Abstract
As a commonly used metal ion, iron(II) (Fe2+) ions pose a potential threat to ecosystems and human health. Therefore, it is particularly important to develop analytical techniques for the rapid and accurate detection of Fe2+ ions. However, the development of near-infrared (NIR) luminescence probes with good photostability for Fe2+ ions remain challenging. In this work, we report a novel iridium(III) complex-based luminescence probe for the sensitive and rapid detection of Fe2+ ions in a solution based on an Fe2+-mediated reduction reaction. This probe is capable of sensitively detecting Fe2+ ions with a limit of detection (LOD) of 0.26 μM. Furthermore, this probe shows high photostability, and its luminescence remains stable under 365 nm irradiation over a time period of 30 min. To our knowledge, this is first iridium(III) complex-based NIR probe for the detection of Fe2+ ions. We believe that this work provides a new method for the detection of Fe2+ ions and has great potential for future applications in water quality testing and human monitoring.
1. Introduction
Iron (Fe) is the second most abundant element in the Earth’s crust and is also an essential nutrient in the human body [1]. In fact, approximately half of the iron in the human body is in the form of Fe2+ ions and Fe3+ [2], which are involved in many important physiological processes in the human body [3], such as oxygen transfer [4], nucleotide synthesis [5], electron transfer [6], and enzymatic reactions [7]. However, the presence of excess redox-active free Fe catalyzes the generation of hydroxide anions and increases the production of reactive oxygen species (ROS) [8,9], and abnormal levels of Fe2+ ions are highly associated with diseases including cancer and inflammation [10]. Therefore, the amount of water-soluble fraction of Fe2+ ions in the body is an important indicator for estimating Fe-induced cellular damage. On the other hand, the release of large amounts of Fe-containing chemicals into the environment may lead to a range of environmental and ecological problems [11]. Therefore, the monitoring and detection of Fe2+ ion levels are crucial in protecting environmental and living systems.
Traditional analytical techniques have been widely developed for the detection of Fe2+ and Fe3+ ions, such as colorimetric [12,13], electrochemical [14], and fluorescent methods [15,16]. Due to the fact that Fe2+ ions are easily oxygenated to Fe3+ ions in aerobic water [17], and numerous biometallic ions can be competitive in binding to common ligands, it is still a challenge to develop probes for the highly selective detection of Fe2+ ions. Reaction-based fluorescence probes offer great potential in overcoming the limitations above, which combine Fe2+-mediated reactions and the advantages of fluorescent techniques, including high sensitivity, high specificity, simple operation, and a fast response time [18]. In particular, the Fe2+-mediated N-oxide reduction strategy, first reported by Nagasawa et al. [19], has been demonstrated as a reliable and selective method to construct Fe2+ ion fluorescence probes. However, most of these probes suffer from narrow Stokes shifts, poor photostability, or a short emission wavelength of below 650 nm.
Recently, NIR iridium(III) complexes have emerged as promising luminescence probes for chemosensing and bioimaging [20] due to their long emission lifetime, high photostability, large Stokes shift, and tunable emissions. Moreover, NIR iridium(III) complex-based probes are highly resistant to background interferences and provide more accurate detection results [21]. These merits offer the possibility of compensating the shortcomings of the traditional Fe2+ ion fluorescence probes. Although a considerable number of NIR iridium(III) complexes have been developed for the detection of metal ions, small molecules, and disease-related proteins [22,23,24,25,26,27], no NIR iridium(III) complex-based probes are available for the detection of Fe2+ ions.
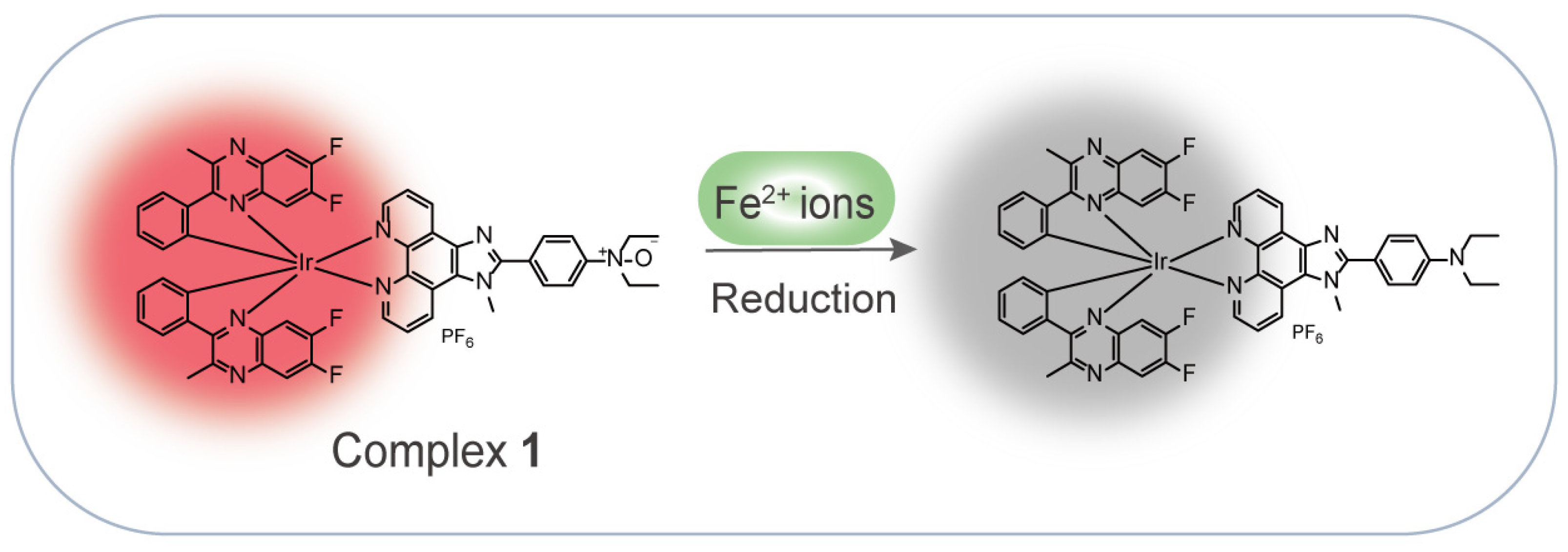
In this work, inspired by the Fe2+-mediated N-oxide reduction reaction, we rationally designed and synthesized a NIR iridium(III) complex for the detection of Fe2+ ions in an aqueous solution (Scheme 1). Complex 1 showed a quenching luminescence upon the addition of Fe2+ ions through the Fe2+-mediated N-oxide moiety reduction of the N^N ligand. Moreover, the complex exhibited a good performance, with high selectivity and high sensitivity for the detection of Fe2+ ions, and a fast response of within 3 min.
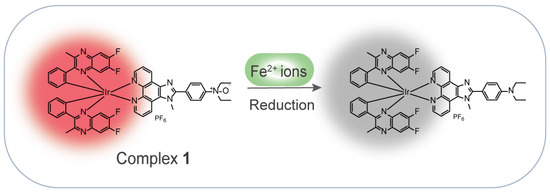
Scheme 1.
Schematic diagram of an iridium(III) complex-based NIR probe for the detection of Fe2+ ions.
2. Results and Discussion
2.1. Design and Synthesis of the Probe for Fe2+ Ions
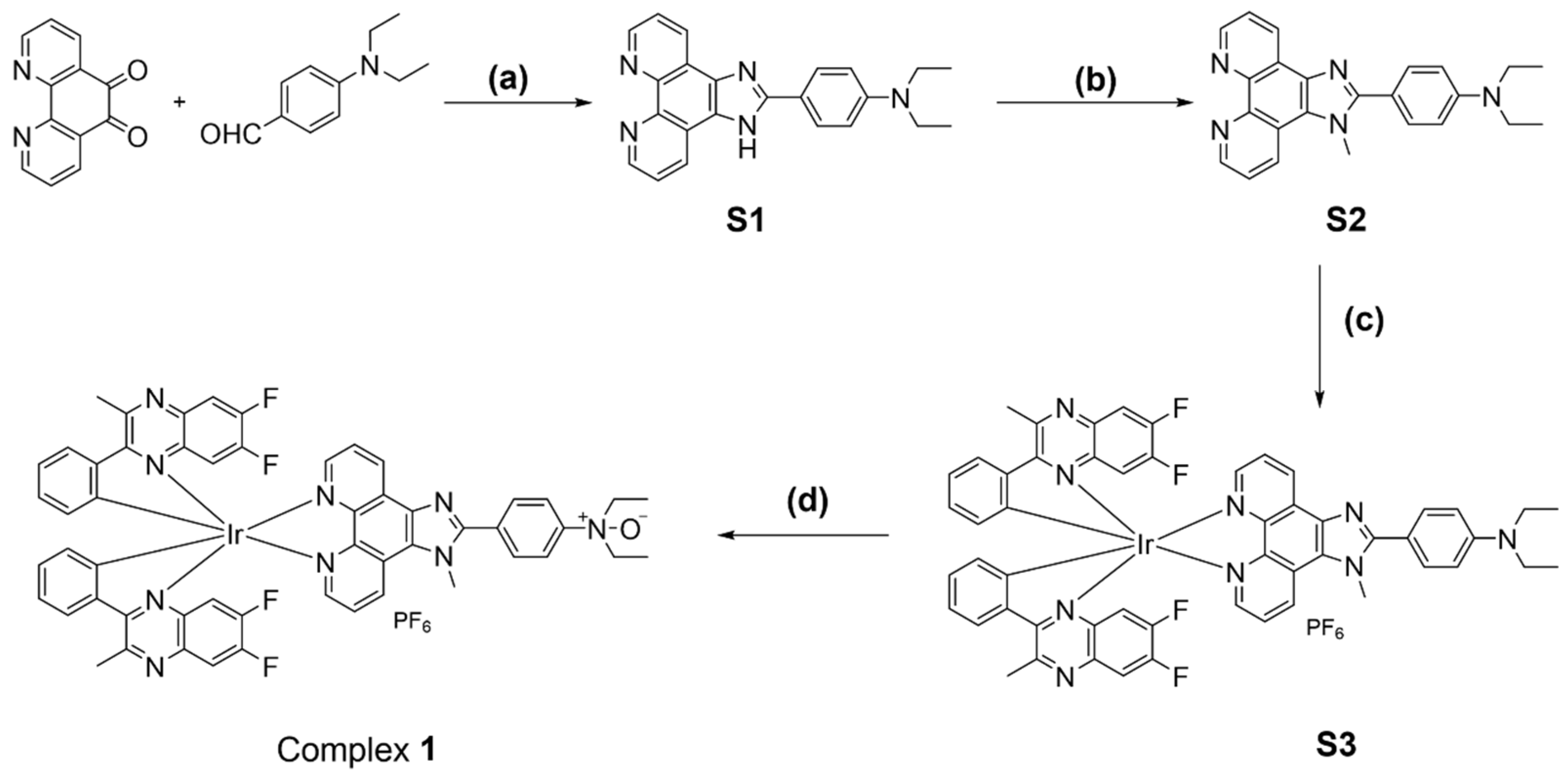
Fe2+ is a common divalent cation that can reduce oxides to their original elements or compounds [28]. This inspired us to design an oxide-containing NIR iridium(III) complex, in which the N^N ligand containing the N-oxide group was responsible for the specific recognition of Fe2+ ions, while 6,7-difluoro-2-methyl-3-phenylquinoxaline was chosen to act as C^N ligand to enable NIR emission (Scheme 1). It was expected that Fe2+ ions would trigger deoxygenation to generate complex S3 and induce a luminescence quenching effect on complex 1. Moreover, the weak complexation of Fe2+ ions with the O atom of the N-oxide might also promote the break of the N–O bond [29]. The complex was directly synthesized in four steps (Scheme 2): 1,10-phenanthroline-5,6-dione and N,N-diethylaminobenzaldehyde were cyclized under the catalyst of ammonium acetate (NH4OAc) to generate ligand S1, which was then substituted with lodomethane (CH3I) in the presence of potassium tert-butoxide (t-BuOK) to produce ligand S2. Ligand S2 coordinated with the chloro-bridged iridium(III) dimer Ir2(dfpq)4Cl2 for the generation of complex S3, which was further oxidized by 3-chloroperoxybenzoic acid (m-CPBA) to afford the desired complex 1. The purity of complex 1 was checked by high-performance liquid chromatography (HPLC), which showed a purity of over 95% (Figure S9). The intermediates and final complex were fully characterized by 1H NMR, 13C NMR, and electrospray ionization mass spectrometry (ESI-MS) (Figures S1–S8).
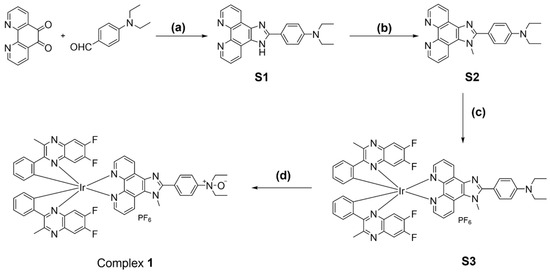
Scheme 2.
Synthesis of complex 1. Reagents and conditions: (a) NH4OAc, HOAc, reflux, overnight, 87%. (b) t-BuOK, CH3I, THF, rt, overnight, N2, 56%. (c) Ir2(dfpq)4Cl2, DCM/MeOH, rt, overnight, then solid NH4PF6 added for another 0.5 h, rt, 58%. (d) m-CPBA, DCM, 0 °C to rt, 75%.
2.2. Photophysical Properties of the Probe
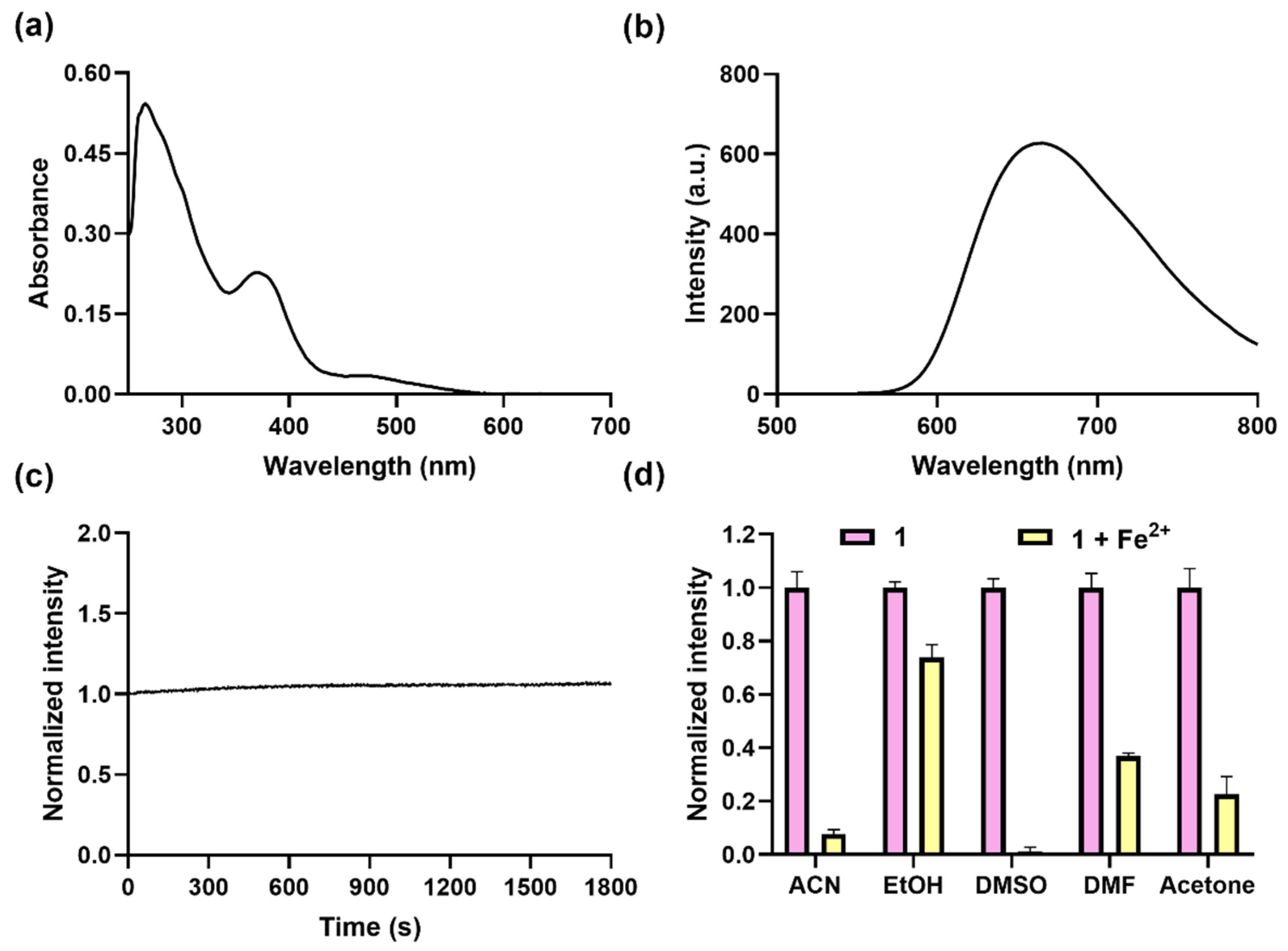
The absorption spectrum of complex 1 was first recorded in dimethyl sulfoxide (DMSO), which showed the assumed ligand-based π−π* transition peak at around 280 nm and a metal-to-ligand charge transfer (MLCT) transition peak at around 365 nm and 480 nm in DMSO (Figure 1a). The excitation and emission spectra showed that complex 1 had the maximum excitation wavelength of 365 nm and the maximum wavelength of 675 nm (Figure 1b and Figure S10). This indicates that complex 1 is a potential NIR probe and has a large Stokes shift of around 195 nm between the 3MLCT absorption and emission, which is much larger than organic dyes [30], making it less interfered with by the excitation light source. In order to assess the photostability, complex 1 was continuously irradiated over a time period of 1800 s at 365 nm (Figure 1c), showing no obvious decrease in luminescence intensity, which confirms its high photostability, ensuring reliable reproducibility and stability in the experimental results.
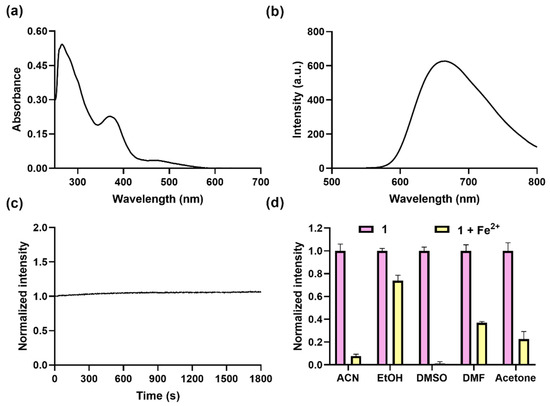
Figure 1.
Optical profiles of complex 1 and its response to Fe2+ ions. Absorption (a) and luminescence spectra (b) of complex 1 (10 µM) in DMSO. (c) Time course of luminescence of complex 1 (10 µM) in DMSO over 1800 s under continuous irradiation at 365 nm. (d) Luminescence at 675 nm of complex 1 (10 μM) for Fe2+ (50 μM) in different solvents, including ACN, EtOH, DMSO, DMF, and acetone; λex was set at 365 nm.
Following the encouraging outcomes delineated above, we assessed the capacity of complex 1 in detecting Fe2+ ions across various solvent media. Complex 1 (10 μM) was added to various solutions, including ACN, ethanol (EtOH), N,N-dimethylformamide (DMF), DMSO, and acetone containing Fe2+ ions (50 μM), respectively; luminescence spectra showed that the best quenching effect was obtained in DMSO (Figure 1d), mainly due to cyclometalated iridium(III) complexes being lipophilic and having better solubility in DMSO and being slight soluble in alcohols [31,32]. In summary, complex 1 has the merits of a large Stokes shift, good photostability, NIR emission, and the best response to Fe2+ ions in DMSO, which is better than that for Fe2+ ion probes with an emission below 600 nm (Table S1).
2.3. Optimization of Detection Conditions
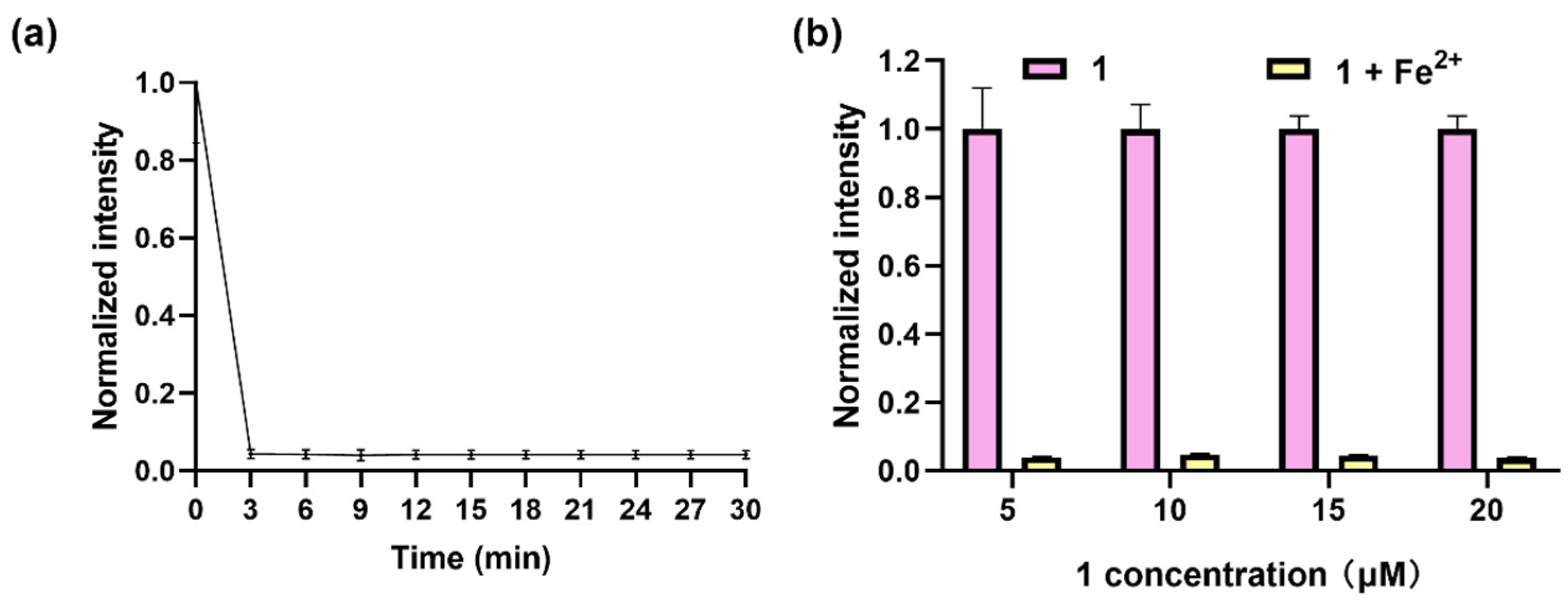
In pursuit of optimal detection performance, a systematic optimization of both the incubation time and the concentration of the complex was conducted. Firstly, the luminescence response of complex 1 (20 μM) to Fe2+ (50 μM) over a time period of 30 min was investigated; the results showed that the luminescence dramatically dropped, reaching a plateau at 3 min (Figure 2a). Notably, this rapid response time contrasts with the behavior exhibited by several previously reported fluorescent probes (Table S1), which typically necessitate incubation periods of around 30 min or longer to achieve a plateau. Further, the luminescence response of complex 1 at different concentrations (5–20 μM) to Fe2+ (50 μM) within 3 min showed that the highest quenching effect was obtained at 20 μM (Figure 2b). Therefore, 3 min of incubation time and 20 μM of complex 1 were chosen for further experiments.
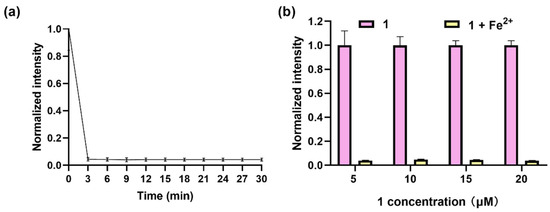
Figure 2.
Optimization of detection parameters. (a) Luminescence of complex 1 (20 μM) for Fe2+ (50 μM) in DMSO within 30 min. (b) Luminescence of complex 1 (5–20 μM) with addition of Fe2+ (50 μM) in DMSO within 3 min. λex was set at 365 nm, and λem was set at 675 nm.
2.4. Sensitivity and Selectivity of Complex 1
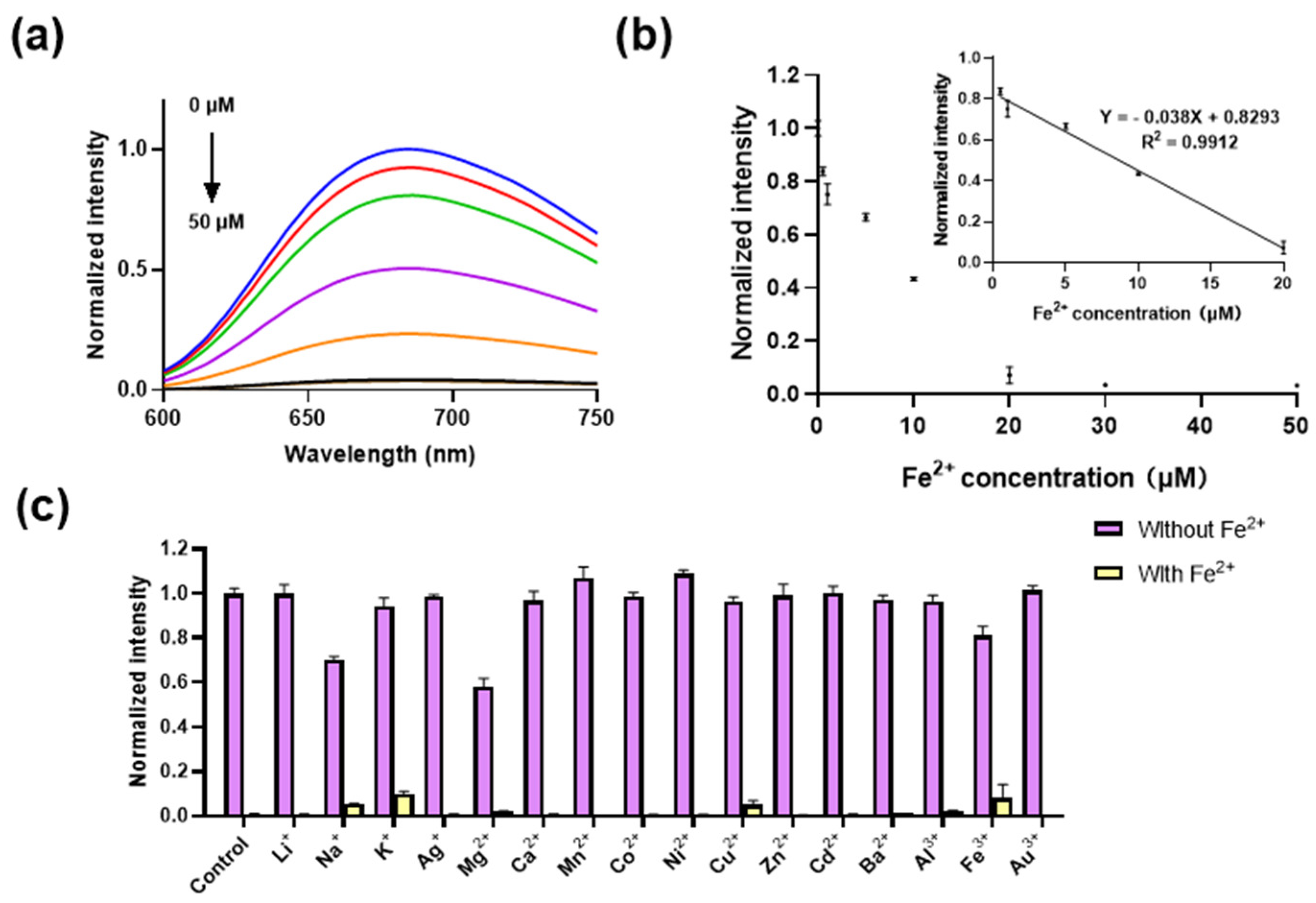
After optimizing the reaction conditions, we investigated the luminescence response of complex 1 to varying concentrations of Fe2+ ions (0–50 μM). It is found that the luminescence of complex 1 decreased gradually with increasing concentrations of Fe2+ ions (Figure 3a, with a good linear relationship obtained between 0.5 and 20 μM (Figure 3b, Inset), obtaining a limit of detection (LOD) of 0.26 μM with a regression equation of Y = −0.038X + 0.8293 according to LOD = 3σ/k, and a sensitivity (Si) of −0.038. This is comparable to most of the reported fluorescence methods and better than the reported colorimetric methods (Table S1).
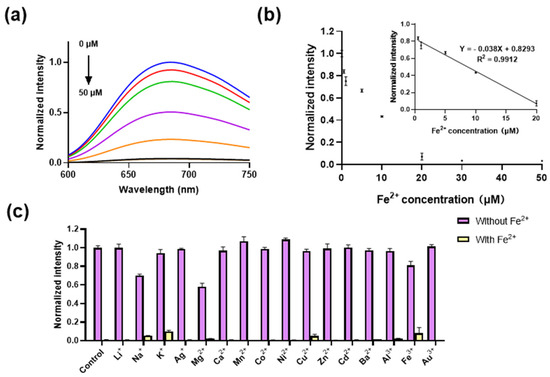
Figure 3.
The sensitivity and selectivity of complex 1. (a) Luminescence spectra of complex 1 (20 μM) with various concentrations of Fe2+ ions (0–50 μM) in DMSO. From blue to black lines represent different concentration of Fe2+ ions (0.5, 1,10, 20, 30, 50 μM). (b) Luminescence of complex 1 (20 μM) with various concentrations of Fe2+ ions (0.5–50 μM) in DMSO. Inset: The linear relationship between luminescence of complex 1 (20 μM) and Fe2+ ion concentrations (0.5–20 μM). (c) Luminescence of complex 1 (10 μM) for Fe2+ (50 μM) in the presence of various metal ions (500 μM) in DMSO. λex was set at 365 nm, and λem was set at 675 nm.
In order to assess the selectivity of complex 1 towards potentially interfering metal ions, the luminescence response of complex 1 (20 μM) to a range of metal ions in DMSO was investigated. The results demonstrated that the luminescent behavior of complex 1 remained consistent upon the introduction of other metal ions (at 10 equiv. concentrations, encompassing Li+, Na+, K+, Ag+, Mg2+, Ca2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Ba2+, Al3+, Fe3+, and Au3+ ions. Conversely, the subsequent addition of Fe2+ ions (50 μM) led to a substantial quenching of the luminescent signal emitted by the probe (Figure 3c). This indicates that the co-existence of other metal ions exerts a negligible influence over the luminescence of the complex toward Fe2+ ions, confirming its excellent selectivity. This attribute confers a distinct advantage over coordination-based Fe2+ ion probes (Table S1), as it minimizes the susceptibility to interference from various other metal ions.
2.5. Mechanism Study
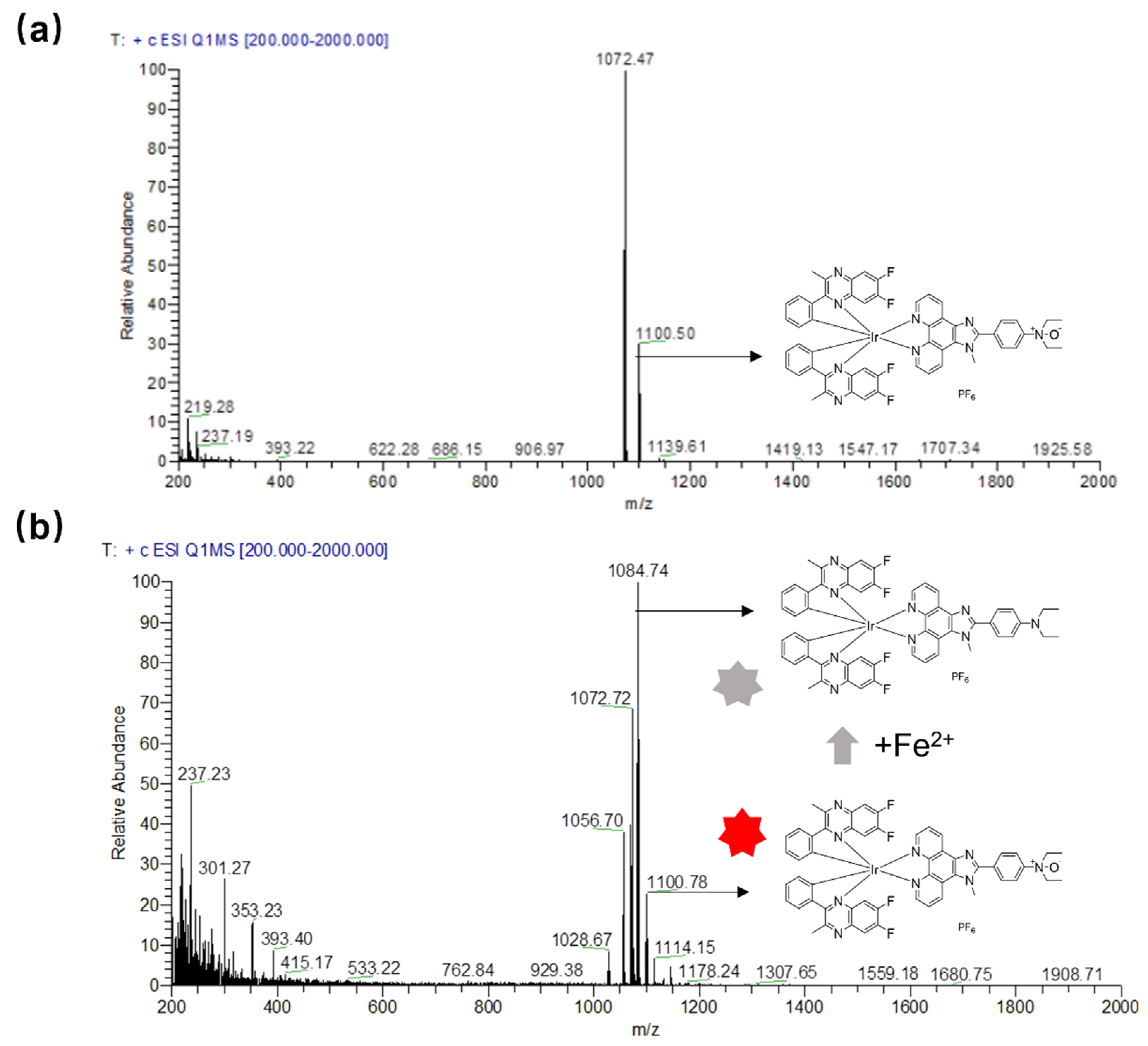
To validate the response mechanism of complex 1 for the detection of Fe2+ ions, ESI-MS was used to confirm the reduction of the N-oxide group by Fe2+ ions to afford control complex S3. The ESI-MS spectrum shows that the presence of Fe2+ ions induced a new m/z peak at 1084.30, corresponding to that of complex S3, while the m/z peak of complex 1 is 1100.74 (Figure 4). In addition, the reaction mechanism was further characterized by HPLC, and the result shows that the presence of Fe2+ ions induced the emergence of a peak with a residual time of around 18.60 min, compared with the residual time of about 14.00 min for complex 1 (Figure S11), which is consistent with complex 1 being more polar than complex S3. These results demonstrate that Fe2+ ions can reduce complex 1 to S3, and the reduction of the N-oxide group to an amino group presumably induces a photoinduced electron transfer (PeT) between the amino group and the iridium(III) complex, which transfers electrons from the donor to the excited state of the iridium(III) complex [33], leading to a strong luminescence quenching effect in complex 1.
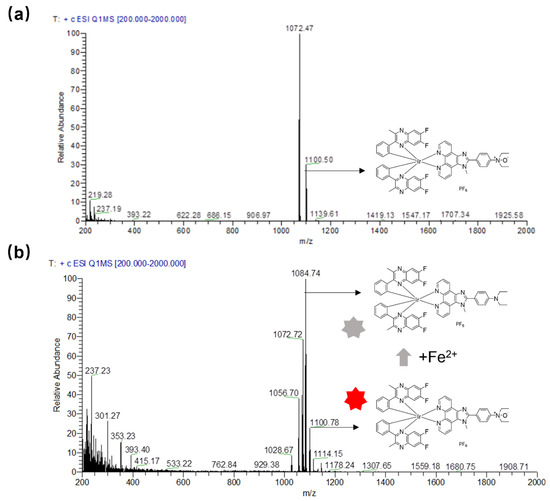
Figure 4.
ESI-MS spectra of complex 1 (a) and complex 1 with the addition of Fe2+ ions (b). The colour change of the red to grey stars indicted the luminescence of complex 1 is quenched.
2.6. The Recovery by Complex 1 of Fe2+ Ions in Water Samples
The contamination associated with Fe2+ ions poses a significant threat to human health, making the practical application of complex 1 in environmental samples highly important. In this study, the feasibility of complex 1 in detecting Fe2+ ions in environment samples was investigated using water from 2% Xi’an Qixiang Lake as a model matrix. Complex 1 (20 μM) was mixed with Fe2+ ions at varying concentrations (5, 10, and 20 μM) in 2% lake-water-spiked DMSO, the recovery rates were found to range from 89.71% to 111.07%, with RSD values of 2.85% to 6.34% (Table S2). This result demonstrates the analytical potential of complex 1 in quantifying Fe2+ ions in environmental matrices.
3. Conclusions
In conclusion, we have successfully designed and synthesized a novel NIR luminescence probe for Fe2+ ion detection in aqueous solution. The complex can respond rapidly to Fe2+ ions within 3 min, exhibiting a high sensitivity, with an LOD of 0.26 μM. Moreover, the complex shows high selectivity for Fe2+ ions over other common metal ions, even in the presence of other interferents, and demonstrates a high photostability, with the luminescence remaining similar even under UV irradiation for 30 min. A further mechanism study demonstrates that the complex detects Fe2+ ions through Fe2+ ion-mediated N-oxide reduction, triggering a PeT process, along with luminescence quenching. In summary, this work provides a new analytical tool for the sensitive and selective detection of Fe2+ ions in aqueous solution, opening up a new avenue for the robust detection of Fe2+ ions. In future, we anticipate that leveraging advanced nanotechnological methodologies will facilitate the further development of this probe into a versatile array of tools [34]; these tools may encompass live imaging applications for the detection of Fe2+ ions within cancer systems, the targeted analysis of Fe2+ ions in cancerous tissues, and precision analyses dedicated to Fe2+ ion quantification in both untreated environmental specimens and blood samples.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/bios14080369/s1, Figures S1–S8, NMR spectra of intermediates and complexes. Figure S9: HPLC analysis of complex 1 (Upper) and complex 1 in the presence of Fe2+ ions (Lower). The absorbance was detected at 254 nm. Figure S10: Excitation spectrum of complex 1 (10 μM) in DMSO. Table S1: Comparison of fluorescence probe/chemosensors for Fe2+ detection. Table S2: Recoveries of complex 1 (20 μM) for the detection of Fe2+ ions in DMSO containing 2% Xi’an Qixiang Lake water. References [35,36,37,38,39] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.W.; methodology, J.W., W.W. (Wanhe Wang) and C.-H.L.; software, W.W. (Wanyi Wang); validation, J.W.; formal analysis, J.L., J.W. and W.W. (Wanhe Wang); investigation, J.W., C.-H.L. and W.W. (Wanhe Wang); resources, J.W. and C.-H.L.; data curation, L.K., J.L. and Z.Z.; writing—original draft preparation, W.W. (Wanyi Wang); writing—review and editing, W.W. (Wanhe Wang); visualization, W.W. (Wanyi Wang), Z.Z. and J.L.; supervision, J.W. and W.W. (Wanhe Wang); project administration, J.W., W.W. (Wanhe Wang) and C.-H.L.; funding acquisition, J.W. and C.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work is sponsored by the Fundamental Research Funds for the Central Universities (D5000230060), the Key Research and Development Program of Shaanxi (2024SF-YBXM-181, 2024SF-YBXM-418), the Natural Science Foundation of Chongqing, China (cstc2021jcyj-msxm2073), the Shaanxi Fundamental Science Research Project for Chemistry & Biology (22JHQ082), the Guangdong Basic and Applied Basic Research Foundation (2021A1515110840, 2023A1515011871), Hainan Province Science and Technology Special Fund (ZDYF2021SHFZ250), the Innovation Capability Support Program of Shaanxi (2023-CX-TD-72), the Science and Technology Development Fund, Macau SAR, China (File nos. 005/2023/SKL, 0020/2022/A1, 0045/2023/AMJ, 0032/2023/RIB2), the University of Macau, Macau SAR, China (File nos. MYRG2020-00017-ICMS, MYRG2022-00137-ICMS, MYRG-GRG2023-00194-ICMS-UMDF), and the State Key Laboratory of Quality Research in Chinese Medicine, the University of Macau, Macau SAR, China (File no. SKL-QRCM-IRG2023-025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data involved in the study are included in the article, and further inquiries can be directly contacted by the corresponding author.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Skjørringe, T.; Møller, L.B.; Moos, T. Impairment of interrelated iron and copper homeostatic mechanisms in brain contributes to the pathogenesis of neurodegenerative disorders. Front. Pharmacol. 2012, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.T.; Reeder, B.J. Oxygen-binding haem proteins. Exp. Physiol. 2008, 93, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Sornjai, W.; Nguyen Van Long, F.; Pion, N.; Pasquer, A.; Saurin, J.C.; Marcel, V.; Diaz, J.J.; Mertani, H.C.; Smith, D.R. Iron and hepcidin mediate human colorectal cancer cell growth. Chem. Biol. Interact. 2020, 319, 109021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A.; Tong, W.H. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 2005, 6, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) redox chemistry in the environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Chen, X.-Z.; Liu, X.-Y.; Wang, M.; Liu, J.-J.; Gao, G.; Zhang, X.-Y.; Sun, R.-Z.; Hou, S.-C.; Wang, H.-M. A highly sensitive multifunctional sensor based on phenylene-acetylene for colorimetric detection of Fe2+ and ratiometric fluorescent detection of Cd2+ and Zn2+. Sens. Actuators B Chem. 2018, 273, 1077–1084. [Google Scholar] [CrossRef]
- Lian, M.; Shi, F.; Cao, Q.; Wang, C.; Li, N.; Li, X.; Zhang, X.; Chen, D. Paper-based colorimetric sensor using bimetallic Nickel-Cobalt selenides nanozyme with artificial neural network-assisted for detection of H2O2 on smartphone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 311, 124038. [Google Scholar] [CrossRef] [PubMed]
- Laglera, L.M.; Monticelli, D. Iron detection and speciation in natural waters by electrochemical techniques: A critical review. Curr. Opin. Electrochem. 2017, 3, 123–129. [Google Scholar] [CrossRef]
- Xing, W.; Xu, H.; Ma, H.; Abedi, S.A.A.; Wang, S.; Zhang, X.; Liu, X.; Xu, H.; Wang, W.; Lou, K. A PET-based fluorescent probe for monitoring labile Fe(II) pools in macrophage activations and ferroptosis. Chem. Commun. 2022, 58, 2979–2982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Tao, X.; Chen, Q.; Yin, D.; Zhang, C. Two AIE-Ligand-Based 2-D Luminescent Metal–Organic Frameworks as Fe3+ Sensors. Inorg. Chem. 2024, 63, 8342–8350. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.L.; Li, P.; Zhang, X.; Han, K. A turn-on fluorescent chemodosimeter based on detelluration for detecting ferrous iron (Fe2+) in living cells. J. Mater. Chem. B 2016, 4, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, P.; Guo, W. Fluorescent probes for iron, heme, and related enzymes. Coord. Chem. Rev. 2021, 429, 213645. [Google Scholar] [CrossRef]
- Hirayama, T.; Okuda, K.; Nagasawa, H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 2013, 4, 1250–1256. [Google Scholar] [CrossRef]
- Jing, S.; Wu, X.; Niu, D.; Wang, J.; Leung, C.H.; Wang, W. Recent advances in organometallic NIR iridium(III) complexes for detection and therapy. Molecules 2024, 29, 256. [Google Scholar] [CrossRef]
- Yoon, S.; Teets, T.S. Red to near-infrared phosphorescent Ir(III) complexes with electron-rich chelating ligands. Chem. Commun. 2021, 57, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Wang, J.; Leung, C.-H.; Wang, W. Imaging mitochondrial palladium species in living cells with a NIR iridium(III) complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 288, 122188. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.; Kong, L.; Wang, L.; Niu, D.; Wang, J.; Leung, C.-H. Synthesis and luminescence monitoring of iridium(III) complex-functionalized gold nanoparticles and their application for determination of gold(III) ions. Microchim. Acta 2023, 190, 171. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, K.; Yuan, X.; Xie, X.; Zhan, Z.; Lv, Y. Novel near-infrared iridium(III) complex for chemiluminescence imaging of hypochlorous acid. Anal. Chem. 2023, 95, 8310–8317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, J.; Yang, K.; Liu, J.; Ma, D.-L.; Leung, C.-H.; Wang, W. Development of a NIR iridium(III) complex for self-calibrated and luminogenic detection of boron trifluoride. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 282, 121658. [Google Scholar] [CrossRef] [PubMed]
- Nao, S.-C.; Kong, L.; Chan, D.S.-H.; Liu, J.; Huang, L.-S.; Wu, L.; Wu, J.; Wong, C.-Y.; Wang, W.; Leung, C.-H. Covalent inhibition of epidermal growth factor receptor using a long-lived iridium(III)-afatinib probe. Int. J. Biol. Macromol. 2024, 259, 129211. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, F.; Song, Y.-Q.; Nao, S.-C.; Chan, D.S.-H.; Wong, C.-Y.; Wang, W.; Leung, C.-H. A glycyrrhetinic acid-iridium(III) conjugate as a theranostic NIR probe for hepatocellular carcinoma with mitochondrial-targeting ability. Eur. J. Med. Chem. 2024, 264, 115995. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Huyan, Y.; Li, H.; Sun, S.; Xu, Y. Reaction-based fluorescent probes for Hg2+, Cu2+ and Fe3+/Fe2+. Coord. Chem. Rev. 2021, 426, 213580. [Google Scholar] [CrossRef]
- BloodChen, Y.; Zhang, H. Complexation facilitated reduction of aromatic N-oxides by aqueous Fe(II)-tiron complex: Reaction kinetics and mechanisms. Environ. Sci. Technol. 2013, 47, 11023–11031. [Google Scholar]
- Colombo, A.; Dragonetti, C.; Guerchais, V.; Hierlinger, C.; Zysman-Colman, E.; Roberto, D. A trip in the nonlinear optical properties of iridium complexes. Coord. Chem. Rev. 2020, 414, 213293. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated iridium(III) complexes for life science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Pettinari, C.; Condello, F.; Petrini, A.; Scopelliti, R.; Riedel, T.; Dyson, P.J. Organometallic rhodium(iii) and iridium(iii) cyclopentadienyl complexes with curcumin and bisdemethoxycurcumin co-ligands. Dalton Trans. 2015, 44, 20523–20531. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Arachchi, D.T.; Wijesekera, G.; De Costa, M.; Senthilnithy, R. Amino and chloro derivatives of 1, 10-phenanthroline as turn-off fluorescence sensors for selective and sensitive detection of Fe(II). J. Photochem. Photobiol. A Chem. 2020, 402, 112805. [Google Scholar]
- Saini, P.; Singh, G.; Kaur, G.; Singh, J.; Singh, H. Copper (I)-catalyzed ‘Quick Click’ generated 1, 2, 3-triazole anthraquinone linkers for selective detection of Fe(II) ions. Inorg. Chem. Commun. 2022, 141, 109524. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mergu, N.; Kumawat, L.K. A new multifunctional rhodamine-derived probe for colorimetric sensing of Cu(II) and Al(III) and fluorometric sensing of Fe(III) in aqueous media. Sens. Actuators B Chem. 2016, 223, 101–113. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Kamaci, M.; Peksel, A. A dual responsive colorimetric sensor based on polyazomethine and ascorbic acid for the detection of Al (III) and Fe (II) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119650. [Google Scholar]
- Yang, C.; Xu, G.; Hou, C.; Peng, L.; Wang, W.; Zhang, H.; Zhang, X. A dual-mode nanoprobe based on silicon nanoparticles and Fe (II)-phenanthroline for the colorimetric and fluorescence determination of nitrite. Microchim. Acta 2023, 190, 318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).