Hsiao, Y.-S., Lai, Y.-J., & Nanda, H. S. (2024). Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications. Biosensors, 14(6), 307. https://doi.org/10.3390/bios14060307









