Quantifying Plant Signaling Pathways by Integrating Luminescence-Based Biosensors and Mathematical Modeling
Abstract
1. Introduction
2. Material and Methods
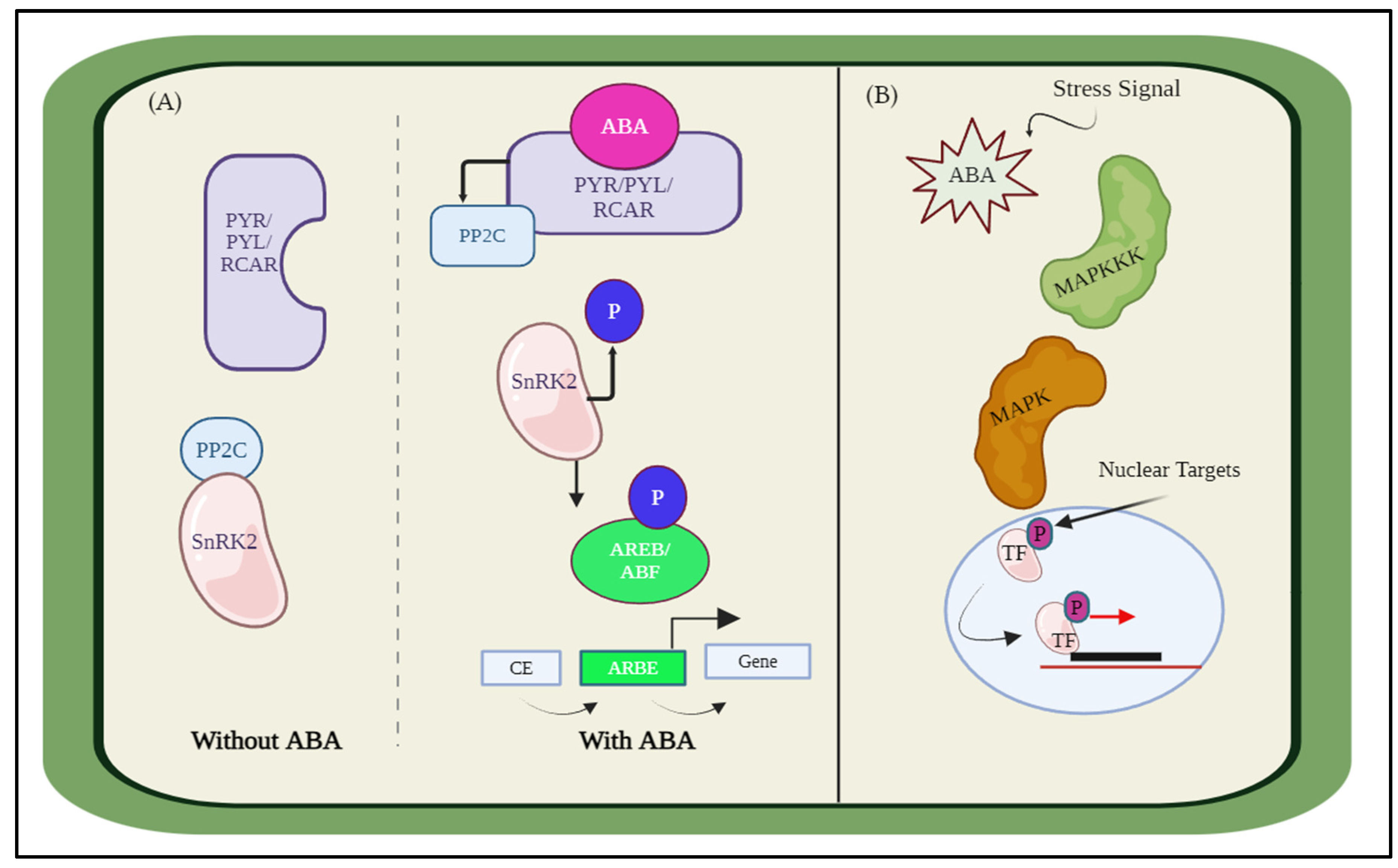
2.1. Simulating ABA Interaction with PP2C/SnRK2/MAPK
- i.
- ii.
- The rate constants “K1”, “K2”, and “K3” are typical parameters used in ODE models [19,20,47] for chemical reactions and biological processes [48]. The constants “K1”, “K2”, and “K3” describe critical processes within our model. “K1” represents the rate constant for ABA production, “K2” accounts for the degradation of ABA, and “K3” describes the rate of ABA binding to and dissociating from its receptor. These constants are crucial for accurately modeling the dynamic behavior of ABA within the plant system.
- iii.
- The interaction constants “kinteract_SNRK2”, “kinteract_PP2C”, and “kinteract_MAPK” represent the interaction rates between the respective proteins. These are often determined experimentally or estimated based on similar systems [49].
- iv.
- The initial concentrations (“CABA_0”, “SNRK2_0”, “PP2C_0”, “MAPK_0”) and the time span (“tspan”) are specific to the system being modeled and can be adjusted as needed.
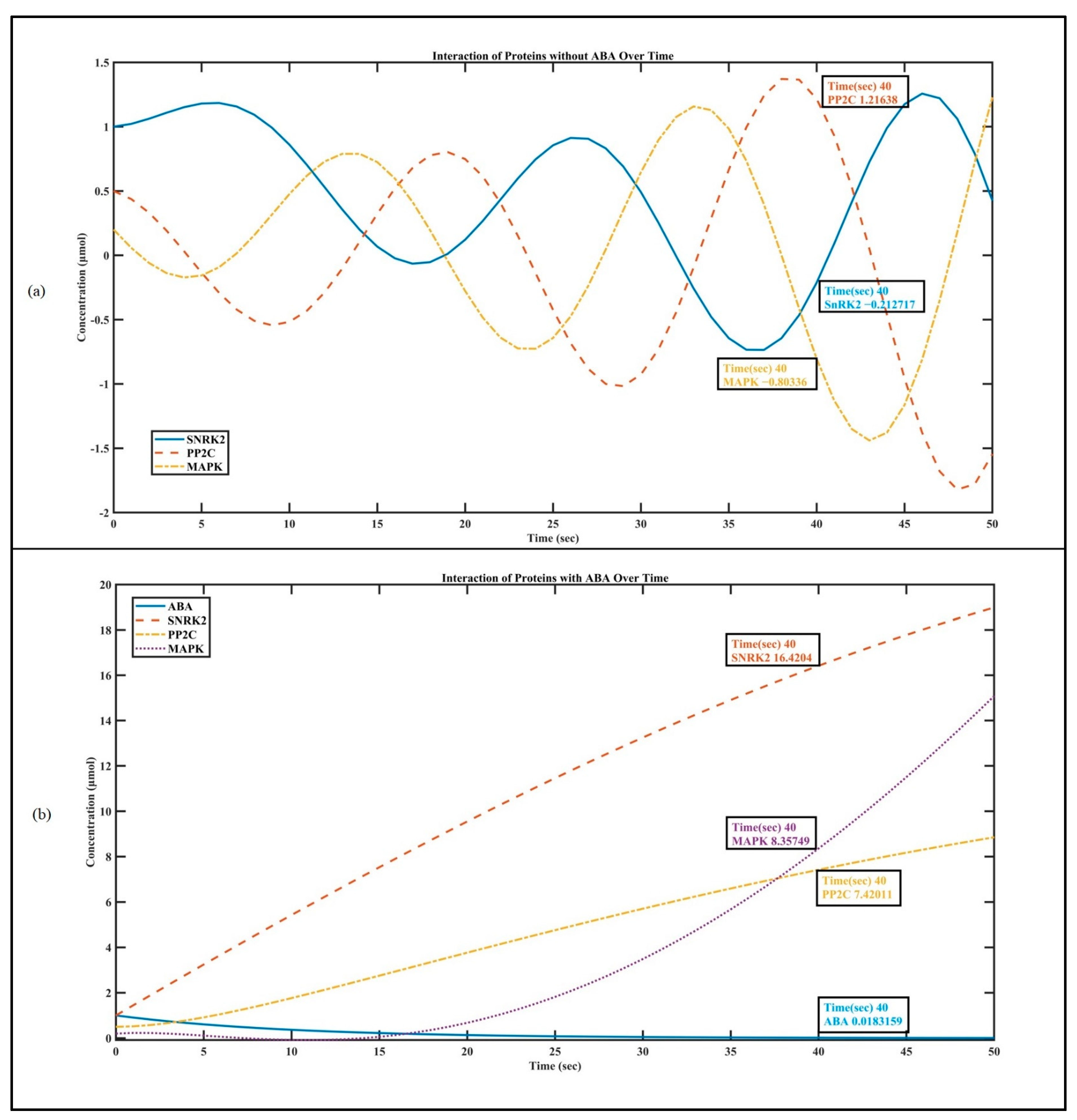
2.1.1. Protein Interaction with ABA
2.1.2. Protein Interaction without ABA
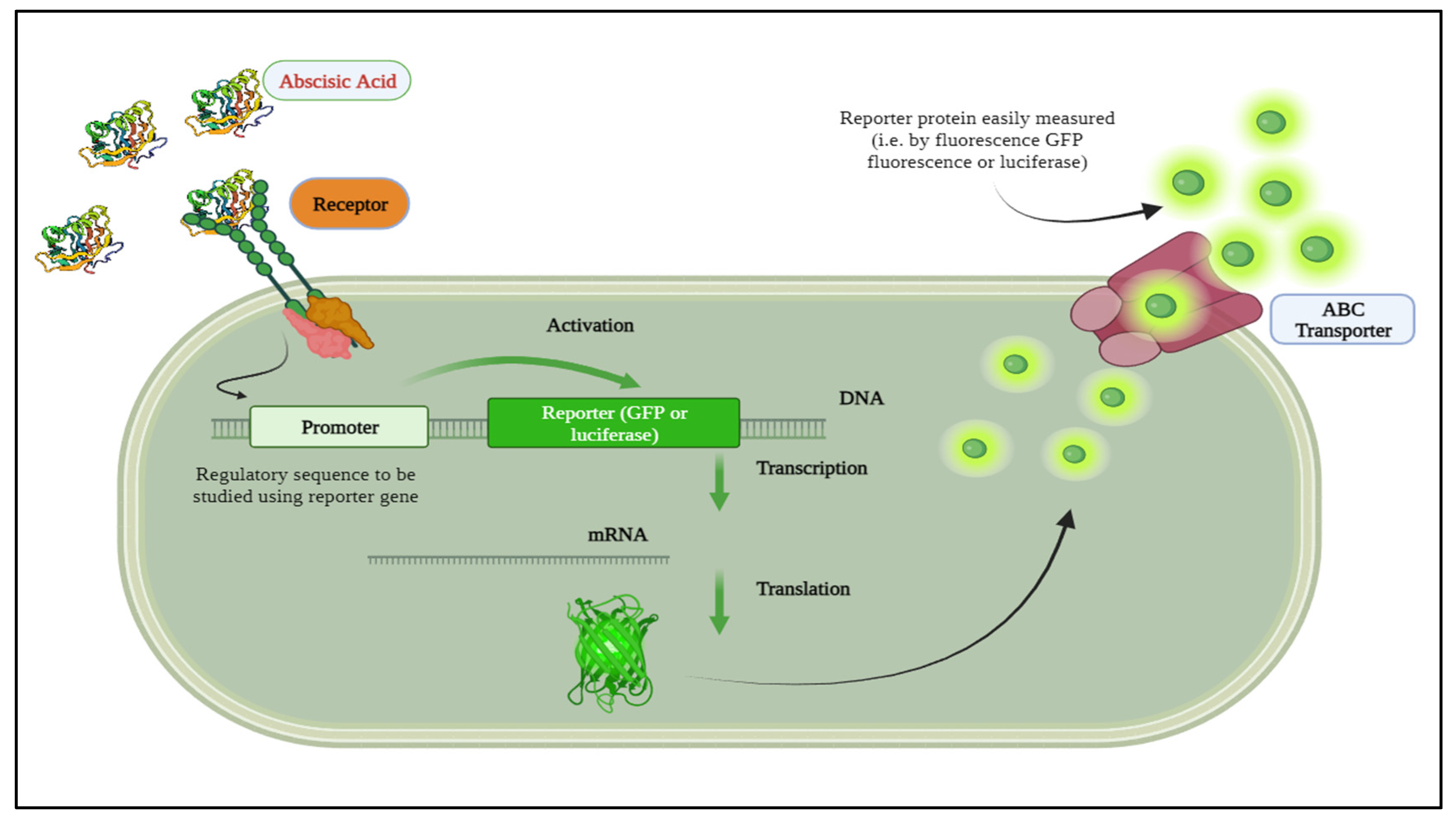
2.2. Integrating Biological Sensors with Mathematical Modeling of Plant Signaling for Improved Understanding of Plant Signaling
Establishing the Relationship between ABA Signaling, Mathematical Model, and Biological Sensor
3. Results
3.1. Visualizing the Interaction of SnRK2, PP2C and MAPK with ABA
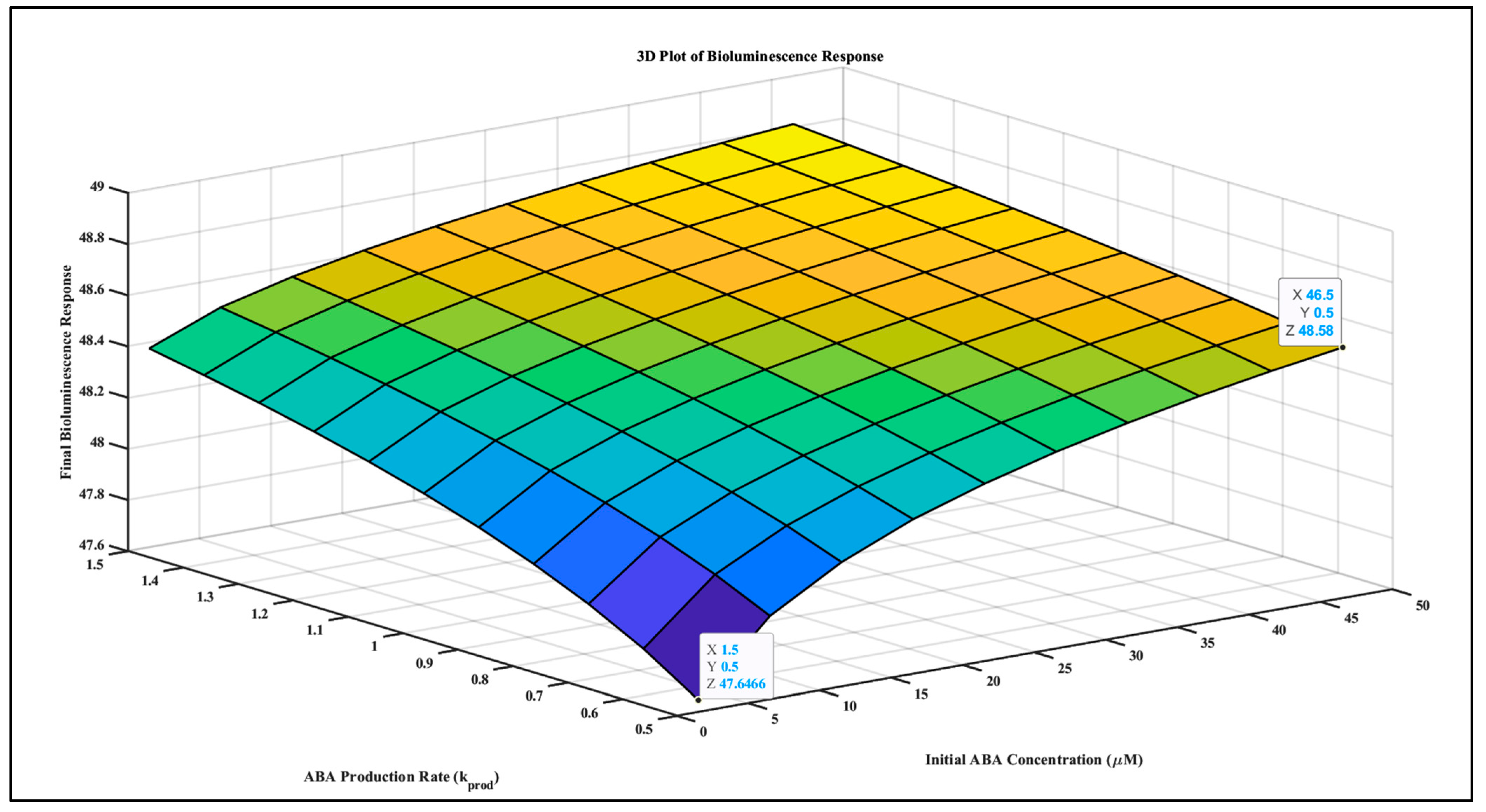
3.2. Simulating the Relationship between ABA Signaling and Bioluminescent Sensors (ABA-Luminescence Model)
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Novaković, L.; Guo, T.; Bacic, A.; Sampathkumar, A.; Johnson, K.L. Hitting the wall—Sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Świeżawska-Boniecka, B.; Duszyn, M.; Kwiatkowski, M.; Szmidt-Jaworska, A.; Jaworski, K. Cross talk between cyclic nucleotides and calcium signaling pathways in plants–achievements and prospects. Front. Plant Sci. 2021, 12, 643560. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E.J. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Wani, S.H.; Razzaq, A.; Skalicky, M.; Samantara, K.; Gupta, S.; Pandita, D.; Goel, S.; Grewal, S.; Hejnak, V.; et al. Abscisic acid: Role in fruit development and ripening. Front. Plant Sci. 2022, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: From experiments to systems modeling, and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Grieneisen, V.A.; Scheres, B. Back to the future: Evolution of computational models in plant morphogenesis. Curr. Opin. Plant Biol. 2009, 12, 606–614. [Google Scholar] [CrossRef]
- Mähönen, A.P.; Tusscher, K.t.; Siligato, R.; Smetana, O.; Díaz-Triviño, S.; Salojärvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. PLETHORA gradient formation mechanism separates auxin responses. Nature 2014, 515, 125–129. [Google Scholar] [CrossRef]
- Samodelov, S.L.; Zurbriggen, M.D. Quantitatively understanding plant signaling: Novel theoretical–experimental approaches. Trends Plant Sci. 2017, 22, 685–704. [Google Scholar] [CrossRef]
- Klipp, E.; Liebermeister, W. Mathematical modeling of intracellular signaling pathways. BMC Neurosci. 2006, 7, S10. [Google Scholar] [CrossRef]
- Karanam, A.; Rappel, W.-J. Boolean modelling in plant biology. Quant. Plant Biol. 2022, 3, e29. [Google Scholar] [CrossRef] [PubMed]
- Smithers, E.T.; Luo, J.; Dyson, R.J. Mathematical principles and models of plant growth mechanics: From cell wall dynamics to tissue morphogenesis. J. Exp. Bot. 2019, 70, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Holzheu, P.; Kummer, U. Computational systems biology of cellular processes in Arabidopsis thaliana: An overview. Cell. Mol. Life Sci. 2020, 77, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; DeAngelis, D.L. An overview of agent-based models in plant biology and ecology. Ann. Bot. 2020, 126, 539–557. [Google Scholar] [CrossRef]
- Bassel, G.W.; Gaudinier, A.; Brady, S.M.; Hennig, L.; Rhee, S.Y.; De Smet, I. Systems analysis of plant functional, transcriptional, physical interaction, and metabolic networks. Plant Cell 2012, 24, 3859–3875. [Google Scholar] [CrossRef]
- Beguerisse-Dıaz, M.; Hernández-Gómez, M.; Lizzul, A.; Barahona, M.; Desikan, R. Compound stress response in stomatal closure: A mathematical model of ABA and ethylene interaction in guard cells. BMC Syst. Biol. 2012, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Assmann, S.M.; Albert, R. Predicting essential components of signal transduction networks: A dynamic model of guard cell abscisic acid signaling. PLoS Biol. 2006, 4, e312. [Google Scholar] [CrossRef]
- Hoops, S.; Hontecillas, R.; Abedi, V.; Leber, A.; Philipson, C.; Carbo, A.; Bassaganya-Riera, J. Chapter 5—Ordinary Differential Equations (ODEs) Based Modeling. In Computational Immunology; Bassaganya-Riera, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 63–78. [Google Scholar]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, D.; Nigam, V.K. Chapter 19—Insight into microbial biosensors: Design, types and applications. In Bioprospecting of Microbial Diversity; Verma, P., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 425–440. [Google Scholar]
- Beltrán, J.; Steiner, P.J.; Bedewitz, M.; Wei, S.; Peterson, F.C.; Li, Z.; Hughes, B.E.; Hartley, Z.; Robertson, N.R.; Medina-Cucurella, A.V.; et al. Rapid biosensor development using plant hormone receptors as reprogrammable scaffolds. Nat. Biotechnol. 2022, 40, 1855–1861. [Google Scholar] [CrossRef]
- Meighen, E.A. Molecular biology of bacterial bioluminescence. Microbiol. Rev. 1991, 55, 123–142. [Google Scholar] [CrossRef]
- Calvache, C.; Vazquez-Vilar, M.; Moreno-Giménez, E.; Orzaez, D. A quantitative autonomous bioluminescence reporter system with a wide dynamic range for Plant Synthetic Biology. Plant Biotechnol. J. 2024, 22, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Stevenson, B.; Lee, B.H.; Zhu, J.K. Screening for gene regulation mutants by bioluminescence imaging. Sci. STKE Signal Transduct. Knowl. Environ. 2002, 2002, pl10. [Google Scholar] [CrossRef] [PubMed]
- de Ruijter, N.C.A.; Verhees, J.; van Leeuwen, W.; van der Krol, A.R. Evaluation and Comparison of the GUS, LUC and GFP Reporter System for Gene Expression Studies in Plants. Plant Biol. 2003, 5, 103–115. [Google Scholar] [CrossRef]
- Dyussembayev, K.; Sambasivam, P.; Bar, I.; Brownlie, J.C.; Shiddiky, M.J.A.; Ford, R. Biosensor Technologies for Early Detection and Quantification of Plant Pathogens. Front. Chem. 2021, 9, 636245. [Google Scholar] [CrossRef] [PubMed]
- Subodh; Ravina; Priyanka; Narang, J.; Mohan, H. Biosensors for phytohormone Abscisic acid and its role in humans: A review. Sens. Int. 2023, 4, 100234. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, G.; Hassan, M.M.; Abraham, P.E.; Mitchell, J.C.; Jacobson, D.; Tuskan, G.A.; Khakhar, A.; Medford, J.; Zhao, C.; et al. Biological and Molecular Components for Genetically Engineering Biosensors in Plants. Biodesign Res. 2022, 2022, 9863496. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, B.; Xu, Y.; Yang, Y.; Ji, J.; Cao, W.; Lu, J.; Ding, J.; Cao, H.; Chu, B.; et al. In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter. Nat. Commun. 2023, 14, 2331. [Google Scholar] [CrossRef]
- Jones, A.M.; Grossmann, G.; Danielson, J.Å.; Sosso, D.; Chen, L.-Q.; Ho, C.-H.; Frommer, W.B. In vivo biochemistry: Applications for small molecule biosensors in plant biology. Curr. Opin. Plant Biol. 2013, 16, 389–395. [Google Scholar] [CrossRef][Green Version]
- Roda, A. Chemiluminescence and Bioluminescence: Past, Present and Future; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Carrasco-Lopez, C.; Lui, N.M.; Schramm, S.; Naumov, P. The elusive relationship between structure and colour emission in beetle luciferases. Nat. Rev. Chem. 2021, 5, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Love, A.C.; Prescher, J.A. Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology. Cell Chem. Biol. 2020, 27, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, C.M.; Prescher, J.A. Bioluminescent Probes for Imaging Biology beyond the Culture Dish. Biochemistry 2017, 56, 5178–5184. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.F.; Geroni, G.M.; Lloyd, D.; Choi, H.; Clark, N.; Pirog, A.; Lees, J.; Porch, A. Bioluminescence of Vibrio fischeri: Bacteria respond quickly and sensitively to pulsed microwave electric (but not magnetic) fields. J. Microbiol. Methods 2019, 24, 051412. [Google Scholar] [CrossRef] [PubMed]
- Strack, R. Building up bioluminescence. Nat. Methods 2019, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Badr, C.E.; Tannous, B.A. Bioluminescence imaging: Progress and applications. Trends Biotechnol. 2011, 29, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Sadanandom, A.; Napier, R.M. Biosensors in plants. Curr. Opin. Plant Biol. 2010, 13, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.M.; Laplaze, L.; Bennett, M.J.; Vernoux, T. Biosensors for phytohormone quantification: Challenges, solutions, and opportunities. Trends Plant Sci. 2013, 18, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Wang, Y.; Liu, X.; Ma, L.; Zhang, Z.; Mu, C.; Zhang, Y.; Peng, L.; Xie, S.; et al. Initiation and amplification of SnRK2 activation in abscisic acid signaling. Nat. Commun. 2021, 12, 2456. [Google Scholar] [CrossRef]
- Danquah, A.; De Zélicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Lolkema, J.S.; Slotboom, D.J. The Hill analysis and co-ion-driven transporter kinetics. J. Gen. Physiol. 2015, 145, 565–574. [Google Scholar] [CrossRef]
- Weiss, J.N. The Hill equation revisited: Uses and misuses. FASEB J. 1997, 11, 835–841. [Google Scholar] [CrossRef]
- Klipp, E.; Liebermeister, W.; Wierling, C.; Kowald, A. Systems Biology: A Textbook; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Schreiber, G.; Haran, G.; Zhou, H.-X. Fundamental aspects of protein—Protein association kinetics. Chem. Rev. 2009, 109, 839–860. [Google Scholar] [CrossRef]
- Zhao, N.; Song, Y.; Xie, X.; Zhu, Z.; Duan, C.; Nong, C.; Wang, H.; Bao, R. Synthetic biology-inspired cell engineering in diagnosis, treatment, and drug development. Signal Transduct. Target. Ther. 2023, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Tindall, M.J.; Porter, S.L.; Maini, P.K.; Gaglia, G.; Armitage, J.P. Overview of Mathematical Approaches Used to Model Bacterial Chemotaxis I: The Single Cell. Bull. Math. Biol. 2008, 70, 1525–1569. [Google Scholar] [CrossRef]
- Mitchell, A.; Pilpel, Y. A mathematical model for adaptive prediction of environmental changes by microorganisms. Proc. Natl. Acad. Sci. USA 2011, 108, 7271–7276. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.H.; Smith, R.W.; Jones, H.J.; Seaton, D.D.; Grima, R.; Halliday, K.J. Mathematical models light up plant signaling. Plant Cell 2014, 26, 5–20. [Google Scholar] [CrossRef]
- Stone, J.D.; Chervin, A.S.; Kranz, D.M. T-cell receptor binding affinities and kinetics: Impact on T-cell activity and specificity. Immunology 2009, 126, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-X.; Bates, P.A. Modeling protein association mechanisms and kinetics. Curr. Opin. Struct. Biol. 2013, 23, 887–893. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef]
- Hewage, K.A.H.; Yang, J.F.; Wang, D.; Hao, G.F.; Yang, G.F.; Zhu, J.K. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef] [PubMed]
- Sirichandra, C.; Gu, D.; Hu, H.C.; Davanture, M.; Lee, S.; Djaoui, M.; Valot, B.; Zivy, M.; Leung, J.; Merlot, S.; et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009, 583, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Naqvi, S.M.Z.A.; Hussain, F.; Awais, M.; Ren, Y.; Wu, J.; Zhang, H.; Zang, Y.; Hu, J. Quantifying Plant Signaling Pathways by Integrating Luminescence-Based Biosensors and Mathematical Modeling. Biosensors 2024, 14, 378. https://doi.org/10.3390/bios14080378
Ahmed S, Naqvi SMZA, Hussain F, Awais M, Ren Y, Wu J, Zhang H, Zang Y, Hu J. Quantifying Plant Signaling Pathways by Integrating Luminescence-Based Biosensors and Mathematical Modeling. Biosensors. 2024; 14(8):378. https://doi.org/10.3390/bios14080378
Chicago/Turabian StyleAhmed, Shakeel, Syed Muhammad Zaigham Abbas Naqvi, Fida Hussain, Muhammad Awais, Yongzhe Ren, Junfeng Wu, Hao Zhang, Yiheng Zang, and Jiandong Hu. 2024. "Quantifying Plant Signaling Pathways by Integrating Luminescence-Based Biosensors and Mathematical Modeling" Biosensors 14, no. 8: 378. https://doi.org/10.3390/bios14080378
APA StyleAhmed, S., Naqvi, S. M. Z. A., Hussain, F., Awais, M., Ren, Y., Wu, J., Zhang, H., Zang, Y., & Hu, J. (2024). Quantifying Plant Signaling Pathways by Integrating Luminescence-Based Biosensors and Mathematical Modeling. Biosensors, 14(8), 378. https://doi.org/10.3390/bios14080378








